User login
Establishing a Rapid Response Team
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

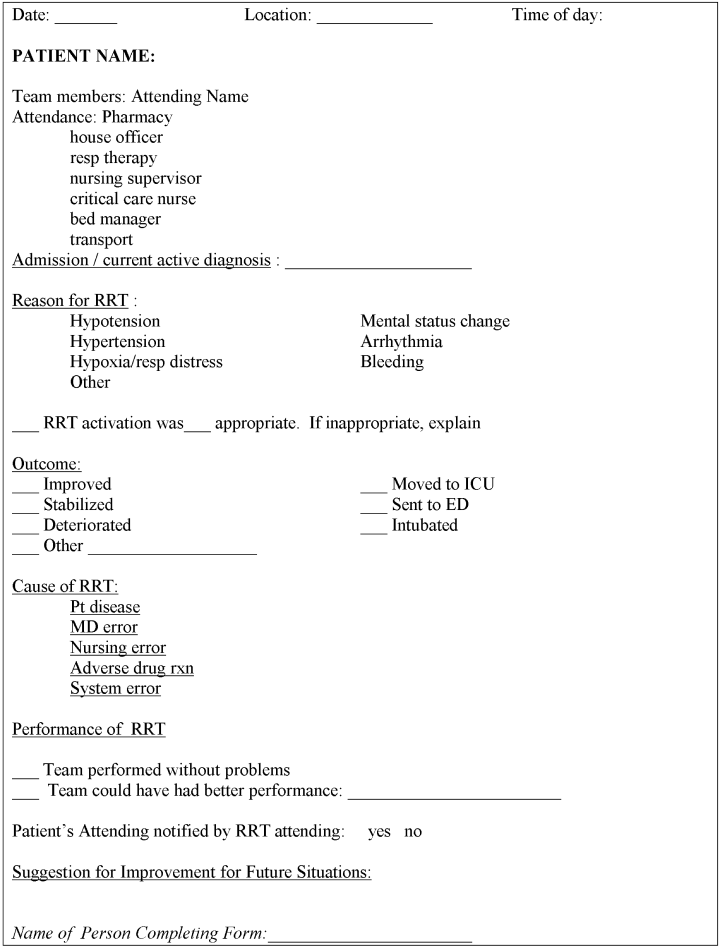
Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.
The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
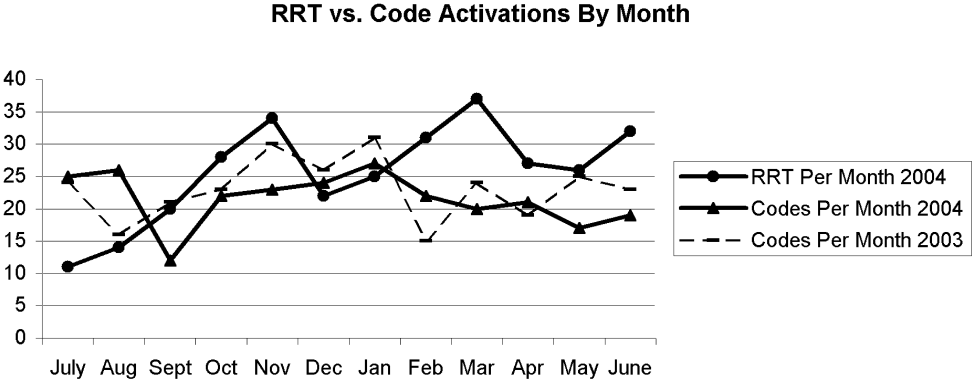
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).
Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
