User login
Focus on menopause
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
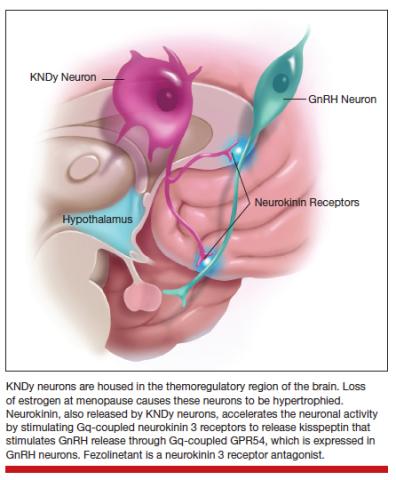
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.