User login
Clinical Progress Note: Myocardial Injury After Noncardiac Surgery
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
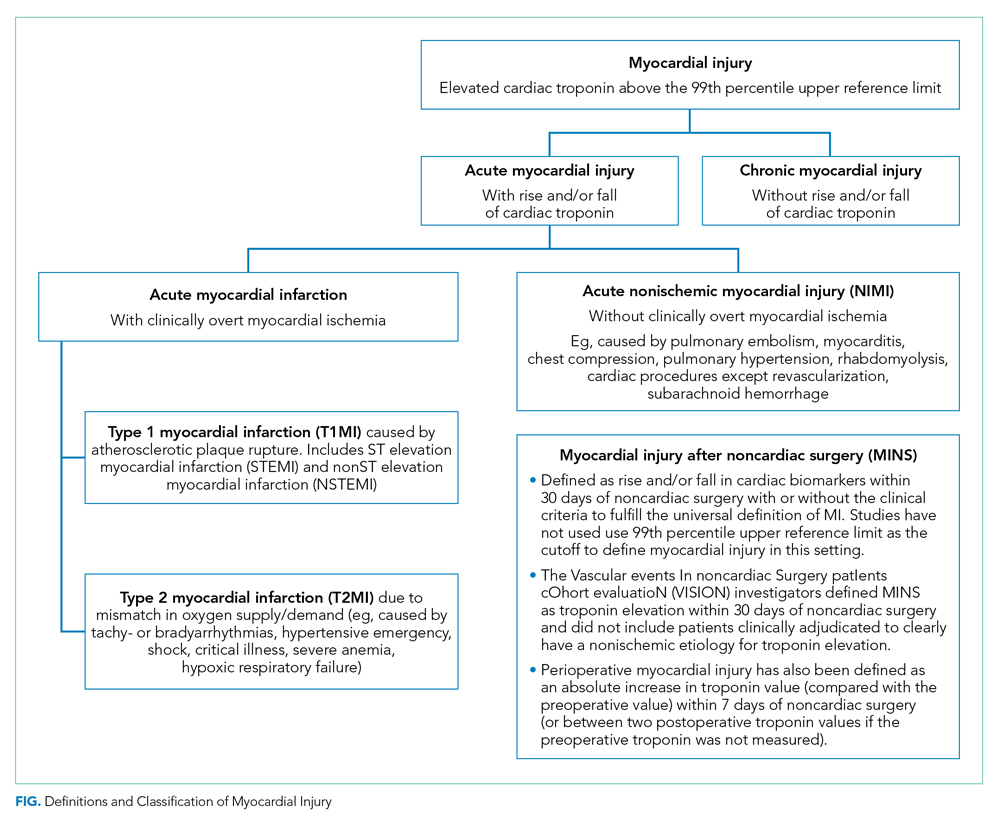
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8
EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
© 2020 Society of Hospital Medicine
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Unscripted
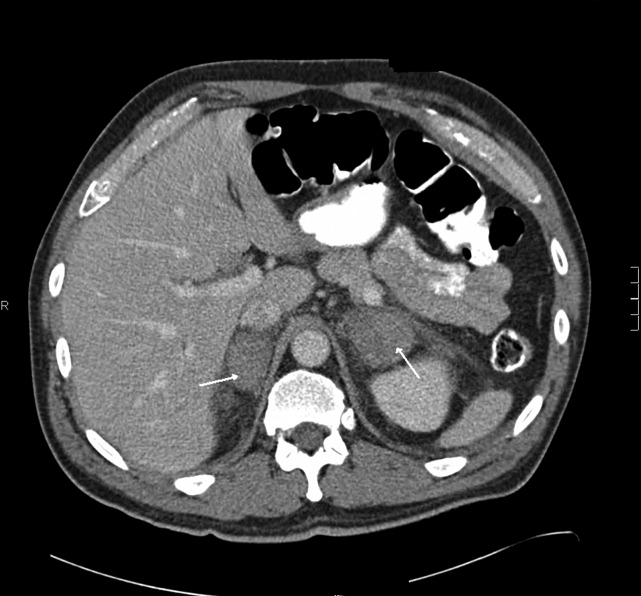
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was 20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal 1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, 5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was 20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal 1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, 5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was 20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal 1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, 5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
Postoperative myocardial infarction and in-hospital mortality predictors in patients undergoing elective noncardiac surgery
Incidence and predictors of postoperative heart failure in patients undergoing elective noncardiac surgery
Bilateral Adrenal Hemorrhage Complication
A 52‐year‐old man presented to the emergency department (ED) from a skilled nursing facility with a complaint of bilateral upper‐quadrant abdominal pain of 48 hours' duration. The pain was sharp, nonradiating, constant, and was associated with nausea, vomiting, and constipation. The patient denied any fever, back pain, dysuria, melena, or hematochezia. In the rehabilitation facility the patient had been initially evaluated for this pain. He was given laxatives and stool softeners for presumed constipation but these measures had not been effective. A computed tomography (CT) scan of the abdomen had only showed stool in the colon and he was sent to the ED for further evaluation.
Apart from severe degenerative joint disease in both his knees he was in good health. He was in the skilled nursing facility (SNF) for rehabilitation for bilateral knee replacement surgery done 9 days prior to this presentation. His postoperative course was unremarkable. He had been maintained on prophylaxis for venous thromboembolism with enoxaparin since postoperative day 1 at a daily dose of 40 mg subcutaneously, and was transferred to the SNF on postoperative day 6 on the same dose. His was receiving oxycodone and Tylenol for pain. He was on no other medications.
Vital signs on presentation revealed a temperature of 97.5F, a heart rate of 100 beats per minute, a respiratory rate of 16 breaths per minute, and a blood pressure of 136/69 mmHg. He was alert and oriented and in mild distress from the abdominal pain. Examination was normal except for tenderness in the upper quadrants of the abdomen though no rigidity or rebound tenderness were noted. Routine chemistries were normal except for sodium of 134 mg/dL. His white count, hemoglobin, hematocrit, and platelet levels were noted to be at 17.5K/L, 10 g/dL, 30%, and 345K/L, respectively, and were stable with regard to his discharge laboratory values. His serum eosinophil level was normal. A complete workup for hypercoagulable state and bleeding disorders including assays for antibodies associated with heparin‐induced thrombocytopenia were negative. He was admitted for further evaluation and treatment.
The patient had another CT scan of the abdomen (Figure 1), which when compared to the one done at the SNF 2 days prior showed markedly enlarged bilateral adrenal glands suggestive of bilateral acute adrenal hemorrhage. The enoxaparin was discontinued and empiric steroid replacement therapy was begun. A random cortisol level was normal but a cosyntropin stimulation test showed an absolute increase in cortisol level of only 0.8 g/dL at both 30 and 60 minutes after administration of 250 g of cosyntropin. An investigation was undertaken to determine if the patient had any prior risk factors for bleeding. There was no evidence of infection and a comprehensive evaluation for bleeding, and coagulation disorders was normal. The bilateral adrenal hemorrhage was attributed to the use of enoxaparin in the postoperative setting. Unfortunately, the patient subsequently developed a deep venous thrombosis in his lower extremity and an inferior vena cava (IVC) filter was placed before discharge. He was doing well 6 months later, and is still continued on glucocorticoid and mineralocorticoid replacement therapy and follows up with endocrinology as an outpatient.
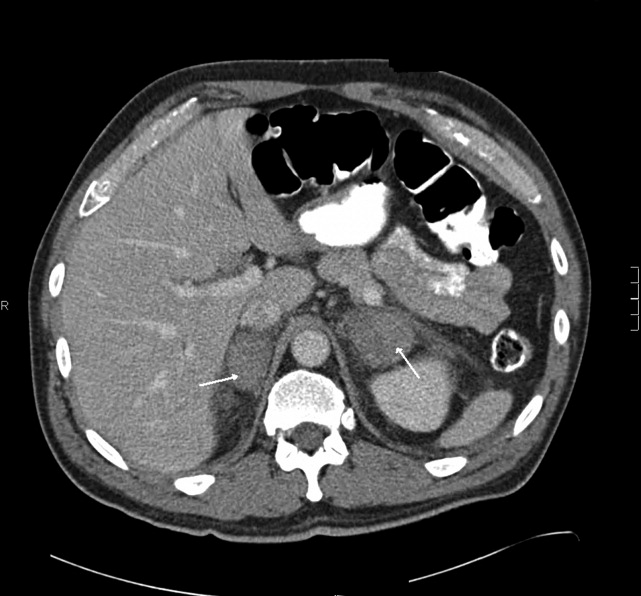
Discussion
Bilateral adrenal hemorrhage is usually associated with massive sepsis from Gram‐negative organisms such as Neisseria meningitides, Pseudomonas aeroginosa, Escherichia coli, and Bacteroides fragilis. Rupert Waterhouse, in 1911, was the first person to describe a patient with severe meningococcal sepsis resulting in acute adrenal hemorrhage and collapse. This was also later described independently by Carl Friderichsen in 1918, and is now referred to as the Waterhouse‐Friderichsen syndrome. Other causes include antiphospholipid antibody syndrome, heparin‐associated thrombocytopenia (HIT), and severe physical stress. Bilateral adrenal hemorrhage can also spontaneously occur in the postoperative period, especially after cardiothoracic or orthopedic surgery. This phenomenon may be related to the frequent use of prophylactic anticoagulants after these types of procedures.
The first case report of bilateral adrenal hemorrhage secondary to use of anticoagulants was described in 1947, and the first case report of successful resuscitation after corticosteroid administration in a patient with bilateral adrenal hemorrhage secondary to anticoagulant use was described by Thorn in 1956.1 A review of the literature demonstrates multiple case reports of adrenal hemorrhage reported in the postoperative period, particularly after joint arthroplasty, and especially after knee replacement surgeries. Most of the recent cases have been associated with use of prophylactic low‐dose heparin or low‐molecular‐weight heparin at the time of adrenal hemorrhage. In a study of 157 case reports of individuals with bilateral hemorrhage (including 22 autopsies), 48 cases were associated with administration of anticoagulants, although the dose and effect were not specified.2 Amador et al.1 showed that out of 4325 autopsies performed from 1949 to 1962 in their institution, 30 cases were found of bilateral hemorrhage, of which 10 were receiving heparin at presumably prophylactic doses; 5 of these patients were also receiving dicumarol.
Mayo Clinic investigators performed a retrospective review of all cases of adrenal hemorrhage over a period of 25 years at their hospital, and found 141 cases of adrenal hemorrhage, of which 78 were bilateral and 63 were unilateral,3 and in 67 patients the condition was diagnosed at autopsy. In this study 14 patients had adrenal hemorrhage in the postoperative period in the absence of lupus anticoagulant or HIT; there was no specific mention in this study of the use of postoperative anticoagulants. Finally, a multicenter case control study was undertaken by Kovacs et al.4 to assess putative risk factors for development of bilateral massive adrenal hemorrhage. In the multivariate analysis, thrombocytopenia, exposure to heparin, and sepsis were found to be strongly associated with risk of hemorrhage. Of 23 patients with bilateral, massive adrenal hemorrhage, 16 had been exposed to heparin, and at least 6 were on exclusively subcutaneous heparin. The authors concluded that heparin exposure was a much bigger risk factor than other coagulopathies, and those exposed to heparin of any route or type for 4 to 6 days and those exposed for more than 6 days were about 17 and 34 times, respectively, more likely to develop bilateral hemorrhage than those who had less than 4 days or no exposure.
The clinical presentation of adrenal insufficiency due to bilateral adrenal hemorrhage is often nonspecific. Symptoms may include abdominal pain, back pain, fever, nausea, vomiting, weakness, obtundation, confusion, and hypotensionall of which are also common postoperative symptoms and can be missed or ignored.5 Rao et al.6 profiled the clinical presentation of 64 cases of bilateral hemorrhage and found the following: abdominal, flank, back, or chest pain (86%); anorexia, nausea, or vomiting (47%); psychiatric symptoms (42%); fever (66%); hypotension recognized before shock episode (19%); and abdominal rigidity or rebound (22%). Adrenal insufficiency becomes clinically evident once 90% of the gland is destroyed. About 50% of patients do not manifest typical laboratory abnormalities, so a high degree of suspicion is necessary to diagnose the condition.3 Also, the laboratory diagnosis of adrenal insufficiency using random cortisol levels is unreliable, as reference ranges in patients experiencing stress (as in the postoperative period) have not been well studied or established. In patients with bilateral hemorrhage postoperatively on prophylactic anticoagulants, the coagulation profile is usually within normal limits and there is typically no evidence of spontaneous bleeding elsewhere. In later stages, the typical laboratory findings of abnormal adrenal function such as hypokalemia, hyponatremia, declining cortisol levels, and an inappropriate response to adrenocorticotropic hormone stimulation test may be seen. A significant drop in hemoglobin secondary to hemorrhage may also be encountered in some patients secondary to the bleed.
CT is the most reliable and extensively used imaging modality for making the diagnosis, although magnetic resonance imaging (MRI) or ultrasound may also be utilized. Early in the course of adrenal hemorrhage, CT findings may be negative, and repeated imaging is appropriate when clinical suspicion is high. The presence of bilateral adrenal enlargement with increased signal attenuation suggests bilateral adrenal hemorrhage. MRI can both characterize adrenal hematomas, and estimate their age.7, 8
Postoperative adrenal hemorrhage and insufficiency is easily treatable and has excellent outcomes; survivors will need lifelong corticosteroid replacement (and usually mineralocorticoid replacement as well). In the Mayo Clinic study, survival was 100% with treatment vs. 17% without treatment. In comparison, sepsis‐induced or stress‐induced adrenal insufficiency has poor outcomes despite adequate treatment (9% survival with treatment vs. 6% survival without treatment).3 Death can occur within hours to days of symptoms if untreated. Treatment includes timely initiation of adrenal hormone replacement and reversal of coagulopathies.
Postoperative venous thromboembolism (VTE) prophylaxis with anticoagulants is the appropriate care in many cases, but, along with the postoperative state itself, also appears to be a risk factor for this unusual condition. Postoperative bilateral adrenal hemorrhage is rare and potentially fatal. Early identification and prompt initiation of steroid replacement therapy and reversal of coagulopathies can prove to be lifesaving. Making this diagnosis can be very challenging, as the clinical presentation and laboratory findings of adrenal hemorrhage are vague and nonspecific and mimic many nonlife threatening postoperative complications. Radiological diagnosis by CT may initially be normal and thus further confound the diagnosis. Hence, providers should remain vigilant for associated complications even with low‐dose prophylactic heparin or low‐molecular‐weight heparin in postoperative patients, and prompt, presumptive treatment with corticosteroids should be started while awaiting confirmation by imaging and laboratory testing.
- .Adrenal hemorrhage during anticoagulant therapy. A clinical and pathological study of ten cases.Ann Intern Med.1965;63(4):559–571.
- ,,,,,.Adrenal hemorrhage in the adult.Medicine.1978;57(3):211–221.
- ,,.Adrenal hemorrhage: a 25‐year experience at the Mayo Clinic.Mayo Clin Proc.2001;76(2):161–168.
- ,,.Bilateral massive adrenal hemorrhage. Assessment of putative risk factors by the case‐control method.Medicine.2001;80(1):45–53.
- .Bilateral massive adrenal hemorrhage.Med Clin North Am.1995;79(1):107–129.
- ,,.Bilateral massive adrenal hemorrhage: early recognition and treatment.Ann Intern Med.1989;110(3):227–235.
- ,,, et al.Imaging of nontraumatic hemorrhage of the adrenal gland.Radiographics.1999;19(4):949–963.
- ,,,,.Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases.J Urol.1995;154(5):1647–1651.
A 52‐year‐old man presented to the emergency department (ED) from a skilled nursing facility with a complaint of bilateral upper‐quadrant abdominal pain of 48 hours' duration. The pain was sharp, nonradiating, constant, and was associated with nausea, vomiting, and constipation. The patient denied any fever, back pain, dysuria, melena, or hematochezia. In the rehabilitation facility the patient had been initially evaluated for this pain. He was given laxatives and stool softeners for presumed constipation but these measures had not been effective. A computed tomography (CT) scan of the abdomen had only showed stool in the colon and he was sent to the ED for further evaluation.
Apart from severe degenerative joint disease in both his knees he was in good health. He was in the skilled nursing facility (SNF) for rehabilitation for bilateral knee replacement surgery done 9 days prior to this presentation. His postoperative course was unremarkable. He had been maintained on prophylaxis for venous thromboembolism with enoxaparin since postoperative day 1 at a daily dose of 40 mg subcutaneously, and was transferred to the SNF on postoperative day 6 on the same dose. His was receiving oxycodone and Tylenol for pain. He was on no other medications.
Vital signs on presentation revealed a temperature of 97.5F, a heart rate of 100 beats per minute, a respiratory rate of 16 breaths per minute, and a blood pressure of 136/69 mmHg. He was alert and oriented and in mild distress from the abdominal pain. Examination was normal except for tenderness in the upper quadrants of the abdomen though no rigidity or rebound tenderness were noted. Routine chemistries were normal except for sodium of 134 mg/dL. His white count, hemoglobin, hematocrit, and platelet levels were noted to be at 17.5K/L, 10 g/dL, 30%, and 345K/L, respectively, and were stable with regard to his discharge laboratory values. His serum eosinophil level was normal. A complete workup for hypercoagulable state and bleeding disorders including assays for antibodies associated with heparin‐induced thrombocytopenia were negative. He was admitted for further evaluation and treatment.
The patient had another CT scan of the abdomen (Figure 1), which when compared to the one done at the SNF 2 days prior showed markedly enlarged bilateral adrenal glands suggestive of bilateral acute adrenal hemorrhage. The enoxaparin was discontinued and empiric steroid replacement therapy was begun. A random cortisol level was normal but a cosyntropin stimulation test showed an absolute increase in cortisol level of only 0.8 g/dL at both 30 and 60 minutes after administration of 250 g of cosyntropin. An investigation was undertaken to determine if the patient had any prior risk factors for bleeding. There was no evidence of infection and a comprehensive evaluation for bleeding, and coagulation disorders was normal. The bilateral adrenal hemorrhage was attributed to the use of enoxaparin in the postoperative setting. Unfortunately, the patient subsequently developed a deep venous thrombosis in his lower extremity and an inferior vena cava (IVC) filter was placed before discharge. He was doing well 6 months later, and is still continued on glucocorticoid and mineralocorticoid replacement therapy and follows up with endocrinology as an outpatient.

Discussion
Bilateral adrenal hemorrhage is usually associated with massive sepsis from Gram‐negative organisms such as Neisseria meningitides, Pseudomonas aeroginosa, Escherichia coli, and Bacteroides fragilis. Rupert Waterhouse, in 1911, was the first person to describe a patient with severe meningococcal sepsis resulting in acute adrenal hemorrhage and collapse. This was also later described independently by Carl Friderichsen in 1918, and is now referred to as the Waterhouse‐Friderichsen syndrome. Other causes include antiphospholipid antibody syndrome, heparin‐associated thrombocytopenia (HIT), and severe physical stress. Bilateral adrenal hemorrhage can also spontaneously occur in the postoperative period, especially after cardiothoracic or orthopedic surgery. This phenomenon may be related to the frequent use of prophylactic anticoagulants after these types of procedures.
The first case report of bilateral adrenal hemorrhage secondary to use of anticoagulants was described in 1947, and the first case report of successful resuscitation after corticosteroid administration in a patient with bilateral adrenal hemorrhage secondary to anticoagulant use was described by Thorn in 1956.1 A review of the literature demonstrates multiple case reports of adrenal hemorrhage reported in the postoperative period, particularly after joint arthroplasty, and especially after knee replacement surgeries. Most of the recent cases have been associated with use of prophylactic low‐dose heparin or low‐molecular‐weight heparin at the time of adrenal hemorrhage. In a study of 157 case reports of individuals with bilateral hemorrhage (including 22 autopsies), 48 cases were associated with administration of anticoagulants, although the dose and effect were not specified.2 Amador et al.1 showed that out of 4325 autopsies performed from 1949 to 1962 in their institution, 30 cases were found of bilateral hemorrhage, of which 10 were receiving heparin at presumably prophylactic doses; 5 of these patients were also receiving dicumarol.
Mayo Clinic investigators performed a retrospective review of all cases of adrenal hemorrhage over a period of 25 years at their hospital, and found 141 cases of adrenal hemorrhage, of which 78 were bilateral and 63 were unilateral,3 and in 67 patients the condition was diagnosed at autopsy. In this study 14 patients had adrenal hemorrhage in the postoperative period in the absence of lupus anticoagulant or HIT; there was no specific mention in this study of the use of postoperative anticoagulants. Finally, a multicenter case control study was undertaken by Kovacs et al.4 to assess putative risk factors for development of bilateral massive adrenal hemorrhage. In the multivariate analysis, thrombocytopenia, exposure to heparin, and sepsis were found to be strongly associated with risk of hemorrhage. Of 23 patients with bilateral, massive adrenal hemorrhage, 16 had been exposed to heparin, and at least 6 were on exclusively subcutaneous heparin. The authors concluded that heparin exposure was a much bigger risk factor than other coagulopathies, and those exposed to heparin of any route or type for 4 to 6 days and those exposed for more than 6 days were about 17 and 34 times, respectively, more likely to develop bilateral hemorrhage than those who had less than 4 days or no exposure.
The clinical presentation of adrenal insufficiency due to bilateral adrenal hemorrhage is often nonspecific. Symptoms may include abdominal pain, back pain, fever, nausea, vomiting, weakness, obtundation, confusion, and hypotensionall of which are also common postoperative symptoms and can be missed or ignored.5 Rao et al.6 profiled the clinical presentation of 64 cases of bilateral hemorrhage and found the following: abdominal, flank, back, or chest pain (86%); anorexia, nausea, or vomiting (47%); psychiatric symptoms (42%); fever (66%); hypotension recognized before shock episode (19%); and abdominal rigidity or rebound (22%). Adrenal insufficiency becomes clinically evident once 90% of the gland is destroyed. About 50% of patients do not manifest typical laboratory abnormalities, so a high degree of suspicion is necessary to diagnose the condition.3 Also, the laboratory diagnosis of adrenal insufficiency using random cortisol levels is unreliable, as reference ranges in patients experiencing stress (as in the postoperative period) have not been well studied or established. In patients with bilateral hemorrhage postoperatively on prophylactic anticoagulants, the coagulation profile is usually within normal limits and there is typically no evidence of spontaneous bleeding elsewhere. In later stages, the typical laboratory findings of abnormal adrenal function such as hypokalemia, hyponatremia, declining cortisol levels, and an inappropriate response to adrenocorticotropic hormone stimulation test may be seen. A significant drop in hemoglobin secondary to hemorrhage may also be encountered in some patients secondary to the bleed.
CT is the most reliable and extensively used imaging modality for making the diagnosis, although magnetic resonance imaging (MRI) or ultrasound may also be utilized. Early in the course of adrenal hemorrhage, CT findings may be negative, and repeated imaging is appropriate when clinical suspicion is high. The presence of bilateral adrenal enlargement with increased signal attenuation suggests bilateral adrenal hemorrhage. MRI can both characterize adrenal hematomas, and estimate their age.7, 8
Postoperative adrenal hemorrhage and insufficiency is easily treatable and has excellent outcomes; survivors will need lifelong corticosteroid replacement (and usually mineralocorticoid replacement as well). In the Mayo Clinic study, survival was 100% with treatment vs. 17% without treatment. In comparison, sepsis‐induced or stress‐induced adrenal insufficiency has poor outcomes despite adequate treatment (9% survival with treatment vs. 6% survival without treatment).3 Death can occur within hours to days of symptoms if untreated. Treatment includes timely initiation of adrenal hormone replacement and reversal of coagulopathies.
Postoperative venous thromboembolism (VTE) prophylaxis with anticoagulants is the appropriate care in many cases, but, along with the postoperative state itself, also appears to be a risk factor for this unusual condition. Postoperative bilateral adrenal hemorrhage is rare and potentially fatal. Early identification and prompt initiation of steroid replacement therapy and reversal of coagulopathies can prove to be lifesaving. Making this diagnosis can be very challenging, as the clinical presentation and laboratory findings of adrenal hemorrhage are vague and nonspecific and mimic many nonlife threatening postoperative complications. Radiological diagnosis by CT may initially be normal and thus further confound the diagnosis. Hence, providers should remain vigilant for associated complications even with low‐dose prophylactic heparin or low‐molecular‐weight heparin in postoperative patients, and prompt, presumptive treatment with corticosteroids should be started while awaiting confirmation by imaging and laboratory testing.
A 52‐year‐old man presented to the emergency department (ED) from a skilled nursing facility with a complaint of bilateral upper‐quadrant abdominal pain of 48 hours' duration. The pain was sharp, nonradiating, constant, and was associated with nausea, vomiting, and constipation. The patient denied any fever, back pain, dysuria, melena, or hematochezia. In the rehabilitation facility the patient had been initially evaluated for this pain. He was given laxatives and stool softeners for presumed constipation but these measures had not been effective. A computed tomography (CT) scan of the abdomen had only showed stool in the colon and he was sent to the ED for further evaluation.
Apart from severe degenerative joint disease in both his knees he was in good health. He was in the skilled nursing facility (SNF) for rehabilitation for bilateral knee replacement surgery done 9 days prior to this presentation. His postoperative course was unremarkable. He had been maintained on prophylaxis for venous thromboembolism with enoxaparin since postoperative day 1 at a daily dose of 40 mg subcutaneously, and was transferred to the SNF on postoperative day 6 on the same dose. His was receiving oxycodone and Tylenol for pain. He was on no other medications.
Vital signs on presentation revealed a temperature of 97.5F, a heart rate of 100 beats per minute, a respiratory rate of 16 breaths per minute, and a blood pressure of 136/69 mmHg. He was alert and oriented and in mild distress from the abdominal pain. Examination was normal except for tenderness in the upper quadrants of the abdomen though no rigidity or rebound tenderness were noted. Routine chemistries were normal except for sodium of 134 mg/dL. His white count, hemoglobin, hematocrit, and platelet levels were noted to be at 17.5K/L, 10 g/dL, 30%, and 345K/L, respectively, and were stable with regard to his discharge laboratory values. His serum eosinophil level was normal. A complete workup for hypercoagulable state and bleeding disorders including assays for antibodies associated with heparin‐induced thrombocytopenia were negative. He was admitted for further evaluation and treatment.
The patient had another CT scan of the abdomen (Figure 1), which when compared to the one done at the SNF 2 days prior showed markedly enlarged bilateral adrenal glands suggestive of bilateral acute adrenal hemorrhage. The enoxaparin was discontinued and empiric steroid replacement therapy was begun. A random cortisol level was normal but a cosyntropin stimulation test showed an absolute increase in cortisol level of only 0.8 g/dL at both 30 and 60 minutes after administration of 250 g of cosyntropin. An investigation was undertaken to determine if the patient had any prior risk factors for bleeding. There was no evidence of infection and a comprehensive evaluation for bleeding, and coagulation disorders was normal. The bilateral adrenal hemorrhage was attributed to the use of enoxaparin in the postoperative setting. Unfortunately, the patient subsequently developed a deep venous thrombosis in his lower extremity and an inferior vena cava (IVC) filter was placed before discharge. He was doing well 6 months later, and is still continued on glucocorticoid and mineralocorticoid replacement therapy and follows up with endocrinology as an outpatient.

Discussion
Bilateral adrenal hemorrhage is usually associated with massive sepsis from Gram‐negative organisms such as Neisseria meningitides, Pseudomonas aeroginosa, Escherichia coli, and Bacteroides fragilis. Rupert Waterhouse, in 1911, was the first person to describe a patient with severe meningococcal sepsis resulting in acute adrenal hemorrhage and collapse. This was also later described independently by Carl Friderichsen in 1918, and is now referred to as the Waterhouse‐Friderichsen syndrome. Other causes include antiphospholipid antibody syndrome, heparin‐associated thrombocytopenia (HIT), and severe physical stress. Bilateral adrenal hemorrhage can also spontaneously occur in the postoperative period, especially after cardiothoracic or orthopedic surgery. This phenomenon may be related to the frequent use of prophylactic anticoagulants after these types of procedures.
The first case report of bilateral adrenal hemorrhage secondary to use of anticoagulants was described in 1947, and the first case report of successful resuscitation after corticosteroid administration in a patient with bilateral adrenal hemorrhage secondary to anticoagulant use was described by Thorn in 1956.1 A review of the literature demonstrates multiple case reports of adrenal hemorrhage reported in the postoperative period, particularly after joint arthroplasty, and especially after knee replacement surgeries. Most of the recent cases have been associated with use of prophylactic low‐dose heparin or low‐molecular‐weight heparin at the time of adrenal hemorrhage. In a study of 157 case reports of individuals with bilateral hemorrhage (including 22 autopsies), 48 cases were associated with administration of anticoagulants, although the dose and effect were not specified.2 Amador et al.1 showed that out of 4325 autopsies performed from 1949 to 1962 in their institution, 30 cases were found of bilateral hemorrhage, of which 10 were receiving heparin at presumably prophylactic doses; 5 of these patients were also receiving dicumarol.
Mayo Clinic investigators performed a retrospective review of all cases of adrenal hemorrhage over a period of 25 years at their hospital, and found 141 cases of adrenal hemorrhage, of which 78 were bilateral and 63 were unilateral,3 and in 67 patients the condition was diagnosed at autopsy. In this study 14 patients had adrenal hemorrhage in the postoperative period in the absence of lupus anticoagulant or HIT; there was no specific mention in this study of the use of postoperative anticoagulants. Finally, a multicenter case control study was undertaken by Kovacs et al.4 to assess putative risk factors for development of bilateral massive adrenal hemorrhage. In the multivariate analysis, thrombocytopenia, exposure to heparin, and sepsis were found to be strongly associated with risk of hemorrhage. Of 23 patients with bilateral, massive adrenal hemorrhage, 16 had been exposed to heparin, and at least 6 were on exclusively subcutaneous heparin. The authors concluded that heparin exposure was a much bigger risk factor than other coagulopathies, and those exposed to heparin of any route or type for 4 to 6 days and those exposed for more than 6 days were about 17 and 34 times, respectively, more likely to develop bilateral hemorrhage than those who had less than 4 days or no exposure.
The clinical presentation of adrenal insufficiency due to bilateral adrenal hemorrhage is often nonspecific. Symptoms may include abdominal pain, back pain, fever, nausea, vomiting, weakness, obtundation, confusion, and hypotensionall of which are also common postoperative symptoms and can be missed or ignored.5 Rao et al.6 profiled the clinical presentation of 64 cases of bilateral hemorrhage and found the following: abdominal, flank, back, or chest pain (86%); anorexia, nausea, or vomiting (47%); psychiatric symptoms (42%); fever (66%); hypotension recognized before shock episode (19%); and abdominal rigidity or rebound (22%). Adrenal insufficiency becomes clinically evident once 90% of the gland is destroyed. About 50% of patients do not manifest typical laboratory abnormalities, so a high degree of suspicion is necessary to diagnose the condition.3 Also, the laboratory diagnosis of adrenal insufficiency using random cortisol levels is unreliable, as reference ranges in patients experiencing stress (as in the postoperative period) have not been well studied or established. In patients with bilateral hemorrhage postoperatively on prophylactic anticoagulants, the coagulation profile is usually within normal limits and there is typically no evidence of spontaneous bleeding elsewhere. In later stages, the typical laboratory findings of abnormal adrenal function such as hypokalemia, hyponatremia, declining cortisol levels, and an inappropriate response to adrenocorticotropic hormone stimulation test may be seen. A significant drop in hemoglobin secondary to hemorrhage may also be encountered in some patients secondary to the bleed.
CT is the most reliable and extensively used imaging modality for making the diagnosis, although magnetic resonance imaging (MRI) or ultrasound may also be utilized. Early in the course of adrenal hemorrhage, CT findings may be negative, and repeated imaging is appropriate when clinical suspicion is high. The presence of bilateral adrenal enlargement with increased signal attenuation suggests bilateral adrenal hemorrhage. MRI can both characterize adrenal hematomas, and estimate their age.7, 8
Postoperative adrenal hemorrhage and insufficiency is easily treatable and has excellent outcomes; survivors will need lifelong corticosteroid replacement (and usually mineralocorticoid replacement as well). In the Mayo Clinic study, survival was 100% with treatment vs. 17% without treatment. In comparison, sepsis‐induced or stress‐induced adrenal insufficiency has poor outcomes despite adequate treatment (9% survival with treatment vs. 6% survival without treatment).3 Death can occur within hours to days of symptoms if untreated. Treatment includes timely initiation of adrenal hormone replacement and reversal of coagulopathies.
Postoperative venous thromboembolism (VTE) prophylaxis with anticoagulants is the appropriate care in many cases, but, along with the postoperative state itself, also appears to be a risk factor for this unusual condition. Postoperative bilateral adrenal hemorrhage is rare and potentially fatal. Early identification and prompt initiation of steroid replacement therapy and reversal of coagulopathies can prove to be lifesaving. Making this diagnosis can be very challenging, as the clinical presentation and laboratory findings of adrenal hemorrhage are vague and nonspecific and mimic many nonlife threatening postoperative complications. Radiological diagnosis by CT may initially be normal and thus further confound the diagnosis. Hence, providers should remain vigilant for associated complications even with low‐dose prophylactic heparin or low‐molecular‐weight heparin in postoperative patients, and prompt, presumptive treatment with corticosteroids should be started while awaiting confirmation by imaging and laboratory testing.
- .Adrenal hemorrhage during anticoagulant therapy. A clinical and pathological study of ten cases.Ann Intern Med.1965;63(4):559–571.
- ,,,,,.Adrenal hemorrhage in the adult.Medicine.1978;57(3):211–221.
- ,,.Adrenal hemorrhage: a 25‐year experience at the Mayo Clinic.Mayo Clin Proc.2001;76(2):161–168.
- ,,.Bilateral massive adrenal hemorrhage. Assessment of putative risk factors by the case‐control method.Medicine.2001;80(1):45–53.
- .Bilateral massive adrenal hemorrhage.Med Clin North Am.1995;79(1):107–129.
- ,,.Bilateral massive adrenal hemorrhage: early recognition and treatment.Ann Intern Med.1989;110(3):227–235.
- ,,, et al.Imaging of nontraumatic hemorrhage of the adrenal gland.Radiographics.1999;19(4):949–963.
- ,,,,.Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases.J Urol.1995;154(5):1647–1651.
- .Adrenal hemorrhage during anticoagulant therapy. A clinical and pathological study of ten cases.Ann Intern Med.1965;63(4):559–571.
- ,,,,,.Adrenal hemorrhage in the adult.Medicine.1978;57(3):211–221.
- ,,.Adrenal hemorrhage: a 25‐year experience at the Mayo Clinic.Mayo Clin Proc.2001;76(2):161–168.
- ,,.Bilateral massive adrenal hemorrhage. Assessment of putative risk factors by the case‐control method.Medicine.2001;80(1):45–53.
- .Bilateral massive adrenal hemorrhage.Med Clin North Am.1995;79(1):107–129.
- ,,.Bilateral massive adrenal hemorrhage: early recognition and treatment.Ann Intern Med.1989;110(3):227–235.
- ,,, et al.Imaging of nontraumatic hemorrhage of the adrenal gland.Radiographics.1999;19(4):949–963.
- ,,,,.Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases.J Urol.1995;154(5):1647–1651.
Are routine preoperative chest radiographs necessary in asymptomatic patients undergoing noncardiothoracic surgery?
In the Literature
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
In the Literature
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH