User login
The experts debate: Perioperative beta-blockade for noncardiac surgery—proven safe or not?
NOTE: The individual co-authors in this debate-based article are responsible only for those views within their respective bylined subsections and those views ascribed to them in the rebuttals and discussion at the end.
Perioperative beta-blockade improves outcomes
By Don Poldermans, MD, PhD
It is my contention that perioperative beta-blockade improves mortality and cardiac outcomes in select high- and intermediate-risk patients undergoing noncardiac surgery. Patients on chronic beta-blocker therapy should have it continued perioperatively. For patients not already on beta-blockade who are at cardiac risk, initiation of low-dose beta-blocker therapy should be considered prior to surgery; such therapy should be started approximately 1 month before surgery, with dose titration to achieve hemodynamic stability. Reports of increased stroke rates with perioperative beta-blockade appear to be due to inappropriate acute administration of high-dose beta-blocker therapy.
THE PHYSIOLOGIC RATIONALE FOR PERIOPERATIVE BETA-BLOCKADE
Perioperative myocardial infarction (MI) can occur by one of two mechanisms, both of which can be attenuated by beta-blockade:
- The stress induced by surgery can cause an asymptomatic coronary plaque to become unstable and rupture, resulting in complete occlusion of a portion of the coronary tree. This type of perioperative MI occurs typically in patients with multiple risk factors for MI absent a critical coronary stenosis. The perioperative risk associated with unstable plaque can be reduced pharmacologically with aspirin, statins, and chronic beta-blocker therapy.
- Alternately, a fixed coronary stenosis can predispose to a mismatch of oxygen demand and supply, leading to myocardial ischemia and infarction. The patient with a fixed coronary lesion typically presents with stable angina pectoris, and the at-risk stenosis is identified through a stress echocardiogram or nuclear scan. The risk conferred by flow-limiting stable plaque can be reduced by coronary revascularization and a short course of beta-blocker therapy prior to surgery.
INITIAL SUPPORTIVE DATA
Mangano and colleagues were the first to evaluate perioperative beta-blockade in a randomized, controlled fashion.1,2 In their study of 200 surgical patients with or at risk for coronary artery disease, oral atenolol administered perioperatively was associated with a 50% reduction (compared with placebo) in the incidence of postoperative myocardial ischemia as measured by three-lead Holter monitoring.2 During 2 years of follow-up, mortality was significantly lower in the atenolol group (10%) than in the placebo group (21%) (P = .019).1
In the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE I), my research group randomized 112 high-risk patients (as identified by dobutamine echocardiography) to standard perioperative care alone or standard perioperative care plus bisoprolol starting 30 days prior to major vascular surgery.3 The dosage of bisoprolol was titrated to achieve a target heart rate of 60 to 70 beats per minute. Thirty days after surgery, the incidence of the primary end point—a composite of death from cardiac causes or nonfatal MI—was reduced from 34% in the standard-care group to 3.4% in the bisoprolol group (P < .001). Thus, in this unblinded study in a population with proven coronary artery disease, beta-blockade clearly improved outcomes.
Additional studies of perioperative beta-blocker use have produced a wide range of outcomes, with most favoring beta-blockade, albeit usually not to a statistically significant degree.4–13 Notably, only some of these trials were randomized, they used various beta-blocker regimens at various doses, they were conducted in patients with varying degrees of cardiac risk, and many had small sample sizes.
What emerged from these trials was the idea that perioperative beta-blockade in patients with coronary artery disease produces an effect similar to that of long-term beta-blockade in reducing the risk of cardiovascular events in post-MI patients and in those with coronary artery disease and heart failure.
THE POISE STUDY AND ITS IMPLICATIONS
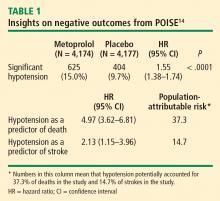
Results of the Perioperative Ischemic Evaluation (POISE) were published in 2008, in which 8,351 noncardiac surgery patients with or at risk of atherosclerotic disease were randomized to placebo or extended-release metoprolol succinate started 2 to 4 hours preoperatively and continued for 30 days.14 Metoprolol was associated with a clear reduction in the primary end point, a composite of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest (5.8% vs 6.9% with placebo; hazard ratio [HR] = 0.84 [95% CI, 0.70–0.99]; P = .0399), but this effect was offset by significant increases in total mortality and stroke incidence in the metoprolol group. Mortality was 3.1% with metoprolol versus 2.3% with placebo (HR = 1.33 [95% CI, 1.03–1.74]; P = .0317), and stroke incidence was 1.0% with metoprolol versus 0.5% with placebo (HR = 2.17 [95% CI, 1.26–3.74]; P = .0053). Cerebral infarction, not bleeding, explained most of the excess mortality with metoprolol.
Of the 60 strokes in POISE, 49 were ischemic in origin, 3 were hemorrhagic, and 8 were of uncertain etiology. Preoperative predictors of stroke were the use of clopidogrel and a history of stroke or transient ischemic attack. Postoperative predictors of stroke included intraoperative bleeding and intraoperative hypotension. These predictors suggest a diseased cerebrovascular tree or unstable hemodynamics during the intraoperative period in the patients who suffered a stroke.
Does dosing explain the rise in mortality and strokes?
Could the fatal outcomes associated with the beta-blocker in POISE be attributed to the dosage of metoprolol? In the study, 100 mg of metoprolol was started immediately prior to surgery, and an additional 100 mg could be given, depending on the hemodynamic response. Maintenance therapy (200 mg/day) was started on the same day, making it possible that a patient could have received as much as 400 mg of metoprolol the day of surgery. The starting dose of metoprolol used in POISE was two to eight times the commonly prescribed dose.
The initial 100-mg dose of metoprolol used in POISE has a similar beta1-receptor blockade potency compared with the 5-mg dose of bisoprolol used in DECREASE I.3 However, in DECREASE I, bisoprolol was initiated 30 days prior to surgery and was titrated, if necessary, according to heart rate. The maintenance dose of bisoprolol was half of the maintenance dose used in POISE. In the later DECREASE trials, the starting dose of bisoprolol was only 2.5 mg. Therefore, there was a huge difference in beta-blocker dosing between POISE and DECREASE.
What about timing of beta-blocker initiation?
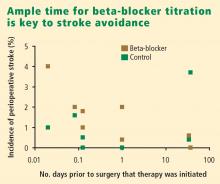
The POISE findings may also be explained in part by the timing of beta-blocker initiation. Whereas bisoprolol was carefully titrated for 30 days before surgery in DECREASE, metoprolol was initiated just before surgery in POISE, and the maximum recommended dose may have been prescribed during the first 24 hours, although subsequent dosing was 200 mg daily, which is 50% of the maximum daily therapeutic dose. This extremely narrow time window for titration may be important, since the beneficial effects of beta-blockade on coronary plaque stability are likely to take weeks to develop.
Reassurance from a large case-control study
My colleagues and I conducted a case-control study from among more than 75,000 patients who underwent noncardiac, nonvascular surgery at our institution, Erasmus Medical Center, from 1991 to 2001.18 The cases were the 989 patients who died in the hospital postoperatively; the controls were 1,879 survivors matched with the cases for age, sex, the year the surgery was performed, and the type of surgery. The incidence of perioperative stroke was 0.5%, which is comparable to the rate found in the literature. Risk factors predictive of stroke were the presence of diabetes, cerebrovascular disease, peripheral arterial disease, atrial fibrillation, coronary artery disease, and hypertension. Notably, no relationship was found between chronic beta-blocker use and stroke.
WHAT ABOUT PATIENTS AT INTERMEDIATE RISK?
Because the effect of perioperative beta-blockade has traditionally been ill defined in surgical patients at intermediate risk of cardiovascular events, the DECREASE study group recently completed a study (DECREASE IV) to assess perioperative bisoprolol in terms of cardiac morbidity and mortality in intermediate-risk patients undergoing elective noncardiovascular surgery.17 Enrollees had a score of 1 to 2 on the Revised Cardiac Risk Index of Lee et al,19 which corresponds to an estimated risk of between 1% and 6% for a perioperative cardiovascular event.17
DECREASE IV also aimed to assess the effect of perioperative fluvastatin, so a 2 x 2 factorial design was used in which the study’s 1,066 patients were randomized to receive bisoprolol, fluvastatin, combination treatment, or combination placebo control. Bisoprolol was initiated up to 30 days prior to surgery, and the 2.5-mg daily starting dosage was titrated according to the patient’s heart rate to achieve a target rate of 50 to 70 beats per minute. Fluvastatin was also started up to 30 days prior to surgery. Patients who received bisoprolol (with or without fluvastatin) had a significant reduction in the 30-day incidence of cardiac death and nonfatal MI compared with those who did not receive bisoprolol (2.1% vs 6.0%; HR = 0.34 [95% CI, 0.17–0.67]; P = .002). Fluvastatin was associated with a favorable trend on this end point, but statistical significance was not achieved (P = .17).17
There was no difference among treatment groups in the incidence of stroke (4 strokes in the 533 patients who received bisoprolol vs 3 strokes in the 533 patients who did not),17 which further suggests that the increased stroke rate seen with beta-blockade in POISE may have been due to dosage, timing of initiation, or both.
CONCLUSIONS
Dose-related hypotension may explain POISE findings
Our understanding of postoperative stroke is incomplete, but it appears that dosing of a beta-blocker can be a contributor, especially with respect to the potential side effect of hypotension during surgery. Keep in mind that the average age of patients in POISE was approximately 70 years and that patients were naïve to beta-blockers. Some may have had asymptomatic left ventricular dysfunction, and we know that starting a beta-blocker at a high dose in such patients may lead to hypotension. At my institution we routinely perform echocardiographic screening of all patients scheduled for surgery, and we have found that more than half of the patients with heart failure have it uncovered only through this screening.
It is not the medicine alone that can cause perioperative hypotension; other factors may induce hypotension, requiring beta-blocker titration and careful monitoring of hemodynamics during surgery.
Advice: Start early and titrate dose; continue chronic beta-blockade
My advice is as follows:
- If a patient is on chronic beta-blocker therapy, do not stop it perioperatively. We have seen devastating outcomes in the Netherlands when patients had their beta-blockers stopped immediately before surgery. Consider adjusting the dose, but do not stop it entirely. If a beta-blocker is on board and the patient develops hypotension or bradycardia during surgery, treat the symptoms and check for sepsis.
- In a patient not on a beta-blocker, consider adding one if the patient is at intermediate or high risk of a cardiac event, but start at a low dosage (ie, 2.5 mg/day for bisoprolol and 25 mg/day for metoprolol). Treatment ideally should be started 30 days preoperatively; in the Netherlands, we have the chance to start well in advance of surgery so we can titrate the dose according to hemodynamics.
- If a beta-blocker is not started because of insufficient time for titration, do not add one to treat tachycardia that develops during surgery, since tachycardia may represent a response to normal defense mechanisms.
Safety of perioperative beta-blocker use has not been adequately demonstrated
By P.J. Devereaux, MD, PhD
I contend that perioperative beta-blockade is a practice not grounded in evidence-based medicine, and its overall safety has increasingly come into question as more data from large, high-quality trials have emerged. I will begin with a historical overview of perioperative beta-blocker use, review the results of the POISE trial (for which I was the co-principal investigator), explore the major questions raised by this trial, and conclude with some take-away messages.
THE HISTORY OF PERIOPERATIVE BETA-BLOCKADE
In the 1970s, physicians were encouraged to hold beta-blockers prior to surgery out of concern that these medications may inhibit the required cardiovascular response when patients developed hypotension, and could thereby lead to serious adverse consequences.
In the 1980s, new research associated tachycardia with perioperative cardiovascular events, leading to proposals to implement perioperative beta-blocker use.
In the 1990s, two randomized trials with a total sample size of 312 patients1,3 suggested that perioperative beta-blockers had a large treatment effect in preventing major cardiovascular events and death. These small trials had several methodological limitations:
- One trial3 was unblinded in a setting in which the vast majority of MIs are clinically silent.
- One trial3 was stopped early—after randomizing only 112 patients—for unexpected large treatment effects.
- One of the studies1 failed to follow intention-to-treat principle.
Nevertheless, guidelines developed at the time by the American College of Cardiology and the American Heart Association (ACC/AHA) recommended the use of perioperative beta-blockers on the basis of the physiological rationale and these two small clinical trials. That recommendation was retained in the latest (2007) update of the ACC/AHA perioperative guidelines.20
In 2006, two clinical trials with a total sample size of 1,417 were completed,4,15 surpassing the total size of previous trials by more than fourfold. These two more recent trials did not suffer from the methodological limitations of earlier trials. These trials showed no benefit of perioperative beta-blocker use; in fact, there was a trend toward worse outcomes in the beta-blocker recipients.4,15 Despite these new data, guidelines committees continued to recommend perioperative beta-blockade.20
THE POISE TRIAL
Study design
This was the context into which the POISE results were released in 2008. POISE was a randomized, controlled, blinded trial of patients 45 years or older scheduled for noncardiac surgery who had, or were at high risk of, atherosclerotic disease.14 The intervention consisted of metoprolol succinate (metoprolol controlled release [CR]) or placebo started 2 to 4 hours preoperatively (if heart rate was ≥ 50 beats per minute and systolic blood pressure [SBP] was ≥ 100 mm Hg) and continued for 30 days. The target dosage of metoprolol was 200 mg once daily. No patients received the recommended maximum dosage of 400 mg over 24 hours. The main outcome measure was a 30-day composite of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest.
We randomized 9,298 patients in a 1:1 ratio to metoprolol or placebo. We encountered data fraud at a number of centers that prompted exclusion of data from 474 patients allocated to metoprolol and 473 allocated to placebo. Therefore, the total number of patients available for the intention-to-treat analysis was 8,351, from 190 centers in 23 countries.
Results
The risk of the primary composite outcome was reduced by 16% (relative reduction) in recipients of metoprolol CR compared with placebo recipients (P = .0399). Significantly fewer nonfatal MIs occurred in the metoprolol CR group than in the placebo group (152 [3.6%] vs 215 [5.1%]; P = .0008), leaving little doubt that perioperative beta-blockade prevents MI.
In contrast, total mortality was increased in the beta-blocker group, with 129 deaths among those assigned to metoprolol CR and 97 among those assigned to placebo (P = .0317), and the incidence of stroke was also significantly greater in the metoprolol CR group (1.0% vs. 0.5%; P = .0053).
Consistency with findings from other trials
The POISE data are consistent with those from a 2008 meta-analysis of high-quality randomized controlled trials in noncardiac surgery patients, which showed a significantly greater risk of death among patients assigned to a beta-blocker than among controls who were not (160 deaths [2.8%] vs 127 deaths [2.3%]; odds ratio [OR] = 1.27 [95% CI, 1.01–1.61]).21 This meta-analysis also found a significantly greater risk of nonfatal stroke in beta-blocker recipients compared with controls (38 [0.7%] vs 17 [0.3%]; OR = 2.16 [95% CI, 1.27–3.68]).
I also contend that the DECREASE IV trial supports the POISE findings in that although few strokes were encountered in DECREASE IV, the trend was in the direction of harm in the beta-blocker group, which had 4 strokes among 533 patients versus 3 strokes among 533 patients not receiving the beta-blocker.17
Predictive role of hypotension
The link between hypotension and death in POISE is consistent with findings from the largest beta-blocker trial undertaken, COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial), in which 45,852 patients with acute MI were randomized to metoprolol or placebo.22 In COMMIT, metoprolol had no effect on 30-day all-cause mortality but significantly reduced the risk of arrhythmic death, a benefit that was countered by a significantly increased risk of death from shock with a beta-blocker in acute MI. Clinically significant hypotension is much more common in the perioperative setting than in acute MI, which may explain the excess number of deaths observed with metoprolol in POISE as opposed to metoprolol’s neutral effect on mortality in COMMIT.
ANSWERING THE CRITICS
Several criticisms have been raised about POISE, as detailed below.
Beta-blocker dose
Some contend that a lower dose of beta-blocker would provide benefit and minimize risk, but this assertion must be supported by evidence from a large clinical trial. The targeted dosage of metoprolol in POISE represents 50% of the maximum daily therapeutic dose. Further, the protocol called for decreasing the dosage to 100 mg/day if SBP dropped to less than 100 mm Hg or if heart rate fell to less than 45 beats per minute.
The two small trials on which guideline recommendations for perioperative beta-blockade are primarily based1,3 had a sample size that was 4% of that in POISE, which calls into question the reliability of their results. The study by Mangano et al used atenolol at a target dosage that was 50% of the maximum daily therapeutic dose,1 the same as with metoprolol in POISE. DECREASE initiated bisoprolol at 25% of the maximum daily therapeutic dose, and allowed for titration to 50% of the maximum daily therapeutic dose.3
As the second largest study of perioperative beta-blockade, the Diabetic Postoperative Mortality and Morbidity (DIPOM) trial enrolled 921 patients who were assigned to placebo or controlled-release metoprolol with a target dosage that was 25% of the maximum daily therapeutic dose.4 The 30-day outcomes from DIPOM showed a trend toward an excess of death and stroke despite using only one-half the dosage in POISE and the same dosage as in DECREASE.
Timing of beta-blocker initiation
Another contention is that earlier beta-blocker initiation would be better. The issue with timing of initiation is not benefit, as POISE showed that starting a beta-blocker hours before surgery results in a reduction in the risk of MI. The issue is whether giving a beta-blocker earlier makes administering the drug safer. Nearly 10% of placebo recipients in POISE developed clinically significant hypotension, which suggests that the titrated dosage of a beta-blocker that appears effective preoperatively is unlikely to inform a safe dose after surgery, when hypotension is common.
The practicality of titrating the dose of beta-blocker prior to surgery also comes into play. Most patients referred to my institution for surgery are seen 1 to 2 weeks in advance, at the earliest. Real-world practice at present simply does not afford us the luxury of seeing patients three to four times before surgery in order to titrate the beta-blocker dose.
POISE did not address chronic beta-blocker therapy
It is important to remember that POISE excluded patients on chronic beta-blocker therapy and thus did not attempt to address the perioperative management of such patients who undergo noncardiac surgery. My suspicion is that perioperative continuation of beta-blockade in these patients is the best course of action, but this too has not been studied robustly, so we need a large controlled trial to confirm that this practice is indeed safe.
CONCLUSIONS
The POISE results suggest that for every 1,000 patients treated, perioperative metoprolol would:
- Prevent 15 MIs, 3 cardiac revascularizations, and 7 new cases of atrial fibrillation
- Result in 8 excess deaths, 5 strokes, 53 cases of clinically significant hypotension, and 42 cases of clinically significant bradycardia.
The central take-away message is that patients are unlikely to want a perioperative beta-blocker if they are unwilling to accept a probable increase in mortality or if they place three times more value on avoiding a perioperative stroke than on avoiding an MI.
It has been 10 years since the recommendation to use perioperatove beta-blockers was incorporated into perioperative practice guidelines. Assuming only 10% of physicians acted on this recommendation, 100 million patients have received a perioperative beta-blocker over this time as a result. If the POISE results are applicable, a full 800,000 of these patients died and another 500,000 suffered perioperative strokes as a result of being given a beta-blocker. This issue is not to be taken lightly, given the evidence to suggest harm.
Though it is possible that an alternative beta-blocker regimen to the one used in POISE may provide benefit without substantial harm, the data suggest this is not probable. The POISE data highlight the risk of making assumptions, as well as the importance of and need for large, high-quality randomized trials in the perioperative setting.
It is time for perioperative medicine to enter the age of evidence-based practice and embrace one of its central tenets: only large trials are reliable when it comes to therapeutic questions.
Rebuttals and discussion
POLDERMANS REBUTTAL: MORE TRANSPARENCY NEEDED IN POISE DOSING DATA
Dr. Poldermans: The initial paper describing the POISE trial design did indeed indicate that it was possible for a patient to receive 400 mg of metoprolol on the first day of treatment. We need to see the actual doses of metoprolol given to all patients in POISE who had a perioperative stroke. If you show me these data, the issue will be much easier to discuss.
Our data from randomized trials are consistent in showing that a titrated dosing regimen using bisoprolol reduces the incidence of postoperative cardiac events with no increase in the number of strokes.
My take-home message is that if you want to use beta-blockers, use them sensibly, use them carefully, and act during surgery. If many of your patients are developing hypotension, then you are doing something wrong.
DEVEREAUX REBUTTAL: A SHIFT IN THINKING IS REQUIRED
Dr. Devereaux: The data from POISE are fully available, and I take issue with Dr. Poldermans’ contention that a patient could have received as much as 400 mg of metoprolol CR on the day of surgery; this was not an option according to protocol. I believe his statement is misleading in the same way that it is misleading to indicate that in the DECREASE trial patients may have received 20 mg of bisoprolol within 24 hours of surgery. It is possible that a patient in DECREASE could have gone to surgery at 2:00 pm and may have taken his or her bisoprolol at 10:00 am that morning. The following morning (in the hospital), it is possible that the patient would have received his or her bisoprolol 10 mg at 7:00 or 8:00 am (ie, 20 mg within 24 hours of surgery). Although this is possible and something similar could have happened within POISE, it does not reflect a patient receiving an effective dose of metoprolol CR 400 mg or bisoprolol 20 mg over a 24-hour period.
I worry about the distortion of reality in perioperative medicine that leads so many of us to believe that randomization is magical despite small sample sizes. Small randomized trials are at profound risk of imbalance between the randomized groups, whether we see it or not, and the results are therefore simply not reliable.
Unless we shift our thinking, we make ourselves susceptible to overconfidence in the benefits of a certain intervention before the data from large clinical trials become available. In the meantime, as we have seen from POISE, an intervention may have negative consequences that are not apparent from small clinical trials.
The reality of excess stroke with perioperative beta-blockers is consistent across all the trials. It does not mean that we cannot find another way to give beta-blockers safely, but if we want to establish safety, we need a large trial that unequivocally demonstrates safety, as opposed to simply using observational data, retrospective cohorts, or comparisons between two nonrandomized trials. Until we have large data sets, it is very difficult to say that we can give beta-blockers safely.
DISCUSSION WITH THE AUDIENCE
Moderator*: Dr. Devereaux, was the hypotension in POISE related to the long-acting beta-blocker itself or to the large dose of if that was used? Similarly, were the strokes a result of the drug itself or of the hypotension?
Dr. Devereaux: I must take issue with your premise that the dose of metoprolol used in POISE was “large.” As I noted, Mangano’s study used its beta-blocker (atenolol) at 50% of its maximum daily therapeutic dose,1 the same proportion used in POISE, and Dr. Poldermans’ own DECREASE trial allowed titration of bisoprolol up to 50% of the maximum daily therapeutic dose.3 The DIPOM trial used half the dose of metoprolol that we used, yet it too yielded a trend toward more death and stroke in the beta-blocker group.4 So it’s not that the dose we used was at some excessive level. At the same time, that does not mean that a smaller dose may not have achieved a similarly significant benefit in cardiac outcomes.
We can’t explain most of the strokes. Because most strokes were ischemic, I suspect that the explanation may lie in the threshold used to define clinically significant hypotension. We used an SBP cutoff of less than 90 mm Hg, but we did not classify large drops in SBP, such as from 180 to 95 mm Hg, as clinically significant hypotension. The high incidence of clinically significant hypotension in the placebo group—about 10%—suggests that hypotension was likely the driving factor for stroke. The beta-blocker exacerbated the hypotension, but its more important effect may have been that it made it harder for the body to overcome the hypotension. That is the exact same signal observed in the COMMIT trial in the setting of acute MI.22
Dr. Poldermans: I’d like to see the intraoperative blood pressure data for the 60 patients who suffered strokes in POISE. We could then find out exactly when the hypotension occurred, what kind of hypotension it was, what the patient’s initial blood pressure reading was, and so on. If we had access to this information, we could determine which occurred first—the hypotension or the stroke.
Dr. Devereaux: Although trials can indicate a signal, they can’t explain with certainty the pathway through which the outcome occurred. For example, we know that beta-blockers prevent MI, but we don’t know how. What’s most impressive about the stroke issue is the consistency across all the perioperative beta-blocker trials: every one shows a direction of excess stroke with beta-blockers.
Question from the audience: The patient groups studied in DECREASE and POISE were different. DECREASE studied a very high-risk vascular surgery group with known coronary artery disease on the basis of echocardiography. POISE included patients undergoing emergency surgery and patients with sepsis. Can you describe the outcomes in POISE solely among the patients who underwent elective vascular surgery, similar to the patients studied in DECREASE?
Dr. Devereaux: In terms of the benefit to bisoprolol in very high-risk patients in DECREASE, remember that it was a study of 112 patients. That’s far too small a trial to establish safety or efficacy. The benefit of perioperative beta-blockade in preventing MI is unequivocal because it’s consistent across all trials. But the real issue is, was it safe?
Interestingly, in POISE, the groups at highest risk looked like they benefited the least, not the most. The notion of targeting high-risk people is not supported by POISE; if anything, the POISE results went in the direction of harm with beta-blockade in high-risk patients. That being said, the P value for interaction is not statistically significant, but it’s heading in the direction of harm. So I wouldn’t take comfort in believing that if we simply target high-risk patients, beta-blockers become safe.
Question from the audience: I believe that the seven or eight studies that showed higher stroke rates with beta-blockers all gave beta-blockade within 24 hours of surgery. Only in DECREASE was it given days and weeks in advance of surgery. Can you comment?
Dr. Poldermans: There’s clearly a relation between the time of beta-blocker initiation and the incidence of stroke. If you look at the randomized trials, you see an increased incidence of stroke in patients in whom beta-blockers are started just prior to surgery but not in patients who are on chronic beta-blockers. In our case-control study,18 we screened more than 185,000 patients for stroke and could not detect an increased incidence of stroke in those on chronic beta-blocker therapy. So stroke indeed has something to do with starting beta-blockers just before surgery.
Dr. Devereaux: In DECREASE IV, bisoprolol was started up to 1 month before surgery, yet there were 4 strokes in the bisoprolol group versus 3 in the control group.17
Dr. Poldermans: Yes, but that difference is not statistically meaningful.
Comment from the audience: I’m uncomfortable with the way Dr. Devereaux stresses the importance of significant findings from large randomized trials but then quibbles about a stroke rate of 4 versus 3, which is not statistically significant. Keep it scientific: either there is or there isn’t a P value that achieves significance.
Though I congratulate you on a great trial, any resident in my program would be fired for pursuing your strategy of perioperative care in POISE, which included using an SBP of 100 mm Hg as the threshold for stopping the beta-blocker regardless of preoperative blood pressure. An SBP of 100 mm Hg is not the definition of hypotension. Most anesthesiologists and perioperative physicians peg the beta-blockade to a reasonable level based on the preoperative blood pressure. They titrate in fluids and titrate in the beta-blocker. Certainly the timing is an issue—we don’t recommend giving it right before the operation.
Dr. Devereaux: I referred to the stroke rates in DECREASE IV because Dr. Poldermans has claimed that the excess in strokes has occurred in all trials but his, yet DECREASE IV was one of his trials and also one in which bisoprolol was started early. I’m not claiming statistical significance between the 3 versus 4 strokes in DECREASE IV, but the stroke trend is in the same direction as in the other trials, so it tells us nothing with regard to safety. My point is, has anyone proven safety? As much as we’d like to imagine that beta-blockers are safe perioperatively—and maybe they are—it has not been proven. The largest trials at present are consistent with a signal toward harm.
It’s easy to criticize the methodology after a trial is done. A number of us came together and thought we had a reasonable protocol for POISE. We found a reduction in the incidence of MI but more strokes and higher mortality with perioperative metoprolol. Does this mean there is not a safe way to give a beta-blocker and derive the benefit? Of course not. But at the moment the evidence does not support a way to give a beta-blocker safely. Do we need to find a way to give it safely? Of course we do.
For example, the design of POISE II is factorial, looking at aspirin versus placebo as well as clonidine versus placebo. There are a number of factors about clonidine that suggest we might be able to achieve the benefits we saw in POISE and avoid the risk, but until we do a large trial, we’re not going to know.
Comment from the audience: It’s important that we clearly understand the conclusion from POISE. It’s not that the administration of beta-blockers is not safe. It’s that the administration of a beta-blocker, as your methodology applied it, was not totally safe. Those are two very different conclusions.
Dr. Devereaux: I would say the conclusion is that the way that beta-blockers are being given has not been proven to be safe. The result is consistent. It’s even consistent with DECREASE IV.
Moderator: I believe in large clinical trials, but they must apply to real-life practice. POISE did not address the practices of many people in this room, particularly regarding the doses of metoprolol used. So it is hard for us to apply the findings in clinical practice. Those of you who design large trials need to think through your trial design very thoroughly, considering the millions of dollars that go into these trials. It’s not fair to clinicians to do a study that may have little clinical relevance.
Dr. Devereaux: Investigators from 190 centers in 23 countries were involved in POISE, and all of them thought we had a reasonable approach and methodology. That doesn’t mean it was perfect, yet the criticisms we are hearing now did not surface while the trial was ongoing.
Comment from the audience: My hospital in Australia contributed to the POISE study. When it started 6 or 7 years ago, the cardiologists were using beta-blockers liberally and haphazardly. It was a huge challenge to convince them that conducting the trial was justifiable—that the case for perioperative beta-blockers had not been absolutely and overwhelmingly proved. They wanted to put beta-blockers in the water supply at the doses we’re talking about.
It would be interesting to do separate analyses of the data from the various countries involved in POISE. In Australia, the percentage of the population on chronic beta-blockers—who therefore would have been ineligible for the trial—is now quite high. Most patients who need to be on beta-blockers long term are on them, whereas that was not the case 15 years ago. The population is changing even while we’re doing the trials. Australian cardiologists are no longer putting every patient on perioperative beta-blockers; they’re thinking about it first.
Dr. Devereaux: A compelling feature of POISE as an international trial is its consistency of outcomes across the planet. No matter where we looked, the outcomes were consistent: in Asia, Europe, North America, and Australia.
Moderator: Would each of you summarize your take-home message for clinicians?
Dr. Devereaux: I urge clinicians to actually read the trials themselves rather than just relying on the advice of guideline writers. It’s important not to allow ourselves to become entrenched in a practice without evidence, just because we’ve done it for so long. If you told your patients the number of MIs prevented and the potential number of excess strokes and deaths, I suspect the average patient would conclude it’s not a great trade-off.
Remember that two-thirds of MIs in the perioperative setting are clinically silent. That doesn’t mean they’re not important, but the strokes, in contrast, are profoundly devastating. One-third of patients with stroke in POISE were dead within 30 days, and of those who survived, 60% were incapacitated, needing help with everyday activities.
I encourage clinicians to read the POISE manuscript with a fresh perspective, regardless of how you’ve practiced until now. Then ask yourself whether you really are comfortable with the safety of perioperative beta-blockers at this time. Of course, that doesn’t mean the evidence won’t change in the future.
Dr. Poldermans: The main imperative is to improve postoperative care. We strongly believe that perioperative beta-blockers work in the general population. If you have a patient who needs to be on a beta-blocker after surgery, why not start it preoperatively? I believe that dosing and timing of initiation are important. If you have the opportunity to start the beta-blocker prior to surgery, do so at a reasonable dose and start early. Patients in whom beta-blockers are started immediately prior to surgery may be worse off, with a higher incidence of stroke.
*Amir K. Jaffer, MD, University of Miami Miller School of Medicine, served as moderator of the debate and the discussion period.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery: Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia: McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery: Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative b-blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Brady AR, Gibbs JS, Greenhalgh RM, et al. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Cucchiara RF, Benefiel DJ, Matteo RS, DeWood M, Albin MS. Evaluation of esmolol in controlling increases in heart rate and blood pressure during endotracheal intubation in patients undergoing carotid endarterectomy. Anesthesiology 1986; 65:528–531.
- Magnusson J, Thulin T, Werner O, Järhult J, Thomson D. Haemodynamic effects of pretreatment with metoprolol in hypertensive patients undergoing surgery. Br J Anaesth 1986; 58:251–260.
- Stone JG, Foëx P, Sear JW, et al. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Jakobsen CJ, Bille S, Ahlburg P, et al. Perioperative metoprolol reduces the frequency of atrial fibrillation after thoracotomy for lung resection. J Cardiothorac Vasc Anesth 1997; 11:746–751.
- Bayliff CD, Massel DR, Inculet RI, et al. Propranolol for the prevention of postoperative arrhythmias in general thoracic surgery. Ann Thorac Surg 1999; 67:182–186.
- Raby KE, Brull SJ, Timimi F, et al. The effect of heart rate control on myocardial ischemia among high-risk patients after vascular surgery. Anesth Analg 1999; 88:477–482.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Urban MK, Markowitz SM, Gordon MA, et al. Postoperative prophylactic administration of beta-adrenergic blockers in patients at risk for myocardial ischemia. Anesth Analg 2000; 90:1257–1261.
- POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Zaugg M, Bestman L, Wacker J, et al. Adrenergic receptor genotype but not perioperative bisoprolol therapy may determine cardiovascular outcome in at-risk patients undergoing surgery with spinal block: the Swiss Beta Blocker in Spinal Anesthesia (BBSA) study. Anesthesiology 2007; 107:33–44.
- Dunkelgrun M, Boersma E, Schouten O, et al. Bisoprolol and fluvastatin for reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiac surgery. A randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- Noordzij PG, Poldermans D, Schouten O, et al. Beta-blockers and statins are individually associated with reduced mortality in patients undergoing noncardiac, nonvascular surgery. Coron Artery Dis 2007; 18:67–72.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:1707–1732.
- Bangalore S, Wetterslev J, Pranesh S, et al. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
NOTE: The individual co-authors in this debate-based article are responsible only for those views within their respective bylined subsections and those views ascribed to them in the rebuttals and discussion at the end.
Perioperative beta-blockade improves outcomes
By Don Poldermans, MD, PhD
It is my contention that perioperative beta-blockade improves mortality and cardiac outcomes in select high- and intermediate-risk patients undergoing noncardiac surgery. Patients on chronic beta-blocker therapy should have it continued perioperatively. For patients not already on beta-blockade who are at cardiac risk, initiation of low-dose beta-blocker therapy should be considered prior to surgery; such therapy should be started approximately 1 month before surgery, with dose titration to achieve hemodynamic stability. Reports of increased stroke rates with perioperative beta-blockade appear to be due to inappropriate acute administration of high-dose beta-blocker therapy.
THE PHYSIOLOGIC RATIONALE FOR PERIOPERATIVE BETA-BLOCKADE
Perioperative myocardial infarction (MI) can occur by one of two mechanisms, both of which can be attenuated by beta-blockade:
- The stress induced by surgery can cause an asymptomatic coronary plaque to become unstable and rupture, resulting in complete occlusion of a portion of the coronary tree. This type of perioperative MI occurs typically in patients with multiple risk factors for MI absent a critical coronary stenosis. The perioperative risk associated with unstable plaque can be reduced pharmacologically with aspirin, statins, and chronic beta-blocker therapy.
- Alternately, a fixed coronary stenosis can predispose to a mismatch of oxygen demand and supply, leading to myocardial ischemia and infarction. The patient with a fixed coronary lesion typically presents with stable angina pectoris, and the at-risk stenosis is identified through a stress echocardiogram or nuclear scan. The risk conferred by flow-limiting stable plaque can be reduced by coronary revascularization and a short course of beta-blocker therapy prior to surgery.
INITIAL SUPPORTIVE DATA
Mangano and colleagues were the first to evaluate perioperative beta-blockade in a randomized, controlled fashion.1,2 In their study of 200 surgical patients with or at risk for coronary artery disease, oral atenolol administered perioperatively was associated with a 50% reduction (compared with placebo) in the incidence of postoperative myocardial ischemia as measured by three-lead Holter monitoring.2 During 2 years of follow-up, mortality was significantly lower in the atenolol group (10%) than in the placebo group (21%) (P = .019).1
In the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE I), my research group randomized 112 high-risk patients (as identified by dobutamine echocardiography) to standard perioperative care alone or standard perioperative care plus bisoprolol starting 30 days prior to major vascular surgery.3 The dosage of bisoprolol was titrated to achieve a target heart rate of 60 to 70 beats per minute. Thirty days after surgery, the incidence of the primary end point—a composite of death from cardiac causes or nonfatal MI—was reduced from 34% in the standard-care group to 3.4% in the bisoprolol group (P < .001). Thus, in this unblinded study in a population with proven coronary artery disease, beta-blockade clearly improved outcomes.
Additional studies of perioperative beta-blocker use have produced a wide range of outcomes, with most favoring beta-blockade, albeit usually not to a statistically significant degree.4–13 Notably, only some of these trials were randomized, they used various beta-blocker regimens at various doses, they were conducted in patients with varying degrees of cardiac risk, and many had small sample sizes.
What emerged from these trials was the idea that perioperative beta-blockade in patients with coronary artery disease produces an effect similar to that of long-term beta-blockade in reducing the risk of cardiovascular events in post-MI patients and in those with coronary artery disease and heart failure.
THE POISE STUDY AND ITS IMPLICATIONS
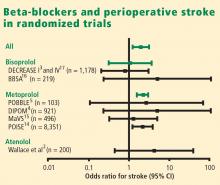
Results of the Perioperative Ischemic Evaluation (POISE) were published in 2008, in which 8,351 noncardiac surgery patients with or at risk of atherosclerotic disease were randomized to placebo or extended-release metoprolol succinate started 2 to 4 hours preoperatively and continued for 30 days.14 Metoprolol was associated with a clear reduction in the primary end point, a composite of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest (5.8% vs 6.9% with placebo; hazard ratio [HR] = 0.84 [95% CI, 0.70–0.99]; P = .0399), but this effect was offset by significant increases in total mortality and stroke incidence in the metoprolol group. Mortality was 3.1% with metoprolol versus 2.3% with placebo (HR = 1.33 [95% CI, 1.03–1.74]; P = .0317), and stroke incidence was 1.0% with metoprolol versus 0.5% with placebo (HR = 2.17 [95% CI, 1.26–3.74]; P = .0053). Cerebral infarction, not bleeding, explained most of the excess mortality with metoprolol.
Of the 60 strokes in POISE, 49 were ischemic in origin, 3 were hemorrhagic, and 8 were of uncertain etiology. Preoperative predictors of stroke were the use of clopidogrel and a history of stroke or transient ischemic attack. Postoperative predictors of stroke included intraoperative bleeding and intraoperative hypotension. These predictors suggest a diseased cerebrovascular tree or unstable hemodynamics during the intraoperative period in the patients who suffered a stroke.
Does dosing explain the rise in mortality and strokes?
Could the fatal outcomes associated with the beta-blocker in POISE be attributed to the dosage of metoprolol? In the study, 100 mg of metoprolol was started immediately prior to surgery, and an additional 100 mg could be given, depending on the hemodynamic response. Maintenance therapy (200 mg/day) was started on the same day, making it possible that a patient could have received as much as 400 mg of metoprolol the day of surgery. The starting dose of metoprolol used in POISE was two to eight times the commonly prescribed dose.
The initial 100-mg dose of metoprolol used in POISE has a similar beta1-receptor blockade potency compared with the 5-mg dose of bisoprolol used in DECREASE I.3 However, in DECREASE I, bisoprolol was initiated 30 days prior to surgery and was titrated, if necessary, according to heart rate. The maintenance dose of bisoprolol was half of the maintenance dose used in POISE. In the later DECREASE trials, the starting dose of bisoprolol was only 2.5 mg. Therefore, there was a huge difference in beta-blocker dosing between POISE and DECREASE.
What about timing of beta-blocker initiation?
The POISE findings may also be explained in part by the timing of beta-blocker initiation. Whereas bisoprolol was carefully titrated for 30 days before surgery in DECREASE, metoprolol was initiated just before surgery in POISE, and the maximum recommended dose may have been prescribed during the first 24 hours, although subsequent dosing was 200 mg daily, which is 50% of the maximum daily therapeutic dose. This extremely narrow time window for titration may be important, since the beneficial effects of beta-blockade on coronary plaque stability are likely to take weeks to develop.
Reassurance from a large case-control study
My colleagues and I conducted a case-control study from among more than 75,000 patients who underwent noncardiac, nonvascular surgery at our institution, Erasmus Medical Center, from 1991 to 2001.18 The cases were the 989 patients who died in the hospital postoperatively; the controls were 1,879 survivors matched with the cases for age, sex, the year the surgery was performed, and the type of surgery. The incidence of perioperative stroke was 0.5%, which is comparable to the rate found in the literature. Risk factors predictive of stroke were the presence of diabetes, cerebrovascular disease, peripheral arterial disease, atrial fibrillation, coronary artery disease, and hypertension. Notably, no relationship was found between chronic beta-blocker use and stroke.
WHAT ABOUT PATIENTS AT INTERMEDIATE RISK?
Because the effect of perioperative beta-blockade has traditionally been ill defined in surgical patients at intermediate risk of cardiovascular events, the DECREASE study group recently completed a study (DECREASE IV) to assess perioperative bisoprolol in terms of cardiac morbidity and mortality in intermediate-risk patients undergoing elective noncardiovascular surgery.17 Enrollees had a score of 1 to 2 on the Revised Cardiac Risk Index of Lee et al,19 which corresponds to an estimated risk of between 1% and 6% for a perioperative cardiovascular event.17
DECREASE IV also aimed to assess the effect of perioperative fluvastatin, so a 2 x 2 factorial design was used in which the study’s 1,066 patients were randomized to receive bisoprolol, fluvastatin, combination treatment, or combination placebo control. Bisoprolol was initiated up to 30 days prior to surgery, and the 2.5-mg daily starting dosage was titrated according to the patient’s heart rate to achieve a target rate of 50 to 70 beats per minute. Fluvastatin was also started up to 30 days prior to surgery. Patients who received bisoprolol (with or without fluvastatin) had a significant reduction in the 30-day incidence of cardiac death and nonfatal MI compared with those who did not receive bisoprolol (2.1% vs 6.0%; HR = 0.34 [95% CI, 0.17–0.67]; P = .002). Fluvastatin was associated with a favorable trend on this end point, but statistical significance was not achieved (P = .17).17
There was no difference among treatment groups in the incidence of stroke (4 strokes in the 533 patients who received bisoprolol vs 3 strokes in the 533 patients who did not),17 which further suggests that the increased stroke rate seen with beta-blockade in POISE may have been due to dosage, timing of initiation, or both.
CONCLUSIONS
Dose-related hypotension may explain POISE findings
Our understanding of postoperative stroke is incomplete, but it appears that dosing of a beta-blocker can be a contributor, especially with respect to the potential side effect of hypotension during surgery. Keep in mind that the average age of patients in POISE was approximately 70 years and that patients were naïve to beta-blockers. Some may have had asymptomatic left ventricular dysfunction, and we know that starting a beta-blocker at a high dose in such patients may lead to hypotension. At my institution we routinely perform echocardiographic screening of all patients scheduled for surgery, and we have found that more than half of the patients with heart failure have it uncovered only through this screening.
It is not the medicine alone that can cause perioperative hypotension; other factors may induce hypotension, requiring beta-blocker titration and careful monitoring of hemodynamics during surgery.
Advice: Start early and titrate dose; continue chronic beta-blockade
My advice is as follows:
- If a patient is on chronic beta-blocker therapy, do not stop it perioperatively. We have seen devastating outcomes in the Netherlands when patients had their beta-blockers stopped immediately before surgery. Consider adjusting the dose, but do not stop it entirely. If a beta-blocker is on board and the patient develops hypotension or bradycardia during surgery, treat the symptoms and check for sepsis.
- In a patient not on a beta-blocker, consider adding one if the patient is at intermediate or high risk of a cardiac event, but start at a low dosage (ie, 2.5 mg/day for bisoprolol and 25 mg/day for metoprolol). Treatment ideally should be started 30 days preoperatively; in the Netherlands, we have the chance to start well in advance of surgery so we can titrate the dose according to hemodynamics.
- If a beta-blocker is not started because of insufficient time for titration, do not add one to treat tachycardia that develops during surgery, since tachycardia may represent a response to normal defense mechanisms.
Safety of perioperative beta-blocker use has not been adequately demonstrated
By P.J. Devereaux, MD, PhD
I contend that perioperative beta-blockade is a practice not grounded in evidence-based medicine, and its overall safety has increasingly come into question as more data from large, high-quality trials have emerged. I will begin with a historical overview of perioperative beta-blocker use, review the results of the POISE trial (for which I was the co-principal investigator), explore the major questions raised by this trial, and conclude with some take-away messages.
THE HISTORY OF PERIOPERATIVE BETA-BLOCKADE
In the 1970s, physicians were encouraged to hold beta-blockers prior to surgery out of concern that these medications may inhibit the required cardiovascular response when patients developed hypotension, and could thereby lead to serious adverse consequences.
In the 1980s, new research associated tachycardia with perioperative cardiovascular events, leading to proposals to implement perioperative beta-blocker use.
In the 1990s, two randomized trials with a total sample size of 312 patients1,3 suggested that perioperative beta-blockers had a large treatment effect in preventing major cardiovascular events and death. These small trials had several methodological limitations:
- One trial3 was unblinded in a setting in which the vast majority of MIs are clinically silent.
- One trial3 was stopped early—after randomizing only 112 patients—for unexpected large treatment effects.
- One of the studies1 failed to follow intention-to-treat principle.
Nevertheless, guidelines developed at the time by the American College of Cardiology and the American Heart Association (ACC/AHA) recommended the use of perioperative beta-blockers on the basis of the physiological rationale and these two small clinical trials. That recommendation was retained in the latest (2007) update of the ACC/AHA perioperative guidelines.20
In 2006, two clinical trials with a total sample size of 1,417 were completed,4,15 surpassing the total size of previous trials by more than fourfold. These two more recent trials did not suffer from the methodological limitations of earlier trials. These trials showed no benefit of perioperative beta-blocker use; in fact, there was a trend toward worse outcomes in the beta-blocker recipients.4,15 Despite these new data, guidelines committees continued to recommend perioperative beta-blockade.20
THE POISE TRIAL
Study design
This was the context into which the POISE results were released in 2008. POISE was a randomized, controlled, blinded trial of patients 45 years or older scheduled for noncardiac surgery who had, or were at high risk of, atherosclerotic disease.14 The intervention consisted of metoprolol succinate (metoprolol controlled release [CR]) or placebo started 2 to 4 hours preoperatively (if heart rate was ≥ 50 beats per minute and systolic blood pressure [SBP] was ≥ 100 mm Hg) and continued for 30 days. The target dosage of metoprolol was 200 mg once daily. No patients received the recommended maximum dosage of 400 mg over 24 hours. The main outcome measure was a 30-day composite of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest.
We randomized 9,298 patients in a 1:1 ratio to metoprolol or placebo. We encountered data fraud at a number of centers that prompted exclusion of data from 474 patients allocated to metoprolol and 473 allocated to placebo. Therefore, the total number of patients available for the intention-to-treat analysis was 8,351, from 190 centers in 23 countries.
Results
The risk of the primary composite outcome was reduced by 16% (relative reduction) in recipients of metoprolol CR compared with placebo recipients (P = .0399). Significantly fewer nonfatal MIs occurred in the metoprolol CR group than in the placebo group (152 [3.6%] vs 215 [5.1%]; P = .0008), leaving little doubt that perioperative beta-blockade prevents MI.
In contrast, total mortality was increased in the beta-blocker group, with 129 deaths among those assigned to metoprolol CR and 97 among those assigned to placebo (P = .0317), and the incidence of stroke was also significantly greater in the metoprolol CR group (1.0% vs. 0.5%; P = .0053).
Consistency with findings from other trials
The POISE data are consistent with those from a 2008 meta-analysis of high-quality randomized controlled trials in noncardiac surgery patients, which showed a significantly greater risk of death among patients assigned to a beta-blocker than among controls who were not (160 deaths [2.8%] vs 127 deaths [2.3%]; odds ratio [OR] = 1.27 [95% CI, 1.01–1.61]).21 This meta-analysis also found a significantly greater risk of nonfatal stroke in beta-blocker recipients compared with controls (38 [0.7%] vs 17 [0.3%]; OR = 2.16 [95% CI, 1.27–3.68]).
I also contend that the DECREASE IV trial supports the POISE findings in that although few strokes were encountered in DECREASE IV, the trend was in the direction of harm in the beta-blocker group, which had 4 strokes among 533 patients versus 3 strokes among 533 patients not receiving the beta-blocker.17
Predictive role of hypotension
The link between hypotension and death in POISE is consistent with findings from the largest beta-blocker trial undertaken, COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial), in which 45,852 patients with acute MI were randomized to metoprolol or placebo.22 In COMMIT, metoprolol had no effect on 30-day all-cause mortality but significantly reduced the risk of arrhythmic death, a benefit that was countered by a significantly increased risk of death from shock with a beta-blocker in acute MI. Clinically significant hypotension is much more common in the perioperative setting than in acute MI, which may explain the excess number of deaths observed with metoprolol in POISE as opposed to metoprolol’s neutral effect on mortality in COMMIT.
ANSWERING THE CRITICS
Several criticisms have been raised about POISE, as detailed below.
Beta-blocker dose
Some contend that a lower dose of beta-blocker would provide benefit and minimize risk, but this assertion must be supported by evidence from a large clinical trial. The targeted dosage of metoprolol in POISE represents 50% of the maximum daily therapeutic dose. Further, the protocol called for decreasing the dosage to 100 mg/day if SBP dropped to less than 100 mm Hg or if heart rate fell to less than 45 beats per minute.
The two small trials on which guideline recommendations for perioperative beta-blockade are primarily based1,3 had a sample size that was 4% of that in POISE, which calls into question the reliability of their results. The study by Mangano et al used atenolol at a target dosage that was 50% of the maximum daily therapeutic dose,1 the same as with metoprolol in POISE. DECREASE initiated bisoprolol at 25% of the maximum daily therapeutic dose, and allowed for titration to 50% of the maximum daily therapeutic dose.3
As the second largest study of perioperative beta-blockade, the Diabetic Postoperative Mortality and Morbidity (DIPOM) trial enrolled 921 patients who were assigned to placebo or controlled-release metoprolol with a target dosage that was 25% of the maximum daily therapeutic dose.4 The 30-day outcomes from DIPOM showed a trend toward an excess of death and stroke despite using only one-half the dosage in POISE and the same dosage as in DECREASE.
Timing of beta-blocker initiation
Another contention is that earlier beta-blocker initiation would be better. The issue with timing of initiation is not benefit, as POISE showed that starting a beta-blocker hours before surgery results in a reduction in the risk of MI. The issue is whether giving a beta-blocker earlier makes administering the drug safer. Nearly 10% of placebo recipients in POISE developed clinically significant hypotension, which suggests that the titrated dosage of a beta-blocker that appears effective preoperatively is unlikely to inform a safe dose after surgery, when hypotension is common.
The practicality of titrating the dose of beta-blocker prior to surgery also comes into play. Most patients referred to my institution for surgery are seen 1 to 2 weeks in advance, at the earliest. Real-world practice at present simply does not afford us the luxury of seeing patients three to four times before surgery in order to titrate the beta-blocker dose.
POISE did not address chronic beta-blocker therapy
It is important to remember that POISE excluded patients on chronic beta-blocker therapy and thus did not attempt to address the perioperative management of such patients who undergo noncardiac surgery. My suspicion is that perioperative continuation of beta-blockade in these patients is the best course of action, but this too has not been studied robustly, so we need a large controlled trial to confirm that this practice is indeed safe.
CONCLUSIONS
The POISE results suggest that for every 1,000 patients treated, perioperative metoprolol would:
- Prevent 15 MIs, 3 cardiac revascularizations, and 7 new cases of atrial fibrillation
- Result in 8 excess deaths, 5 strokes, 53 cases of clinically significant hypotension, and 42 cases of clinically significant bradycardia.
The central take-away message is that patients are unlikely to want a perioperative beta-blocker if they are unwilling to accept a probable increase in mortality or if they place three times more value on avoiding a perioperative stroke than on avoiding an MI.
It has been 10 years since the recommendation to use perioperatove beta-blockers was incorporated into perioperative practice guidelines. Assuming only 10% of physicians acted on this recommendation, 100 million patients have received a perioperative beta-blocker over this time as a result. If the POISE results are applicable, a full 800,000 of these patients died and another 500,000 suffered perioperative strokes as a result of being given a beta-blocker. This issue is not to be taken lightly, given the evidence to suggest harm.
Though it is possible that an alternative beta-blocker regimen to the one used in POISE may provide benefit without substantial harm, the data suggest this is not probable. The POISE data highlight the risk of making assumptions, as well as the importance of and need for large, high-quality randomized trials in the perioperative setting.
It is time for perioperative medicine to enter the age of evidence-based practice and embrace one of its central tenets: only large trials are reliable when it comes to therapeutic questions.
Rebuttals and discussion
POLDERMANS REBUTTAL: MORE TRANSPARENCY NEEDED IN POISE DOSING DATA
Dr. Poldermans: The initial paper describing the POISE trial design did indeed indicate that it was possible for a patient to receive 400 mg of metoprolol on the first day of treatment. We need to see the actual doses of metoprolol given to all patients in POISE who had a perioperative stroke. If you show me these data, the issue will be much easier to discuss.
Our data from randomized trials are consistent in showing that a titrated dosing regimen using bisoprolol reduces the incidence of postoperative cardiac events with no increase in the number of strokes.
My take-home message is that if you want to use beta-blockers, use them sensibly, use them carefully, and act during surgery. If many of your patients are developing hypotension, then you are doing something wrong.
DEVEREAUX REBUTTAL: A SHIFT IN THINKING IS REQUIRED
Dr. Devereaux: The data from POISE are fully available, and I take issue with Dr. Poldermans’ contention that a patient could have received as much as 400 mg of metoprolol CR on the day of surgery; this was not an option according to protocol. I believe his statement is misleading in the same way that it is misleading to indicate that in the DECREASE trial patients may have received 20 mg of bisoprolol within 24 hours of surgery. It is possible that a patient in DECREASE could have gone to surgery at 2:00 pm and may have taken his or her bisoprolol at 10:00 am that morning. The following morning (in the hospital), it is possible that the patient would have received his or her bisoprolol 10 mg at 7:00 or 8:00 am (ie, 20 mg within 24 hours of surgery). Although this is possible and something similar could have happened within POISE, it does not reflect a patient receiving an effective dose of metoprolol CR 400 mg or bisoprolol 20 mg over a 24-hour period.
I worry about the distortion of reality in perioperative medicine that leads so many of us to believe that randomization is magical despite small sample sizes. Small randomized trials are at profound risk of imbalance between the randomized groups, whether we see it or not, and the results are therefore simply not reliable.
Unless we shift our thinking, we make ourselves susceptible to overconfidence in the benefits of a certain intervention before the data from large clinical trials become available. In the meantime, as we have seen from POISE, an intervention may have negative consequences that are not apparent from small clinical trials.
The reality of excess stroke with perioperative beta-blockers is consistent across all the trials. It does not mean that we cannot find another way to give beta-blockers safely, but if we want to establish safety, we need a large trial that unequivocally demonstrates safety, as opposed to simply using observational data, retrospective cohorts, or comparisons between two nonrandomized trials. Until we have large data sets, it is very difficult to say that we can give beta-blockers safely.
DISCUSSION WITH THE AUDIENCE
Moderator*: Dr. Devereaux, was the hypotension in POISE related to the long-acting beta-blocker itself or to the large dose of if that was used? Similarly, were the strokes a result of the drug itself or of the hypotension?
Dr. Devereaux: I must take issue with your premise that the dose of metoprolol used in POISE was “large.” As I noted, Mangano’s study used its beta-blocker (atenolol) at 50% of its maximum daily therapeutic dose,1 the same proportion used in POISE, and Dr. Poldermans’ own DECREASE trial allowed titration of bisoprolol up to 50% of the maximum daily therapeutic dose.3 The DIPOM trial used half the dose of metoprolol that we used, yet it too yielded a trend toward more death and stroke in the beta-blocker group.4 So it’s not that the dose we used was at some excessive level. At the same time, that does not mean that a smaller dose may not have achieved a similarly significant benefit in cardiac outcomes.
We can’t explain most of the strokes. Because most strokes were ischemic, I suspect that the explanation may lie in the threshold used to define clinically significant hypotension. We used an SBP cutoff of less than 90 mm Hg, but we did not classify large drops in SBP, such as from 180 to 95 mm Hg, as clinically significant hypotension. The high incidence of clinically significant hypotension in the placebo group—about 10%—suggests that hypotension was likely the driving factor for stroke. The beta-blocker exacerbated the hypotension, but its more important effect may have been that it made it harder for the body to overcome the hypotension. That is the exact same signal observed in the COMMIT trial in the setting of acute MI.22
Dr. Poldermans: I’d like to see the intraoperative blood pressure data for the 60 patients who suffered strokes in POISE. We could then find out exactly when the hypotension occurred, what kind of hypotension it was, what the patient’s initial blood pressure reading was, and so on. If we had access to this information, we could determine which occurred first—the hypotension or the stroke.
Dr. Devereaux: Although trials can indicate a signal, they can’t explain with certainty the pathway through which the outcome occurred. For example, we know that beta-blockers prevent MI, but we don’t know how. What’s most impressive about the stroke issue is the consistency across all the perioperative beta-blocker trials: every one shows a direction of excess stroke with beta-blockers.
Question from the audience: The patient groups studied in DECREASE and POISE were different. DECREASE studied a very high-risk vascular surgery group with known coronary artery disease on the basis of echocardiography. POISE included patients undergoing emergency surgery and patients with sepsis. Can you describe the outcomes in POISE solely among the patients who underwent elective vascular surgery, similar to the patients studied in DECREASE?
Dr. Devereaux: In terms of the benefit to bisoprolol in very high-risk patients in DECREASE, remember that it was a study of 112 patients. That’s far too small a trial to establish safety or efficacy. The benefit of perioperative beta-blockade in preventing MI is unequivocal because it’s consistent across all trials. But the real issue is, was it safe?
Interestingly, in POISE, the groups at highest risk looked like they benefited the least, not the most. The notion of targeting high-risk people is not supported by POISE; if anything, the POISE results went in the direction of harm with beta-blockade in high-risk patients. That being said, the P value for interaction is not statistically significant, but it’s heading in the direction of harm. So I wouldn’t take comfort in believing that if we simply target high-risk patients, beta-blockers become safe.
Question from the audience: I believe that the seven or eight studies that showed higher stroke rates with beta-blockers all gave beta-blockade within 24 hours of surgery. Only in DECREASE was it given days and weeks in advance of surgery. Can you comment?
Dr. Poldermans: There’s clearly a relation between the time of beta-blocker initiation and the incidence of stroke. If you look at the randomized trials, you see an increased incidence of stroke in patients in whom beta-blockers are started just prior to surgery but not in patients who are on chronic beta-blockers. In our case-control study,18 we screened more than 185,000 patients for stroke and could not detect an increased incidence of stroke in those on chronic beta-blocker therapy. So stroke indeed has something to do with starting beta-blockers just before surgery.
Dr. Devereaux: In DECREASE IV, bisoprolol was started up to 1 month before surgery, yet there were 4 strokes in the bisoprolol group versus 3 in the control group.17
Dr. Poldermans: Yes, but that difference is not statistically meaningful.
Comment from the audience: I’m uncomfortable with the way Dr. Devereaux stresses the importance of significant findings from large randomized trials but then quibbles about a stroke rate of 4 versus 3, which is not statistically significant. Keep it scientific: either there is or there isn’t a P value that achieves significance.
Though I congratulate you on a great trial, any resident in my program would be fired for pursuing your strategy of perioperative care in POISE, which included using an SBP of 100 mm Hg as the threshold for stopping the beta-blocker regardless of preoperative blood pressure. An SBP of 100 mm Hg is not the definition of hypotension. Most anesthesiologists and perioperative physicians peg the beta-blockade to a reasonable level based on the preoperative blood pressure. They titrate in fluids and titrate in the beta-blocker. Certainly the timing is an issue—we don’t recommend giving it right before the operation.
Dr. Devereaux: I referred to the stroke rates in DECREASE IV because Dr. Poldermans has claimed that the excess in strokes has occurred in all trials but his, yet DECREASE IV was one of his trials and also one in which bisoprolol was started early. I’m not claiming statistical significance between the 3 versus 4 strokes in DECREASE IV, but the stroke trend is in the same direction as in the other trials, so it tells us nothing with regard to safety. My point is, has anyone proven safety? As much as we’d like to imagine that beta-blockers are safe perioperatively—and maybe they are—it has not been proven. The largest trials at present are consistent with a signal toward harm.
It’s easy to criticize the methodology after a trial is done. A number of us came together and thought we had a reasonable protocol for POISE. We found a reduction in the incidence of MI but more strokes and higher mortality with perioperative metoprolol. Does this mean there is not a safe way to give a beta-blocker and derive the benefit? Of course not. But at the moment the evidence does not support a way to give a beta-blocker safely. Do we need to find a way to give it safely? Of course we do.
For example, the design of POISE II is factorial, looking at aspirin versus placebo as well as clonidine versus placebo. There are a number of factors about clonidine that suggest we might be able to achieve the benefits we saw in POISE and avoid the risk, but until we do a large trial, we’re not going to know.
Comment from the audience: It’s important that we clearly understand the conclusion from POISE. It’s not that the administration of beta-blockers is not safe. It’s that the administration of a beta-blocker, as your methodology applied it, was not totally safe. Those are two very different conclusions.
Dr. Devereaux: I would say the conclusion is that the way that beta-blockers are being given has not been proven to be safe. The result is consistent. It’s even consistent with DECREASE IV.
Moderator: I believe in large clinical trials, but they must apply to real-life practice. POISE did not address the practices of many people in this room, particularly regarding the doses of metoprolol used. So it is hard for us to apply the findings in clinical practice. Those of you who design large trials need to think through your trial design very thoroughly, considering the millions of dollars that go into these trials. It’s not fair to clinicians to do a study that may have little clinical relevance.
Dr. Devereaux: Investigators from 190 centers in 23 countries were involved in POISE, and all of them thought we had a reasonable approach and methodology. That doesn’t mean it was perfect, yet the criticisms we are hearing now did not surface while the trial was ongoing.
Comment from the audience: My hospital in Australia contributed to the POISE study. When it started 6 or 7 years ago, the cardiologists were using beta-blockers liberally and haphazardly. It was a huge challenge to convince them that conducting the trial was justifiable—that the case for perioperative beta-blockers had not been absolutely and overwhelmingly proved. They wanted to put beta-blockers in the water supply at the doses we’re talking about.
It would be interesting to do separate analyses of the data from the various countries involved in POISE. In Australia, the percentage of the population on chronic beta-blockers—who therefore would have been ineligible for the trial—is now quite high. Most patients who need to be on beta-blockers long term are on them, whereas that was not the case 15 years ago. The population is changing even while we’re doing the trials. Australian cardiologists are no longer putting every patient on perioperative beta-blockers; they’re thinking about it first.
Dr. Devereaux: A compelling feature of POISE as an international trial is its consistency of outcomes across the planet. No matter where we looked, the outcomes were consistent: in Asia, Europe, North America, and Australia.
Moderator: Would each of you summarize your take-home message for clinicians?
Dr. Devereaux: I urge clinicians to actually read the trials themselves rather than just relying on the advice of guideline writers. It’s important not to allow ourselves to become entrenched in a practice without evidence, just because we’ve done it for so long. If you told your patients the number of MIs prevented and the potential number of excess strokes and deaths, I suspect the average patient would conclude it’s not a great trade-off.
Remember that two-thirds of MIs in the perioperative setting are clinically silent. That doesn’t mean they’re not important, but the strokes, in contrast, are profoundly devastating. One-third of patients with stroke in POISE were dead within 30 days, and of those who survived, 60% were incapacitated, needing help with everyday activities.
I encourage clinicians to read the POISE manuscript with a fresh perspective, regardless of how you’ve practiced until now. Then ask yourself whether you really are comfortable with the safety of perioperative beta-blockers at this time. Of course, that doesn’t mean the evidence won’t change in the future.
Dr. Poldermans: The main imperative is to improve postoperative care. We strongly believe that perioperative beta-blockers work in the general population. If you have a patient who needs to be on a beta-blocker after surgery, why not start it preoperatively? I believe that dosing and timing of initiation are important. If you have the opportunity to start the beta-blocker prior to surgery, do so at a reasonable dose and start early. Patients in whom beta-blockers are started immediately prior to surgery may be worse off, with a higher incidence of stroke.
*Amir K. Jaffer, MD, University of Miami Miller School of Medicine, served as moderator of the debate and the discussion period.
NOTE: The individual co-authors in this debate-based article are responsible only for those views within their respective bylined subsections and those views ascribed to them in the rebuttals and discussion at the end.
Perioperative beta-blockade improves outcomes
By Don Poldermans, MD, PhD
It is my contention that perioperative beta-blockade improves mortality and cardiac outcomes in select high- and intermediate-risk patients undergoing noncardiac surgery. Patients on chronic beta-blocker therapy should have it continued perioperatively. For patients not already on beta-blockade who are at cardiac risk, initiation of low-dose beta-blocker therapy should be considered prior to surgery; such therapy should be started approximately 1 month before surgery, with dose titration to achieve hemodynamic stability. Reports of increased stroke rates with perioperative beta-blockade appear to be due to inappropriate acute administration of high-dose beta-blocker therapy.
THE PHYSIOLOGIC RATIONALE FOR PERIOPERATIVE BETA-BLOCKADE
Perioperative myocardial infarction (MI) can occur by one of two mechanisms, both of which can be attenuated by beta-blockade:
- The stress induced by surgery can cause an asymptomatic coronary plaque to become unstable and rupture, resulting in complete occlusion of a portion of the coronary tree. This type of perioperative MI occurs typically in patients with multiple risk factors for MI absent a critical coronary stenosis. The perioperative risk associated with unstable plaque can be reduced pharmacologically with aspirin, statins, and chronic beta-blocker therapy.
- Alternately, a fixed coronary stenosis can predispose to a mismatch of oxygen demand and supply, leading to myocardial ischemia and infarction. The patient with a fixed coronary lesion typically presents with stable angina pectoris, and the at-risk stenosis is identified through a stress echocardiogram or nuclear scan. The risk conferred by flow-limiting stable plaque can be reduced by coronary revascularization and a short course of beta-blocker therapy prior to surgery.
INITIAL SUPPORTIVE DATA
Mangano and colleagues were the first to evaluate perioperative beta-blockade in a randomized, controlled fashion.1,2 In their study of 200 surgical patients with or at risk for coronary artery disease, oral atenolol administered perioperatively was associated with a 50% reduction (compared with placebo) in the incidence of postoperative myocardial ischemia as measured by three-lead Holter monitoring.2 During 2 years of follow-up, mortality was significantly lower in the atenolol group (10%) than in the placebo group (21%) (P = .019).1
In the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE I), my research group randomized 112 high-risk patients (as identified by dobutamine echocardiography) to standard perioperative care alone or standard perioperative care plus bisoprolol starting 30 days prior to major vascular surgery.3 The dosage of bisoprolol was titrated to achieve a target heart rate of 60 to 70 beats per minute. Thirty days after surgery, the incidence of the primary end point—a composite of death from cardiac causes or nonfatal MI—was reduced from 34% in the standard-care group to 3.4% in the bisoprolol group (P < .001). Thus, in this unblinded study in a population with proven coronary artery disease, beta-blockade clearly improved outcomes.
Additional studies of perioperative beta-blocker use have produced a wide range of outcomes, with most favoring beta-blockade, albeit usually not to a statistically significant degree.4–13 Notably, only some of these trials were randomized, they used various beta-blocker regimens at various doses, they were conducted in patients with varying degrees of cardiac risk, and many had small sample sizes.
What emerged from these trials was the idea that perioperative beta-blockade in patients with coronary artery disease produces an effect similar to that of long-term beta-blockade in reducing the risk of cardiovascular events in post-MI patients and in those with coronary artery disease and heart failure.
THE POISE STUDY AND ITS IMPLICATIONS
Results of the Perioperative Ischemic Evaluation (POISE) were published in 2008, in which 8,351 noncardiac surgery patients with or at risk of atherosclerotic disease were randomized to placebo or extended-release metoprolol succinate started 2 to 4 hours preoperatively and continued for 30 days.14 Metoprolol was associated with a clear reduction in the primary end point, a composite of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest (5.8% vs 6.9% with placebo; hazard ratio [HR] = 0.84 [95% CI, 0.70–0.99]; P = .0399), but this effect was offset by significant increases in total mortality and stroke incidence in the metoprolol group. Mortality was 3.1% with metoprolol versus 2.3% with placebo (HR = 1.33 [95% CI, 1.03–1.74]; P = .0317), and stroke incidence was 1.0% with metoprolol versus 0.5% with placebo (HR = 2.17 [95% CI, 1.26–3.74]; P = .0053). Cerebral infarction, not bleeding, explained most of the excess mortality with metoprolol.
Of the 60 strokes in POISE, 49 were ischemic in origin, 3 were hemorrhagic, and 8 were of uncertain etiology. Preoperative predictors of stroke were the use of clopidogrel and a history of stroke or transient ischemic attack. Postoperative predictors of stroke included intraoperative bleeding and intraoperative hypotension. These predictors suggest a diseased cerebrovascular tree or unstable hemodynamics during the intraoperative period in the patients who suffered a stroke.
Does dosing explain the rise in mortality and strokes?
Could the fatal outcomes associated with the beta-blocker in POISE be attributed to the dosage of metoprolol? In the study, 100 mg of metoprolol was started immediately prior to surgery, and an additional 100 mg could be given, depending on the hemodynamic response. Maintenance therapy (200 mg/day) was started on the same day, making it possible that a patient could have received as much as 400 mg of metoprolol the day of surgery. The starting dose of metoprolol used in POISE was two to eight times the commonly prescribed dose.
The initial 100-mg dose of metoprolol used in POISE has a similar beta1-receptor blockade potency compared with the 5-mg dose of bisoprolol used in DECREASE I.3 However, in DECREASE I, bisoprolol was initiated 30 days prior to surgery and was titrated, if necessary, according to heart rate. The maintenance dose of bisoprolol was half of the maintenance dose used in POISE. In the later DECREASE trials, the starting dose of bisoprolol was only 2.5 mg. Therefore, there was a huge difference in beta-blocker dosing between POISE and DECREASE.
What about timing of beta-blocker initiation?
The POISE findings may also be explained in part by the timing of beta-blocker initiation. Whereas bisoprolol was carefully titrated for 30 days before surgery in DECREASE, metoprolol was initiated just before surgery in POISE, and the maximum recommended dose may have been prescribed during the first 24 hours, although subsequent dosing was 200 mg daily, which is 50% of the maximum daily therapeutic dose. This extremely narrow time window for titration may be important, since the beneficial effects of beta-blockade on coronary plaque stability are likely to take weeks to develop.
Reassurance from a large case-control study
My colleagues and I conducted a case-control study from among more than 75,000 patients who underwent noncardiac, nonvascular surgery at our institution, Erasmus Medical Center, from 1991 to 2001.18 The cases were the 989 patients who died in the hospital postoperatively; the controls were 1,879 survivors matched with the cases for age, sex, the year the surgery was performed, and the type of surgery. The incidence of perioperative stroke was 0.5%, which is comparable to the rate found in the literature. Risk factors predictive of stroke were the presence of diabetes, cerebrovascular disease, peripheral arterial disease, atrial fibrillation, coronary artery disease, and hypertension. Notably, no relationship was found between chronic beta-blocker use and stroke.
WHAT ABOUT PATIENTS AT INTERMEDIATE RISK?
Because the effect of perioperative beta-blockade has traditionally been ill defined in surgical patients at intermediate risk of cardiovascular events, the DECREASE study group recently completed a study (DECREASE IV) to assess perioperative bisoprolol in terms of cardiac morbidity and mortality in intermediate-risk patients undergoing elective noncardiovascular surgery.17 Enrollees had a score of 1 to 2 on the Revised Cardiac Risk Index of Lee et al,19 which corresponds to an estimated risk of between 1% and 6% for a perioperative cardiovascular event.17
DECREASE IV also aimed to assess the effect of perioperative fluvastatin, so a 2 x 2 factorial design was used in which the study’s 1,066 patients were randomized to receive bisoprolol, fluvastatin, combination treatment, or combination placebo control. Bisoprolol was initiated up to 30 days prior to surgery, and the 2.5-mg daily starting dosage was titrated according to the patient’s heart rate to achieve a target rate of 50 to 70 beats per minute. Fluvastatin was also started up to 30 days prior to surgery. Patients who received bisoprolol (with or without fluvastatin) had a significant reduction in the 30-day incidence of cardiac death and nonfatal MI compared with those who did not receive bisoprolol (2.1% vs 6.0%; HR = 0.34 [95% CI, 0.17–0.67]; P = .002). Fluvastatin was associated with a favorable trend on this end point, but statistical significance was not achieved (P = .17).17
There was no difference among treatment groups in the incidence of stroke (4 strokes in the 533 patients who received bisoprolol vs 3 strokes in the 533 patients who did not),17 which further suggests that the increased stroke rate seen with beta-blockade in POISE may have been due to dosage, timing of initiation, or both.
CONCLUSIONS
Dose-related hypotension may explain POISE findings
Our understanding of postoperative stroke is incomplete, but it appears that dosing of a beta-blocker can be a contributor, especially with respect to the potential side effect of hypotension during surgery. Keep in mind that the average age of patients in POISE was approximately 70 years and that patients were naïve to beta-blockers. Some may have had asymptomatic left ventricular dysfunction, and we know that starting a beta-blocker at a high dose in such patients may lead to hypotension. At my institution we routinely perform echocardiographic screening of all patients scheduled for surgery, and we have found that more than half of the patients with heart failure have it uncovered only through this screening.
It is not the medicine alone that can cause perioperative hypotension; other factors may induce hypotension, requiring beta-blocker titration and careful monitoring of hemodynamics during surgery.
Advice: Start early and titrate dose; continue chronic beta-blockade
My advice is as follows:
- If a patient is on chronic beta-blocker therapy, do not stop it perioperatively. We have seen devastating outcomes in the Netherlands when patients had their beta-blockers stopped immediately before surgery. Consider adjusting the dose, but do not stop it entirely. If a beta-blocker is on board and the patient develops hypotension or bradycardia during surgery, treat the symptoms and check for sepsis.
- In a patient not on a beta-blocker, consider adding one if the patient is at intermediate or high risk of a cardiac event, but start at a low dosage (ie, 2.5 mg/day for bisoprolol and 25 mg/day for metoprolol). Treatment ideally should be started 30 days preoperatively; in the Netherlands, we have the chance to start well in advance of surgery so we can titrate the dose according to hemodynamics.
- If a beta-blocker is not started because of insufficient time for titration, do not add one to treat tachycardia that develops during surgery, since tachycardia may represent a response to normal defense mechanisms.
Safety of perioperative beta-blocker use has not been adequately demonstrated
By P.J. Devereaux, MD, PhD
I contend that perioperative beta-blockade is a practice not grounded in evidence-based medicine, and its overall safety has increasingly come into question as more data from large, high-quality trials have emerged. I will begin with a historical overview of perioperative beta-blocker use, review the results of the POISE trial (for which I was the co-principal investigator), explore the major questions raised by this trial, and conclude with some take-away messages.
THE HISTORY OF PERIOPERATIVE BETA-BLOCKADE
In the 1970s, physicians were encouraged to hold beta-blockers prior to surgery out of concern that these medications may inhibit the required cardiovascular response when patients developed hypotension, and could thereby lead to serious adverse consequences.
In the 1980s, new research associated tachycardia with perioperative cardiovascular events, leading to proposals to implement perioperative beta-blocker use.
In the 1990s, two randomized trials with a total sample size of 312 patients1,3 suggested that perioperative beta-blockers had a large treatment effect in preventing major cardiovascular events and death. These small trials had several methodological limitations:
- One trial3 was unblinded in a setting in which the vast majority of MIs are clinically silent.
- One trial3 was stopped early—after randomizing only 112 patients—for unexpected large treatment effects.
- One of the studies1 failed to follow intention-to-treat principle.
Nevertheless, guidelines developed at the time by the American College of Cardiology and the American Heart Association (ACC/AHA) recommended the use of perioperative beta-blockers on the basis of the physiological rationale and these two small clinical trials. That recommendation was retained in the latest (2007) update of the ACC/AHA perioperative guidelines.20
In 2006, two clinical trials with a total sample size of 1,417 were completed,4,15 surpassing the total size of previous trials by more than fourfold. These two more recent trials did not suffer from the methodological limitations of earlier trials. These trials showed no benefit of perioperative beta-blocker use; in fact, there was a trend toward worse outcomes in the beta-blocker recipients.4,15 Despite these new data, guidelines committees continued to recommend perioperative beta-blockade.20
THE POISE TRIAL
Study design
This was the context into which the POISE results were released in 2008. POISE was a randomized, controlled, blinded trial of patients 45 years or older scheduled for noncardiac surgery who had, or were at high risk of, atherosclerotic disease.14 The intervention consisted of metoprolol succinate (metoprolol controlled release [CR]) or placebo started 2 to 4 hours preoperatively (if heart rate was ≥ 50 beats per minute and systolic blood pressure [SBP] was ≥ 100 mm Hg) and continued for 30 days. The target dosage of metoprolol was 200 mg once daily. No patients received the recommended maximum dosage of 400 mg over 24 hours. The main outcome measure was a 30-day composite of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest.
We randomized 9,298 patients in a 1:1 ratio to metoprolol or placebo. We encountered data fraud at a number of centers that prompted exclusion of data from 474 patients allocated to metoprolol and 473 allocated to placebo. Therefore, the total number of patients available for the intention-to-treat analysis was 8,351, from 190 centers in 23 countries.
Results
The risk of the primary composite outcome was reduced by 16% (relative reduction) in recipients of metoprolol CR compared with placebo recipients (P = .0399). Significantly fewer nonfatal MIs occurred in the metoprolol CR group than in the placebo group (152 [3.6%] vs 215 [5.1%]; P = .0008), leaving little doubt that perioperative beta-blockade prevents MI.
In contrast, total mortality was increased in the beta-blocker group, with 129 deaths among those assigned to metoprolol CR and 97 among those assigned to placebo (P = .0317), and the incidence of stroke was also significantly greater in the metoprolol CR group (1.0% vs. 0.5%; P = .0053).
Consistency with findings from other trials
The POISE data are consistent with those from a 2008 meta-analysis of high-quality randomized controlled trials in noncardiac surgery patients, which showed a significantly greater risk of death among patients assigned to a beta-blocker than among controls who were not (160 deaths [2.8%] vs 127 deaths [2.3%]; odds ratio [OR] = 1.27 [95% CI, 1.01–1.61]).21 This meta-analysis also found a significantly greater risk of nonfatal stroke in beta-blocker recipients compared with controls (38 [0.7%] vs 17 [0.3%]; OR = 2.16 [95% CI, 1.27–3.68]).
I also contend that the DECREASE IV trial supports the POISE findings in that although few strokes were encountered in DECREASE IV, the trend was in the direction of harm in the beta-blocker group, which had 4 strokes among 533 patients versus 3 strokes among 533 patients not receiving the beta-blocker.17
Predictive role of hypotension
The link between hypotension and death in POISE is consistent with findings from the largest beta-blocker trial undertaken, COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial), in which 45,852 patients with acute MI were randomized to metoprolol or placebo.22 In COMMIT, metoprolol had no effect on 30-day all-cause mortality but significantly reduced the risk of arrhythmic death, a benefit that was countered by a significantly increased risk of death from shock with a beta-blocker in acute MI. Clinically significant hypotension is much more common in the perioperative setting than in acute MI, which may explain the excess number of deaths observed with metoprolol in POISE as opposed to metoprolol’s neutral effect on mortality in COMMIT.
ANSWERING THE CRITICS
Several criticisms have been raised about POISE, as detailed below.
Beta-blocker dose
Some contend that a lower dose of beta-blocker would provide benefit and minimize risk, but this assertion must be supported by evidence from a large clinical trial. The targeted dosage of metoprolol in POISE represents 50% of the maximum daily therapeutic dose. Further, the protocol called for decreasing the dosage to 100 mg/day if SBP dropped to less than 100 mm Hg or if heart rate fell to less than 45 beats per minute.
The two small trials on which guideline recommendations for perioperative beta-blockade are primarily based1,3 had a sample size that was 4% of that in POISE, which calls into question the reliability of their results. The study by Mangano et al used atenolol at a target dosage that was 50% of the maximum daily therapeutic dose,1 the same as with metoprolol in POISE. DECREASE initiated bisoprolol at 25% of the maximum daily therapeutic dose, and allowed for titration to 50% of the maximum daily therapeutic dose.3
As the second largest study of perioperative beta-blockade, the Diabetic Postoperative Mortality and Morbidity (DIPOM) trial enrolled 921 patients who were assigned to placebo or controlled-release metoprolol with a target dosage that was 25% of the maximum daily therapeutic dose.4 The 30-day outcomes from DIPOM showed a trend toward an excess of death and stroke despite using only one-half the dosage in POISE and the same dosage as in DECREASE.
Timing of beta-blocker initiation
Another contention is that earlier beta-blocker initiation would be better. The issue with timing of initiation is not benefit, as POISE showed that starting a beta-blocker hours before surgery results in a reduction in the risk of MI. The issue is whether giving a beta-blocker earlier makes administering the drug safer. Nearly 10% of placebo recipients in POISE developed clinically significant hypotension, which suggests that the titrated dosage of a beta-blocker that appears effective preoperatively is unlikely to inform a safe dose after surgery, when hypotension is common.
The practicality of titrating the dose of beta-blocker prior to surgery also comes into play. Most patients referred to my institution for surgery are seen 1 to 2 weeks in advance, at the earliest. Real-world practice at present simply does not afford us the luxury of seeing patients three to four times before surgery in order to titrate the beta-blocker dose.
POISE did not address chronic beta-blocker therapy
It is important to remember that POISE excluded patients on chronic beta-blocker therapy and thus did not attempt to address the perioperative management of such patients who undergo noncardiac surgery. My suspicion is that perioperative continuation of beta-blockade in these patients is the best course of action, but this too has not been studied robustly, so we need a large controlled trial to confirm that this practice is indeed safe.
CONCLUSIONS
The POISE results suggest that for every 1,000 patients treated, perioperative metoprolol would:
- Prevent 15 MIs, 3 cardiac revascularizations, and 7 new cases of atrial fibrillation
- Result in 8 excess deaths, 5 strokes, 53 cases of clinically significant hypotension, and 42 cases of clinically significant bradycardia.
The central take-away message is that patients are unlikely to want a perioperative beta-blocker if they are unwilling to accept a probable increase in mortality or if they place three times more value on avoiding a perioperative stroke than on avoiding an MI.
It has been 10 years since the recommendation to use perioperatove beta-blockers was incorporated into perioperative practice guidelines. Assuming only 10% of physicians acted on this recommendation, 100 million patients have received a perioperative beta-blocker over this time as a result. If the POISE results are applicable, a full 800,000 of these patients died and another 500,000 suffered perioperative strokes as a result of being given a beta-blocker. This issue is not to be taken lightly, given the evidence to suggest harm.
Though it is possible that an alternative beta-blocker regimen to the one used in POISE may provide benefit without substantial harm, the data suggest this is not probable. The POISE data highlight the risk of making assumptions, as well as the importance of and need for large, high-quality randomized trials in the perioperative setting.
It is time for perioperative medicine to enter the age of evidence-based practice and embrace one of its central tenets: only large trials are reliable when it comes to therapeutic questions.
Rebuttals and discussion
POLDERMANS REBUTTAL: MORE TRANSPARENCY NEEDED IN POISE DOSING DATA
Dr. Poldermans: The initial paper describing the POISE trial design did indeed indicate that it was possible for a patient to receive 400 mg of metoprolol on the first day of treatment. We need to see the actual doses of metoprolol given to all patients in POISE who had a perioperative stroke. If you show me these data, the issue will be much easier to discuss.
Our data from randomized trials are consistent in showing that a titrated dosing regimen using bisoprolol reduces the incidence of postoperative cardiac events with no increase in the number of strokes.
My take-home message is that if you want to use beta-blockers, use them sensibly, use them carefully, and act during surgery. If many of your patients are developing hypotension, then you are doing something wrong.
DEVEREAUX REBUTTAL: A SHIFT IN THINKING IS REQUIRED
Dr. Devereaux: The data from POISE are fully available, and I take issue with Dr. Poldermans’ contention that a patient could have received as much as 400 mg of metoprolol CR on the day of surgery; this was not an option according to protocol. I believe his statement is misleading in the same way that it is misleading to indicate that in the DECREASE trial patients may have received 20 mg of bisoprolol within 24 hours of surgery. It is possible that a patient in DECREASE could have gone to surgery at 2:00 pm and may have taken his or her bisoprolol at 10:00 am that morning. The following morning (in the hospital), it is possible that the patient would have received his or her bisoprolol 10 mg at 7:00 or 8:00 am (ie, 20 mg within 24 hours of surgery). Although this is possible and something similar could have happened within POISE, it does not reflect a patient receiving an effective dose of metoprolol CR 400 mg or bisoprolol 20 mg over a 24-hour period.
I worry about the distortion of reality in perioperative medicine that leads so many of us to believe that randomization is magical despite small sample sizes. Small randomized trials are at profound risk of imbalance between the randomized groups, whether we see it or not, and the results are therefore simply not reliable.
Unless we shift our thinking, we make ourselves susceptible to overconfidence in the benefits of a certain intervention before the data from large clinical trials become available. In the meantime, as we have seen from POISE, an intervention may have negative consequences that are not apparent from small clinical trials.
The reality of excess stroke with perioperative beta-blockers is consistent across all the trials. It does not mean that we cannot find another way to give beta-blockers safely, but if we want to establish safety, we need a large trial that unequivocally demonstrates safety, as opposed to simply using observational data, retrospective cohorts, or comparisons between two nonrandomized trials. Until we have large data sets, it is very difficult to say that we can give beta-blockers safely.
DISCUSSION WITH THE AUDIENCE
Moderator*: Dr. Devereaux, was the hypotension in POISE related to the long-acting beta-blocker itself or to the large dose of if that was used? Similarly, were the strokes a result of the drug itself or of the hypotension?
Dr. Devereaux: I must take issue with your premise that the dose of metoprolol used in POISE was “large.” As I noted, Mangano’s study used its beta-blocker (atenolol) at 50% of its maximum daily therapeutic dose,1 the same proportion used in POISE, and Dr. Poldermans’ own DECREASE trial allowed titration of bisoprolol up to 50% of the maximum daily therapeutic dose.3 The DIPOM trial used half the dose of metoprolol that we used, yet it too yielded a trend toward more death and stroke in the beta-blocker group.4 So it’s not that the dose we used was at some excessive level. At the same time, that does not mean that a smaller dose may not have achieved a similarly significant benefit in cardiac outcomes.
We can’t explain most of the strokes. Because most strokes were ischemic, I suspect that the explanation may lie in the threshold used to define clinically significant hypotension. We used an SBP cutoff of less than 90 mm Hg, but we did not classify large drops in SBP, such as from 180 to 95 mm Hg, as clinically significant hypotension. The high incidence of clinically significant hypotension in the placebo group—about 10%—suggests that hypotension was likely the driving factor for stroke. The beta-blocker exacerbated the hypotension, but its more important effect may have been that it made it harder for the body to overcome the hypotension. That is the exact same signal observed in the COMMIT trial in the setting of acute MI.22
Dr. Poldermans: I’d like to see the intraoperative blood pressure data for the 60 patients who suffered strokes in POISE. We could then find out exactly when the hypotension occurred, what kind of hypotension it was, what the patient’s initial blood pressure reading was, and so on. If we had access to this information, we could determine which occurred first—the hypotension or the stroke.
Dr. Devereaux: Although trials can indicate a signal, they can’t explain with certainty the pathway through which the outcome occurred. For example, we know that beta-blockers prevent MI, but we don’t know how. What’s most impressive about the stroke issue is the consistency across all the perioperative beta-blocker trials: every one shows a direction of excess stroke with beta-blockers.
Question from the audience: The patient groups studied in DECREASE and POISE were different. DECREASE studied a very high-risk vascular surgery group with known coronary artery disease on the basis of echocardiography. POISE included patients undergoing emergency surgery and patients with sepsis. Can you describe the outcomes in POISE solely among the patients who underwent elective vascular surgery, similar to the patients studied in DECREASE?
Dr. Devereaux: In terms of the benefit to bisoprolol in very high-risk patients in DECREASE, remember that it was a study of 112 patients. That’s far too small a trial to establish safety or efficacy. The benefit of perioperative beta-blockade in preventing MI is unequivocal because it’s consistent across all trials. But the real issue is, was it safe?
Interestingly, in POISE, the groups at highest risk looked like they benefited the least, not the most. The notion of targeting high-risk people is not supported by POISE; if anything, the POISE results went in the direction of harm with beta-blockade in high-risk patients. That being said, the P value for interaction is not statistically significant, but it’s heading in the direction of harm. So I wouldn’t take comfort in believing that if we simply target high-risk patients, beta-blockers become safe.
Question from the audience: I believe that the seven or eight studies that showed higher stroke rates with beta-blockers all gave beta-blockade within 24 hours of surgery. Only in DECREASE was it given days and weeks in advance of surgery. Can you comment?
Dr. Poldermans: There’s clearly a relation between the time of beta-blocker initiation and the incidence of stroke. If you look at the randomized trials, you see an increased incidence of stroke in patients in whom beta-blockers are started just prior to surgery but not in patients who are on chronic beta-blockers. In our case-control study,18 we screened more than 185,000 patients for stroke and could not detect an increased incidence of stroke in those on chronic beta-blocker therapy. So stroke indeed has something to do with starting beta-blockers just before surgery.
Dr. Devereaux: In DECREASE IV, bisoprolol was started up to 1 month before surgery, yet there were 4 strokes in the bisoprolol group versus 3 in the control group.17
Dr. Poldermans: Yes, but that difference is not statistically meaningful.
Comment from the audience: I’m uncomfortable with the way Dr. Devereaux stresses the importance of significant findings from large randomized trials but then quibbles about a stroke rate of 4 versus 3, which is not statistically significant. Keep it scientific: either there is or there isn’t a P value that achieves significance.
Though I congratulate you on a great trial, any resident in my program would be fired for pursuing your strategy of perioperative care in POISE, which included using an SBP of 100 mm Hg as the threshold for stopping the beta-blocker regardless of preoperative blood pressure. An SBP of 100 mm Hg is not the definition of hypotension. Most anesthesiologists and perioperative physicians peg the beta-blockade to a reasonable level based on the preoperative blood pressure. They titrate in fluids and titrate in the beta-blocker. Certainly the timing is an issue—we don’t recommend giving it right before the operation.
Dr. Devereaux: I referred to the stroke rates in DECREASE IV because Dr. Poldermans has claimed that the excess in strokes has occurred in all trials but his, yet DECREASE IV was one of his trials and also one in which bisoprolol was started early. I’m not claiming statistical significance between the 3 versus 4 strokes in DECREASE IV, but the stroke trend is in the same direction as in the other trials, so it tells us nothing with regard to safety. My point is, has anyone proven safety? As much as we’d like to imagine that beta-blockers are safe perioperatively—and maybe they are—it has not been proven. The largest trials at present are consistent with a signal toward harm.
It’s easy to criticize the methodology after a trial is done. A number of us came together and thought we had a reasonable protocol for POISE. We found a reduction in the incidence of MI but more strokes and higher mortality with perioperative metoprolol. Does this mean there is not a safe way to give a beta-blocker and derive the benefit? Of course not. But at the moment the evidence does not support a way to give a beta-blocker safely. Do we need to find a way to give it safely? Of course we do.
For example, the design of POISE II is factorial, looking at aspirin versus placebo as well as clonidine versus placebo. There are a number of factors about clonidine that suggest we might be able to achieve the benefits we saw in POISE and avoid the risk, but until we do a large trial, we’re not going to know.
Comment from the audience: It’s important that we clearly understand the conclusion from POISE. It’s not that the administration of beta-blockers is not safe. It’s that the administration of a beta-blocker, as your methodology applied it, was not totally safe. Those are two very different conclusions.
Dr. Devereaux: I would say the conclusion is that the way that beta-blockers are being given has not been proven to be safe. The result is consistent. It’s even consistent with DECREASE IV.
Moderator: I believe in large clinical trials, but they must apply to real-life practice. POISE did not address the practices of many people in this room, particularly regarding the doses of metoprolol used. So it is hard for us to apply the findings in clinical practice. Those of you who design large trials need to think through your trial design very thoroughly, considering the millions of dollars that go into these trials. It’s not fair to clinicians to do a study that may have little clinical relevance.
Dr. Devereaux: Investigators from 190 centers in 23 countries were involved in POISE, and all of them thought we had a reasonable approach and methodology. That doesn’t mean it was perfect, yet the criticisms we are hearing now did not surface while the trial was ongoing.
Comment from the audience: My hospital in Australia contributed to the POISE study. When it started 6 or 7 years ago, the cardiologists were using beta-blockers liberally and haphazardly. It was a huge challenge to convince them that conducting the trial was justifiable—that the case for perioperative beta-blockers had not been absolutely and overwhelmingly proved. They wanted to put beta-blockers in the water supply at the doses we’re talking about.
It would be interesting to do separate analyses of the data from the various countries involved in POISE. In Australia, the percentage of the population on chronic beta-blockers—who therefore would have been ineligible for the trial—is now quite high. Most patients who need to be on beta-blockers long term are on them, whereas that was not the case 15 years ago. The population is changing even while we’re doing the trials. Australian cardiologists are no longer putting every patient on perioperative beta-blockers; they’re thinking about it first.
Dr. Devereaux: A compelling feature of POISE as an international trial is its consistency of outcomes across the planet. No matter where we looked, the outcomes were consistent: in Asia, Europe, North America, and Australia.
Moderator: Would each of you summarize your take-home message for clinicians?
Dr. Devereaux: I urge clinicians to actually read the trials themselves rather than just relying on the advice of guideline writers. It’s important not to allow ourselves to become entrenched in a practice without evidence, just because we’ve done it for so long. If you told your patients the number of MIs prevented and the potential number of excess strokes and deaths, I suspect the average patient would conclude it’s not a great trade-off.
Remember that two-thirds of MIs in the perioperative setting are clinically silent. That doesn’t mean they’re not important, but the strokes, in contrast, are profoundly devastating. One-third of patients with stroke in POISE were dead within 30 days, and of those who survived, 60% were incapacitated, needing help with everyday activities.
I encourage clinicians to read the POISE manuscript with a fresh perspective, regardless of how you’ve practiced until now. Then ask yourself whether you really are comfortable with the safety of perioperative beta-blockers at this time. Of course, that doesn’t mean the evidence won’t change in the future.
Dr. Poldermans: The main imperative is to improve postoperative care. We strongly believe that perioperative beta-blockers work in the general population. If you have a patient who needs to be on a beta-blocker after surgery, why not start it preoperatively? I believe that dosing and timing of initiation are important. If you have the opportunity to start the beta-blocker prior to surgery, do so at a reasonable dose and start early. Patients in whom beta-blockers are started immediately prior to surgery may be worse off, with a higher incidence of stroke.
*Amir K. Jaffer, MD, University of Miami Miller School of Medicine, served as moderator of the debate and the discussion period.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery: Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia: McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery: Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative b-blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Brady AR, Gibbs JS, Greenhalgh RM, et al. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Cucchiara RF, Benefiel DJ, Matteo RS, DeWood M, Albin MS. Evaluation of esmolol in controlling increases in heart rate and blood pressure during endotracheal intubation in patients undergoing carotid endarterectomy. Anesthesiology 1986; 65:528–531.
- Magnusson J, Thulin T, Werner O, Järhult J, Thomson D. Haemodynamic effects of pretreatment with metoprolol in hypertensive patients undergoing surgery. Br J Anaesth 1986; 58:251–260.
- Stone JG, Foëx P, Sear JW, et al. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Jakobsen CJ, Bille S, Ahlburg P, et al. Perioperative metoprolol reduces the frequency of atrial fibrillation after thoracotomy for lung resection. J Cardiothorac Vasc Anesth 1997; 11:746–751.
- Bayliff CD, Massel DR, Inculet RI, et al. Propranolol for the prevention of postoperative arrhythmias in general thoracic surgery. Ann Thorac Surg 1999; 67:182–186.
- Raby KE, Brull SJ, Timimi F, et al. The effect of heart rate control on myocardial ischemia among high-risk patients after vascular surgery. Anesth Analg 1999; 88:477–482.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Urban MK, Markowitz SM, Gordon MA, et al. Postoperative prophylactic administration of beta-adrenergic blockers in patients at risk for myocardial ischemia. Anesth Analg 2000; 90:1257–1261.
- POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Zaugg M, Bestman L, Wacker J, et al. Adrenergic receptor genotype but not perioperative bisoprolol therapy may determine cardiovascular outcome in at-risk patients undergoing surgery with spinal block: the Swiss Beta Blocker in Spinal Anesthesia (BBSA) study. Anesthesiology 2007; 107:33–44.
- Dunkelgrun M, Boersma E, Schouten O, et al. Bisoprolol and fluvastatin for reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiac surgery. A randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- Noordzij PG, Poldermans D, Schouten O, et al. Beta-blockers and statins are individually associated with reduced mortality in patients undergoing noncardiac, nonvascular surgery. Coron Artery Dis 2007; 18:67–72.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:1707–1732.
- Bangalore S, Wetterslev J, Pranesh S, et al. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery: Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia: McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery: Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative b-blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Brady AR, Gibbs JS, Greenhalgh RM, et al. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Cucchiara RF, Benefiel DJ, Matteo RS, DeWood M, Albin MS. Evaluation of esmolol in controlling increases in heart rate and blood pressure during endotracheal intubation in patients undergoing carotid endarterectomy. Anesthesiology 1986; 65:528–531.
- Magnusson J, Thulin T, Werner O, Järhult J, Thomson D. Haemodynamic effects of pretreatment with metoprolol in hypertensive patients undergoing surgery. Br J Anaesth 1986; 58:251–260.
- Stone JG, Foëx P, Sear JW, et al. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Jakobsen CJ, Bille S, Ahlburg P, et al. Perioperative metoprolol reduces the frequency of atrial fibrillation after thoracotomy for lung resection. J Cardiothorac Vasc Anesth 1997; 11:746–751.
- Bayliff CD, Massel DR, Inculet RI, et al. Propranolol for the prevention of postoperative arrhythmias in general thoracic surgery. Ann Thorac Surg 1999; 67:182–186.
- Raby KE, Brull SJ, Timimi F, et al. The effect of heart rate control on myocardial ischemia among high-risk patients after vascular surgery. Anesth Analg 1999; 88:477–482.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Urban MK, Markowitz SM, Gordon MA, et al. Postoperative prophylactic administration of beta-adrenergic blockers in patients at risk for myocardial ischemia. Anesth Analg 2000; 90:1257–1261.
- POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Zaugg M, Bestman L, Wacker J, et al. Adrenergic receptor genotype but not perioperative bisoprolol therapy may determine cardiovascular outcome in at-risk patients undergoing surgery with spinal block: the Swiss Beta Blocker in Spinal Anesthesia (BBSA) study. Anesthesiology 2007; 107:33–44.
- Dunkelgrun M, Boersma E, Schouten O, et al. Bisoprolol and fluvastatin for reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiac surgery. A randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- Noordzij PG, Poldermans D, Schouten O, et al. Beta-blockers and statins are individually associated with reduced mortality in patients undergoing noncardiac, nonvascular surgery. Coron Artery Dis 2007; 18:67–72.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:1707–1732.
- Bangalore S, Wetterslev J, Pranesh S, et al. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.