User login
Causes of Unplanned ICU Transfers
Two national surveys indicate that 14% to 28% of patients admitted to intensive care units (ICU's) are unplanned transfers (i.e., moving a patient to the ICU from other areas in the hospital providing lower intensity care due to an unanticipated change in the patient's clinical status), and that the most common reason for unplanned transfers is respiratory insufficiency/failure.1, 2 Patients suffering adverse events during a hospitalization are more likely to have an unplanned ICU transfer and patients requiring unplanned transfers have a higher mortality.35 Accordingly, the Joint Commission has identified improved recognition and response to changes in a patient's condition as a national patient safety goal,6 and Rapid Response Teams (RRTs) have been advocated to deal with these changes,7 although recent studies question the effectiveness of RRTs.811
We sought to classify the causes of unplanned, in‐hospital transfers to a medical ICU (MICU) with the idea of identifying common problems in care that might be addressed by process improvement activities. We also sought to determine the fraction of patients requiring an unplanned MICU transfer that had evidence of clinical deterioration prior to the time of transfer and whether, in retrospect, different or earlier interventions might have prevented the transfer. Our hypotheses were that (1) most unplanned MICU transfers occurred as a result of errors in care, (2) most were preceded by clinical deterioration within 12 hours prior to the transfer, and (3) most were preventable.
Methods
We conducted a retrospective cohort study of patients transferring to the MICU from non‐ICU Medicine units at Denver Health, a university‐affiliated, public safety net hospital. All adult patients between 18 to 89 years of age, who were admitted to the Medicine service between June, 2005 and May, 2006 were included in the study. Exclusion criteria included patients who (1) transferred from outside hospitals, (2) transferred from nonMedicine units within Denver Health, (3) were admitted directly to the MICU from the emergency department (ED), (4) were prisoners, (5) were readmitted to the MICU during the same hospitalization, (6) were known to be pregnant, or (7) were planned MICU transfers following invasive procedures (eg, elective cardiac catheterization, defibrillator placement, ablations). Patients readmitted to the MICU were excluded because of the difficulty distinguishing between premature transfer from the MICU or potential problems in care that might have occurred prior to the time of transfer from those occurring during follow‐up care on the Medicine floor services.
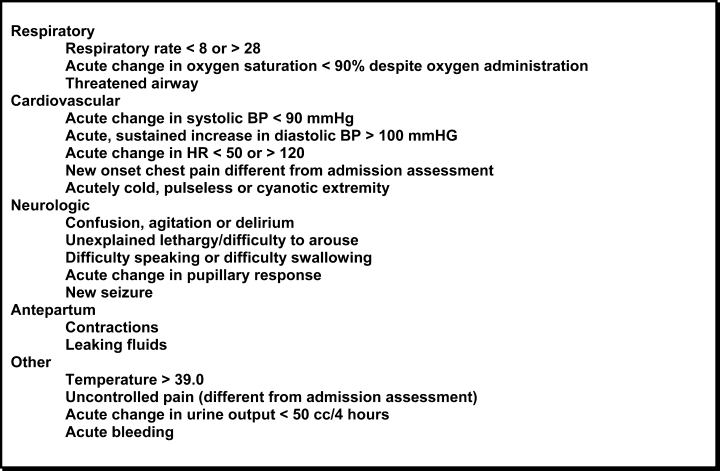
Computerized medical records of eligible patients were searched for demographic information and for admitting and transfer diagnoses (with the latter being categorized using a taxonomy we developed for classifying unplanned transfers, Table 1). Three independent observers (all of whom were board certified in Internal Medicine and had been practicing as Hospitalists at our institution for a minimum of three years) retrospectively reviewed each patient's hospital record to determine the cause of the unplanned transfer using this taxonomy. All three also made a judgment as to whether deterioration was evident at any time within the 12 hours preceding the unplanned transfer on the basis of clinical criteria used as our hospital's rapid response triggers (Table 2). When clinical triggers were found, each of the reviewers independently judged whether the unplanned transfer might have been prevented had different or earlier interventions been instituted. Each reviewer was blinded to the results of the other two.
|
| 1. Errors in triage from the Emergency Department |
| A. Diagnostic errors (conditions that were overlooked at the time of admission but explained the chief complaint). |
| B. Inadequate assessment (new diagnosis established after more extensive evaluation that could have been performed at the time of admission). |
| C. Overlooked severity (patients meeting MICU admission criteria at the time of admission from the ED). |
| 2. Worsening of condition for which the patient was admitted |
| A. Errors with assessment or treatment (evaluation or treatment that was not thought to be standard of care for the admitting diagnosis). |
| 1. Delayed (could reasonably have been instituted earlier) |
| 2. Incorrect (not thought to represent standard of care) |
| 3. Inadequate (correct, but insufficient for the admitting diagnosis) |
| B. Spontaneous worsening (worsening of the problem for which the patients were admitted to the point of requiring MICU transfer for which no specific cause could be identified) |
| 3. Development of a new problem |
| A. Iatrogenic (thought to be caused by a diagnostic or therapeutic intervention) |
| B. Spontaneous (no specific cause could be identified) |
| 4. Critical laboratory values (laboratory values needing frequent monitoring of patient and/or blood draws) |
| A. Respiratory |
| Respiratory rate <8 or >28/minute |
| Acute change in oxygen saturation to <90% despite oxygen administration |
| Threatened airway |
| B. Cardiovascular |
| Acute change in systolic blood pressure to <90 mmHg |
| Acute, sustained increase in diastolic blood pressure to >110 mmHg |
| Acute change in heart rate to <50 or >120 beats/minute |
| New onset chest pain or chest pain different than on admission assessment |
| Acutely cold and pulseless extremity. |
| C. Neurological |
| Confusion, agitation or delirium |
| Unexplained lethargy/difficult to arouse |
| Difficulty speaking or swallowing |
| Acute change in pupillary response |
| New seizure |
| D. Other |
| Temperature >39.0 Celsius |
| Uncontrolled pain (if different than admission pain assessment) |
| Acute change in urine output <50 mL/4 hours |
| Acute bleeding (bleeding with a change in vitals, urine output or mental status) |
All analyses were done using SAS Enterprise Guide 4.1, SAS Institute, Cary, NC. Data are presented as mean (standard deviation [SD]). Interobserver agreement was measured by calculating a statistic. values were interpreted by using the guidelines suggested by Landis and colleagues.12 A chi‐square test was used to seek associations between baseline characteristics, reasons for MICU transfer and mortality. P < 0.05 was considered to be statistically significant. The Colorado Multiple Institutional Review Board approved the research protocol.
Results
Over the period of the study the Medicine floor services had 4468 admissions of which 152 met the inclusion criteria for having an unplanned MICU transfer (Table 3). The most common admitting diagnoses were heart failure (12%) and community acquired pneumonia (9%). The most common diagnoses to which the unplanned MICU transfers were attributed were respiratory failure (27%) and sepsis (9%) (Table 4). Seven cardiopulmonary arrests were successfully resuscitated and transferred to the MICU. Throughout the period of the study, no patients were admitted to non‐MICU units because the MICU was at full capacity. Additionally the investigators did not find any inordinate delays in transfer to the ICU while waiting for a bed.
| |
| Age (years) mean (SD) | 52 14 |
| Gender (male:female) | |
| Number | 95:57 |
| % | 63:37 |
| Race, n (%) | |
| White, non‐Hispanic | 54 (35) |
| White, Hispanic | 59 (39) |
| Black | 30 (20) |
| Other | 9 (6) |
| Primary language, n (%) | |
| English | 131 (86) |
| Spanish | 17 (11) |
| Other | 4 (3) |
| Length of stay prior to transfer (hours) (median, IQR) | 46, 89 |
| Admitting diagnosis, n (%) | |
| Acute decompensated heart failure (systolic/diastolic) | 18 (12) |
| Community acquired pneumonia | 13 (9) |
| Suspected acute coronary syndrome | 9 (6) |
| Delirium | 8 (5) |
| Acute kidney injury | 8 (5) |
| Abdominal pain | 8 (5) |
| Respiratory failure | 6 (4) |
| |
| Respiratory failure (cardiogenic/non‐cardiogenic) | 41 (27) |
| Sepsis | 14 (9) |
| Hypotension | 13 (9) |
| Gastrointestinal bleeding | 12 (8) |
| Tachyarrhythmia | 9 (6) |
| Cardiac arrest | 7 (5) |
| Hypertensive emergency | 7 (5) |
| Acute coronary syndrome | 7 (5) |
A total of 51 patients (34%) were transferred within the first 24 hours of admission. The most common diagnoses resulting in transfer in this group were respiratory failure, hypertensive emergency, hypotension, gastrointestinal bleed, and acute coronary syndrome. The remaining 101 patients (66%) were transferred from two to 15 days following admission for a variety of problems but respiratory failure was most common (34 patients, 22%).
Worsening of the problem for which the patients were initially admitted accounted for the unplanned transfers of 73 patients (48%) (Table 5). Development of a new problem unrelated to the admitting diagnosis accounted for the transfer in 59 patients (39%). Five patients were transferred to the ICU for a critical laboratory value that required a closer monitoring of the patient or needed more frequent lab draws that could not be achieved on the floor.
| Causes | n (%) |
|---|---|
| |
| 1. Errors in triage from the emergency department: | 15 (10) |
| A. Diagnostic errors: | 1 (0.7) |
| B. Inadequate assessment: | 0 (0) |
| C. Overlooked severity: | 14 (9) |
| 2. Worsening of condition for which the patient was admitted: | 73 (48) |
| A. Problems with assessment or treatment: | 5 (3) |
| 1. Delayed | 1 (0.7) |
| 2. Incorrect | 1 (0.7) |
| 3. Inadequate | 3 (2) |
| B. Spontaneous worsening | 68 (45) |
| 3. Development of a new problem | 59 (39) |
| A. Iatrogenic | 9 (6) |
| B. Spontaneous | 50 (33) |
| 4. Critical laboratory values | 5 (3) |
Errors in care were thought to be present in 29 patients (19% of the unplanned transfers). For 15 of these (52%) the error involved incorrect triage from the ED as 14 of the 15 patients met MICU admission criteria at the time they were triaged to non‐MICU units (Table 6). The remaining patient had a dissecting aortic aneurysm that was not considered while he was being evaluated for acute chest pain. All these patients were transferred to the ICU within 24 hours of their admission and the reviewers agreed that all could have been prevented if existing diagnostic and admission algorithms were followed.
|
| Hemodynamic instability requiring vasopressor agents, continued aggressive fluid resuscitation, or central venous/pulmonary artery catheter monitoring or balloon pump |
| Acute respiratory failure with ongoing or impending need for ventilatory support (either invasively or non‐ invasively). |
| Gastrointestinal bleeding meeting ICU admission criteria (>2 clinical risk factors and Rockall score >3 per Gastrointestinal Bleeding Protocol) |
| Cardiac chest pains associated with two of the three criteria |
| Ongoing ischemic chest pain |
| Enzyme elevation |
| ST segment depression <0.5 mm in 2 consecutives leads or transient ST‐segment elevation |
| Chest pain requiring IV nitroglycerin infusion. |
| Complex cardiac arrhythmia requiring close monitoring and/or intravenous infusion therapy |
| Temporary pacemaker. |
| Hypertensive crisis with end‐organ dysfunction or aortic dissection requiring intravenous treatment. |
| Massive hemoptysis (>500 cc/24 hours) |
| Acute neurological dysfunction requiring one of |
| ICP monitoring, |
| Acute respiratory failure with impending need for ventilatory support |
| Hourly neurological checks. |
| Status epilepticus |
| Post‐operative patients requiring hemodynamic monitoring/ventilator support of extensive nursing care. |
| Severe metabolic disorder or intoxication requiring frequent monitoring and/or intravenous infusion therapy that cannot be administered on a floor. |
| Multiple trauma, including severe head and spine trauma |
| Other indication (please specify) |
Of the remaining 14 patients thought to have errors in care, nine were classified as the development of a new, iatrogenic problem (ie, opiate or benzodiazepine overdose occurring during treatment for pain and/or anxiety in 3, volume overload in 2, insulin‐induced hypoglycemia, antibiotic associated reaction, ‐blocker overdose and acute renal failure from over‐diuresis in one each) and five occurred because the patient's admitting problem worsened because treatment was thought to be either delayed, incorrect, or inadequate (Table 5). The reviewers all agreed that the unplanned transfers could have been prevented in eight of the 14 patients who developed iatrogenic problems if existing algorithms were followed or if an earlier or different intervention had occurred. The reviewers did not agree about whether the unplanned transfer could have been prevented in one patient who developed an iatrogenic problem and in all five patients whose underlying condition worsened. Accordingly, in sum, the reviewers felt that 23 of the 152 unplanned transfers (15%) could have been prevented.
In addition to trying to determine how many of the unplanned MICU transfers could have been prevented, we also investigated the utility of rapid response triggers in alerting the physicians and nurses of impending deteriorations in status and whether earlier recognition of this deterioration might have prevented the transfers. Of the 152 unplanned transfers, 106 (70%) had one or more rapid response triggers within the preceding 12 hours. All three reviewers agreed and concluded that in 94 (89%) of these, the unplanned transfer could not have been prevented, even with different or earlier interventions. For five patients (5% of the 106) all reviewers agreed and concluded that earlier intervention might have averted the subsequent transfer. For the other seven patients (6%), no consensus was reached. If we assume that, for all of these latter seven, earlier or different intervention might have averted the unplanned transfer, a maximum of 12 unplanned transfers (11% of the 106) might have been prevented by having a system of care that employed regularly assessing rapid response triggers and acting on them when recognized.
The interobserver reliability for the three reviewers was moderate to almost perfect with = 0.60, 95% confidence interval (CI) (0.31, 0.88); = 0.90, 95% CI (0.71, 1); = 0.55, 95% CI (0.26, 0.84).
A total of 27 (18%) of the patients with unplanned transfers died in the MICU. During this same time period 91 of 1511 patients (6%) admitted directly from the ED to the MICU died (P < 0.05). Mortality was lower for patients transferred within 24 hours of admission compared to those transferred > 24 hours after admission (4% vs. 22% mortality, respectively, P < 0.05; 95% CI, 0.09‐0.89). We found no difference in mortality as a function of time of admission or time of transfer implying that differences in staffing, or the availability of various services, did not contribute to the unplanned transfers.
Discussion
The important findings of this study were that (1) 19% of unplanned, in‐hospital transfers from Medicine floor services to the MICU seemed to result from apparent errors in care, (2) 15% of the unplanned transfers were potentially preventable, (3) the majority of the errors in care involved inappropriate triage of patients from the ED to the non‐MICU units, (4) 106 (70%) of the patients requiring unplanned transfers developed rapid response criteria within 12 hours prior to the transfer, but on review of these (5) the transfer was thought to be preventable in only a maximum of 12 (11%).
We designed our study in part to find specific errors that commonly resulted in unplanned MICU transfers with the idea that, if these could be identified, they might be corrected, thereby improving care. Contrary to our hypothesis we found that only 29 (19%) of the unplanned transfers seemed to result from errors in care. Of these, however, half were attributable to overlooking that patients met our own institution's MICU admission criteria at the time they were triaged to non‐MICU units. This result is consistent with Walter et al.13 finding that while 88% of MICUs in academic health centers had written MICU admission criteria, only 25% used these criteria on a regular basis. Hospital mortality is likely lower for patients meeting MICU admission criteria when they are appropriately and expeditiously triaged.1418 Accordingly, developing mechanisms by which patients are routinely screened for meeting MICU admission criteria could and should reduce this source of error and improve patient outcomes.
Nine of the remaining 14 errors in care resulted from what the chart reviewers concluded was overly aggressive treatment; either excess fluid resuscitation or excess treatment of pain or anxiety. It is not clear that these represent correctable errors in care, however, as hypotensive patients require fluid resuscitation, and patients with pain or anxiety should receive analgesics or anxiolytics and it is not reasonable to expect that these interventions will be appropriately titrated in every instance. Nonetheless, our reviewers all agreed that, in eight of these patients, different interventions could have prevented the unplanned transfer.
Since 41 (27%) of the unplanned transfers were for respiratory failure, we reviewed each of these patients' records seeking evidence suggesting that the problem might have resulted from excessive use of fluids, narcotics, or anxiolytics. By retrospective analysis only six such cases could be identified. Most were due to worsening of the problem for which the patient was admitted.
Consistent with our hypothesis the majority of patients requiring unplanned MICU transfers (106/152, 70%) developed rapid response clinical triggers within the 12 hours preceding transfer, as has been previously demonstrated by Hillman et al.7 and others.8‐10, 19 Our reviewers tried to determine whether earlier or different interventions might have prevented the deterioration and the resulting unplanned transfer. Interestingly, in the large majority (94/106, 89%) they concluded that nothing different could have been done and that the transfer could not have been avoided. While this observation contrasts with our hypothesis, it is consistent with two studies questioning the utility of RRTs in preventing unplanned ICU transfers.9, 10 In addition some patients may ultimately need an ICU transfer despite receiving appropriate interventions as it is impossible to prevent an ICU transfer in every patient. Conversely, just because a patient meets a rapid response criteria does not mean that the patient needs a higher level of care or an ICU transfer as some can be safely managed on the floor.
Our study has a number of potential limitations. The data came from a single teaching hospital and we only assessed patients admitted to General Internal Medicine units and transferred to a MICU. Accordingly, our results might not generalize to other hospitals (teaching or nonteaching), to other services or to other types of ICUs. We found, however, that (1) unplanned transfers accounted for 10% of the total admissions to our MICU, a similar fraction as reported by Angus et al.1 in 2006; (2) respiratory failure/emnsufficiency and sepsis were the most common diagnoses leading to unplanned transfers as previously reported by Groeger et al.2 and Hillman et al.5; (3) mortality was increased in patients requiring unplanned transfer, as noted by Escarce and Kelley3 and Hillman et al.5; and (4) patients who were transferred to the MICU within 24 hours of admission had better outcomes than those who were transferred later, as reported by Goldhill et al.4 Accordingly, our patient population seems quite similar to others in the literature.
Since we did not use objective criteria to assign patients to each of the categories itemized in Table 5 we could have misclassified patients with respect to the cause for their unplanned MICU transfer. Despite this shortcoming, however, the scores among our independent reviewers were moderate to almost perfect suggesting misclassification did not occur commonly.
Our retrospective study design may have underestimated the utility of RRTs as we had no way of knowing the outcomes of patients who met rapid response criteria and had interventions that prevented unplanned MICU transfers.
In summary, approximately 15% of unplanned MICU transfers seem to be preventable and approximately one‐fifth seem to result from errors in care, the majority of which are errors in triage from the ED. While the large majority of unplanned transfers were preceded by clinical deterioration within the preceding 12 hours, manifested by the presence of rapid response triggers, the large majority of these do not seem to be preventable. From these findings we suggest that unplanned transfers could be reduced by more closely screening patients for the presence of defined MICU admission criteria at the time of admission from the ED, by recognizing that fluid resuscitation and control of pain and/or anxiety can have adverse effects and by monitoring patients receiving these interventions more closely.
- ,,,,,.Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34(4):1016–1024.
- ,,, et al.Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization.Crit Care Med.1993;21(2):279–291.
- ,.Admission source to the medical intensive care unit predicts hospital death independent of APACHE II score.JAMA.1990;264(18):2389–2394.
- ,,,.The longer patients are in hospital before Intensive Care admission the higher their mortality.Intensive Care Med.2004;30(10):1908–1913.
- ,,,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28(11):1629–1634.
- Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission Hospital Accreditation Program, National Patient Safety Goals, Goal 16; 2008. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed May2010.
- ,,, et al.MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365(9477):2091–2097.
- ,,,,,.Rapid response systems: a systematic review.Crit Care Med.2007;35(5):1238–1243.
- ,,,,,.Hospital‐wide code rates and mortality before and after implementation of a rapid response team.JAMA.2008;300(21):2506–2513.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,,,,.Rapid response teams: a systematic review and meta‐analysis.Arch Intern Med.2010;170(1):18–26.
- ,.The measurement of observer agreement for categorical data.Biometrics.1977;33(1):159–174.
- ,,.How decisions are made to admit patients to medical intensive care units (MICUs): A survey of MICU directors at academic medical centers across the United States.Crit Care Med.2008;36:414–420.
- ,,.Mortality among appropriately referred patients refused admission to intensive‐care units.Lancet.1997;350:7–12.
- ,,,,,.Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome.Intensive Care Med.2001;27:1459–1465.
- ,,,,,for the Values, Ethics and Rationing in Critical Care (VERICC) Task Force. Rationing critical care beds: A systematic review.Crit Care Med.2004;32:1588–1597.
- ,,, et al.Survival of critically ill patients hospitalized in and out of intensive care.Crit Care Med.2007;35:449–457.
- ,,,,,for the DELAY‐ED study group. Impact of delayed transfer of critically ill patients form the emergency department to the intensive care unit.Crit Care Med.2007;35:1477–1483.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31(6):343–348.
Two national surveys indicate that 14% to 28% of patients admitted to intensive care units (ICU's) are unplanned transfers (i.e., moving a patient to the ICU from other areas in the hospital providing lower intensity care due to an unanticipated change in the patient's clinical status), and that the most common reason for unplanned transfers is respiratory insufficiency/failure.1, 2 Patients suffering adverse events during a hospitalization are more likely to have an unplanned ICU transfer and patients requiring unplanned transfers have a higher mortality.35 Accordingly, the Joint Commission has identified improved recognition and response to changes in a patient's condition as a national patient safety goal,6 and Rapid Response Teams (RRTs) have been advocated to deal with these changes,7 although recent studies question the effectiveness of RRTs.811
We sought to classify the causes of unplanned, in‐hospital transfers to a medical ICU (MICU) with the idea of identifying common problems in care that might be addressed by process improvement activities. We also sought to determine the fraction of patients requiring an unplanned MICU transfer that had evidence of clinical deterioration prior to the time of transfer and whether, in retrospect, different or earlier interventions might have prevented the transfer. Our hypotheses were that (1) most unplanned MICU transfers occurred as a result of errors in care, (2) most were preceded by clinical deterioration within 12 hours prior to the transfer, and (3) most were preventable.
Methods
We conducted a retrospective cohort study of patients transferring to the MICU from non‐ICU Medicine units at Denver Health, a university‐affiliated, public safety net hospital. All adult patients between 18 to 89 years of age, who were admitted to the Medicine service between June, 2005 and May, 2006 were included in the study. Exclusion criteria included patients who (1) transferred from outside hospitals, (2) transferred from nonMedicine units within Denver Health, (3) were admitted directly to the MICU from the emergency department (ED), (4) were prisoners, (5) were readmitted to the MICU during the same hospitalization, (6) were known to be pregnant, or (7) were planned MICU transfers following invasive procedures (eg, elective cardiac catheterization, defibrillator placement, ablations). Patients readmitted to the MICU were excluded because of the difficulty distinguishing between premature transfer from the MICU or potential problems in care that might have occurred prior to the time of transfer from those occurring during follow‐up care on the Medicine floor services.
Computerized medical records of eligible patients were searched for demographic information and for admitting and transfer diagnoses (with the latter being categorized using a taxonomy we developed for classifying unplanned transfers, Table 1). Three independent observers (all of whom were board certified in Internal Medicine and had been practicing as Hospitalists at our institution for a minimum of three years) retrospectively reviewed each patient's hospital record to determine the cause of the unplanned transfer using this taxonomy. All three also made a judgment as to whether deterioration was evident at any time within the 12 hours preceding the unplanned transfer on the basis of clinical criteria used as our hospital's rapid response triggers (Table 2). When clinical triggers were found, each of the reviewers independently judged whether the unplanned transfer might have been prevented had different or earlier interventions been instituted. Each reviewer was blinded to the results of the other two.
|
| 1. Errors in triage from the Emergency Department |
| A. Diagnostic errors (conditions that were overlooked at the time of admission but explained the chief complaint). |
| B. Inadequate assessment (new diagnosis established after more extensive evaluation that could have been performed at the time of admission). |
| C. Overlooked severity (patients meeting MICU admission criteria at the time of admission from the ED). |
| 2. Worsening of condition for which the patient was admitted |
| A. Errors with assessment or treatment (evaluation or treatment that was not thought to be standard of care for the admitting diagnosis). |
| 1. Delayed (could reasonably have been instituted earlier) |
| 2. Incorrect (not thought to represent standard of care) |
| 3. Inadequate (correct, but insufficient for the admitting diagnosis) |
| B. Spontaneous worsening (worsening of the problem for which the patients were admitted to the point of requiring MICU transfer for which no specific cause could be identified) |
| 3. Development of a new problem |
| A. Iatrogenic (thought to be caused by a diagnostic or therapeutic intervention) |
| B. Spontaneous (no specific cause could be identified) |
| 4. Critical laboratory values (laboratory values needing frequent monitoring of patient and/or blood draws) |
| A. Respiratory |
| Respiratory rate <8 or >28/minute |
| Acute change in oxygen saturation to <90% despite oxygen administration |
| Threatened airway |
| B. Cardiovascular |
| Acute change in systolic blood pressure to <90 mmHg |
| Acute, sustained increase in diastolic blood pressure to >110 mmHg |
| Acute change in heart rate to <50 or >120 beats/minute |
| New onset chest pain or chest pain different than on admission assessment |
| Acutely cold and pulseless extremity. |
| C. Neurological |
| Confusion, agitation or delirium |
| Unexplained lethargy/difficult to arouse |
| Difficulty speaking or swallowing |
| Acute change in pupillary response |
| New seizure |
| D. Other |
| Temperature >39.0 Celsius |
| Uncontrolled pain (if different than admission pain assessment) |
| Acute change in urine output <50 mL/4 hours |
| Acute bleeding (bleeding with a change in vitals, urine output or mental status) |
All analyses were done using SAS Enterprise Guide 4.1, SAS Institute, Cary, NC. Data are presented as mean (standard deviation [SD]). Interobserver agreement was measured by calculating a statistic. values were interpreted by using the guidelines suggested by Landis and colleagues.12 A chi‐square test was used to seek associations between baseline characteristics, reasons for MICU transfer and mortality. P < 0.05 was considered to be statistically significant. The Colorado Multiple Institutional Review Board approved the research protocol.
Results
Over the period of the study the Medicine floor services had 4468 admissions of which 152 met the inclusion criteria for having an unplanned MICU transfer (Table 3). The most common admitting diagnoses were heart failure (12%) and community acquired pneumonia (9%). The most common diagnoses to which the unplanned MICU transfers were attributed were respiratory failure (27%) and sepsis (9%) (Table 4). Seven cardiopulmonary arrests were successfully resuscitated and transferred to the MICU. Throughout the period of the study, no patients were admitted to non‐MICU units because the MICU was at full capacity. Additionally the investigators did not find any inordinate delays in transfer to the ICU while waiting for a bed.
| |
| Age (years) mean (SD) | 52 14 |
| Gender (male:female) | |
| Number | 95:57 |
| % | 63:37 |
| Race, n (%) | |
| White, non‐Hispanic | 54 (35) |
| White, Hispanic | 59 (39) |
| Black | 30 (20) |
| Other | 9 (6) |
| Primary language, n (%) | |
| English | 131 (86) |
| Spanish | 17 (11) |
| Other | 4 (3) |
| Length of stay prior to transfer (hours) (median, IQR) | 46, 89 |
| Admitting diagnosis, n (%) | |
| Acute decompensated heart failure (systolic/diastolic) | 18 (12) |
| Community acquired pneumonia | 13 (9) |
| Suspected acute coronary syndrome | 9 (6) |
| Delirium | 8 (5) |
| Acute kidney injury | 8 (5) |
| Abdominal pain | 8 (5) |
| Respiratory failure | 6 (4) |
| |
| Respiratory failure (cardiogenic/non‐cardiogenic) | 41 (27) |
| Sepsis | 14 (9) |
| Hypotension | 13 (9) |
| Gastrointestinal bleeding | 12 (8) |
| Tachyarrhythmia | 9 (6) |
| Cardiac arrest | 7 (5) |
| Hypertensive emergency | 7 (5) |
| Acute coronary syndrome | 7 (5) |
A total of 51 patients (34%) were transferred within the first 24 hours of admission. The most common diagnoses resulting in transfer in this group were respiratory failure, hypertensive emergency, hypotension, gastrointestinal bleed, and acute coronary syndrome. The remaining 101 patients (66%) were transferred from two to 15 days following admission for a variety of problems but respiratory failure was most common (34 patients, 22%).
Worsening of the problem for which the patients were initially admitted accounted for the unplanned transfers of 73 patients (48%) (Table 5). Development of a new problem unrelated to the admitting diagnosis accounted for the transfer in 59 patients (39%). Five patients were transferred to the ICU for a critical laboratory value that required a closer monitoring of the patient or needed more frequent lab draws that could not be achieved on the floor.
| Causes | n (%) |
|---|---|
| |
| 1. Errors in triage from the emergency department: | 15 (10) |
| A. Diagnostic errors: | 1 (0.7) |
| B. Inadequate assessment: | 0 (0) |
| C. Overlooked severity: | 14 (9) |
| 2. Worsening of condition for which the patient was admitted: | 73 (48) |
| A. Problems with assessment or treatment: | 5 (3) |
| 1. Delayed | 1 (0.7) |
| 2. Incorrect | 1 (0.7) |
| 3. Inadequate | 3 (2) |
| B. Spontaneous worsening | 68 (45) |
| 3. Development of a new problem | 59 (39) |
| A. Iatrogenic | 9 (6) |
| B. Spontaneous | 50 (33) |
| 4. Critical laboratory values | 5 (3) |
Errors in care were thought to be present in 29 patients (19% of the unplanned transfers). For 15 of these (52%) the error involved incorrect triage from the ED as 14 of the 15 patients met MICU admission criteria at the time they were triaged to non‐MICU units (Table 6). The remaining patient had a dissecting aortic aneurysm that was not considered while he was being evaluated for acute chest pain. All these patients were transferred to the ICU within 24 hours of their admission and the reviewers agreed that all could have been prevented if existing diagnostic and admission algorithms were followed.
|
| Hemodynamic instability requiring vasopressor agents, continued aggressive fluid resuscitation, or central venous/pulmonary artery catheter monitoring or balloon pump |
| Acute respiratory failure with ongoing or impending need for ventilatory support (either invasively or non‐ invasively). |
| Gastrointestinal bleeding meeting ICU admission criteria (>2 clinical risk factors and Rockall score >3 per Gastrointestinal Bleeding Protocol) |
| Cardiac chest pains associated with two of the three criteria |
| Ongoing ischemic chest pain |
| Enzyme elevation |
| ST segment depression <0.5 mm in 2 consecutives leads or transient ST‐segment elevation |
| Chest pain requiring IV nitroglycerin infusion. |
| Complex cardiac arrhythmia requiring close monitoring and/or intravenous infusion therapy |
| Temporary pacemaker. |
| Hypertensive crisis with end‐organ dysfunction or aortic dissection requiring intravenous treatment. |
| Massive hemoptysis (>500 cc/24 hours) |
| Acute neurological dysfunction requiring one of |
| ICP monitoring, |
| Acute respiratory failure with impending need for ventilatory support |
| Hourly neurological checks. |
| Status epilepticus |
| Post‐operative patients requiring hemodynamic monitoring/ventilator support of extensive nursing care. |
| Severe metabolic disorder or intoxication requiring frequent monitoring and/or intravenous infusion therapy that cannot be administered on a floor. |
| Multiple trauma, including severe head and spine trauma |
| Other indication (please specify) |
Of the remaining 14 patients thought to have errors in care, nine were classified as the development of a new, iatrogenic problem (ie, opiate or benzodiazepine overdose occurring during treatment for pain and/or anxiety in 3, volume overload in 2, insulin‐induced hypoglycemia, antibiotic associated reaction, ‐blocker overdose and acute renal failure from over‐diuresis in one each) and five occurred because the patient's admitting problem worsened because treatment was thought to be either delayed, incorrect, or inadequate (Table 5). The reviewers all agreed that the unplanned transfers could have been prevented in eight of the 14 patients who developed iatrogenic problems if existing algorithms were followed or if an earlier or different intervention had occurred. The reviewers did not agree about whether the unplanned transfer could have been prevented in one patient who developed an iatrogenic problem and in all five patients whose underlying condition worsened. Accordingly, in sum, the reviewers felt that 23 of the 152 unplanned transfers (15%) could have been prevented.
In addition to trying to determine how many of the unplanned MICU transfers could have been prevented, we also investigated the utility of rapid response triggers in alerting the physicians and nurses of impending deteriorations in status and whether earlier recognition of this deterioration might have prevented the transfers. Of the 152 unplanned transfers, 106 (70%) had one or more rapid response triggers within the preceding 12 hours. All three reviewers agreed and concluded that in 94 (89%) of these, the unplanned transfer could not have been prevented, even with different or earlier interventions. For five patients (5% of the 106) all reviewers agreed and concluded that earlier intervention might have averted the subsequent transfer. For the other seven patients (6%), no consensus was reached. If we assume that, for all of these latter seven, earlier or different intervention might have averted the unplanned transfer, a maximum of 12 unplanned transfers (11% of the 106) might have been prevented by having a system of care that employed regularly assessing rapid response triggers and acting on them when recognized.
The interobserver reliability for the three reviewers was moderate to almost perfect with = 0.60, 95% confidence interval (CI) (0.31, 0.88); = 0.90, 95% CI (0.71, 1); = 0.55, 95% CI (0.26, 0.84).
A total of 27 (18%) of the patients with unplanned transfers died in the MICU. During this same time period 91 of 1511 patients (6%) admitted directly from the ED to the MICU died (P < 0.05). Mortality was lower for patients transferred within 24 hours of admission compared to those transferred > 24 hours after admission (4% vs. 22% mortality, respectively, P < 0.05; 95% CI, 0.09‐0.89). We found no difference in mortality as a function of time of admission or time of transfer implying that differences in staffing, or the availability of various services, did not contribute to the unplanned transfers.
Discussion
The important findings of this study were that (1) 19% of unplanned, in‐hospital transfers from Medicine floor services to the MICU seemed to result from apparent errors in care, (2) 15% of the unplanned transfers were potentially preventable, (3) the majority of the errors in care involved inappropriate triage of patients from the ED to the non‐MICU units, (4) 106 (70%) of the patients requiring unplanned transfers developed rapid response criteria within 12 hours prior to the transfer, but on review of these (5) the transfer was thought to be preventable in only a maximum of 12 (11%).
We designed our study in part to find specific errors that commonly resulted in unplanned MICU transfers with the idea that, if these could be identified, they might be corrected, thereby improving care. Contrary to our hypothesis we found that only 29 (19%) of the unplanned transfers seemed to result from errors in care. Of these, however, half were attributable to overlooking that patients met our own institution's MICU admission criteria at the time they were triaged to non‐MICU units. This result is consistent with Walter et al.13 finding that while 88% of MICUs in academic health centers had written MICU admission criteria, only 25% used these criteria on a regular basis. Hospital mortality is likely lower for patients meeting MICU admission criteria when they are appropriately and expeditiously triaged.1418 Accordingly, developing mechanisms by which patients are routinely screened for meeting MICU admission criteria could and should reduce this source of error and improve patient outcomes.
Nine of the remaining 14 errors in care resulted from what the chart reviewers concluded was overly aggressive treatment; either excess fluid resuscitation or excess treatment of pain or anxiety. It is not clear that these represent correctable errors in care, however, as hypotensive patients require fluid resuscitation, and patients with pain or anxiety should receive analgesics or anxiolytics and it is not reasonable to expect that these interventions will be appropriately titrated in every instance. Nonetheless, our reviewers all agreed that, in eight of these patients, different interventions could have prevented the unplanned transfer.
Since 41 (27%) of the unplanned transfers were for respiratory failure, we reviewed each of these patients' records seeking evidence suggesting that the problem might have resulted from excessive use of fluids, narcotics, or anxiolytics. By retrospective analysis only six such cases could be identified. Most were due to worsening of the problem for which the patient was admitted.
Consistent with our hypothesis the majority of patients requiring unplanned MICU transfers (106/152, 70%) developed rapid response clinical triggers within the 12 hours preceding transfer, as has been previously demonstrated by Hillman et al.7 and others.8‐10, 19 Our reviewers tried to determine whether earlier or different interventions might have prevented the deterioration and the resulting unplanned transfer. Interestingly, in the large majority (94/106, 89%) they concluded that nothing different could have been done and that the transfer could not have been avoided. While this observation contrasts with our hypothesis, it is consistent with two studies questioning the utility of RRTs in preventing unplanned ICU transfers.9, 10 In addition some patients may ultimately need an ICU transfer despite receiving appropriate interventions as it is impossible to prevent an ICU transfer in every patient. Conversely, just because a patient meets a rapid response criteria does not mean that the patient needs a higher level of care or an ICU transfer as some can be safely managed on the floor.
Our study has a number of potential limitations. The data came from a single teaching hospital and we only assessed patients admitted to General Internal Medicine units and transferred to a MICU. Accordingly, our results might not generalize to other hospitals (teaching or nonteaching), to other services or to other types of ICUs. We found, however, that (1) unplanned transfers accounted for 10% of the total admissions to our MICU, a similar fraction as reported by Angus et al.1 in 2006; (2) respiratory failure/emnsufficiency and sepsis were the most common diagnoses leading to unplanned transfers as previously reported by Groeger et al.2 and Hillman et al.5; (3) mortality was increased in patients requiring unplanned transfer, as noted by Escarce and Kelley3 and Hillman et al.5; and (4) patients who were transferred to the MICU within 24 hours of admission had better outcomes than those who were transferred later, as reported by Goldhill et al.4 Accordingly, our patient population seems quite similar to others in the literature.
Since we did not use objective criteria to assign patients to each of the categories itemized in Table 5 we could have misclassified patients with respect to the cause for their unplanned MICU transfer. Despite this shortcoming, however, the scores among our independent reviewers were moderate to almost perfect suggesting misclassification did not occur commonly.
Our retrospective study design may have underestimated the utility of RRTs as we had no way of knowing the outcomes of patients who met rapid response criteria and had interventions that prevented unplanned MICU transfers.
In summary, approximately 15% of unplanned MICU transfers seem to be preventable and approximately one‐fifth seem to result from errors in care, the majority of which are errors in triage from the ED. While the large majority of unplanned transfers were preceded by clinical deterioration within the preceding 12 hours, manifested by the presence of rapid response triggers, the large majority of these do not seem to be preventable. From these findings we suggest that unplanned transfers could be reduced by more closely screening patients for the presence of defined MICU admission criteria at the time of admission from the ED, by recognizing that fluid resuscitation and control of pain and/or anxiety can have adverse effects and by monitoring patients receiving these interventions more closely.
Two national surveys indicate that 14% to 28% of patients admitted to intensive care units (ICU's) are unplanned transfers (i.e., moving a patient to the ICU from other areas in the hospital providing lower intensity care due to an unanticipated change in the patient's clinical status), and that the most common reason for unplanned transfers is respiratory insufficiency/failure.1, 2 Patients suffering adverse events during a hospitalization are more likely to have an unplanned ICU transfer and patients requiring unplanned transfers have a higher mortality.35 Accordingly, the Joint Commission has identified improved recognition and response to changes in a patient's condition as a national patient safety goal,6 and Rapid Response Teams (RRTs) have been advocated to deal with these changes,7 although recent studies question the effectiveness of RRTs.811
We sought to classify the causes of unplanned, in‐hospital transfers to a medical ICU (MICU) with the idea of identifying common problems in care that might be addressed by process improvement activities. We also sought to determine the fraction of patients requiring an unplanned MICU transfer that had evidence of clinical deterioration prior to the time of transfer and whether, in retrospect, different or earlier interventions might have prevented the transfer. Our hypotheses were that (1) most unplanned MICU transfers occurred as a result of errors in care, (2) most were preceded by clinical deterioration within 12 hours prior to the transfer, and (3) most were preventable.
Methods
We conducted a retrospective cohort study of patients transferring to the MICU from non‐ICU Medicine units at Denver Health, a university‐affiliated, public safety net hospital. All adult patients between 18 to 89 years of age, who were admitted to the Medicine service between June, 2005 and May, 2006 were included in the study. Exclusion criteria included patients who (1) transferred from outside hospitals, (2) transferred from nonMedicine units within Denver Health, (3) were admitted directly to the MICU from the emergency department (ED), (4) were prisoners, (5) were readmitted to the MICU during the same hospitalization, (6) were known to be pregnant, or (7) were planned MICU transfers following invasive procedures (eg, elective cardiac catheterization, defibrillator placement, ablations). Patients readmitted to the MICU were excluded because of the difficulty distinguishing between premature transfer from the MICU or potential problems in care that might have occurred prior to the time of transfer from those occurring during follow‐up care on the Medicine floor services.
Computerized medical records of eligible patients were searched for demographic information and for admitting and transfer diagnoses (with the latter being categorized using a taxonomy we developed for classifying unplanned transfers, Table 1). Three independent observers (all of whom were board certified in Internal Medicine and had been practicing as Hospitalists at our institution for a minimum of three years) retrospectively reviewed each patient's hospital record to determine the cause of the unplanned transfer using this taxonomy. All three also made a judgment as to whether deterioration was evident at any time within the 12 hours preceding the unplanned transfer on the basis of clinical criteria used as our hospital's rapid response triggers (Table 2). When clinical triggers were found, each of the reviewers independently judged whether the unplanned transfer might have been prevented had different or earlier interventions been instituted. Each reviewer was blinded to the results of the other two.
|
| 1. Errors in triage from the Emergency Department |
| A. Diagnostic errors (conditions that were overlooked at the time of admission but explained the chief complaint). |
| B. Inadequate assessment (new diagnosis established after more extensive evaluation that could have been performed at the time of admission). |
| C. Overlooked severity (patients meeting MICU admission criteria at the time of admission from the ED). |
| 2. Worsening of condition for which the patient was admitted |
| A. Errors with assessment or treatment (evaluation or treatment that was not thought to be standard of care for the admitting diagnosis). |
| 1. Delayed (could reasonably have been instituted earlier) |
| 2. Incorrect (not thought to represent standard of care) |
| 3. Inadequate (correct, but insufficient for the admitting diagnosis) |
| B. Spontaneous worsening (worsening of the problem for which the patients were admitted to the point of requiring MICU transfer for which no specific cause could be identified) |
| 3. Development of a new problem |
| A. Iatrogenic (thought to be caused by a diagnostic or therapeutic intervention) |
| B. Spontaneous (no specific cause could be identified) |
| 4. Critical laboratory values (laboratory values needing frequent monitoring of patient and/or blood draws) |
| A. Respiratory |
| Respiratory rate <8 or >28/minute |
| Acute change in oxygen saturation to <90% despite oxygen administration |
| Threatened airway |
| B. Cardiovascular |
| Acute change in systolic blood pressure to <90 mmHg |
| Acute, sustained increase in diastolic blood pressure to >110 mmHg |
| Acute change in heart rate to <50 or >120 beats/minute |
| New onset chest pain or chest pain different than on admission assessment |
| Acutely cold and pulseless extremity. |
| C. Neurological |
| Confusion, agitation or delirium |
| Unexplained lethargy/difficult to arouse |
| Difficulty speaking or swallowing |
| Acute change in pupillary response |
| New seizure |
| D. Other |
| Temperature >39.0 Celsius |
| Uncontrolled pain (if different than admission pain assessment) |
| Acute change in urine output <50 mL/4 hours |
| Acute bleeding (bleeding with a change in vitals, urine output or mental status) |
All analyses were done using SAS Enterprise Guide 4.1, SAS Institute, Cary, NC. Data are presented as mean (standard deviation [SD]). Interobserver agreement was measured by calculating a statistic. values were interpreted by using the guidelines suggested by Landis and colleagues.12 A chi‐square test was used to seek associations between baseline characteristics, reasons for MICU transfer and mortality. P < 0.05 was considered to be statistically significant. The Colorado Multiple Institutional Review Board approved the research protocol.
Results
Over the period of the study the Medicine floor services had 4468 admissions of which 152 met the inclusion criteria for having an unplanned MICU transfer (Table 3). The most common admitting diagnoses were heart failure (12%) and community acquired pneumonia (9%). The most common diagnoses to which the unplanned MICU transfers were attributed were respiratory failure (27%) and sepsis (9%) (Table 4). Seven cardiopulmonary arrests were successfully resuscitated and transferred to the MICU. Throughout the period of the study, no patients were admitted to non‐MICU units because the MICU was at full capacity. Additionally the investigators did not find any inordinate delays in transfer to the ICU while waiting for a bed.
| |
| Age (years) mean (SD) | 52 14 |
| Gender (male:female) | |
| Number | 95:57 |
| % | 63:37 |
| Race, n (%) | |
| White, non‐Hispanic | 54 (35) |
| White, Hispanic | 59 (39) |
| Black | 30 (20) |
| Other | 9 (6) |
| Primary language, n (%) | |
| English | 131 (86) |
| Spanish | 17 (11) |
| Other | 4 (3) |
| Length of stay prior to transfer (hours) (median, IQR) | 46, 89 |
| Admitting diagnosis, n (%) | |
| Acute decompensated heart failure (systolic/diastolic) | 18 (12) |
| Community acquired pneumonia | 13 (9) |
| Suspected acute coronary syndrome | 9 (6) |
| Delirium | 8 (5) |
| Acute kidney injury | 8 (5) |
| Abdominal pain | 8 (5) |
| Respiratory failure | 6 (4) |
| |
| Respiratory failure (cardiogenic/non‐cardiogenic) | 41 (27) |
| Sepsis | 14 (9) |
| Hypotension | 13 (9) |
| Gastrointestinal bleeding | 12 (8) |
| Tachyarrhythmia | 9 (6) |
| Cardiac arrest | 7 (5) |
| Hypertensive emergency | 7 (5) |
| Acute coronary syndrome | 7 (5) |
A total of 51 patients (34%) were transferred within the first 24 hours of admission. The most common diagnoses resulting in transfer in this group were respiratory failure, hypertensive emergency, hypotension, gastrointestinal bleed, and acute coronary syndrome. The remaining 101 patients (66%) were transferred from two to 15 days following admission for a variety of problems but respiratory failure was most common (34 patients, 22%).
Worsening of the problem for which the patients were initially admitted accounted for the unplanned transfers of 73 patients (48%) (Table 5). Development of a new problem unrelated to the admitting diagnosis accounted for the transfer in 59 patients (39%). Five patients were transferred to the ICU for a critical laboratory value that required a closer monitoring of the patient or needed more frequent lab draws that could not be achieved on the floor.
| Causes | n (%) |
|---|---|
| |
| 1. Errors in triage from the emergency department: | 15 (10) |
| A. Diagnostic errors: | 1 (0.7) |
| B. Inadequate assessment: | 0 (0) |
| C. Overlooked severity: | 14 (9) |
| 2. Worsening of condition for which the patient was admitted: | 73 (48) |
| A. Problems with assessment or treatment: | 5 (3) |
| 1. Delayed | 1 (0.7) |
| 2. Incorrect | 1 (0.7) |
| 3. Inadequate | 3 (2) |
| B. Spontaneous worsening | 68 (45) |
| 3. Development of a new problem | 59 (39) |
| A. Iatrogenic | 9 (6) |
| B. Spontaneous | 50 (33) |
| 4. Critical laboratory values | 5 (3) |
Errors in care were thought to be present in 29 patients (19% of the unplanned transfers). For 15 of these (52%) the error involved incorrect triage from the ED as 14 of the 15 patients met MICU admission criteria at the time they were triaged to non‐MICU units (Table 6). The remaining patient had a dissecting aortic aneurysm that was not considered while he was being evaluated for acute chest pain. All these patients were transferred to the ICU within 24 hours of their admission and the reviewers agreed that all could have been prevented if existing diagnostic and admission algorithms were followed.
|
| Hemodynamic instability requiring vasopressor agents, continued aggressive fluid resuscitation, or central venous/pulmonary artery catheter monitoring or balloon pump |
| Acute respiratory failure with ongoing or impending need for ventilatory support (either invasively or non‐ invasively). |
| Gastrointestinal bleeding meeting ICU admission criteria (>2 clinical risk factors and Rockall score >3 per Gastrointestinal Bleeding Protocol) |
| Cardiac chest pains associated with two of the three criteria |
| Ongoing ischemic chest pain |
| Enzyme elevation |
| ST segment depression <0.5 mm in 2 consecutives leads or transient ST‐segment elevation |
| Chest pain requiring IV nitroglycerin infusion. |
| Complex cardiac arrhythmia requiring close monitoring and/or intravenous infusion therapy |
| Temporary pacemaker. |
| Hypertensive crisis with end‐organ dysfunction or aortic dissection requiring intravenous treatment. |
| Massive hemoptysis (>500 cc/24 hours) |
| Acute neurological dysfunction requiring one of |
| ICP monitoring, |
| Acute respiratory failure with impending need for ventilatory support |
| Hourly neurological checks. |
| Status epilepticus |
| Post‐operative patients requiring hemodynamic monitoring/ventilator support of extensive nursing care. |
| Severe metabolic disorder or intoxication requiring frequent monitoring and/or intravenous infusion therapy that cannot be administered on a floor. |
| Multiple trauma, including severe head and spine trauma |
| Other indication (please specify) |
Of the remaining 14 patients thought to have errors in care, nine were classified as the development of a new, iatrogenic problem (ie, opiate or benzodiazepine overdose occurring during treatment for pain and/or anxiety in 3, volume overload in 2, insulin‐induced hypoglycemia, antibiotic associated reaction, ‐blocker overdose and acute renal failure from over‐diuresis in one each) and five occurred because the patient's admitting problem worsened because treatment was thought to be either delayed, incorrect, or inadequate (Table 5). The reviewers all agreed that the unplanned transfers could have been prevented in eight of the 14 patients who developed iatrogenic problems if existing algorithms were followed or if an earlier or different intervention had occurred. The reviewers did not agree about whether the unplanned transfer could have been prevented in one patient who developed an iatrogenic problem and in all five patients whose underlying condition worsened. Accordingly, in sum, the reviewers felt that 23 of the 152 unplanned transfers (15%) could have been prevented.
In addition to trying to determine how many of the unplanned MICU transfers could have been prevented, we also investigated the utility of rapid response triggers in alerting the physicians and nurses of impending deteriorations in status and whether earlier recognition of this deterioration might have prevented the transfers. Of the 152 unplanned transfers, 106 (70%) had one or more rapid response triggers within the preceding 12 hours. All three reviewers agreed and concluded that in 94 (89%) of these, the unplanned transfer could not have been prevented, even with different or earlier interventions. For five patients (5% of the 106) all reviewers agreed and concluded that earlier intervention might have averted the subsequent transfer. For the other seven patients (6%), no consensus was reached. If we assume that, for all of these latter seven, earlier or different intervention might have averted the unplanned transfer, a maximum of 12 unplanned transfers (11% of the 106) might have been prevented by having a system of care that employed regularly assessing rapid response triggers and acting on them when recognized.
The interobserver reliability for the three reviewers was moderate to almost perfect with = 0.60, 95% confidence interval (CI) (0.31, 0.88); = 0.90, 95% CI (0.71, 1); = 0.55, 95% CI (0.26, 0.84).
A total of 27 (18%) of the patients with unplanned transfers died in the MICU. During this same time period 91 of 1511 patients (6%) admitted directly from the ED to the MICU died (P < 0.05). Mortality was lower for patients transferred within 24 hours of admission compared to those transferred > 24 hours after admission (4% vs. 22% mortality, respectively, P < 0.05; 95% CI, 0.09‐0.89). We found no difference in mortality as a function of time of admission or time of transfer implying that differences in staffing, or the availability of various services, did not contribute to the unplanned transfers.
Discussion
The important findings of this study were that (1) 19% of unplanned, in‐hospital transfers from Medicine floor services to the MICU seemed to result from apparent errors in care, (2) 15% of the unplanned transfers were potentially preventable, (3) the majority of the errors in care involved inappropriate triage of patients from the ED to the non‐MICU units, (4) 106 (70%) of the patients requiring unplanned transfers developed rapid response criteria within 12 hours prior to the transfer, but on review of these (5) the transfer was thought to be preventable in only a maximum of 12 (11%).
We designed our study in part to find specific errors that commonly resulted in unplanned MICU transfers with the idea that, if these could be identified, they might be corrected, thereby improving care. Contrary to our hypothesis we found that only 29 (19%) of the unplanned transfers seemed to result from errors in care. Of these, however, half were attributable to overlooking that patients met our own institution's MICU admission criteria at the time they were triaged to non‐MICU units. This result is consistent with Walter et al.13 finding that while 88% of MICUs in academic health centers had written MICU admission criteria, only 25% used these criteria on a regular basis. Hospital mortality is likely lower for patients meeting MICU admission criteria when they are appropriately and expeditiously triaged.1418 Accordingly, developing mechanisms by which patients are routinely screened for meeting MICU admission criteria could and should reduce this source of error and improve patient outcomes.
Nine of the remaining 14 errors in care resulted from what the chart reviewers concluded was overly aggressive treatment; either excess fluid resuscitation or excess treatment of pain or anxiety. It is not clear that these represent correctable errors in care, however, as hypotensive patients require fluid resuscitation, and patients with pain or anxiety should receive analgesics or anxiolytics and it is not reasonable to expect that these interventions will be appropriately titrated in every instance. Nonetheless, our reviewers all agreed that, in eight of these patients, different interventions could have prevented the unplanned transfer.
Since 41 (27%) of the unplanned transfers were for respiratory failure, we reviewed each of these patients' records seeking evidence suggesting that the problem might have resulted from excessive use of fluids, narcotics, or anxiolytics. By retrospective analysis only six such cases could be identified. Most were due to worsening of the problem for which the patient was admitted.
Consistent with our hypothesis the majority of patients requiring unplanned MICU transfers (106/152, 70%) developed rapid response clinical triggers within the 12 hours preceding transfer, as has been previously demonstrated by Hillman et al.7 and others.8‐10, 19 Our reviewers tried to determine whether earlier or different interventions might have prevented the deterioration and the resulting unplanned transfer. Interestingly, in the large majority (94/106, 89%) they concluded that nothing different could have been done and that the transfer could not have been avoided. While this observation contrasts with our hypothesis, it is consistent with two studies questioning the utility of RRTs in preventing unplanned ICU transfers.9, 10 In addition some patients may ultimately need an ICU transfer despite receiving appropriate interventions as it is impossible to prevent an ICU transfer in every patient. Conversely, just because a patient meets a rapid response criteria does not mean that the patient needs a higher level of care or an ICU transfer as some can be safely managed on the floor.
Our study has a number of potential limitations. The data came from a single teaching hospital and we only assessed patients admitted to General Internal Medicine units and transferred to a MICU. Accordingly, our results might not generalize to other hospitals (teaching or nonteaching), to other services or to other types of ICUs. We found, however, that (1) unplanned transfers accounted for 10% of the total admissions to our MICU, a similar fraction as reported by Angus et al.1 in 2006; (2) respiratory failure/emnsufficiency and sepsis were the most common diagnoses leading to unplanned transfers as previously reported by Groeger et al.2 and Hillman et al.5; (3) mortality was increased in patients requiring unplanned transfer, as noted by Escarce and Kelley3 and Hillman et al.5; and (4) patients who were transferred to the MICU within 24 hours of admission had better outcomes than those who were transferred later, as reported by Goldhill et al.4 Accordingly, our patient population seems quite similar to others in the literature.
Since we did not use objective criteria to assign patients to each of the categories itemized in Table 5 we could have misclassified patients with respect to the cause for their unplanned MICU transfer. Despite this shortcoming, however, the scores among our independent reviewers were moderate to almost perfect suggesting misclassification did not occur commonly.
Our retrospective study design may have underestimated the utility of RRTs as we had no way of knowing the outcomes of patients who met rapid response criteria and had interventions that prevented unplanned MICU transfers.
In summary, approximately 15% of unplanned MICU transfers seem to be preventable and approximately one‐fifth seem to result from errors in care, the majority of which are errors in triage from the ED. While the large majority of unplanned transfers were preceded by clinical deterioration within the preceding 12 hours, manifested by the presence of rapid response triggers, the large majority of these do not seem to be preventable. From these findings we suggest that unplanned transfers could be reduced by more closely screening patients for the presence of defined MICU admission criteria at the time of admission from the ED, by recognizing that fluid resuscitation and control of pain and/or anxiety can have adverse effects and by monitoring patients receiving these interventions more closely.
- ,,,,,.Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34(4):1016–1024.
- ,,, et al.Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization.Crit Care Med.1993;21(2):279–291.
- ,.Admission source to the medical intensive care unit predicts hospital death independent of APACHE II score.JAMA.1990;264(18):2389–2394.
- ,,,.The longer patients are in hospital before Intensive Care admission the higher their mortality.Intensive Care Med.2004;30(10):1908–1913.
- ,,,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28(11):1629–1634.
- Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission Hospital Accreditation Program, National Patient Safety Goals, Goal 16; 2008. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed May2010.
- ,,, et al.MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365(9477):2091–2097.
- ,,,,,.Rapid response systems: a systematic review.Crit Care Med.2007;35(5):1238–1243.
- ,,,,,.Hospital‐wide code rates and mortality before and after implementation of a rapid response team.JAMA.2008;300(21):2506–2513.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,,,,.Rapid response teams: a systematic review and meta‐analysis.Arch Intern Med.2010;170(1):18–26.
- ,.The measurement of observer agreement for categorical data.Biometrics.1977;33(1):159–174.
- ,,.How decisions are made to admit patients to medical intensive care units (MICUs): A survey of MICU directors at academic medical centers across the United States.Crit Care Med.2008;36:414–420.
- ,,.Mortality among appropriately referred patients refused admission to intensive‐care units.Lancet.1997;350:7–12.
- ,,,,,.Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome.Intensive Care Med.2001;27:1459–1465.
- ,,,,,for the Values, Ethics and Rationing in Critical Care (VERICC) Task Force. Rationing critical care beds: A systematic review.Crit Care Med.2004;32:1588–1597.
- ,,, et al.Survival of critically ill patients hospitalized in and out of intensive care.Crit Care Med.2007;35:449–457.
- ,,,,,for the DELAY‐ED study group. Impact of delayed transfer of critically ill patients form the emergency department to the intensive care unit.Crit Care Med.2007;35:1477–1483.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31(6):343–348.
- ,,,,,.Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34(4):1016–1024.
- ,,, et al.Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization.Crit Care Med.1993;21(2):279–291.
- ,.Admission source to the medical intensive care unit predicts hospital death independent of APACHE II score.JAMA.1990;264(18):2389–2394.
- ,,,.The longer patients are in hospital before Intensive Care admission the higher their mortality.Intensive Care Med.2004;30(10):1908–1913.
- ,,,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28(11):1629–1634.
- Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission Hospital Accreditation Program, National Patient Safety Goals, Goal 16; 2008. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed May2010.
- ,,, et al.MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365(9477):2091–2097.
- ,,,,,.Rapid response systems: a systematic review.Crit Care Med.2007;35(5):1238–1243.
- ,,,,,.Hospital‐wide code rates and mortality before and after implementation of a rapid response team.JAMA.2008;300(21):2506–2513.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,,,,.Rapid response teams: a systematic review and meta‐analysis.Arch Intern Med.2010;170(1):18–26.
- ,.The measurement of observer agreement for categorical data.Biometrics.1977;33(1):159–174.
- ,,.How decisions are made to admit patients to medical intensive care units (MICUs): A survey of MICU directors at academic medical centers across the United States.Crit Care Med.2008;36:414–420.
- ,,.Mortality among appropriately referred patients refused admission to intensive‐care units.Lancet.1997;350:7–12.
- ,,,,,.Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome.Intensive Care Med.2001;27:1459–1465.
- ,,,,,for the Values, Ethics and Rationing in Critical Care (VERICC) Task Force. Rationing critical care beds: A systematic review.Crit Care Med.2004;32:1588–1597.
- ,,, et al.Survival of critically ill patients hospitalized in and out of intensive care.Crit Care Med.2007;35:449–457.
- ,,,,,for the DELAY‐ED study group. Impact of delayed transfer of critically ill patients form the emergency department to the intensive care unit.Crit Care Med.2007;35:1477–1483.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31(6):343–348.
Copyright © 2010 Society of Hospital Medicine
Effectiveness of Course to Teach Handoffs
Communication failures are well‐recognized as causes of medical errors.1, 2 Specifically, handoffs of patient care responsibilities, which are increasingly prevalent in academic medical centers,3 have been cited as the most frequent cause of teamwork breakdown resulting in the harmful medical errors found in malpractice claims.1 The Institute of Medicine has recently identified patient handoffs as the moment where patient care errors are most likely to occur.4 A survey of 125 U.S. medical schools, however, found that only 8% specifically taught students how to hand off patient care.3
In July 2003, the American Council of Graduate Medical Education (ACGME) mandated that residency programs decrease resident work hours to improve patient care and safety by reducing fatigue,5 and a recent Institute of Medicine report suggests that they be decreased even further.4 Studies examining outcomes during the first 2 years after reducing duty hours did not find reductions in risk‐adjusted mortality.68 One proposed explanation for this lack of improvement is that the reduction in fatigue‐related medical errors is being offset by discontinuity of care with due to the increased number of patient handoffs resulting from shortened duty hours,911 one recent study found that omission of key information during patient sign outs frequently resulted in adverse patient care outcomes.12
In 2007, the Joint Commission developed a new National Patient Safety Goal that requires organizations to improve communication between caregivers.13 We recently developed an approach by which Internal Medicine residents hand off patient care using a structured process, written and verbal templates, formal training about handoffs, and direct attending supervision.14 Because fourth‐year medical students perform the duties of interns when working as subinterns, we recognized that education about handoffs should occur prior to the time students became interns. Accordingly, we developed a course designed to teach patient handoffs to medical students at the transition between their third and fourth years of training.
Setting
The Handoff Selective was developed by faculty of Denver Health and the University of Colorado Denver School of Medicine.
Program Description
The Selective was first offered in April 2007 as part of an Integrated Clinician's Course (ICC), a 2‐week course for students beginning their fourth year, which starts in April at the University of Colorado. The ICC includes both mandatory and selective sessions that are focused on developing clinical skills and preparing them for their subinternships. The Handoff Selective was conducted in a computerized teaching laboratory, lasted a total of 2 hours and consisted of 2 parts. Each of the 5 Denver Health Hospital Medicine faculty members versed in handoff education taught 2 sessions of 6 to 8 students.
Part 1: Didactic
During the first hour of class, the faculty presented a lecture that summarized the relevant literature on handoffs and explained the importance of the topic. The objectives of the didactic were to: (1) understand the importance of handoffs; (2) explore different communication elements and structures; (3) gain exposure to handoffs outside of healthcare; and (4) learn a structure for handoffs of patient care in hospitalized patients.
We used 3 video clips of handoffs from 2 football games to demonstrate the importance of practice, training, and 2‐way communications in handoffs. The first video clip showed a runner trying to make a spontaneous handoff while being tackled. The receiver was not expecting the handoff and was preoccupied with blocking another player. This attempted handoff resulted in a fumble, which we related to an adverse patient event.
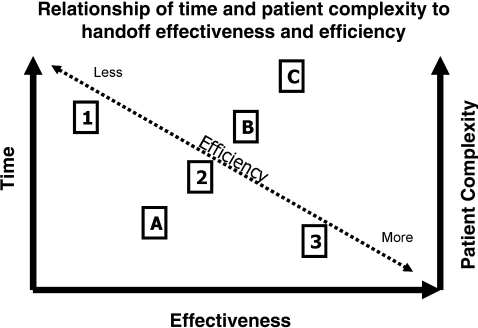
The next 2 video clips showed 2 complex, seldom used, but well‐known football handoffsthe hook and lateral and the Statue of Liberty. Both handoffs were successfully executed presumably as a result of education, practice and the active participation of both players (handing off and receiving) in the process. We then related the teaching and practicing of complex communication to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO; now simply the Joint Commission) data suggesting that most sentinel events have their root cause in communication and training failures.2
Basic communication elements and process structures were then explored using scenarios from everyday life and evidence from fields outside of medicine. We emphasized that structures for communication (modes, vehicles, and settings) must be chosen according to the occasion and that handoffs are common and important in all occupations. In discussing modes (verbal, written, or nonverbal), vehicles (paper, telephone, or e‐mail), and settings (face‐to face, virtual, or disconnected), we emphasized that the most effective structures for communication (verbal, face‐to face meetings, with written materials and other visual aids at the patient's bedside) were also the most time‐consuming (Figure 1). While our standard for resident handoffs is a face‐to‐face verbal interaction with preprinted written materials as an aid, we also emphasized that for complex patients (eg, mental status changes, concern for an acute abdomen) more robust communication is often needed. Accordingly, a more time‐consuming bedside handoff with simultaneous, focused physical exam and history‐taking by both oncoming and off‐going providers may be most appropriate.
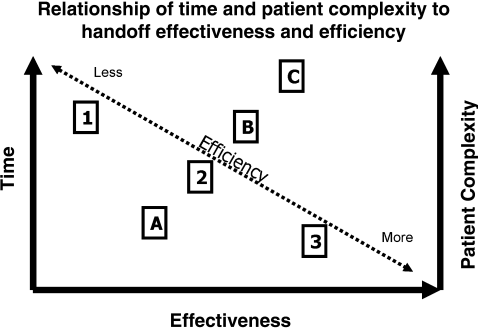
As real‐life examples, we asked our students to communicate a happy birthday wish to their mother, who lives in another state. Almost uniformly, in addition to a written aid (birthday card), they choose the telephone as a vehicle for their verbal mode in a virtual setting with 2‐way communication possible. In contrast, when asked to propose marriage to a significant other in another state, students felt that a face‐to‐face meeting with verbal and nonverbal (ie, ring) modes was appropriate. This time‐consuming mode of communication was felt to be necessary to create a sentiment of importance and avert any possible miscommunication.
The didactic session concluded by demonstrating how to use standardized written and verbal templates for handoffs of the care of a hospitalized patient. We explore the differentiation between written and verbal handoffs in our discussion below.
Part 2: Practicum
The second hour was devoted to practicing handoffs as a group. The faculty developed 6 case scenarios that differed with respect to diagnosis, length of stay, active medical issues, and anticipated discharge (Table 1). The scenarios included extensive admission information as well as evolving issues for each patient that were specific to the day of the intended handoff. Students were given Microsoft Word table‐based handoff templates to use when creating written sign‐outs for their patients. Verbal handoffs were performed between students and sign‐outs were exchanged. The faculty then role‐played cross‐cover calls that were specific for each scenario to test the students' inclusion of integral information in their handoffs and their ability to create contingency plans.
| Diagnosis | LOS | Active Issues | Cross‐Cover |
|---|---|---|---|
| |||
| CP | 1 | CP, HTN, DM | CP, HTN, headache |
| GIB | 1 | GIB, alcohol withdrawal | Poor response to red call transfusion, coagulopathy |
| Acute pancreatitis | 2 | Pain, possible pancreatic abscess | Fever, agitation, hypoxia |
| CHF | 2 | CHF, DM, nausea | Lack of diuresis, CP, hypoglycemia |
| Acute kidney injury | 3 | None, ready for discharge | HTN, hyperglycemia |
| Community acquired pneumonia | 3 | Anxiety, discharge pending | Confusion, emesis with hypoxia |
Program Evaluation
We developed a 2‐part survey to evaluate the effectiveness of the Selective and to solicit feedback about the didactic and practicum portions of the course. The first part of the survey (Table 2) contained 16 items to assess the students' knowledge of, and attitudes toward handing off patient care, along with their comfort with the handoff process. Responses to this section were scored using a 5‐point Likert scale with 1 indicating strongly disagree and 5 indicating strongly agree. This part of the survey was administered both prior to and after the Selective.
| Competency | Selective | |
|---|---|---|
| Before | After | |
| ||
| I know how to hand off patients | 2.3 0.8 | 4.2 0.6* |
| I know how to make contingency plans for my patients | 2.1 0.8 | 3.9 0.7* |
| I know what a read‐back is | 2.3 1.3 | 4.4 0.9* |
| I know how to perform a read‐back | 2.0 1.2 | 4.2 0.9* |
| I know when to perform a read‐back | 1.6 0.8 | 4.1 1.0* |
| I am efficient at communicating patient information | 2.2 0.9 | 3.6 0.7* |
| I am effective at communicating patient information | 2.2 0.8 | 3.8 0.6* |
| I know a standard written structure for handoffs | 2.1 1.1 | 4.4 0.6* |
| I know a standard verbal structure for handoffs | 2.0 1.1 | 4.2 0.6* |
| I can choose appropriate modes of communication | 2.7 1.1 | 4.4 0.6* |
| I can choose appropriate vehicles of communication | 2.6 1.1 | 4.5 0.6* |
| I can choose appropriate settings for communication | 2.9 1.1 | 4.4 0.6* |
| Handoffs are well taught in my medical school | 1.6 0.8 | 3.5 1.0* |
| Standardization is important in handoffs | 4.3 0.9 | 4.6 0.5 |
| Handoffs are safer with attending supervision | 3.7 1.0 | 3.9 0.8 |
| I feel comfortable cross‐covering on patients | 1.6 0.7 | 3.0 1.0* |
The second part (Table 3) contained 12 items and was designed to evaluate the perceived usefulness of the different components of the class. This section was only administered at the end of the Selective. It utilized a 4‐point Likert scale with 1 indicating that the component was not useful at all, and 4 indicating that it was extremely useful. The first 6 items of the second section allowed students to evaluate the didactic portion of the handoff. The second 6 items allowed students to evaluate the practicum. Responses to all 12 items were then combined to determine an overall composite usefulness for the Selective.
| Useful [n (%)] | |
|---|---|
| |
| Overall composite usefulness | 578 (92) |
| Didactic composite usefulness | 254 (84) |
| Using fumble video clips for discussing handoffs | 32 (64)* |
| Discussion of modes of communication | 46 (88) |
| Discussion of vehicles of communication | 46 (88) |
| Discussion of settings of communication | 48 (96) |
| Choosing handoff structures for nonhealthcare handoffs | 37 (71)* |
| Discussing handoffs in industries outside of healthcare | 45 (94) |
| Practicum composite usefulness | 324(100) |
| Role playing | 54 (100) |
| Patient handoff scenarios | 54 (100) |
| Completing computerized templates | 54 (100) |
| Delivering handoffs to peer | 54 (100) |
| Receiving handoffs from peer | 54 (100) |
| Cross‐cover questions and discussion | 54 (100) |
The Selective was also evaluated qualitatively through the use of open‐ended, written comments that were solicited at the end of the survey. All surveys were administered anonymously.
Data Analysis
Student paired t test was used to compare continuous variables recorded before and after the Selective. A chi‐square test was used to assess the students' perception of the usefulness of the didactic vs. the practicum methods of teaching handoffs.
All analyses were performed using SAS (version 8.1; SAS Institute, Inc., Cary, NC). Bonferroni corrections were used for multiple comparisons such that P values of <0.003 and <0.004 were considered to be significant for continuous and categorical variables, respectively. All data are reported as mean standard deviation (SD).
The survey was approved by our local Institutional Review Board.
Results
More students chose the Selective than we had capacity to accommodate (60 of a class of 150). The pre‐ and postcourse survey response rate was 56 of 60 (93%) and 58 of 60 (97%), respectively. After the Selective, the mean score in response to whether handoffs are well taught in medical school increased from 1.6 to 3.5 (P < 0.003). Our students' self‐perceived skills and knowledge about handoffs improved after the Selective (Table 2). The greatest changes in perceived knowledge occurred in questions regarding the what, how, and when of read‐backs, and the knowledge of standard verbal and written handoff structures. The responses to the survey elements which assessed our students' attitudes regarding the importance of standardization and whether they felt handoffs were safer with faculty supervision did not change after the Selective (Table 2).
A total of 92% of the students felt that the course was extremely useful or useful. The role‐playing activity was thought to be more helpful than the didactic, but 84% of the students still rated the didactic portion as useful or extremely useful (Table 3). The element which was the least well received in the didactic portion was the use of video clips to demonstrate successful and unsuccessful (fumbled) college football handoffs, although the majority (64%) of students still found it useful.
The major theme generated from the comments section of the survey was that the Selective should be a required course.
Discussion
We know of no previously published literature that has addressed teaching handoffs to medical students. Horwitz et al.15 developed a sign‐out curriculum for Internal Medicine residents and found that none of their house‐staff had any previous training in handoffs during medical school, consistent with the finding that only 8% of U.S. medical schools provided formal instruction on handoffs.3 Prior to taking the Selective, our students had no knowledge of verbal or written templates for patient handoffs, although both before and after the course they felt that standardization was an important component of the process.
A number of verbal structures for handing off patient care have been described in the literature and there is not a consensus as to which functions best. Perhaps the most cited verbal communication format is SBAR (ie, situation, background, assessment and recommendation).16, 17 This tool was developed by Leonard et al.18 specifically for use by nurses to provide 1‐way communication to physicians pertaining to a change in patient status. We considered teaching the SBAR approach to the students but felt that it did not provide a suitable structure for handoffs because the transfer of care is not generally an event‐based situation and the literature on handoffs indicates that an optimal verbal system includes 2‐way communication.
Additional mnemonics for handoffs found in the literature include SIGNOUT (ie, Sick or DNR, Identifying information, General hospital course, New events of the day, Overall health status, Upcoming possibilities with plan, and Tasks to complete),14 I PASS the BATON (ie, Introduction, Patient, Assessment, Situation, Safety, Background, Actions, Timing, Ownership, Next)19 and the SAIF‐IR system (see boxed text).14
Verbal Structure for Patient Handoffs: SAIF‐IR
Off‐going provider performs a SAIF handoff:
Summary statement(s)
Active issues
If‐then contingency planning
Follow‐up activities
On‐coming provider makes the handoff SAIF‐IR:
Interactive questioning
Read‐backs
We developed the SAIF‐IR mnemonic to maximize efficiency and effectiveness while differentiating the verbal portion of the handoff from the written and incorporating 2‐way communication into its structure. In the Summary statement, we emphasize that this is not a history of present illness. We ask our students to summarize, in 1 to 3 sentences, the patient's presentation and working diagnosis. When discussing patient issues, we ask our students to only verbalize Active issues, although the written template has inactive, chronic issues listed. Here, we also ask our students to express their level of concern for the active issues and patient in general. If‐then's and Follow‐ups are usually verbalized together. Based on the offgoing provider's knowledge of the patient, we encourage the offgoing provider to anticipate potential problems and advise the oncoming provider on potential responses. Much of this advice is difficult to express in the written format and thus may not be found on the written handoff when the verbal handoff occurs. We encourage oncoming providers to take notes on the preprinted handoff sheet as part of the handoff process.
Through Interactive questioning and Read‐backs, we train our students and house‐staff to use the active listening techniques used outside of healthcare, in settings such as nuclear power plants and National Aeronautics and Space Administration mission control, where poor handoff communication may also result in safety concerns and adverse events.20 Interactive questioning allows the oncoming provider to correct or clarify any information given by the off‐going provider. Read‐backs are a method of confirming follow‐up activity or contingency plans. Together, the SAIF‐IR mnemonic builds a 2‐way communication structure into the patient handoff with both offgoing and oncoming providers having predefined roles.
Much of the information on our written handoff (patient identifying information, medications, language preference, code status, admission date) is not verbalized unless it is part of the active issues or the if‐then, follow‐ups (ie, medication titration for a patient admitted with an acute coronary syndrome or cor status in a patient newly made comfort care). By not reading extraneous information, we seek to emphasize the Active issues as well as the If‐then, Follow‐ups. We feel this emphasis maximizes the effectiveness of the handoff, while the purposeful nonverbalization of written materials such as identifying information maximizes its efficiency. Future work may examine which verbal and written structures for patient handoffs most benefit patient care and workflow through standard communication.
While our students found the Handoff Selective to be useful and to improve their self‐perceived ability to perform handoffs, we were not able to determine whether our program affected downstream outcomes such as adverse events relating to failures in handoff communication. Additionally, since we only taught and evaluated our Selective at the University of Colorado Denver School of Medicine, the response of our students may not generalize to other medical schools. Multicentered, prospective, randomized controlled trials may determine whether handoff education programs are successful in reducing patient adverse events related to transfers of care.
While handoffs occur frequently and are increasingly recognized as a vulnerable time in patient care, little is known about how to effectively teach handoffs to medical students during their clinical years. We developed a formal course to teach the importance of handoffs and how the process should be conducted. Our students reported that the Handoff Selective we developed improved their knowledge about the process and their perception of their ability to perform handoffs in a time‐appropriate and effective manner. In response to the feedback we received from our students, the Handoff Selective is the only course in the ICC that has been made mandatory for all students.
- , , .Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79:186–194.
- Root causes of sentinel events. The Joint Commission. Available at: http://www.jointcommission.org/NR/rdonlyres/FA465646‐5F5F‐4543‐AC8F‐E8AF6571E372/0/root_cause_se.jpg Accessed October2009.
- , , , et al.Lost in translation: challenges‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- Institute of Medicine.Resident Duty Hours: Enhancing Sleep, Supervision and Safety.Washington, DC:National Academies Press;2008.
- ACGME duty hours. Accreditation Council for Graduate Medical Education. http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October2009.
- , , , et al.Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME duty hour reform.JAMA.2007;298(9):975–983.
- , , , et al.Mortality among patient in VA hospitals in the first 2 years following ACGME duty hour reform.JAMA.2007;298(9):984–992.
- , , , .Changes in outcomes for internal medicine patients after work‐hour regulations.Ann Intern Med.2007;147(2):1–7.
- , , , et al.Transfers of patient care between house staff on internal medicine wards.Arch Intern Med.2006;166:1173–1177.
- , , , .Medical errors involving trainees.Arch Intern Med.2007;167(19):2030–2036.
- .Reducing resident work hours: unproven assumptions and unforeseen outcomes.Ann Intern Med.2006;140:814–815.
- , , et al.Consequences of inadequate sign‐out for patient care.Arch Intern Med.2008;168(16):1755–1760.
- JCAHO Handoff Communication. National patient safety goal. The Joint Commission. http://www.jointcommission.org/GeneralPublic/NPSG/07_npsgs.htm. Accessed October2009.
- , , , et al.A structured handoff program for interns.Acad Med.2009;84:347–352.
- , , .Development and implementation of an oral sign out skills curriculum.J Gen Intern Med.2007;22(10):1470–1474.
- , , , et al.Managing discontinuity in academic medical centers: strategies for a safe and effective sign out.J Hosp Med.2006;1:257–266.
- , , , .A theoretical framework and competency based approach to improving handoffs.Qual Saf Health Care.2008;17:11–14.
- , , .The human factor: the critical importance of effective teamwork in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- University HealthSystem Consortium Best Practice Recommendation: Patient Handoff Communication. White Paper. May 2006.Oak Brook, IL:University HealthSystem Consortium;2006.
- , , , , .Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
Communication failures are well‐recognized as causes of medical errors.1, 2 Specifically, handoffs of patient care responsibilities, which are increasingly prevalent in academic medical centers,3 have been cited as the most frequent cause of teamwork breakdown resulting in the harmful medical errors found in malpractice claims.1 The Institute of Medicine has recently identified patient handoffs as the moment where patient care errors are most likely to occur.4 A survey of 125 U.S. medical schools, however, found that only 8% specifically taught students how to hand off patient care.3
In July 2003, the American Council of Graduate Medical Education (ACGME) mandated that residency programs decrease resident work hours to improve patient care and safety by reducing fatigue,5 and a recent Institute of Medicine report suggests that they be decreased even further.4 Studies examining outcomes during the first 2 years after reducing duty hours did not find reductions in risk‐adjusted mortality.68 One proposed explanation for this lack of improvement is that the reduction in fatigue‐related medical errors is being offset by discontinuity of care with due to the increased number of patient handoffs resulting from shortened duty hours,911 one recent study found that omission of key information during patient sign outs frequently resulted in adverse patient care outcomes.12
In 2007, the Joint Commission developed a new National Patient Safety Goal that requires organizations to improve communication between caregivers.13 We recently developed an approach by which Internal Medicine residents hand off patient care using a structured process, written and verbal templates, formal training about handoffs, and direct attending supervision.14 Because fourth‐year medical students perform the duties of interns when working as subinterns, we recognized that education about handoffs should occur prior to the time students became interns. Accordingly, we developed a course designed to teach patient handoffs to medical students at the transition between their third and fourth years of training.
Setting
The Handoff Selective was developed by faculty of Denver Health and the University of Colorado Denver School of Medicine.
Program Description
The Selective was first offered in April 2007 as part of an Integrated Clinician's Course (ICC), a 2‐week course for students beginning their fourth year, which starts in April at the University of Colorado. The ICC includes both mandatory and selective sessions that are focused on developing clinical skills and preparing them for their subinternships. The Handoff Selective was conducted in a computerized teaching laboratory, lasted a total of 2 hours and consisted of 2 parts. Each of the 5 Denver Health Hospital Medicine faculty members versed in handoff education taught 2 sessions of 6 to 8 students.
Part 1: Didactic
During the first hour of class, the faculty presented a lecture that summarized the relevant literature on handoffs and explained the importance of the topic. The objectives of the didactic were to: (1) understand the importance of handoffs; (2) explore different communication elements and structures; (3) gain exposure to handoffs outside of healthcare; and (4) learn a structure for handoffs of patient care in hospitalized patients.
We used 3 video clips of handoffs from 2 football games to demonstrate the importance of practice, training, and 2‐way communications in handoffs. The first video clip showed a runner trying to make a spontaneous handoff while being tackled. The receiver was not expecting the handoff and was preoccupied with blocking another player. This attempted handoff resulted in a fumble, which we related to an adverse patient event.
The next 2 video clips showed 2 complex, seldom used, but well‐known football handoffsthe hook and lateral and the Statue of Liberty. Both handoffs were successfully executed presumably as a result of education, practice and the active participation of both players (handing off and receiving) in the process. We then related the teaching and practicing of complex communication to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO; now simply the Joint Commission) data suggesting that most sentinel events have their root cause in communication and training failures.2
Basic communication elements and process structures were then explored using scenarios from everyday life and evidence from fields outside of medicine. We emphasized that structures for communication (modes, vehicles, and settings) must be chosen according to the occasion and that handoffs are common and important in all occupations. In discussing modes (verbal, written, or nonverbal), vehicles (paper, telephone, or e‐mail), and settings (face‐to face, virtual, or disconnected), we emphasized that the most effective structures for communication (verbal, face‐to face meetings, with written materials and other visual aids at the patient's bedside) were also the most time‐consuming (Figure 1). While our standard for resident handoffs is a face‐to‐face verbal interaction with preprinted written materials as an aid, we also emphasized that for complex patients (eg, mental status changes, concern for an acute abdomen) more robust communication is often needed. Accordingly, a more time‐consuming bedside handoff with simultaneous, focused physical exam and history‐taking by both oncoming and off‐going providers may be most appropriate.

As real‐life examples, we asked our students to communicate a happy birthday wish to their mother, who lives in another state. Almost uniformly, in addition to a written aid (birthday card), they choose the telephone as a vehicle for their verbal mode in a virtual setting with 2‐way communication possible. In contrast, when asked to propose marriage to a significant other in another state, students felt that a face‐to‐face meeting with verbal and nonverbal (ie, ring) modes was appropriate. This time‐consuming mode of communication was felt to be necessary to create a sentiment of importance and avert any possible miscommunication.
The didactic session concluded by demonstrating how to use standardized written and verbal templates for handoffs of the care of a hospitalized patient. We explore the differentiation between written and verbal handoffs in our discussion below.
Part 2: Practicum
The second hour was devoted to practicing handoffs as a group. The faculty developed 6 case scenarios that differed with respect to diagnosis, length of stay, active medical issues, and anticipated discharge (Table 1). The scenarios included extensive admission information as well as evolving issues for each patient that were specific to the day of the intended handoff. Students were given Microsoft Word table‐based handoff templates to use when creating written sign‐outs for their patients. Verbal handoffs were performed between students and sign‐outs were exchanged. The faculty then role‐played cross‐cover calls that were specific for each scenario to test the students' inclusion of integral information in their handoffs and their ability to create contingency plans.
| Diagnosis | LOS | Active Issues | Cross‐Cover |
|---|---|---|---|
| |||
| CP | 1 | CP, HTN, DM | CP, HTN, headache |
| GIB | 1 | GIB, alcohol withdrawal | Poor response to red call transfusion, coagulopathy |
| Acute pancreatitis | 2 | Pain, possible pancreatic abscess | Fever, agitation, hypoxia |
| CHF | 2 | CHF, DM, nausea | Lack of diuresis, CP, hypoglycemia |
| Acute kidney injury | 3 | None, ready for discharge | HTN, hyperglycemia |
| Community acquired pneumonia | 3 | Anxiety, discharge pending | Confusion, emesis with hypoxia |
Program Evaluation
We developed a 2‐part survey to evaluate the effectiveness of the Selective and to solicit feedback about the didactic and practicum portions of the course. The first part of the survey (Table 2) contained 16 items to assess the students' knowledge of, and attitudes toward handing off patient care, along with their comfort with the handoff process. Responses to this section were scored using a 5‐point Likert scale with 1 indicating strongly disagree and 5 indicating strongly agree. This part of the survey was administered both prior to and after the Selective.
| Competency | Selective | |
|---|---|---|
| Before | After | |
| ||
| I know how to hand off patients | 2.3 0.8 | 4.2 0.6* |
| I know how to make contingency plans for my patients | 2.1 0.8 | 3.9 0.7* |
| I know what a read‐back is | 2.3 1.3 | 4.4 0.9* |
| I know how to perform a read‐back | 2.0 1.2 | 4.2 0.9* |
| I know when to perform a read‐back | 1.6 0.8 | 4.1 1.0* |
| I am efficient at communicating patient information | 2.2 0.9 | 3.6 0.7* |
| I am effective at communicating patient information | 2.2 0.8 | 3.8 0.6* |
| I know a standard written structure for handoffs | 2.1 1.1 | 4.4 0.6* |
| I know a standard verbal structure for handoffs | 2.0 1.1 | 4.2 0.6* |
| I can choose appropriate modes of communication | 2.7 1.1 | 4.4 0.6* |
| I can choose appropriate vehicles of communication | 2.6 1.1 | 4.5 0.6* |
| I can choose appropriate settings for communication | 2.9 1.1 | 4.4 0.6* |
| Handoffs are well taught in my medical school | 1.6 0.8 | 3.5 1.0* |
| Standardization is important in handoffs | 4.3 0.9 | 4.6 0.5 |
| Handoffs are safer with attending supervision | 3.7 1.0 | 3.9 0.8 |
| I feel comfortable cross‐covering on patients | 1.6 0.7 | 3.0 1.0* |
The second part (Table 3) contained 12 items and was designed to evaluate the perceived usefulness of the different components of the class. This section was only administered at the end of the Selective. It utilized a 4‐point Likert scale with 1 indicating that the component was not useful at all, and 4 indicating that it was extremely useful. The first 6 items of the second section allowed students to evaluate the didactic portion of the handoff. The second 6 items allowed students to evaluate the practicum. Responses to all 12 items were then combined to determine an overall composite usefulness for the Selective.
| Useful [n (%)] | |
|---|---|
| |
| Overall composite usefulness | 578 (92) |
| Didactic composite usefulness | 254 (84) |
| Using fumble video clips for discussing handoffs | 32 (64)* |
| Discussion of modes of communication | 46 (88) |
| Discussion of vehicles of communication | 46 (88) |
| Discussion of settings of communication | 48 (96) |
| Choosing handoff structures for nonhealthcare handoffs | 37 (71)* |
| Discussing handoffs in industries outside of healthcare | 45 (94) |
| Practicum composite usefulness | 324(100) |
| Role playing | 54 (100) |
| Patient handoff scenarios | 54 (100) |
| Completing computerized templates | 54 (100) |
| Delivering handoffs to peer | 54 (100) |
| Receiving handoffs from peer | 54 (100) |
| Cross‐cover questions and discussion | 54 (100) |
The Selective was also evaluated qualitatively through the use of open‐ended, written comments that were solicited at the end of the survey. All surveys were administered anonymously.
Data Analysis
Student paired t test was used to compare continuous variables recorded before and after the Selective. A chi‐square test was used to assess the students' perception of the usefulness of the didactic vs. the practicum methods of teaching handoffs.
All analyses were performed using SAS (version 8.1; SAS Institute, Inc., Cary, NC). Bonferroni corrections were used for multiple comparisons such that P values of <0.003 and <0.004 were considered to be significant for continuous and categorical variables, respectively. All data are reported as mean standard deviation (SD).
The survey was approved by our local Institutional Review Board.
Results
More students chose the Selective than we had capacity to accommodate (60 of a class of 150). The pre‐ and postcourse survey response rate was 56 of 60 (93%) and 58 of 60 (97%), respectively. After the Selective, the mean score in response to whether handoffs are well taught in medical school increased from 1.6 to 3.5 (P < 0.003). Our students' self‐perceived skills and knowledge about handoffs improved after the Selective (Table 2). The greatest changes in perceived knowledge occurred in questions regarding the what, how, and when of read‐backs, and the knowledge of standard verbal and written handoff structures. The responses to the survey elements which assessed our students' attitudes regarding the importance of standardization and whether they felt handoffs were safer with faculty supervision did not change after the Selective (Table 2).
A total of 92% of the students felt that the course was extremely useful or useful. The role‐playing activity was thought to be more helpful than the didactic, but 84% of the students still rated the didactic portion as useful or extremely useful (Table 3). The element which was the least well received in the didactic portion was the use of video clips to demonstrate successful and unsuccessful (fumbled) college football handoffs, although the majority (64%) of students still found it useful.
The major theme generated from the comments section of the survey was that the Selective should be a required course.
Discussion
We know of no previously published literature that has addressed teaching handoffs to medical students. Horwitz et al.15 developed a sign‐out curriculum for Internal Medicine residents and found that none of their house‐staff had any previous training in handoffs during medical school, consistent with the finding that only 8% of U.S. medical schools provided formal instruction on handoffs.3 Prior to taking the Selective, our students had no knowledge of verbal or written templates for patient handoffs, although both before and after the course they felt that standardization was an important component of the process.
A number of verbal structures for handing off patient care have been described in the literature and there is not a consensus as to which functions best. Perhaps the most cited verbal communication format is SBAR (ie, situation, background, assessment and recommendation).16, 17 This tool was developed by Leonard et al.18 specifically for use by nurses to provide 1‐way communication to physicians pertaining to a change in patient status. We considered teaching the SBAR approach to the students but felt that it did not provide a suitable structure for handoffs because the transfer of care is not generally an event‐based situation and the literature on handoffs indicates that an optimal verbal system includes 2‐way communication.
Additional mnemonics for handoffs found in the literature include SIGNOUT (ie, Sick or DNR, Identifying information, General hospital course, New events of the day, Overall health status, Upcoming possibilities with plan, and Tasks to complete),14 I PASS the BATON (ie, Introduction, Patient, Assessment, Situation, Safety, Background, Actions, Timing, Ownership, Next)19 and the SAIF‐IR system (see boxed text).14
Verbal Structure for Patient Handoffs: SAIF‐IR
Off‐going provider performs a SAIF handoff:
Summary statement(s)
Active issues
If‐then contingency planning
Follow‐up activities
On‐coming provider makes the handoff SAIF‐IR:
Interactive questioning
Read‐backs
We developed the SAIF‐IR mnemonic to maximize efficiency and effectiveness while differentiating the verbal portion of the handoff from the written and incorporating 2‐way communication into its structure. In the Summary statement, we emphasize that this is not a history of present illness. We ask our students to summarize, in 1 to 3 sentences, the patient's presentation and working diagnosis. When discussing patient issues, we ask our students to only verbalize Active issues, although the written template has inactive, chronic issues listed. Here, we also ask our students to express their level of concern for the active issues and patient in general. If‐then's and Follow‐ups are usually verbalized together. Based on the offgoing provider's knowledge of the patient, we encourage the offgoing provider to anticipate potential problems and advise the oncoming provider on potential responses. Much of this advice is difficult to express in the written format and thus may not be found on the written handoff when the verbal handoff occurs. We encourage oncoming providers to take notes on the preprinted handoff sheet as part of the handoff process.
Through Interactive questioning and Read‐backs, we train our students and house‐staff to use the active listening techniques used outside of healthcare, in settings such as nuclear power plants and National Aeronautics and Space Administration mission control, where poor handoff communication may also result in safety concerns and adverse events.20 Interactive questioning allows the oncoming provider to correct or clarify any information given by the off‐going provider. Read‐backs are a method of confirming follow‐up activity or contingency plans. Together, the SAIF‐IR mnemonic builds a 2‐way communication structure into the patient handoff with both offgoing and oncoming providers having predefined roles.
Much of the information on our written handoff (patient identifying information, medications, language preference, code status, admission date) is not verbalized unless it is part of the active issues or the if‐then, follow‐ups (ie, medication titration for a patient admitted with an acute coronary syndrome or cor status in a patient newly made comfort care). By not reading extraneous information, we seek to emphasize the Active issues as well as the If‐then, Follow‐ups. We feel this emphasis maximizes the effectiveness of the handoff, while the purposeful nonverbalization of written materials such as identifying information maximizes its efficiency. Future work may examine which verbal and written structures for patient handoffs most benefit patient care and workflow through standard communication.
While our students found the Handoff Selective to be useful and to improve their self‐perceived ability to perform handoffs, we were not able to determine whether our program affected downstream outcomes such as adverse events relating to failures in handoff communication. Additionally, since we only taught and evaluated our Selective at the University of Colorado Denver School of Medicine, the response of our students may not generalize to other medical schools. Multicentered, prospective, randomized controlled trials may determine whether handoff education programs are successful in reducing patient adverse events related to transfers of care.
While handoffs occur frequently and are increasingly recognized as a vulnerable time in patient care, little is known about how to effectively teach handoffs to medical students during their clinical years. We developed a formal course to teach the importance of handoffs and how the process should be conducted. Our students reported that the Handoff Selective we developed improved their knowledge about the process and their perception of their ability to perform handoffs in a time‐appropriate and effective manner. In response to the feedback we received from our students, the Handoff Selective is the only course in the ICC that has been made mandatory for all students.
Communication failures are well‐recognized as causes of medical errors.1, 2 Specifically, handoffs of patient care responsibilities, which are increasingly prevalent in academic medical centers,3 have been cited as the most frequent cause of teamwork breakdown resulting in the harmful medical errors found in malpractice claims.1 The Institute of Medicine has recently identified patient handoffs as the moment where patient care errors are most likely to occur.4 A survey of 125 U.S. medical schools, however, found that only 8% specifically taught students how to hand off patient care.3
In July 2003, the American Council of Graduate Medical Education (ACGME) mandated that residency programs decrease resident work hours to improve patient care and safety by reducing fatigue,5 and a recent Institute of Medicine report suggests that they be decreased even further.4 Studies examining outcomes during the first 2 years after reducing duty hours did not find reductions in risk‐adjusted mortality.68 One proposed explanation for this lack of improvement is that the reduction in fatigue‐related medical errors is being offset by discontinuity of care with due to the increased number of patient handoffs resulting from shortened duty hours,911 one recent study found that omission of key information during patient sign outs frequently resulted in adverse patient care outcomes.12
In 2007, the Joint Commission developed a new National Patient Safety Goal that requires organizations to improve communication between caregivers.13 We recently developed an approach by which Internal Medicine residents hand off patient care using a structured process, written and verbal templates, formal training about handoffs, and direct attending supervision.14 Because fourth‐year medical students perform the duties of interns when working as subinterns, we recognized that education about handoffs should occur prior to the time students became interns. Accordingly, we developed a course designed to teach patient handoffs to medical students at the transition between their third and fourth years of training.
Setting
The Handoff Selective was developed by faculty of Denver Health and the University of Colorado Denver School of Medicine.
Program Description
The Selective was first offered in April 2007 as part of an Integrated Clinician's Course (ICC), a 2‐week course for students beginning their fourth year, which starts in April at the University of Colorado. The ICC includes both mandatory and selective sessions that are focused on developing clinical skills and preparing them for their subinternships. The Handoff Selective was conducted in a computerized teaching laboratory, lasted a total of 2 hours and consisted of 2 parts. Each of the 5 Denver Health Hospital Medicine faculty members versed in handoff education taught 2 sessions of 6 to 8 students.
Part 1: Didactic
During the first hour of class, the faculty presented a lecture that summarized the relevant literature on handoffs and explained the importance of the topic. The objectives of the didactic were to: (1) understand the importance of handoffs; (2) explore different communication elements and structures; (3) gain exposure to handoffs outside of healthcare; and (4) learn a structure for handoffs of patient care in hospitalized patients.
We used 3 video clips of handoffs from 2 football games to demonstrate the importance of practice, training, and 2‐way communications in handoffs. The first video clip showed a runner trying to make a spontaneous handoff while being tackled. The receiver was not expecting the handoff and was preoccupied with blocking another player. This attempted handoff resulted in a fumble, which we related to an adverse patient event.
The next 2 video clips showed 2 complex, seldom used, but well‐known football handoffsthe hook and lateral and the Statue of Liberty. Both handoffs were successfully executed presumably as a result of education, practice and the active participation of both players (handing off and receiving) in the process. We then related the teaching and practicing of complex communication to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO; now simply the Joint Commission) data suggesting that most sentinel events have their root cause in communication and training failures.2
Basic communication elements and process structures were then explored using scenarios from everyday life and evidence from fields outside of medicine. We emphasized that structures for communication (modes, vehicles, and settings) must be chosen according to the occasion and that handoffs are common and important in all occupations. In discussing modes (verbal, written, or nonverbal), vehicles (paper, telephone, or e‐mail), and settings (face‐to face, virtual, or disconnected), we emphasized that the most effective structures for communication (verbal, face‐to face meetings, with written materials and other visual aids at the patient's bedside) were also the most time‐consuming (Figure 1). While our standard for resident handoffs is a face‐to‐face verbal interaction with preprinted written materials as an aid, we also emphasized that for complex patients (eg, mental status changes, concern for an acute abdomen) more robust communication is often needed. Accordingly, a more time‐consuming bedside handoff with simultaneous, focused physical exam and history‐taking by both oncoming and off‐going providers may be most appropriate.

As real‐life examples, we asked our students to communicate a happy birthday wish to their mother, who lives in another state. Almost uniformly, in addition to a written aid (birthday card), they choose the telephone as a vehicle for their verbal mode in a virtual setting with 2‐way communication possible. In contrast, when asked to propose marriage to a significant other in another state, students felt that a face‐to‐face meeting with verbal and nonverbal (ie, ring) modes was appropriate. This time‐consuming mode of communication was felt to be necessary to create a sentiment of importance and avert any possible miscommunication.
The didactic session concluded by demonstrating how to use standardized written and verbal templates for handoffs of the care of a hospitalized patient. We explore the differentiation between written and verbal handoffs in our discussion below.
Part 2: Practicum
The second hour was devoted to practicing handoffs as a group. The faculty developed 6 case scenarios that differed with respect to diagnosis, length of stay, active medical issues, and anticipated discharge (Table 1). The scenarios included extensive admission information as well as evolving issues for each patient that were specific to the day of the intended handoff. Students were given Microsoft Word table‐based handoff templates to use when creating written sign‐outs for their patients. Verbal handoffs were performed between students and sign‐outs were exchanged. The faculty then role‐played cross‐cover calls that were specific for each scenario to test the students' inclusion of integral information in their handoffs and their ability to create contingency plans.
| Diagnosis | LOS | Active Issues | Cross‐Cover |
|---|---|---|---|
| |||
| CP | 1 | CP, HTN, DM | CP, HTN, headache |
| GIB | 1 | GIB, alcohol withdrawal | Poor response to red call transfusion, coagulopathy |
| Acute pancreatitis | 2 | Pain, possible pancreatic abscess | Fever, agitation, hypoxia |
| CHF | 2 | CHF, DM, nausea | Lack of diuresis, CP, hypoglycemia |
| Acute kidney injury | 3 | None, ready for discharge | HTN, hyperglycemia |
| Community acquired pneumonia | 3 | Anxiety, discharge pending | Confusion, emesis with hypoxia |
Program Evaluation
We developed a 2‐part survey to evaluate the effectiveness of the Selective and to solicit feedback about the didactic and practicum portions of the course. The first part of the survey (Table 2) contained 16 items to assess the students' knowledge of, and attitudes toward handing off patient care, along with their comfort with the handoff process. Responses to this section were scored using a 5‐point Likert scale with 1 indicating strongly disagree and 5 indicating strongly agree. This part of the survey was administered both prior to and after the Selective.
| Competency | Selective | |
|---|---|---|
| Before | After | |
| ||
| I know how to hand off patients | 2.3 0.8 | 4.2 0.6* |
| I know how to make contingency plans for my patients | 2.1 0.8 | 3.9 0.7* |
| I know what a read‐back is | 2.3 1.3 | 4.4 0.9* |
| I know how to perform a read‐back | 2.0 1.2 | 4.2 0.9* |
| I know when to perform a read‐back | 1.6 0.8 | 4.1 1.0* |
| I am efficient at communicating patient information | 2.2 0.9 | 3.6 0.7* |
| I am effective at communicating patient information | 2.2 0.8 | 3.8 0.6* |
| I know a standard written structure for handoffs | 2.1 1.1 | 4.4 0.6* |
| I know a standard verbal structure for handoffs | 2.0 1.1 | 4.2 0.6* |
| I can choose appropriate modes of communication | 2.7 1.1 | 4.4 0.6* |
| I can choose appropriate vehicles of communication | 2.6 1.1 | 4.5 0.6* |
| I can choose appropriate settings for communication | 2.9 1.1 | 4.4 0.6* |
| Handoffs are well taught in my medical school | 1.6 0.8 | 3.5 1.0* |
| Standardization is important in handoffs | 4.3 0.9 | 4.6 0.5 |
| Handoffs are safer with attending supervision | 3.7 1.0 | 3.9 0.8 |
| I feel comfortable cross‐covering on patients | 1.6 0.7 | 3.0 1.0* |
The second part (Table 3) contained 12 items and was designed to evaluate the perceived usefulness of the different components of the class. This section was only administered at the end of the Selective. It utilized a 4‐point Likert scale with 1 indicating that the component was not useful at all, and 4 indicating that it was extremely useful. The first 6 items of the second section allowed students to evaluate the didactic portion of the handoff. The second 6 items allowed students to evaluate the practicum. Responses to all 12 items were then combined to determine an overall composite usefulness for the Selective.
| Useful [n (%)] | |
|---|---|
| |
| Overall composite usefulness | 578 (92) |
| Didactic composite usefulness | 254 (84) |
| Using fumble video clips for discussing handoffs | 32 (64)* |
| Discussion of modes of communication | 46 (88) |
| Discussion of vehicles of communication | 46 (88) |
| Discussion of settings of communication | 48 (96) |
| Choosing handoff structures for nonhealthcare handoffs | 37 (71)* |
| Discussing handoffs in industries outside of healthcare | 45 (94) |
| Practicum composite usefulness | 324(100) |
| Role playing | 54 (100) |
| Patient handoff scenarios | 54 (100) |
| Completing computerized templates | 54 (100) |
| Delivering handoffs to peer | 54 (100) |
| Receiving handoffs from peer | 54 (100) |
| Cross‐cover questions and discussion | 54 (100) |
The Selective was also evaluated qualitatively through the use of open‐ended, written comments that were solicited at the end of the survey. All surveys were administered anonymously.
Data Analysis
Student paired t test was used to compare continuous variables recorded before and after the Selective. A chi‐square test was used to assess the students' perception of the usefulness of the didactic vs. the practicum methods of teaching handoffs.
All analyses were performed using SAS (version 8.1; SAS Institute, Inc., Cary, NC). Bonferroni corrections were used for multiple comparisons such that P values of <0.003 and <0.004 were considered to be significant for continuous and categorical variables, respectively. All data are reported as mean standard deviation (SD).
The survey was approved by our local Institutional Review Board.
Results
More students chose the Selective than we had capacity to accommodate (60 of a class of 150). The pre‐ and postcourse survey response rate was 56 of 60 (93%) and 58 of 60 (97%), respectively. After the Selective, the mean score in response to whether handoffs are well taught in medical school increased from 1.6 to 3.5 (P < 0.003). Our students' self‐perceived skills and knowledge about handoffs improved after the Selective (Table 2). The greatest changes in perceived knowledge occurred in questions regarding the what, how, and when of read‐backs, and the knowledge of standard verbal and written handoff structures. The responses to the survey elements which assessed our students' attitudes regarding the importance of standardization and whether they felt handoffs were safer with faculty supervision did not change after the Selective (Table 2).
A total of 92% of the students felt that the course was extremely useful or useful. The role‐playing activity was thought to be more helpful than the didactic, but 84% of the students still rated the didactic portion as useful or extremely useful (Table 3). The element which was the least well received in the didactic portion was the use of video clips to demonstrate successful and unsuccessful (fumbled) college football handoffs, although the majority (64%) of students still found it useful.
The major theme generated from the comments section of the survey was that the Selective should be a required course.
Discussion
We know of no previously published literature that has addressed teaching handoffs to medical students. Horwitz et al.15 developed a sign‐out curriculum for Internal Medicine residents and found that none of their house‐staff had any previous training in handoffs during medical school, consistent with the finding that only 8% of U.S. medical schools provided formal instruction on handoffs.3 Prior to taking the Selective, our students had no knowledge of verbal or written templates for patient handoffs, although both before and after the course they felt that standardization was an important component of the process.
A number of verbal structures for handing off patient care have been described in the literature and there is not a consensus as to which functions best. Perhaps the most cited verbal communication format is SBAR (ie, situation, background, assessment and recommendation).16, 17 This tool was developed by Leonard et al.18 specifically for use by nurses to provide 1‐way communication to physicians pertaining to a change in patient status. We considered teaching the SBAR approach to the students but felt that it did not provide a suitable structure for handoffs because the transfer of care is not generally an event‐based situation and the literature on handoffs indicates that an optimal verbal system includes 2‐way communication.
Additional mnemonics for handoffs found in the literature include SIGNOUT (ie, Sick or DNR, Identifying information, General hospital course, New events of the day, Overall health status, Upcoming possibilities with plan, and Tasks to complete),14 I PASS the BATON (ie, Introduction, Patient, Assessment, Situation, Safety, Background, Actions, Timing, Ownership, Next)19 and the SAIF‐IR system (see boxed text).14
Verbal Structure for Patient Handoffs: SAIF‐IR
Off‐going provider performs a SAIF handoff:
Summary statement(s)
Active issues
If‐then contingency planning
Follow‐up activities
On‐coming provider makes the handoff SAIF‐IR:
Interactive questioning
Read‐backs
We developed the SAIF‐IR mnemonic to maximize efficiency and effectiveness while differentiating the verbal portion of the handoff from the written and incorporating 2‐way communication into its structure. In the Summary statement, we emphasize that this is not a history of present illness. We ask our students to summarize, in 1 to 3 sentences, the patient's presentation and working diagnosis. When discussing patient issues, we ask our students to only verbalize Active issues, although the written template has inactive, chronic issues listed. Here, we also ask our students to express their level of concern for the active issues and patient in general. If‐then's and Follow‐ups are usually verbalized together. Based on the offgoing provider's knowledge of the patient, we encourage the offgoing provider to anticipate potential problems and advise the oncoming provider on potential responses. Much of this advice is difficult to express in the written format and thus may not be found on the written handoff when the verbal handoff occurs. We encourage oncoming providers to take notes on the preprinted handoff sheet as part of the handoff process.
Through Interactive questioning and Read‐backs, we train our students and house‐staff to use the active listening techniques used outside of healthcare, in settings such as nuclear power plants and National Aeronautics and Space Administration mission control, where poor handoff communication may also result in safety concerns and adverse events.20 Interactive questioning allows the oncoming provider to correct or clarify any information given by the off‐going provider. Read‐backs are a method of confirming follow‐up activity or contingency plans. Together, the SAIF‐IR mnemonic builds a 2‐way communication structure into the patient handoff with both offgoing and oncoming providers having predefined roles.
Much of the information on our written handoff (patient identifying information, medications, language preference, code status, admission date) is not verbalized unless it is part of the active issues or the if‐then, follow‐ups (ie, medication titration for a patient admitted with an acute coronary syndrome or cor status in a patient newly made comfort care). By not reading extraneous information, we seek to emphasize the Active issues as well as the If‐then, Follow‐ups. We feel this emphasis maximizes the effectiveness of the handoff, while the purposeful nonverbalization of written materials such as identifying information maximizes its efficiency. Future work may examine which verbal and written structures for patient handoffs most benefit patient care and workflow through standard communication.
While our students found the Handoff Selective to be useful and to improve their self‐perceived ability to perform handoffs, we were not able to determine whether our program affected downstream outcomes such as adverse events relating to failures in handoff communication. Additionally, since we only taught and evaluated our Selective at the University of Colorado Denver School of Medicine, the response of our students may not generalize to other medical schools. Multicentered, prospective, randomized controlled trials may determine whether handoff education programs are successful in reducing patient adverse events related to transfers of care.
While handoffs occur frequently and are increasingly recognized as a vulnerable time in patient care, little is known about how to effectively teach handoffs to medical students during their clinical years. We developed a formal course to teach the importance of handoffs and how the process should be conducted. Our students reported that the Handoff Selective we developed improved their knowledge about the process and their perception of their ability to perform handoffs in a time‐appropriate and effective manner. In response to the feedback we received from our students, the Handoff Selective is the only course in the ICC that has been made mandatory for all students.
- , , .Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79:186–194.
- Root causes of sentinel events. The Joint Commission. Available at: http://www.jointcommission.org/NR/rdonlyres/FA465646‐5F5F‐4543‐AC8F‐E8AF6571E372/0/root_cause_se.jpg Accessed October2009.
- , , , et al.Lost in translation: challenges‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- Institute of Medicine.Resident Duty Hours: Enhancing Sleep, Supervision and Safety.Washington, DC:National Academies Press;2008.
- ACGME duty hours. Accreditation Council for Graduate Medical Education. http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October2009.
- , , , et al.Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME duty hour reform.JAMA.2007;298(9):975–983.
- , , , et al.Mortality among patient in VA hospitals in the first 2 years following ACGME duty hour reform.JAMA.2007;298(9):984–992.
- , , , .Changes in outcomes for internal medicine patients after work‐hour regulations.Ann Intern Med.2007;147(2):1–7.
- , , , et al.Transfers of patient care between house staff on internal medicine wards.Arch Intern Med.2006;166:1173–1177.
- , , , .Medical errors involving trainees.Arch Intern Med.2007;167(19):2030–2036.
- .Reducing resident work hours: unproven assumptions and unforeseen outcomes.Ann Intern Med.2006;140:814–815.
- , , et al.Consequences of inadequate sign‐out for patient care.Arch Intern Med.2008;168(16):1755–1760.
- JCAHO Handoff Communication. National patient safety goal. The Joint Commission. http://www.jointcommission.org/GeneralPublic/NPSG/07_npsgs.htm. Accessed October2009.
- , , , et al.A structured handoff program for interns.Acad Med.2009;84:347–352.
- , , .Development and implementation of an oral sign out skills curriculum.J Gen Intern Med.2007;22(10):1470–1474.
- , , , et al.Managing discontinuity in academic medical centers: strategies for a safe and effective sign out.J Hosp Med.2006;1:257–266.
- , , , .A theoretical framework and competency based approach to improving handoffs.Qual Saf Health Care.2008;17:11–14.
- , , .The human factor: the critical importance of effective teamwork in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- University HealthSystem Consortium Best Practice Recommendation: Patient Handoff Communication. White Paper. May 2006.Oak Brook, IL:University HealthSystem Consortium;2006.
- , , , , .Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- , , .Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79:186–194.
- Root causes of sentinel events. The Joint Commission. Available at: http://www.jointcommission.org/NR/rdonlyres/FA465646‐5F5F‐4543‐AC8F‐E8AF6571E372/0/root_cause_se.jpg Accessed October2009.
- , , , et al.Lost in translation: challenges‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- Institute of Medicine.Resident Duty Hours: Enhancing Sleep, Supervision and Safety.Washington, DC:National Academies Press;2008.
- ACGME duty hours. Accreditation Council for Graduate Medical Education. http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October2009.
- , , , et al.Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME duty hour reform.JAMA.2007;298(9):975–983.
- , , , et al.Mortality among patient in VA hospitals in the first 2 years following ACGME duty hour reform.JAMA.2007;298(9):984–992.
- , , , .Changes in outcomes for internal medicine patients after work‐hour regulations.Ann Intern Med.2007;147(2):1–7.
- , , , et al.Transfers of patient care between house staff on internal medicine wards.Arch Intern Med.2006;166:1173–1177.
- , , , .Medical errors involving trainees.Arch Intern Med.2007;167(19):2030–2036.
- .Reducing resident work hours: unproven assumptions and unforeseen outcomes.Ann Intern Med.2006;140:814–815.
- , , et al.Consequences of inadequate sign‐out for patient care.Arch Intern Med.2008;168(16):1755–1760.
- JCAHO Handoff Communication. National patient safety goal. The Joint Commission. http://www.jointcommission.org/GeneralPublic/NPSG/07_npsgs.htm. Accessed October2009.
- , , , et al.A structured handoff program for interns.Acad Med.2009;84:347–352.
- , , .Development and implementation of an oral sign out skills curriculum.J Gen Intern Med.2007;22(10):1470–1474.
- , , , et al.Managing discontinuity in academic medical centers: strategies for a safe and effective sign out.J Hosp Med.2006;1:257–266.
- , , , .A theoretical framework and competency based approach to improving handoffs.Qual Saf Health Care.2008;17:11–14.
- , , .The human factor: the critical importance of effective teamwork in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- University HealthSystem Consortium Best Practice Recommendation: Patient Handoff Communication. White Paper. May 2006.Oak Brook, IL:University HealthSystem Consortium;2006.
- , , , , .Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
Copyright © 2010 Society of Hospital Medicine
In response to: A quality conundrum: Well done but not enough—Quality improvement conundrums: Looking back before moving forward
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
Rapid Response: A QI Conundrum
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.
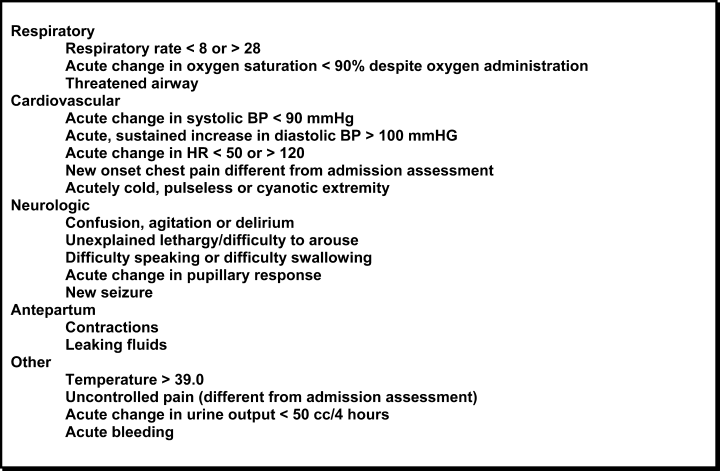
Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.

Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.

Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.
Intimate Partner Violence
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.
| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.
No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.

| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.

No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.

| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.

No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
Copyright © 2008 Society of Hospital Medicine
Time for Health Education of Hospitalized Patients
Educating patients about smoking cessation when they are hospitalized for acute coronary syndrome results in a 57% 1‐year quit rate.1 This rate is far higher than the typical 15%‐30% 1‐year quit rates observed with smoking cessation programs administered in the outpatient setting24 and suggests that hospitalized patients may be uniquely motivated to respond to health education.
Kerzman and colleagues5 found that 42% of hospitalized patients expressed a wish to receive more comprehensive counseling about their medications before being discharged from the hospital. And although the Joint Commission on Hospital Accreditation and the Centers for Medicare and Medicaid Services have established core quality measures mandating that patients hospitalized with congestive heart failure receive education as one component of a high‐quality discharge process,6, 7 approximately one third of patients nationally do not receive adequate patient education.8
Barber‐Parker9 suggested that because patient acuity in hospitals was so high and patients were so commonly absent from their nursing units for testing and treatment, there was little time available for health education during their hospitalization. Anecdotal observations in our institution suggested, however, that adult patients hospitalized on the general Internal Medicine service spent much of their day doing little more than lying in bed watching television. Accordingly, we hypothesized that considerable time might be available for patient education during a hospitalization. We therefore sought to quantify the fraction of time patients were not involved in treatment activities, diagnostic testing, or other evaluations and to determine whether during these times they wanted and were feeling well enough to participate in educational activities. We also sought to determine what patients wanted to know about their health problems and what types of educational activities they most preferred.
MATERIALS AND METHODS
We conducted a time‐motion and survey study from June 25, 2005, to August 15, 2005, at Denver Health Medical Center, an academic public safety‐net hospital affiliated with the University of Colorado School of Medicine. All patients older than 18 years of age who spoke English or Spanish and were admitted to the general Internal Medicine service were candidates for enrollment. Exclusion criteria were being admitted to the intensive care unit, having an inability to communicate, being in contact precautions, and being previously enrolled. The study was approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained for all study participants.
At 8:00 AM, all patients admitted during the previous 15 hours were assigned a random number from a random number table and were approached for consent in numeric order. With 2 data collectors working daily, a maximum of 12 patients could be enrolled each day. Consenting subjects who passed a vision test were given the Test of Functional Health Literacy in Adults at the time of enrollment and a written questionnaire (in either English or Spanish) on a daily basis for a maximum of 6 days. Some of these patients also participated in a structured interview that was designed to elicit their views on health education topics and formats for education of hospitalized patients. Others, again determined by random number, were subjects of a time‐motion study.
Demographic data collected included age, sex, language, race, comorbidities, insurance status, and discharge diagnosis.
Time‐Motion Study
Patients were observed from 8:00 AM to noon and from 1:00 to 5:00 PM7 days a week. Data were collected using TimerPro on a Dell Axim A5 pocket PC and imported daily into an Excel spreadsheet. Observations were categorized as downtime, busy time, or provider time and subcategorized as summarized in Table 1.
| First level | Second level | Third level |
|---|---|---|
| Downtime | Alone | TV |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone | ||
| Other | ||
| Friends/family | TV | |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone/talk | ||
| Other | ||
| Provider | Physician | |
| Nurse | ||
| Physician and nurse | ||
| Physician and other | ||
| Other | ||
| Busy | ADL | |
| Meal | ||
| Out of room | ||
| Other |
Questionnaire
We were unable to find a validated questionnaire in the literature that was designed to assess patient opinion or level of interest in educational activities during a hospitalization. Accordingly, we developed our own using a 5‐point Likert scale (Box 1). Two outcomes researchers with expertise in using questionnaires for clinical research independently reviewed the questionnaire to establish face validity.
Box 1. Daily Questionnaire on In‐Hospital Health Education
The following statements were read to the patients on a daily basis and answered using the following scale: 1 = Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree
I feel well enough today to learn about my illness or my health.
I want to learn more about my illness or my health today.
I have time to learn about my health today.
It is important to me to learn more about my illness or health while in the hospital.
Interview
All patients were asked the open‐ended questions listed in Box 2, and the entire interview was recorded on audiotape for subsequent analysis.
Box 2. Interview Questions with Probes for Educational Preferences
What things related to your health would you like to learn more about while you are in the hospital? (list up to three topics in order of impotance to you).
How can we help you learn more about your illness or health while in the hospital?
Who should do the teaching (eg, an MD, a nurse, a dietician, a medical student, peers, physical therapists, respiratory therapists)?
Who else should be present (eg, patients with similar illness, family, no one)?
How should this teaching be done (eg, didactic sessions, hands‐on, video tape, pre‐ and post‐testing)?
Data Analysis
All analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC). A P value < 0.05 was considered significant.
Time‐Motion Data
Mixed‐effects general linear models (growth curve or repeated measures), using SAS Proc Mixed, were used to test whether the proportions of downtime, busy time, and provider time differed by day of hospitalization. Linear growth curve models were used to test whether a linear trend was present. If not, repeated‐measures models were used to obtain estimates by day of hospitalization.
Questionnaire Data
Mixed‐effects general linear models (growth curve or repeated measures) were used to determine whether patient responses differed by day of hospitalization, as described above.
Interview Data
Tape recordings were reviewed in depth to code participant responses to the structured questions. We utilized the template style of analysis, coding segments of the interviews and identifying illustrative quotes whenever possible. Key patterns and themes were summarized along with specific patient preferences regarding topics of interest and learning opportunities while in the hospital and after discharge.
RESULTS
Patient selection is described in Figure 1, and patient demographics are summarized in Tables 2 and 3.
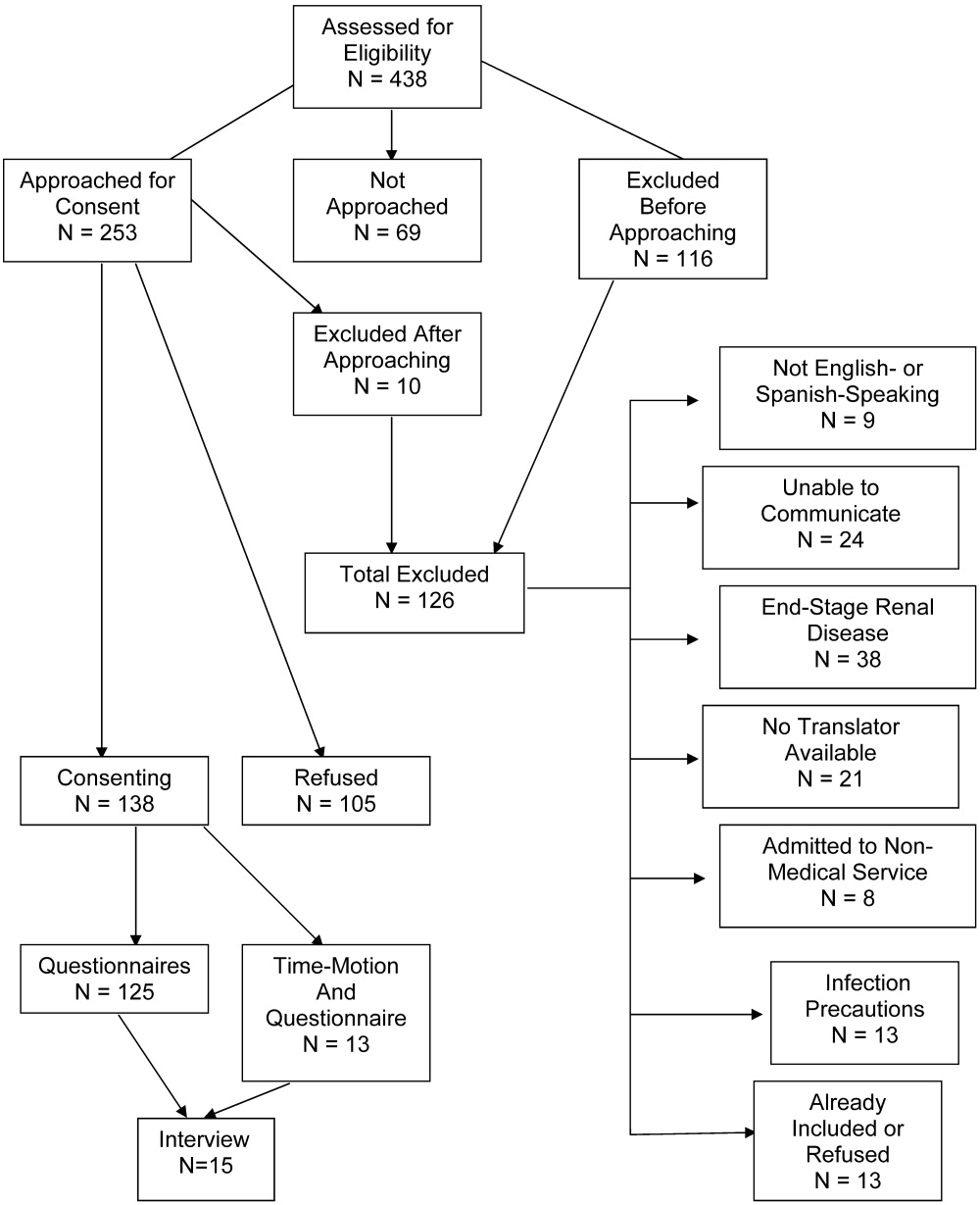
| Demographic | Time‐motion | Interview | Questionnaire |
|---|---|---|---|
| |||
| Study subjects | 13 | 15 | 125 |
| Sex | |||
| Male | 6 (46) | 7 (47) | 61 (49) |
| Female | 7 (54) | 8 (53) | 64 (51) |
| Age (years), median (IQR) | 47 (20) | 51 (20.5) | 51 (18) |
| Race/ethnicity | |||
| White, non‐Hispanic | 6 (46) | 5 (33) | 46 (37) |
| Black/African American | 3 (23) | 4 (27) | 27 (22) |
| American Indian | 0 (0) | 0 (0) | 1 (1) |
| Hispanic | 4 (31) | 6 (40) | 51 (41) |
| Primary language | |||
| English | 12 (92) | 14 (93) | 109 (87) |
| Spanish | 1 (8) | 1 (7) | 16 (13) |
| Health literacy* | |||
| Adequate | 3 (75) | 9 (82) | 60 (71) |
| Marginal | 1 (25) | 0 (0) | 6 (7) |
| Inadequate | 0 (0) | 2 (18) | 18 (22) |
| Insurance status | |||
| Self‐pay | 3 (23) | 1 (7) | 24 (19) |
| Medicaid | 1 (8) | 4 (27) | 19 (15) |
| Medicare | 3 (23) | 0 (0) | 2 (2) |
| Colorado Indigent Care Program | 3 (23) | 7 (47) | 51 (41) |
| Private | 2 (15) | 1 (7) | 5 (4) |
| Other | 1 (8) | 2 (14) | 24 (18) |
| Time‐motion | Interview | Questionnaire | |
|---|---|---|---|
| Study subjects (n) | 13 | 15 | 125 |
| Discharge diagnoses (selected) | |||
| Coronary artery disease (including angina) | 1 (8) | 2 (13) | 24 (19) |
| Congestive heart failure | 1 (8) | 1 (7) | 4 (3) |
| Upper gastrointestinal bleeding, gastritis, reflux | 2 (15) | 4 (27) | 14 (11) |
| Syncope | 2 (15) | 0 | 5 (4) |
| Acute renal failure | 0 | 0 | 5 (4) |
| Pancreatitis | 0 | 1 (7) | 6 (5) |
| Venous thromboembolism | 2 (15) | 1 (7) | 3 (2) |
| Chronic obstructive pulmonary disease | 0 | 0 | 4 (3) |
| Diabetic ketoacidosis | 1 (8) | 1 (7) | 3 (2) |
| Pyelonephritis | 0 | 0 | 5 (4) |
| Pneumonia | 2 (15) | 1 (7) | 5 (4) |
| Comorbidities | |||
| Diabetes | 5 (38) | 9 (60) | 41 (33) |
| Hypertension | 1 (8) | 9 (60) | 55 (44) |
| Dyslipidemia | 6 (46) | 2 (13) | 26 (21) |
| Tobacco | 5 (38) | 7 (47) | 55 (44) |
| Chronic obstructive pulmonary disease | 2 (15) | 4 (27) | 15 (12) |
| Congestive heart failure | 2 (15) | 2 (13) | 13 (10) |
| Coronary heart disease | 3 (23) | 3 (20) | 21 (17) |
Time‐Motion Study
Thirteen patients were studied. Of the 315 patient‐hours observed, 71% were categorized as downtime, 15% as provider time, and 14% as busy time. The proportion of downtime ranged from a low of 0.65 (SE 0.04) on hospital Day 2 to a high of 0.76 (SE 0.06) on hospital Day 4, but the differences in downtime proportions by day did not reach statistical significance (P = .65; Fig. 2). The lowest percentage of downtime observed in any patient on any day was 39%. The 125 hours of downtime observed consisted of 1317 separate blocks of time, 80% of which were less than 15 minutes in duration, 14% of which were 15 to 30 minutes in duration, and 6% of which exceeded 30 minutes in duration.
Thirty‐six full days of observation, defined as greater than 7 hours of observation in 1 day, were used to assess the amount of time spent with providers. Of the 60 minutes/day (IQR = 44) that patients spent with health care providers, 21 minutes/day (IQR = 34) was spent with phlebotomists, physical or occupational therapists, dieticians, or social workers, 25 minutes/day (IQR = 25) was spent with patients' nurses, and a median of only 9 minutes/day (IQR = 11) was spent with their physicians.
Questionnaire
A total of 311 questionnaires were administered to the 138 consenting participants. Irrespective of the day of testing, 79% to 97% strongly agreed or agreed with the 4 statements (Fig. 3). In response to the first statementI feel well enough to learnpatient scores increased steadily over the 6 days of hospitalization patients were surveyed (coefficient = 0.15, P = .004). On hospital day 1, the mean score was 3.85 (SE 0.08), and by day 6 the mean score had increased to 4.75 (SE 0.08) However, there was no significant change over time in patients' desire to learn, self‐perceived time available to learn, or importance placed on learning during their hospital stay.

Interview
Fifteen interviews were conducted. Representative comments are presented in Table 4. Responses generally indicated that the patients had anxieties and uncertainties about their health and safety after discharge. Most participants wanted to know more about the condition for which they had been hospitalized, including information pertaining to management, prevention, etiology, and prognosis of their disease. Diabetic patients asked for information on insulin dosing, nutrition, and the effect of the disease on their bodies.
| Theme | Sample quotes |
|---|---|
| Preferred topics | |
| Self‐management | I need to know what to do when I go home, how to take care of it. Medical peopledon't give enough information to the patient and patient's family so they can help themselves. You need to encourage patients to help themselves, take some responsibility for themselves. |
| Prevention of disease recurrence or progression | It's okay to tell people that they have something and give them medicine, but also tell them what they can do to prevent it or make it less painful. I've known about bronchitis for many years, but didn't know it would affect my heart. |
| What's happening to me? | Am I going to diehow long? |
| Just fix me | I came to the hospital to get fixed, not educated. I'm results‐oriented, not cause‐oriented. |
| Preferred learning methods | |
| One‐to‐one didactics with MDs | I'd like one‐to‐one time with someone who has the time to listen. One to one with doctors who can explain what can happen, what to take, what not to take. |
| Family involvement | Get the family involved so the family understands the limitations of the person, how medications affect them. To say a person has a heart condition is a very vague statement. If they [family] understand more, it's better. |
| Groups | A group of people with similar illnessI like groups where everyone listens. I'd participate in groups at the hospital but not at home. |
| Video | Hospital TV is not meeting my needs. |
| Printed material | A doctor or nurse tell me what's going on and then also handouts on dietary and nutrition. |
| Electronic learning | I learned a lot through the encyclopedia of family health care, and through Web sites |
Patients preferred to pick their own topics for education rather than having topics chosen for them. Patients also showed in interest in prevention. One diabetic patient wanted to know how to prevent her children from becoming diabetic, and a cardiac patient wanted education on heart disease prevention. Other recurrent themes included the desire to know what was causing their illness and information about prognosis.
Almost all the patients were interested in more than one type of learning experience. The most frequently cited preference was to have a doctor or other knowledgeable health care professional answer questions specific to their individual situation. Video and group learning were each mentioned by approximately half the participants. Most patients thought that having family present during educational discussions was important.
Video was the most frequently mentioned learning tool, and patients thought it would be useful to have this modality available in the hospital as well as the home. Two patients expressed interest in computerized learning (one of whom had used health Web sites before his hospitalization). Most patients wanted handouts or reading material in addition to other methods of communicating information. Although many patients said they felt comfortable discussing their problems in group settings, some did not.
DISCUSSION
The important findings of this study are that hospitalized patients have a substantial amount of time available for health education and a considerable willingness and interest in participating in health educational activities. We found that although there was a great deal of time available on all days of hospitalization studied, patients felt increasingly well enough to participate in educational activities through their hospital stay.
We are unaware of other studies that have attempted to quantify the amount of time hospitalized patients are available for educational activities or whether they feel capable of participating in these activities. McBride10 found that 95% of hospitalized patients supported a health‐promoting hospital and that almost 80% wanted to modify at least 1 aspect of their lifestyle. Martin and colleagues11 found that patient satisfaction was improved by a patient‐centered unit incorporating dedicated nursing staff to promote patient involvement and provide personalized care and education. Barber‐Parker9 suggested that high patient acuity, short durations of hospitalization, and lack of patient availability because of testing and treatment limited the opportunities that patients had for health education during their hospitalization. These conclusions were reached on the basis of surveys of nurses' perceptions, however, rather than on direct observations or assessments of patients' perceptions.
Our findings suggest that many types of patient educational approaches may be needed to achieve maximal effectiveness and that regardless of the specific approach employed, the focus should be on the primary reason for a patient's hospitalization, what the hospitalization meant, why it happened and what the patient can do to prevent hospitalization from occurring in the future.
Transitions in care have been identified as periods in which communication lapses occur and outcomes can be adversely affected.12 A recent study by Epstein and colleagues13 found that almost 12% of patients had new or worsening symptoms of disease within the first few days after discharge from the hospital and that 22% either did not pick up their medications or understand how to take them (consistent with the observations of Kerzman and colleagues).5 The most common action taken in response to these findings was nurse‐mediated patient education. Our study indicates there is potential for further educational processes in hospitals, which may improve the safety of transitions from a hospital setting to outpatient care.
Although many disease management programs have been studied in the outpatient setting,1417 very few have been extended into hospitals. Accordingly, hospitalists are ideally suited to develop and implement disease management programs in concert with outpatient efforts.18 Our study suggests there is an underutilized opportunity for hospital‐based physicians and other health care providers to work with patients at a time when they are uniquely focused on their own health and free from many of the time constraints of their normal lives.
Although JCAHO mandates that hospitalized patients receive education and training specific to the patient's needs and as appropriate to the care, treatment and services provided,19 there is a paucity of data describing the educational processes in US hospitals. Johansson and colleagues20 conducted a survey in a Finnish hospital where patient education is also mandated. Written materials were given to about 55% of the patients. Demonstration and practice were used with about one third, whereas the Internet and videotapes were used for fewer than 10%. Although patients underwent educational activities throughout their hospitalization, and most were satisfied with the process, Johansson and colleagues found that only 59% felt that what they knew about their care was sufficient, almost a third felt they did not know enough about the side effects of their medical care, and almost half felt they did not have sufficient input into what they were being taught.
Although we found a large amount of time that might be used for patient education during a hospitalization, this time was commonly limited to 15‐minute blocks, as has been noted previously.9 This observation implies that educational activities should be designed so they can be conducted over short periods and/or stopped for short periods when interruptions occur or that the processes of care during a hospitalization should be altered to create larger blocks of continuous time available for educational activities.
A number of issues could have biased our results. Only 66% of the patients who were approached to participate agreed to do so. Because those declining may have been sicker and because sicker patients may require more diagnostic testing or more invasive treatment, we may have overestimated both the amount of downtime available and the willingness of patients to participate in educational activities. If we assume, however, that all patients who refused to participate either disagreed or strongly disagreed with the statements in the questionnaire regarding their interest in educational activities, the fraction of patients agreeing or strongly agreeing with idea that they were well enough and interested would still be 57% to 75% of the population sampled. Accordingly, this potential bias, if it occurred, would not alter our conclusions.
The time‐motion studies were only performed between 8:00 AM and 5:00 PM, such that the resulting data do not reflect any diagnostic testing, therapeutic interventions, or contact with health care providers that occurred at other times. This may have contributed to the strikingly small amount of time that patients spent with their physicians and nurses. If patient time after 5:00 PM and before 8:00 AM had been observed and included, it is likely that the absolute amount of time spent with physicians and nurses would increase, whereas the overall proportion of patient time spent with providers would decrease.
We were also only able to collect data on 13 time‐motion subjects. This limited sample size from a single institution may not be representative of all hospitalized non‐ICU patients on general medical wards. Accordingly, we make no claims that our data can be generalized to the entire population of patients admitted to non‐ICU medical services. However, the results of our surveys, which sampled a much broader patient population and supported our time‐motion findings, suggest that our time‐motion findings are likely to be representative of significant underutilized time and motivation for patient education in the hospital setting.
Also, it is important to note that although the time‐motion studies were only done with 13 patients, these studies are extremely labor intensive and are rarely done with much larger samples. In addition, the SDs on the data collected from the time‐motion studies were quite small. It is possible that if a larger sample were studied, the percentage of free time might be larger or smaller than what we observed for the 13 patients we studied. However, it would be quite unlikely that the amount of free time would be so small (eg, 10%‐15%) that it would invalidate our conclusion that considerable time is available for patient education over and above what currently occurs in most hospital settings.
A patient's self‐perception of his or her ability to learn may not reflect that patient's true cognitive readiness to do so. JCAHO requirements mandate that nurses be trained to assess patients for their ability to learn and to do so as part of the admission process. After reviewing all day 1 patient responses to our questionnaires, in no instance did a nurse assess a patient as having a barrier to learning when the patient had reported feeling well enough to learn. Accordingly, although we performed no direct tests of patients' ability to learn, this retrospective independent assessment did not suggest that patients systematically overestimated their ability to learn.
Finding that hospitalized patients are unoccupied for approximately 70% of their daytime hours and that most patients are both highly motivated to learn and have few barriers to doing so indicates that educational activities during hospitalizations have substantial potential for expansion. The current structure for educating hospitalized patients should be supplemented to take these findings into account.
Acknowledgements
We thank Dr. John Steiner and Dr. Sheena Bull for their assistance in study design as well as the development of the questionnaire and interview tools. We also thank Carolyn Nowels for her assistance with the qualitative data analysis. The assistance of Dr. Bull and Dr. Steiner was made possible through NHLBI grant U01HL079160, and funding for data collection was made possible by the University of Colorado at Denver and Health Sciences Center Department of Medicine, Division of General Internal Medicine small grants program.
- ,.Randomized controlled trial of smoking cessation intervention after admission for coronary heart disease.BMJ.2003;327:1254–1257.
- .Treatment of tobacco use and dependence.N Engl J Med.2002;346:506–512.
- ,, et al.A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report.JAMA.2000;283:3244–3254.
- ,,, et al.National Institutes of Health state‐of‐the science conference statement: tobacco use: prevention, cessation and control.Ann Intern Med.2006;145:839–844.
- ,,.What do discharged patients know about their medications?Patient Educ Couns.2005;56:276–282.
- Joint Commission on Accreditation of Healthcare Organizations.A Comprehensive Review for the Development and Testing for National Implementation of Hospital Core Measures.2006:1–40.
- .ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/ American Heart Association task force on practice guidelines.J Am Coll Cardiol.2005;46:1–82.
- JCAHO data. Available: https://cimprod.uhc.edu/CoreMeasures/Products/DownloadSystem/WebPages/ViewReportsDownloadList.aspx.
- .Integrating patient teaching into bedside patient care: a participant‐observation study of hospital nurses.Patient Educ Couns.2002;48:107–113.
- .Health promotion in the acute hospital setting: the receptivity of adult in‐patients.Patient Educ Couns.2004;54:73–78.
- ,,,,,.Randomized trial of a patient‐centered hospital unit.Patient Educ Couns.1998;34:125–133.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. HYPERLINK “javascript:AL_get(this,%20'jour',%20'JAMA.');”JAMA.2007;297:831–841.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2:58–68.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288:2469–2475.
- ,,.Effectiveness of self‐management training in type 2 diabetes.Diabetes Care.2001;24:561–587.
- ,,.Quality of life assessment after patient education a randomized controlled study on asthma and chronic obstructive pulmonary disease.Am J Respir Crit Care Med.2001;95:56–63.
- ,.The effects of a health educational and exercise program for older adults with osteoarthritis for the hip or knee.J Rheumatol.1997;24:1378–1383.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- The Joint Commission on Accreditation of Healthcare Organizations Comprehensive Accreditation Manual for Hospitals: The Official Handbook. January2007. p.152
- ,,,.Need for change in patient education: a Finnish survey from the patient's perspective.Patient Educ Couns.2003;51:239–245.
Educating patients about smoking cessation when they are hospitalized for acute coronary syndrome results in a 57% 1‐year quit rate.1 This rate is far higher than the typical 15%‐30% 1‐year quit rates observed with smoking cessation programs administered in the outpatient setting24 and suggests that hospitalized patients may be uniquely motivated to respond to health education.
Kerzman and colleagues5 found that 42% of hospitalized patients expressed a wish to receive more comprehensive counseling about their medications before being discharged from the hospital. And although the Joint Commission on Hospital Accreditation and the Centers for Medicare and Medicaid Services have established core quality measures mandating that patients hospitalized with congestive heart failure receive education as one component of a high‐quality discharge process,6, 7 approximately one third of patients nationally do not receive adequate patient education.8
Barber‐Parker9 suggested that because patient acuity in hospitals was so high and patients were so commonly absent from their nursing units for testing and treatment, there was little time available for health education during their hospitalization. Anecdotal observations in our institution suggested, however, that adult patients hospitalized on the general Internal Medicine service spent much of their day doing little more than lying in bed watching television. Accordingly, we hypothesized that considerable time might be available for patient education during a hospitalization. We therefore sought to quantify the fraction of time patients were not involved in treatment activities, diagnostic testing, or other evaluations and to determine whether during these times they wanted and were feeling well enough to participate in educational activities. We also sought to determine what patients wanted to know about their health problems and what types of educational activities they most preferred.
MATERIALS AND METHODS
We conducted a time‐motion and survey study from June 25, 2005, to August 15, 2005, at Denver Health Medical Center, an academic public safety‐net hospital affiliated with the University of Colorado School of Medicine. All patients older than 18 years of age who spoke English or Spanish and were admitted to the general Internal Medicine service were candidates for enrollment. Exclusion criteria were being admitted to the intensive care unit, having an inability to communicate, being in contact precautions, and being previously enrolled. The study was approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained for all study participants.
At 8:00 AM, all patients admitted during the previous 15 hours were assigned a random number from a random number table and were approached for consent in numeric order. With 2 data collectors working daily, a maximum of 12 patients could be enrolled each day. Consenting subjects who passed a vision test were given the Test of Functional Health Literacy in Adults at the time of enrollment and a written questionnaire (in either English or Spanish) on a daily basis for a maximum of 6 days. Some of these patients also participated in a structured interview that was designed to elicit their views on health education topics and formats for education of hospitalized patients. Others, again determined by random number, were subjects of a time‐motion study.
Demographic data collected included age, sex, language, race, comorbidities, insurance status, and discharge diagnosis.
Time‐Motion Study
Patients were observed from 8:00 AM to noon and from 1:00 to 5:00 PM7 days a week. Data were collected using TimerPro on a Dell Axim A5 pocket PC and imported daily into an Excel spreadsheet. Observations were categorized as downtime, busy time, or provider time and subcategorized as summarized in Table 1.
| First level | Second level | Third level |
|---|---|---|
| Downtime | Alone | TV |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone | ||
| Other | ||
| Friends/family | TV | |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone/talk | ||
| Other | ||
| Provider | Physician | |
| Nurse | ||
| Physician and nurse | ||
| Physician and other | ||
| Other | ||
| Busy | ADL | |
| Meal | ||
| Out of room | ||
| Other |
Questionnaire
We were unable to find a validated questionnaire in the literature that was designed to assess patient opinion or level of interest in educational activities during a hospitalization. Accordingly, we developed our own using a 5‐point Likert scale (Box 1). Two outcomes researchers with expertise in using questionnaires for clinical research independently reviewed the questionnaire to establish face validity.
Box 1. Daily Questionnaire on In‐Hospital Health Education
The following statements were read to the patients on a daily basis and answered using the following scale: 1 = Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree
I feel well enough today to learn about my illness or my health.
I want to learn more about my illness or my health today.
I have time to learn about my health today.
It is important to me to learn more about my illness or health while in the hospital.
Interview
All patients were asked the open‐ended questions listed in Box 2, and the entire interview was recorded on audiotape for subsequent analysis.
Box 2. Interview Questions with Probes for Educational Preferences
What things related to your health would you like to learn more about while you are in the hospital? (list up to three topics in order of impotance to you).
How can we help you learn more about your illness or health while in the hospital?
Who should do the teaching (eg, an MD, a nurse, a dietician, a medical student, peers, physical therapists, respiratory therapists)?
Who else should be present (eg, patients with similar illness, family, no one)?
How should this teaching be done (eg, didactic sessions, hands‐on, video tape, pre‐ and post‐testing)?
Data Analysis
All analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC). A P value < 0.05 was considered significant.
Time‐Motion Data
Mixed‐effects general linear models (growth curve or repeated measures), using SAS Proc Mixed, were used to test whether the proportions of downtime, busy time, and provider time differed by day of hospitalization. Linear growth curve models were used to test whether a linear trend was present. If not, repeated‐measures models were used to obtain estimates by day of hospitalization.
Questionnaire Data
Mixed‐effects general linear models (growth curve or repeated measures) were used to determine whether patient responses differed by day of hospitalization, as described above.
Interview Data
Tape recordings were reviewed in depth to code participant responses to the structured questions. We utilized the template style of analysis, coding segments of the interviews and identifying illustrative quotes whenever possible. Key patterns and themes were summarized along with specific patient preferences regarding topics of interest and learning opportunities while in the hospital and after discharge.
RESULTS
Patient selection is described in Figure 1, and patient demographics are summarized in Tables 2 and 3.

| Demographic | Time‐motion | Interview | Questionnaire |
|---|---|---|---|
| |||
| Study subjects | 13 | 15 | 125 |
| Sex | |||
| Male | 6 (46) | 7 (47) | 61 (49) |
| Female | 7 (54) | 8 (53) | 64 (51) |
| Age (years), median (IQR) | 47 (20) | 51 (20.5) | 51 (18) |
| Race/ethnicity | |||
| White, non‐Hispanic | 6 (46) | 5 (33) | 46 (37) |
| Black/African American | 3 (23) | 4 (27) | 27 (22) |
| American Indian | 0 (0) | 0 (0) | 1 (1) |
| Hispanic | 4 (31) | 6 (40) | 51 (41) |
| Primary language | |||
| English | 12 (92) | 14 (93) | 109 (87) |
| Spanish | 1 (8) | 1 (7) | 16 (13) |
| Health literacy* | |||
| Adequate | 3 (75) | 9 (82) | 60 (71) |
| Marginal | 1 (25) | 0 (0) | 6 (7) |
| Inadequate | 0 (0) | 2 (18) | 18 (22) |
| Insurance status | |||
| Self‐pay | 3 (23) | 1 (7) | 24 (19) |
| Medicaid | 1 (8) | 4 (27) | 19 (15) |
| Medicare | 3 (23) | 0 (0) | 2 (2) |
| Colorado Indigent Care Program | 3 (23) | 7 (47) | 51 (41) |
| Private | 2 (15) | 1 (7) | 5 (4) |
| Other | 1 (8) | 2 (14) | 24 (18) |
| Time‐motion | Interview | Questionnaire | |
|---|---|---|---|
| Study subjects (n) | 13 | 15 | 125 |
| Discharge diagnoses (selected) | |||
| Coronary artery disease (including angina) | 1 (8) | 2 (13) | 24 (19) |
| Congestive heart failure | 1 (8) | 1 (7) | 4 (3) |
| Upper gastrointestinal bleeding, gastritis, reflux | 2 (15) | 4 (27) | 14 (11) |
| Syncope | 2 (15) | 0 | 5 (4) |
| Acute renal failure | 0 | 0 | 5 (4) |
| Pancreatitis | 0 | 1 (7) | 6 (5) |
| Venous thromboembolism | 2 (15) | 1 (7) | 3 (2) |
| Chronic obstructive pulmonary disease | 0 | 0 | 4 (3) |
| Diabetic ketoacidosis | 1 (8) | 1 (7) | 3 (2) |
| Pyelonephritis | 0 | 0 | 5 (4) |
| Pneumonia | 2 (15) | 1 (7) | 5 (4) |
| Comorbidities | |||
| Diabetes | 5 (38) | 9 (60) | 41 (33) |
| Hypertension | 1 (8) | 9 (60) | 55 (44) |
| Dyslipidemia | 6 (46) | 2 (13) | 26 (21) |
| Tobacco | 5 (38) | 7 (47) | 55 (44) |
| Chronic obstructive pulmonary disease | 2 (15) | 4 (27) | 15 (12) |
| Congestive heart failure | 2 (15) | 2 (13) | 13 (10) |
| Coronary heart disease | 3 (23) | 3 (20) | 21 (17) |
Time‐Motion Study
Thirteen patients were studied. Of the 315 patient‐hours observed, 71% were categorized as downtime, 15% as provider time, and 14% as busy time. The proportion of downtime ranged from a low of 0.65 (SE 0.04) on hospital Day 2 to a high of 0.76 (SE 0.06) on hospital Day 4, but the differences in downtime proportions by day did not reach statistical significance (P = .65; Fig. 2). The lowest percentage of downtime observed in any patient on any day was 39%. The 125 hours of downtime observed consisted of 1317 separate blocks of time, 80% of which were less than 15 minutes in duration, 14% of which were 15 to 30 minutes in duration, and 6% of which exceeded 30 minutes in duration.

Thirty‐six full days of observation, defined as greater than 7 hours of observation in 1 day, were used to assess the amount of time spent with providers. Of the 60 minutes/day (IQR = 44) that patients spent with health care providers, 21 minutes/day (IQR = 34) was spent with phlebotomists, physical or occupational therapists, dieticians, or social workers, 25 minutes/day (IQR = 25) was spent with patients' nurses, and a median of only 9 minutes/day (IQR = 11) was spent with their physicians.
Questionnaire
A total of 311 questionnaires were administered to the 138 consenting participants. Irrespective of the day of testing, 79% to 97% strongly agreed or agreed with the 4 statements (Fig. 3). In response to the first statementI feel well enough to learnpatient scores increased steadily over the 6 days of hospitalization patients were surveyed (coefficient = 0.15, P = .004). On hospital day 1, the mean score was 3.85 (SE 0.08), and by day 6 the mean score had increased to 4.75 (SE 0.08) However, there was no significant change over time in patients' desire to learn, self‐perceived time available to learn, or importance placed on learning during their hospital stay.

Interview
Fifteen interviews were conducted. Representative comments are presented in Table 4. Responses generally indicated that the patients had anxieties and uncertainties about their health and safety after discharge. Most participants wanted to know more about the condition for which they had been hospitalized, including information pertaining to management, prevention, etiology, and prognosis of their disease. Diabetic patients asked for information on insulin dosing, nutrition, and the effect of the disease on their bodies.
| Theme | Sample quotes |
|---|---|
| Preferred topics | |
| Self‐management | I need to know what to do when I go home, how to take care of it. Medical peopledon't give enough information to the patient and patient's family so they can help themselves. You need to encourage patients to help themselves, take some responsibility for themselves. |
| Prevention of disease recurrence or progression | It's okay to tell people that they have something and give them medicine, but also tell them what they can do to prevent it or make it less painful. I've known about bronchitis for many years, but didn't know it would affect my heart. |
| What's happening to me? | Am I going to diehow long? |
| Just fix me | I came to the hospital to get fixed, not educated. I'm results‐oriented, not cause‐oriented. |
| Preferred learning methods | |
| One‐to‐one didactics with MDs | I'd like one‐to‐one time with someone who has the time to listen. One to one with doctors who can explain what can happen, what to take, what not to take. |
| Family involvement | Get the family involved so the family understands the limitations of the person, how medications affect them. To say a person has a heart condition is a very vague statement. If they [family] understand more, it's better. |
| Groups | A group of people with similar illnessI like groups where everyone listens. I'd participate in groups at the hospital but not at home. |
| Video | Hospital TV is not meeting my needs. |
| Printed material | A doctor or nurse tell me what's going on and then also handouts on dietary and nutrition. |
| Electronic learning | I learned a lot through the encyclopedia of family health care, and through Web sites |
Patients preferred to pick their own topics for education rather than having topics chosen for them. Patients also showed in interest in prevention. One diabetic patient wanted to know how to prevent her children from becoming diabetic, and a cardiac patient wanted education on heart disease prevention. Other recurrent themes included the desire to know what was causing their illness and information about prognosis.
Almost all the patients were interested in more than one type of learning experience. The most frequently cited preference was to have a doctor or other knowledgeable health care professional answer questions specific to their individual situation. Video and group learning were each mentioned by approximately half the participants. Most patients thought that having family present during educational discussions was important.
Video was the most frequently mentioned learning tool, and patients thought it would be useful to have this modality available in the hospital as well as the home. Two patients expressed interest in computerized learning (one of whom had used health Web sites before his hospitalization). Most patients wanted handouts or reading material in addition to other methods of communicating information. Although many patients said they felt comfortable discussing their problems in group settings, some did not.
DISCUSSION
The important findings of this study are that hospitalized patients have a substantial amount of time available for health education and a considerable willingness and interest in participating in health educational activities. We found that although there was a great deal of time available on all days of hospitalization studied, patients felt increasingly well enough to participate in educational activities through their hospital stay.
We are unaware of other studies that have attempted to quantify the amount of time hospitalized patients are available for educational activities or whether they feel capable of participating in these activities. McBride10 found that 95% of hospitalized patients supported a health‐promoting hospital and that almost 80% wanted to modify at least 1 aspect of their lifestyle. Martin and colleagues11 found that patient satisfaction was improved by a patient‐centered unit incorporating dedicated nursing staff to promote patient involvement and provide personalized care and education. Barber‐Parker9 suggested that high patient acuity, short durations of hospitalization, and lack of patient availability because of testing and treatment limited the opportunities that patients had for health education during their hospitalization. These conclusions were reached on the basis of surveys of nurses' perceptions, however, rather than on direct observations or assessments of patients' perceptions.
Our findings suggest that many types of patient educational approaches may be needed to achieve maximal effectiveness and that regardless of the specific approach employed, the focus should be on the primary reason for a patient's hospitalization, what the hospitalization meant, why it happened and what the patient can do to prevent hospitalization from occurring in the future.
Transitions in care have been identified as periods in which communication lapses occur and outcomes can be adversely affected.12 A recent study by Epstein and colleagues13 found that almost 12% of patients had new or worsening symptoms of disease within the first few days after discharge from the hospital and that 22% either did not pick up their medications or understand how to take them (consistent with the observations of Kerzman and colleagues).5 The most common action taken in response to these findings was nurse‐mediated patient education. Our study indicates there is potential for further educational processes in hospitals, which may improve the safety of transitions from a hospital setting to outpatient care.
Although many disease management programs have been studied in the outpatient setting,1417 very few have been extended into hospitals. Accordingly, hospitalists are ideally suited to develop and implement disease management programs in concert with outpatient efforts.18 Our study suggests there is an underutilized opportunity for hospital‐based physicians and other health care providers to work with patients at a time when they are uniquely focused on their own health and free from many of the time constraints of their normal lives.
Although JCAHO mandates that hospitalized patients receive education and training specific to the patient's needs and as appropriate to the care, treatment and services provided,19 there is a paucity of data describing the educational processes in US hospitals. Johansson and colleagues20 conducted a survey in a Finnish hospital where patient education is also mandated. Written materials were given to about 55% of the patients. Demonstration and practice were used with about one third, whereas the Internet and videotapes were used for fewer than 10%. Although patients underwent educational activities throughout their hospitalization, and most were satisfied with the process, Johansson and colleagues found that only 59% felt that what they knew about their care was sufficient, almost a third felt they did not know enough about the side effects of their medical care, and almost half felt they did not have sufficient input into what they were being taught.
Although we found a large amount of time that might be used for patient education during a hospitalization, this time was commonly limited to 15‐minute blocks, as has been noted previously.9 This observation implies that educational activities should be designed so they can be conducted over short periods and/or stopped for short periods when interruptions occur or that the processes of care during a hospitalization should be altered to create larger blocks of continuous time available for educational activities.
A number of issues could have biased our results. Only 66% of the patients who were approached to participate agreed to do so. Because those declining may have been sicker and because sicker patients may require more diagnostic testing or more invasive treatment, we may have overestimated both the amount of downtime available and the willingness of patients to participate in educational activities. If we assume, however, that all patients who refused to participate either disagreed or strongly disagreed with the statements in the questionnaire regarding their interest in educational activities, the fraction of patients agreeing or strongly agreeing with idea that they were well enough and interested would still be 57% to 75% of the population sampled. Accordingly, this potential bias, if it occurred, would not alter our conclusions.
The time‐motion studies were only performed between 8:00 AM and 5:00 PM, such that the resulting data do not reflect any diagnostic testing, therapeutic interventions, or contact with health care providers that occurred at other times. This may have contributed to the strikingly small amount of time that patients spent with their physicians and nurses. If patient time after 5:00 PM and before 8:00 AM had been observed and included, it is likely that the absolute amount of time spent with physicians and nurses would increase, whereas the overall proportion of patient time spent with providers would decrease.
We were also only able to collect data on 13 time‐motion subjects. This limited sample size from a single institution may not be representative of all hospitalized non‐ICU patients on general medical wards. Accordingly, we make no claims that our data can be generalized to the entire population of patients admitted to non‐ICU medical services. However, the results of our surveys, which sampled a much broader patient population and supported our time‐motion findings, suggest that our time‐motion findings are likely to be representative of significant underutilized time and motivation for patient education in the hospital setting.
Also, it is important to note that although the time‐motion studies were only done with 13 patients, these studies are extremely labor intensive and are rarely done with much larger samples. In addition, the SDs on the data collected from the time‐motion studies were quite small. It is possible that if a larger sample were studied, the percentage of free time might be larger or smaller than what we observed for the 13 patients we studied. However, it would be quite unlikely that the amount of free time would be so small (eg, 10%‐15%) that it would invalidate our conclusion that considerable time is available for patient education over and above what currently occurs in most hospital settings.
A patient's self‐perception of his or her ability to learn may not reflect that patient's true cognitive readiness to do so. JCAHO requirements mandate that nurses be trained to assess patients for their ability to learn and to do so as part of the admission process. After reviewing all day 1 patient responses to our questionnaires, in no instance did a nurse assess a patient as having a barrier to learning when the patient had reported feeling well enough to learn. Accordingly, although we performed no direct tests of patients' ability to learn, this retrospective independent assessment did not suggest that patients systematically overestimated their ability to learn.
Finding that hospitalized patients are unoccupied for approximately 70% of their daytime hours and that most patients are both highly motivated to learn and have few barriers to doing so indicates that educational activities during hospitalizations have substantial potential for expansion. The current structure for educating hospitalized patients should be supplemented to take these findings into account.
Acknowledgements
We thank Dr. John Steiner and Dr. Sheena Bull for their assistance in study design as well as the development of the questionnaire and interview tools. We also thank Carolyn Nowels for her assistance with the qualitative data analysis. The assistance of Dr. Bull and Dr. Steiner was made possible through NHLBI grant U01HL079160, and funding for data collection was made possible by the University of Colorado at Denver and Health Sciences Center Department of Medicine, Division of General Internal Medicine small grants program.
Educating patients about smoking cessation when they are hospitalized for acute coronary syndrome results in a 57% 1‐year quit rate.1 This rate is far higher than the typical 15%‐30% 1‐year quit rates observed with smoking cessation programs administered in the outpatient setting24 and suggests that hospitalized patients may be uniquely motivated to respond to health education.
Kerzman and colleagues5 found that 42% of hospitalized patients expressed a wish to receive more comprehensive counseling about their medications before being discharged from the hospital. And although the Joint Commission on Hospital Accreditation and the Centers for Medicare and Medicaid Services have established core quality measures mandating that patients hospitalized with congestive heart failure receive education as one component of a high‐quality discharge process,6, 7 approximately one third of patients nationally do not receive adequate patient education.8
Barber‐Parker9 suggested that because patient acuity in hospitals was so high and patients were so commonly absent from their nursing units for testing and treatment, there was little time available for health education during their hospitalization. Anecdotal observations in our institution suggested, however, that adult patients hospitalized on the general Internal Medicine service spent much of their day doing little more than lying in bed watching television. Accordingly, we hypothesized that considerable time might be available for patient education during a hospitalization. We therefore sought to quantify the fraction of time patients were not involved in treatment activities, diagnostic testing, or other evaluations and to determine whether during these times they wanted and were feeling well enough to participate in educational activities. We also sought to determine what patients wanted to know about their health problems and what types of educational activities they most preferred.
MATERIALS AND METHODS
We conducted a time‐motion and survey study from June 25, 2005, to August 15, 2005, at Denver Health Medical Center, an academic public safety‐net hospital affiliated with the University of Colorado School of Medicine. All patients older than 18 years of age who spoke English or Spanish and were admitted to the general Internal Medicine service were candidates for enrollment. Exclusion criteria were being admitted to the intensive care unit, having an inability to communicate, being in contact precautions, and being previously enrolled. The study was approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained for all study participants.
At 8:00 AM, all patients admitted during the previous 15 hours were assigned a random number from a random number table and were approached for consent in numeric order. With 2 data collectors working daily, a maximum of 12 patients could be enrolled each day. Consenting subjects who passed a vision test were given the Test of Functional Health Literacy in Adults at the time of enrollment and a written questionnaire (in either English or Spanish) on a daily basis for a maximum of 6 days. Some of these patients also participated in a structured interview that was designed to elicit their views on health education topics and formats for education of hospitalized patients. Others, again determined by random number, were subjects of a time‐motion study.
Demographic data collected included age, sex, language, race, comorbidities, insurance status, and discharge diagnosis.
Time‐Motion Study
Patients were observed from 8:00 AM to noon and from 1:00 to 5:00 PM7 days a week. Data were collected using TimerPro on a Dell Axim A5 pocket PC and imported daily into an Excel spreadsheet. Observations were categorized as downtime, busy time, or provider time and subcategorized as summarized in Table 1.
| First level | Second level | Third level |
|---|---|---|
| Downtime | Alone | TV |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone | ||
| Other | ||
| Friends/family | TV | |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone/talk | ||
| Other | ||
| Provider | Physician | |
| Nurse | ||
| Physician and nurse | ||
| Physician and other | ||
| Other | ||
| Busy | ADL | |
| Meal | ||
| Out of room | ||
| Other |
Questionnaire
We were unable to find a validated questionnaire in the literature that was designed to assess patient opinion or level of interest in educational activities during a hospitalization. Accordingly, we developed our own using a 5‐point Likert scale (Box 1). Two outcomes researchers with expertise in using questionnaires for clinical research independently reviewed the questionnaire to establish face validity.
Box 1. Daily Questionnaire on In‐Hospital Health Education
The following statements were read to the patients on a daily basis and answered using the following scale: 1 = Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree
I feel well enough today to learn about my illness or my health.
I want to learn more about my illness or my health today.
I have time to learn about my health today.
It is important to me to learn more about my illness or health while in the hospital.
Interview
All patients were asked the open‐ended questions listed in Box 2, and the entire interview was recorded on audiotape for subsequent analysis.
Box 2. Interview Questions with Probes for Educational Preferences
What things related to your health would you like to learn more about while you are in the hospital? (list up to three topics in order of impotance to you).
How can we help you learn more about your illness or health while in the hospital?
Who should do the teaching (eg, an MD, a nurse, a dietician, a medical student, peers, physical therapists, respiratory therapists)?
Who else should be present (eg, patients with similar illness, family, no one)?
How should this teaching be done (eg, didactic sessions, hands‐on, video tape, pre‐ and post‐testing)?
Data Analysis
All analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC). A P value < 0.05 was considered significant.
Time‐Motion Data
Mixed‐effects general linear models (growth curve or repeated measures), using SAS Proc Mixed, were used to test whether the proportions of downtime, busy time, and provider time differed by day of hospitalization. Linear growth curve models were used to test whether a linear trend was present. If not, repeated‐measures models were used to obtain estimates by day of hospitalization.
Questionnaire Data
Mixed‐effects general linear models (growth curve or repeated measures) were used to determine whether patient responses differed by day of hospitalization, as described above.
Interview Data
Tape recordings were reviewed in depth to code participant responses to the structured questions. We utilized the template style of analysis, coding segments of the interviews and identifying illustrative quotes whenever possible. Key patterns and themes were summarized along with specific patient preferences regarding topics of interest and learning opportunities while in the hospital and after discharge.
RESULTS
Patient selection is described in Figure 1, and patient demographics are summarized in Tables 2 and 3.

| Demographic | Time‐motion | Interview | Questionnaire |
|---|---|---|---|
| |||
| Study subjects | 13 | 15 | 125 |
| Sex | |||
| Male | 6 (46) | 7 (47) | 61 (49) |
| Female | 7 (54) | 8 (53) | 64 (51) |
| Age (years), median (IQR) | 47 (20) | 51 (20.5) | 51 (18) |
| Race/ethnicity | |||
| White, non‐Hispanic | 6 (46) | 5 (33) | 46 (37) |
| Black/African American | 3 (23) | 4 (27) | 27 (22) |
| American Indian | 0 (0) | 0 (0) | 1 (1) |
| Hispanic | 4 (31) | 6 (40) | 51 (41) |
| Primary language | |||
| English | 12 (92) | 14 (93) | 109 (87) |
| Spanish | 1 (8) | 1 (7) | 16 (13) |
| Health literacy* | |||
| Adequate | 3 (75) | 9 (82) | 60 (71) |
| Marginal | 1 (25) | 0 (0) | 6 (7) |
| Inadequate | 0 (0) | 2 (18) | 18 (22) |
| Insurance status | |||
| Self‐pay | 3 (23) | 1 (7) | 24 (19) |
| Medicaid | 1 (8) | 4 (27) | 19 (15) |
| Medicare | 3 (23) | 0 (0) | 2 (2) |
| Colorado Indigent Care Program | 3 (23) | 7 (47) | 51 (41) |
| Private | 2 (15) | 1 (7) | 5 (4) |
| Other | 1 (8) | 2 (14) | 24 (18) |
| Time‐motion | Interview | Questionnaire | |
|---|---|---|---|
| Study subjects (n) | 13 | 15 | 125 |
| Discharge diagnoses (selected) | |||
| Coronary artery disease (including angina) | 1 (8) | 2 (13) | 24 (19) |
| Congestive heart failure | 1 (8) | 1 (7) | 4 (3) |
| Upper gastrointestinal bleeding, gastritis, reflux | 2 (15) | 4 (27) | 14 (11) |
| Syncope | 2 (15) | 0 | 5 (4) |
| Acute renal failure | 0 | 0 | 5 (4) |
| Pancreatitis | 0 | 1 (7) | 6 (5) |
| Venous thromboembolism | 2 (15) | 1 (7) | 3 (2) |
| Chronic obstructive pulmonary disease | 0 | 0 | 4 (3) |
| Diabetic ketoacidosis | 1 (8) | 1 (7) | 3 (2) |
| Pyelonephritis | 0 | 0 | 5 (4) |
| Pneumonia | 2 (15) | 1 (7) | 5 (4) |
| Comorbidities | |||
| Diabetes | 5 (38) | 9 (60) | 41 (33) |
| Hypertension | 1 (8) | 9 (60) | 55 (44) |
| Dyslipidemia | 6 (46) | 2 (13) | 26 (21) |
| Tobacco | 5 (38) | 7 (47) | 55 (44) |
| Chronic obstructive pulmonary disease | 2 (15) | 4 (27) | 15 (12) |
| Congestive heart failure | 2 (15) | 2 (13) | 13 (10) |
| Coronary heart disease | 3 (23) | 3 (20) | 21 (17) |
Time‐Motion Study
Thirteen patients were studied. Of the 315 patient‐hours observed, 71% were categorized as downtime, 15% as provider time, and 14% as busy time. The proportion of downtime ranged from a low of 0.65 (SE 0.04) on hospital Day 2 to a high of 0.76 (SE 0.06) on hospital Day 4, but the differences in downtime proportions by day did not reach statistical significance (P = .65; Fig. 2). The lowest percentage of downtime observed in any patient on any day was 39%. The 125 hours of downtime observed consisted of 1317 separate blocks of time, 80% of which were less than 15 minutes in duration, 14% of which were 15 to 30 minutes in duration, and 6% of which exceeded 30 minutes in duration.

Thirty‐six full days of observation, defined as greater than 7 hours of observation in 1 day, were used to assess the amount of time spent with providers. Of the 60 minutes/day (IQR = 44) that patients spent with health care providers, 21 minutes/day (IQR = 34) was spent with phlebotomists, physical or occupational therapists, dieticians, or social workers, 25 minutes/day (IQR = 25) was spent with patients' nurses, and a median of only 9 minutes/day (IQR = 11) was spent with their physicians.
Questionnaire
A total of 311 questionnaires were administered to the 138 consenting participants. Irrespective of the day of testing, 79% to 97% strongly agreed or agreed with the 4 statements (Fig. 3). In response to the first statementI feel well enough to learnpatient scores increased steadily over the 6 days of hospitalization patients were surveyed (coefficient = 0.15, P = .004). On hospital day 1, the mean score was 3.85 (SE 0.08), and by day 6 the mean score had increased to 4.75 (SE 0.08) However, there was no significant change over time in patients' desire to learn, self‐perceived time available to learn, or importance placed on learning during their hospital stay.

Interview
Fifteen interviews were conducted. Representative comments are presented in Table 4. Responses generally indicated that the patients had anxieties and uncertainties about their health and safety after discharge. Most participants wanted to know more about the condition for which they had been hospitalized, including information pertaining to management, prevention, etiology, and prognosis of their disease. Diabetic patients asked for information on insulin dosing, nutrition, and the effect of the disease on their bodies.
| Theme | Sample quotes |
|---|---|
| Preferred topics | |
| Self‐management | I need to know what to do when I go home, how to take care of it. Medical peopledon't give enough information to the patient and patient's family so they can help themselves. You need to encourage patients to help themselves, take some responsibility for themselves. |
| Prevention of disease recurrence or progression | It's okay to tell people that they have something and give them medicine, but also tell them what they can do to prevent it or make it less painful. I've known about bronchitis for many years, but didn't know it would affect my heart. |
| What's happening to me? | Am I going to diehow long? |
| Just fix me | I came to the hospital to get fixed, not educated. I'm results‐oriented, not cause‐oriented. |
| Preferred learning methods | |
| One‐to‐one didactics with MDs | I'd like one‐to‐one time with someone who has the time to listen. One to one with doctors who can explain what can happen, what to take, what not to take. |
| Family involvement | Get the family involved so the family understands the limitations of the person, how medications affect them. To say a person has a heart condition is a very vague statement. If they [family] understand more, it's better. |
| Groups | A group of people with similar illnessI like groups where everyone listens. I'd participate in groups at the hospital but not at home. |
| Video | Hospital TV is not meeting my needs. |
| Printed material | A doctor or nurse tell me what's going on and then also handouts on dietary and nutrition. |
| Electronic learning | I learned a lot through the encyclopedia of family health care, and through Web sites |
Patients preferred to pick their own topics for education rather than having topics chosen for them. Patients also showed in interest in prevention. One diabetic patient wanted to know how to prevent her children from becoming diabetic, and a cardiac patient wanted education on heart disease prevention. Other recurrent themes included the desire to know what was causing their illness and information about prognosis.
Almost all the patients were interested in more than one type of learning experience. The most frequently cited preference was to have a doctor or other knowledgeable health care professional answer questions specific to their individual situation. Video and group learning were each mentioned by approximately half the participants. Most patients thought that having family present during educational discussions was important.
Video was the most frequently mentioned learning tool, and patients thought it would be useful to have this modality available in the hospital as well as the home. Two patients expressed interest in computerized learning (one of whom had used health Web sites before his hospitalization). Most patients wanted handouts or reading material in addition to other methods of communicating information. Although many patients said they felt comfortable discussing their problems in group settings, some did not.
DISCUSSION
The important findings of this study are that hospitalized patients have a substantial amount of time available for health education and a considerable willingness and interest in participating in health educational activities. We found that although there was a great deal of time available on all days of hospitalization studied, patients felt increasingly well enough to participate in educational activities through their hospital stay.
We are unaware of other studies that have attempted to quantify the amount of time hospitalized patients are available for educational activities or whether they feel capable of participating in these activities. McBride10 found that 95% of hospitalized patients supported a health‐promoting hospital and that almost 80% wanted to modify at least 1 aspect of their lifestyle. Martin and colleagues11 found that patient satisfaction was improved by a patient‐centered unit incorporating dedicated nursing staff to promote patient involvement and provide personalized care and education. Barber‐Parker9 suggested that high patient acuity, short durations of hospitalization, and lack of patient availability because of testing and treatment limited the opportunities that patients had for health education during their hospitalization. These conclusions were reached on the basis of surveys of nurses' perceptions, however, rather than on direct observations or assessments of patients' perceptions.
Our findings suggest that many types of patient educational approaches may be needed to achieve maximal effectiveness and that regardless of the specific approach employed, the focus should be on the primary reason for a patient's hospitalization, what the hospitalization meant, why it happened and what the patient can do to prevent hospitalization from occurring in the future.
Transitions in care have been identified as periods in which communication lapses occur and outcomes can be adversely affected.12 A recent study by Epstein and colleagues13 found that almost 12% of patients had new or worsening symptoms of disease within the first few days after discharge from the hospital and that 22% either did not pick up their medications or understand how to take them (consistent with the observations of Kerzman and colleagues).5 The most common action taken in response to these findings was nurse‐mediated patient education. Our study indicates there is potential for further educational processes in hospitals, which may improve the safety of transitions from a hospital setting to outpatient care.
Although many disease management programs have been studied in the outpatient setting,1417 very few have been extended into hospitals. Accordingly, hospitalists are ideally suited to develop and implement disease management programs in concert with outpatient efforts.18 Our study suggests there is an underutilized opportunity for hospital‐based physicians and other health care providers to work with patients at a time when they are uniquely focused on their own health and free from many of the time constraints of their normal lives.
Although JCAHO mandates that hospitalized patients receive education and training specific to the patient's needs and as appropriate to the care, treatment and services provided,19 there is a paucity of data describing the educational processes in US hospitals. Johansson and colleagues20 conducted a survey in a Finnish hospital where patient education is also mandated. Written materials were given to about 55% of the patients. Demonstration and practice were used with about one third, whereas the Internet and videotapes were used for fewer than 10%. Although patients underwent educational activities throughout their hospitalization, and most were satisfied with the process, Johansson and colleagues found that only 59% felt that what they knew about their care was sufficient, almost a third felt they did not know enough about the side effects of their medical care, and almost half felt they did not have sufficient input into what they were being taught.
Although we found a large amount of time that might be used for patient education during a hospitalization, this time was commonly limited to 15‐minute blocks, as has been noted previously.9 This observation implies that educational activities should be designed so they can be conducted over short periods and/or stopped for short periods when interruptions occur or that the processes of care during a hospitalization should be altered to create larger blocks of continuous time available for educational activities.
A number of issues could have biased our results. Only 66% of the patients who were approached to participate agreed to do so. Because those declining may have been sicker and because sicker patients may require more diagnostic testing or more invasive treatment, we may have overestimated both the amount of downtime available and the willingness of patients to participate in educational activities. If we assume, however, that all patients who refused to participate either disagreed or strongly disagreed with the statements in the questionnaire regarding their interest in educational activities, the fraction of patients agreeing or strongly agreeing with idea that they were well enough and interested would still be 57% to 75% of the population sampled. Accordingly, this potential bias, if it occurred, would not alter our conclusions.
The time‐motion studies were only performed between 8:00 AM and 5:00 PM, such that the resulting data do not reflect any diagnostic testing, therapeutic interventions, or contact with health care providers that occurred at other times. This may have contributed to the strikingly small amount of time that patients spent with their physicians and nurses. If patient time after 5:00 PM and before 8:00 AM had been observed and included, it is likely that the absolute amount of time spent with physicians and nurses would increase, whereas the overall proportion of patient time spent with providers would decrease.
We were also only able to collect data on 13 time‐motion subjects. This limited sample size from a single institution may not be representative of all hospitalized non‐ICU patients on general medical wards. Accordingly, we make no claims that our data can be generalized to the entire population of patients admitted to non‐ICU medical services. However, the results of our surveys, which sampled a much broader patient population and supported our time‐motion findings, suggest that our time‐motion findings are likely to be representative of significant underutilized time and motivation for patient education in the hospital setting.
Also, it is important to note that although the time‐motion studies were only done with 13 patients, these studies are extremely labor intensive and are rarely done with much larger samples. In addition, the SDs on the data collected from the time‐motion studies were quite small. It is possible that if a larger sample were studied, the percentage of free time might be larger or smaller than what we observed for the 13 patients we studied. However, it would be quite unlikely that the amount of free time would be so small (eg, 10%‐15%) that it would invalidate our conclusion that considerable time is available for patient education over and above what currently occurs in most hospital settings.
A patient's self‐perception of his or her ability to learn may not reflect that patient's true cognitive readiness to do so. JCAHO requirements mandate that nurses be trained to assess patients for their ability to learn and to do so as part of the admission process. After reviewing all day 1 patient responses to our questionnaires, in no instance did a nurse assess a patient as having a barrier to learning when the patient had reported feeling well enough to learn. Accordingly, although we performed no direct tests of patients' ability to learn, this retrospective independent assessment did not suggest that patients systematically overestimated their ability to learn.
Finding that hospitalized patients are unoccupied for approximately 70% of their daytime hours and that most patients are both highly motivated to learn and have few barriers to doing so indicates that educational activities during hospitalizations have substantial potential for expansion. The current structure for educating hospitalized patients should be supplemented to take these findings into account.
Acknowledgements
We thank Dr. John Steiner and Dr. Sheena Bull for their assistance in study design as well as the development of the questionnaire and interview tools. We also thank Carolyn Nowels for her assistance with the qualitative data analysis. The assistance of Dr. Bull and Dr. Steiner was made possible through NHLBI grant U01HL079160, and funding for data collection was made possible by the University of Colorado at Denver and Health Sciences Center Department of Medicine, Division of General Internal Medicine small grants program.
- ,.Randomized controlled trial of smoking cessation intervention after admission for coronary heart disease.BMJ.2003;327:1254–1257.
- .Treatment of tobacco use and dependence.N Engl J Med.2002;346:506–512.
- ,, et al.A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report.JAMA.2000;283:3244–3254.
- ,,, et al.National Institutes of Health state‐of‐the science conference statement: tobacco use: prevention, cessation and control.Ann Intern Med.2006;145:839–844.
- ,,.What do discharged patients know about their medications?Patient Educ Couns.2005;56:276–282.
- Joint Commission on Accreditation of Healthcare Organizations.A Comprehensive Review for the Development and Testing for National Implementation of Hospital Core Measures.2006:1–40.
- .ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/ American Heart Association task force on practice guidelines.J Am Coll Cardiol.2005;46:1–82.
- JCAHO data. Available: https://cimprod.uhc.edu/CoreMeasures/Products/DownloadSystem/WebPages/ViewReportsDownloadList.aspx.
- .Integrating patient teaching into bedside patient care: a participant‐observation study of hospital nurses.Patient Educ Couns.2002;48:107–113.
- .Health promotion in the acute hospital setting: the receptivity of adult in‐patients.Patient Educ Couns.2004;54:73–78.
- ,,,,,.Randomized trial of a patient‐centered hospital unit.Patient Educ Couns.1998;34:125–133.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. HYPERLINK “javascript:AL_get(this,%20'jour',%20'JAMA.');”JAMA.2007;297:831–841.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2:58–68.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288:2469–2475.
- ,,.Effectiveness of self‐management training in type 2 diabetes.Diabetes Care.2001;24:561–587.
- ,,.Quality of life assessment after patient education a randomized controlled study on asthma and chronic obstructive pulmonary disease.Am J Respir Crit Care Med.2001;95:56–63.
- ,.The effects of a health educational and exercise program for older adults with osteoarthritis for the hip or knee.J Rheumatol.1997;24:1378–1383.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- The Joint Commission on Accreditation of Healthcare Organizations Comprehensive Accreditation Manual for Hospitals: The Official Handbook. January2007. p.152
- ,,,.Need for change in patient education: a Finnish survey from the patient's perspective.Patient Educ Couns.2003;51:239–245.
- ,.Randomized controlled trial of smoking cessation intervention after admission for coronary heart disease.BMJ.2003;327:1254–1257.
- .Treatment of tobacco use and dependence.N Engl J Med.2002;346:506–512.
- ,, et al.A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report.JAMA.2000;283:3244–3254.
- ,,, et al.National Institutes of Health state‐of‐the science conference statement: tobacco use: prevention, cessation and control.Ann Intern Med.2006;145:839–844.
- ,,.What do discharged patients know about their medications?Patient Educ Couns.2005;56:276–282.
- Joint Commission on Accreditation of Healthcare Organizations.A Comprehensive Review for the Development and Testing for National Implementation of Hospital Core Measures.2006:1–40.
- .ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/ American Heart Association task force on practice guidelines.J Am Coll Cardiol.2005;46:1–82.
- JCAHO data. Available: https://cimprod.uhc.edu/CoreMeasures/Products/DownloadSystem/WebPages/ViewReportsDownloadList.aspx.
- .Integrating patient teaching into bedside patient care: a participant‐observation study of hospital nurses.Patient Educ Couns.2002;48:107–113.
- .Health promotion in the acute hospital setting: the receptivity of adult in‐patients.Patient Educ Couns.2004;54:73–78.
- ,,,,,.Randomized trial of a patient‐centered hospital unit.Patient Educ Couns.1998;34:125–133.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. HYPERLINK “javascript:AL_get(this,%20'jour',%20'JAMA.');”JAMA.2007;297:831–841.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2:58–68.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288:2469–2475.
- ,,.Effectiveness of self‐management training in type 2 diabetes.Diabetes Care.2001;24:561–587.
- ,,.Quality of life assessment after patient education a randomized controlled study on asthma and chronic obstructive pulmonary disease.Am J Respir Crit Care Med.2001;95:56–63.
- ,.The effects of a health educational and exercise program for older adults with osteoarthritis for the hip or knee.J Rheumatol.1997;24:1378–1383.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- The Joint Commission on Accreditation of Healthcare Organizations Comprehensive Accreditation Manual for Hospitals: The Official Handbook. January2007. p.152
- ,,,.Need for change in patient education: a Finnish survey from the patient's perspective.Patient Educ Couns.2003;51:239–245.
Copyright © 2008 Society of Hospital Medicine
Improving Cost Effectiveness of Blood Cultures
Because as many as 90% of all blood cultures grow no organisms1 developing rules that predict which patients are at the lowest risk of having bacteremia could improve the utilization of this test and markedly reduce its cost. Of the approximate 10% of cultures that do grow organisms, only about half represent true bacteremia (ie, true positives), whereas the other half are considered contaminants (ie, false positives)2; the latter are known to increase both the cost and duration of care.3 Accordingly, reducing the number of contaminants could also reduce the cost of care. We assessed which of these two strategies would be the most cost effective. Although only 6% of the blood cultures obtained at our hospital represented contaminants, their associated cost was more than twice that associated with the 87% of cultures that were true negatives.
METHODS
We conducted a retrospective review of microbiological results and hospital records of patients for whom blood cultures were obtained in January 2002 at Denver Health Medical Center, a 400‐bed university‐affiliated public safety net hospital. The study was given exempt status by the Colorado Multiple Institutional Review Board. Patients were identified using a preexisting laboratory database.
We adopted the definitions used by Bates et al.3 for the inclusion and exclusion criteria and the definition of a blood culture episode so that we could apply the financial data presented by these authors to our results. Briefly, a blood culture set was defined as a single venipuncture, regardless of the number of bottles sent for culturing, and a blood culture episode was defined as the 48‐hour period beginning when a blood culture was drawn. All sets within the same 48‐hour period were considered part of the same episode. Cultures that grew bacteria were classified as either true positive, representing bacteremia, or false positive, representing contaminants. Determination of whether a patient had a true‐positive culture versus a contaminant was made in a weekly conference attended by the chief of the Infectious Disease Division, an Infectious Disease fellow, and at least one microbiologist, during which the species of organism cultured and the associated clinical data for each patient were considered. Organisms considered to indicate false positives included diphtheroids, Bacillus sp, Propionibacterium sp, coagulase‐negative staphylococci, and micrococci. All other organisms were considered true positives in the setting of appropriate clinical criteria as specified by the CDC guidelines.4 Hospital charges and lengths of stay were obtained from our institutional database.
The cost associated with a true‐negative blood culture was determined by summing the charges for phlebotomy and microbiological testing obtained from the January 2005 Denver Health hospital charge master and applying the cost‐to‐charge ratio reported on the Medicare Cost Report for inpatient services (not including the costs of physician salaries and benefits).
The cost of a false positive was determined two ways: (a) adjusting the data reported by Bates et al.3 for changes in the Consumer Price Index5 and (b) comparing the actual hospital charges of the patients in our sample who had false‐positive cultures with those who did not (adjusting both by the hospital's inpatient cost‐to‐charge ratio, again not including the cost of physician salaries and benefits).
The length of stay and cost of care for patients with true‐ and false‐positive blood cultures were compared by chi‐square analysis. P < .05 was considered statistically significant. The data were not normally distributed and, as such, are presented as medians and interquartile ranges.
RESULTS
Table 1 summarizes the interpretation of the 939 blood cultures drawn in January 2002. Only 6 culture sets (0.6%) could not be classified. The positive predictive value of a positive blood culture was only 53%.
| Blood cultures | Bacteremia | ||
|---|---|---|---|
| Number positive (%) | Number negative (%) | Total | |
| Number positive (%) | 62 (7) | 56 (6) | 118 (13) |
| Number negative (%) | 0 (0) | 815 (87) | 815 (87) |
| Total | 62 (7) | 871 (93) | 933 (100) |
Laboratory charges for patients with true‐negative and false‐positive blood cultures in January 2002 are shown in Table 2. Annualized, the associated charges were $1,781,292, and the costs were $748,143.
| Charge ($) | Tests (N) | Total ($) | |
|---|---|---|---|
| True‐negative cultures | |||
| Phlebotomy | $13.25 | ||
| Microbiology | $147.50 | ||
| Subtotal | $160.75 | 815 | $131,011 |
| False‐positive cultures | |||
| Phlebotomy | $13.25 | ||
| Microbiology | $147.50 | ||
| Identification | $60.75 | ||
| Sensitivity | $89.75 | ||
| Subtotal | $311.25 | 56 | $17,430 |
| $148,441 |
Bates et al.3 found that false‐positive blood cultures increased the length of hospital stay by 4.5 days and increased total charges by $4385 over those for patients with no contaminants. This adjusted to $6878 in 2005 according to the Consumer Price Index.5 After grouping our blood cultures into episodes as defined by Bates et al. (Table 3), we had 41 episodes of contaminated blood cultures that would annualize to charges of $3,383,976 and costs of $1,421,270 after applying the cost‐to‐charge ratio.
| Blood cultures | Bacteremia | ||
|---|---|---|---|
| Number positive (%) | Number negative (%) | Total | |
| |||
| Number positive (%) | 39 (9) | 41 (10) | 80 (19) |
| Number negative (%) | 0 (0) | 335 (81) | 335 (81) |
| Total | 39 (9) | 376 (91) | 415 (100) |
The median length of hospital stay and total charges for the patients with true‐negative and false‐positive blood cultures at Denver Health in January 2002 are summarized in Table 4. Using this approach, patients with false‐positive blood cultures at our institution added 1450‐2200 extra hospital days and accrued additional charges of $4,305,000 and costs of $1,808,100.
| Length of stay (days) | Interquartile range (days) | Total Charges ($) | Interquartile Range ($) | |
|---|---|---|---|---|
| ||||
| True negative | 5 | 2‐12 | $15,158 | $7,007‐$40,270 |
| False positive | 8a | 4‐13.5 | $23,908a | $14,083‐$52,031 |
| Difference | 3 | $8,750 | ||
DISCUSSION
The important finding of this study is that, despite there being nearly 15 times as many true‐negative blood cultures as false‐positive ones, the savings generated by reducing contaminants would be approximately twice that saved by reducing the true negatives (eg, a 50% reduction in the rate of contamination would reduce the total number of false‐positive episodes by 246 annually, saving $710,635‐$904,050, whereas reducing the true negatives by 50% would only save approximately $375,000.
There is no independent gold standard for evaluating the operating characteristics of a blood culture.6 Data from a series of repeated blood cultures represent the closest surrogate. Weinstein et al.7 drew at least 3 sets of cultures from 282 bacteremic patients and noted that bacteremia was documented in 91.5% of the first cultures, in 99.3% in 1 of the first 2 cultures, and in 99.6% in 1 of the first 3 cultures. Because 2 blood culture sets are drawn routinely, the difference between those 2 (if negative) and a third (if it represents a true positive) is 0.3% and would represent a false‐negative culture rate. Given that the true‐negative rate of blood cultures is 87%‐90%,1 the potential 0.3% false‐negative rate would not affect our analysis, and as such, we chose to ignore it. Accordingly, all sets of blood cultures with no growth were classified as true negatives.
Although we cannot show a cause‐and‐effect relationship between false‐positive cultures and the charges associated resulting from them, a recent study suggested that much of the excess length of stay of such patients is attributable to the false‐positive culture itself.8
Because health care costs have exceeded increases in general goods and services, adjusting the results of Bates et al.3 using the Consumer Price Index likely underestimated the projected cost of the false‐positive cultures. This limitation likely accounts for the observation that the difference in actual charges for our patients between those who did and those who did not have false‐positive blood cultures was greater than the cost of these false‐positive cultures as estimated by extrapolating from the data of Bates et al.3 Given the magnitude of the financial difference we observed, however, we suggest that this difference is not of sufficient size to alter our conclusion.
Physicians working at Denver Health are directly employed by the hospital, and the cost of physician salaries and benefits is included in the cost‐to‐charge ratio reported in our Medicare Cost Report. For purposes of this study, however, we elected to utilize a cost‐to‐charge ratio that was exclusive of physician salaries and benefits (ie, 0.42 rather than 0.66) because most hospitals in the United States do not employ their physicians. Accordingly, the costs we present underestimate the true cost to our institution by approximately 32% but are more representative of the costs of services provided by most hospitals in the United States.
Recent studies have shown that the rate of false‐positive cultures is higher when blood is drawn from indwelling catheters than when it is obtained by peripheral venipuncture.9, 10 The rates we cite from the literature2 and from our own institution (Table 1) are aggregate data that include samples drawn from both sites. Separating these would not alter our conclusion that a 50% reduction in false positives would save approximately twice as much as a 50% reduction in false negatives. These studies do, however, identify an important method for reducing false positives: sampling by venipuncture whenever possible, and only drawing through a catheter under very limited circumstances.
There are additional factors that favor a strategy of reducing contaminants over one that attempts to reduce the number of true‐negative cultures. First, reducing the total number of true‐negative blood cultures by 50% would require a very ambitious prediction rule that did not reduce the number of true positives to any meaningful extent. Prediction rules to reduce blood culture testing have been developed for patients with community‐acquired pneumonia, but the rules only reduced the number of cultures by 37% and, more importantly, left 11% of true bacteremias undetected.8 Reducing contaminants would have no effect on the detection of true positives, whereas any prediction rule would inevitably increase the risk of missing true bacteremia in at least a fraction of patients. Second, methods aimed at reducing contaminants can be implemented immediately, whereas deriving a prediction rule would take years to develop and test before it could be utilized. Third, implementing prediction rules may be difficult because many physicians prefer to rely on their clinical impressions.11
Reducing contaminants would require improving the technique by which blood cultures are obtained, with the objective of shifting a portion of false positives to true negatives. This might be accomplished in many ways: increasing the time spent on antiseptic scrubbing, improving the ways in which antiseptic devices are used, waiting for the antiseptic to air‐dry completely, choosing the antiseptic that is most effective in trials, drawing blood by venipuncture instead of through an indwelling catheter, limiting the number of venipuncture attempts before requiring a second site to be prepared, requiring all cultures be drawn by trained phlebotomists, and reducing phlebotomist turnover, among others. Denver Health has a 4‐page set of directions for phlebotomists to follow when obtaining blood cultures. Accordingly, there are numerous places the process could break down. Although having 2 phlebotomists involved (ie, one to perform the procedure and the other to observe and guide the first, assuring that all the appropriate steps are followed) might be considered an extraordinary step, our findings suggest the potential saving to the institution could far outweigh the additional personnel expense resulting from such an approach. Other potential solutions we have considered but not tested include providing a monthly salary bonus to the phlebotomist with the lowest contamination rate or giving bonuses to every phlebotomist who achieves a zero contamination rate.
In summary, we have concluded that the resource utilization associated with obtaining blood cultures can best be improved by reducing the small percentage of cultures that represent contaminants rather than by developing rules to reduce the much larger number of true negatives. The magnitude of the potential savings resulting from reducing contaminants is sufficiently large to warrant expending additional resources to accomplish this task.
- .Clinically relevant, cost‐effective clinical microbiology. Strategies to decrease unnecessary testing.Am J Clin Path.1997;107:154–167.
- ,,, et al.The clinical significance of positive blood cultures in the 1990s: A prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults.Clin Infect Dis.1997;24:584–602.
- ,,.Contaminant blood cultures and resource utilization: the true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,.Surveillance of nosocomial infection. In:Mayhall CG, ed.Hospital Epidemiology and Infection Control.3rd ed.Philadelphia:Lippincott Williams 106:246–253.
- ,,,.The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations.Rev Infect Dis.1983;5:35–70.
- ,,,.Predicting bacteremia in patients with community acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,,.Clinical utility of blood cultures drawn from central venous or arterial catheters in critically ill surgical patients.Crit Care Med.2002;30:7–13.
- ,,.Comparison of contamination rates of catheter‐drawn and peripheral blood cultures.J Hosp Infect.2005;60:118–121.
- ,,,,.Physician response to a prediction rule for the triage of emergency department patients with chest pain.J Gen Intern Med.1994;9:241–247.
Because as many as 90% of all blood cultures grow no organisms1 developing rules that predict which patients are at the lowest risk of having bacteremia could improve the utilization of this test and markedly reduce its cost. Of the approximate 10% of cultures that do grow organisms, only about half represent true bacteremia (ie, true positives), whereas the other half are considered contaminants (ie, false positives)2; the latter are known to increase both the cost and duration of care.3 Accordingly, reducing the number of contaminants could also reduce the cost of care. We assessed which of these two strategies would be the most cost effective. Although only 6% of the blood cultures obtained at our hospital represented contaminants, their associated cost was more than twice that associated with the 87% of cultures that were true negatives.
METHODS
We conducted a retrospective review of microbiological results and hospital records of patients for whom blood cultures were obtained in January 2002 at Denver Health Medical Center, a 400‐bed university‐affiliated public safety net hospital. The study was given exempt status by the Colorado Multiple Institutional Review Board. Patients were identified using a preexisting laboratory database.
We adopted the definitions used by Bates et al.3 for the inclusion and exclusion criteria and the definition of a blood culture episode so that we could apply the financial data presented by these authors to our results. Briefly, a blood culture set was defined as a single venipuncture, regardless of the number of bottles sent for culturing, and a blood culture episode was defined as the 48‐hour period beginning when a blood culture was drawn. All sets within the same 48‐hour period were considered part of the same episode. Cultures that grew bacteria were classified as either true positive, representing bacteremia, or false positive, representing contaminants. Determination of whether a patient had a true‐positive culture versus a contaminant was made in a weekly conference attended by the chief of the Infectious Disease Division, an Infectious Disease fellow, and at least one microbiologist, during which the species of organism cultured and the associated clinical data for each patient were considered. Organisms considered to indicate false positives included diphtheroids, Bacillus sp, Propionibacterium sp, coagulase‐negative staphylococci, and micrococci. All other organisms were considered true positives in the setting of appropriate clinical criteria as specified by the CDC guidelines.4 Hospital charges and lengths of stay were obtained from our institutional database.
The cost associated with a true‐negative blood culture was determined by summing the charges for phlebotomy and microbiological testing obtained from the January 2005 Denver Health hospital charge master and applying the cost‐to‐charge ratio reported on the Medicare Cost Report for inpatient services (not including the costs of physician salaries and benefits).
The cost of a false positive was determined two ways: (a) adjusting the data reported by Bates et al.3 for changes in the Consumer Price Index5 and (b) comparing the actual hospital charges of the patients in our sample who had false‐positive cultures with those who did not (adjusting both by the hospital's inpatient cost‐to‐charge ratio, again not including the cost of physician salaries and benefits).
The length of stay and cost of care for patients with true‐ and false‐positive blood cultures were compared by chi‐square analysis. P < .05 was considered statistically significant. The data were not normally distributed and, as such, are presented as medians and interquartile ranges.
RESULTS
Table 1 summarizes the interpretation of the 939 blood cultures drawn in January 2002. Only 6 culture sets (0.6%) could not be classified. The positive predictive value of a positive blood culture was only 53%.
| Blood cultures | Bacteremia | ||
|---|---|---|---|
| Number positive (%) | Number negative (%) | Total | |
| Number positive (%) | 62 (7) | 56 (6) | 118 (13) |
| Number negative (%) | 0 (0) | 815 (87) | 815 (87) |
| Total | 62 (7) | 871 (93) | 933 (100) |
Laboratory charges for patients with true‐negative and false‐positive blood cultures in January 2002 are shown in Table 2. Annualized, the associated charges were $1,781,292, and the costs were $748,143.
| Charge ($) | Tests (N) | Total ($) | |
|---|---|---|---|
| True‐negative cultures | |||
| Phlebotomy | $13.25 | ||
| Microbiology | $147.50 | ||
| Subtotal | $160.75 | 815 | $131,011 |
| False‐positive cultures | |||
| Phlebotomy | $13.25 | ||
| Microbiology | $147.50 | ||
| Identification | $60.75 | ||
| Sensitivity | $89.75 | ||
| Subtotal | $311.25 | 56 | $17,430 |
| $148,441 |
Bates et al.3 found that false‐positive blood cultures increased the length of hospital stay by 4.5 days and increased total charges by $4385 over those for patients with no contaminants. This adjusted to $6878 in 2005 according to the Consumer Price Index.5 After grouping our blood cultures into episodes as defined by Bates et al. (Table 3), we had 41 episodes of contaminated blood cultures that would annualize to charges of $3,383,976 and costs of $1,421,270 after applying the cost‐to‐charge ratio.
| Blood cultures | Bacteremia | ||
|---|---|---|---|
| Number positive (%) | Number negative (%) | Total | |
| |||
| Number positive (%) | 39 (9) | 41 (10) | 80 (19) |
| Number negative (%) | 0 (0) | 335 (81) | 335 (81) |
| Total | 39 (9) | 376 (91) | 415 (100) |
The median length of hospital stay and total charges for the patients with true‐negative and false‐positive blood cultures at Denver Health in January 2002 are summarized in Table 4. Using this approach, patients with false‐positive blood cultures at our institution added 1450‐2200 extra hospital days and accrued additional charges of $4,305,000 and costs of $1,808,100.
| Length of stay (days) | Interquartile range (days) | Total Charges ($) | Interquartile Range ($) | |
|---|---|---|---|---|
| ||||
| True negative | 5 | 2‐12 | $15,158 | $7,007‐$40,270 |
| False positive | 8a | 4‐13.5 | $23,908a | $14,083‐$52,031 |
| Difference | 3 | $8,750 | ||
DISCUSSION
The important finding of this study is that, despite there being nearly 15 times as many true‐negative blood cultures as false‐positive ones, the savings generated by reducing contaminants would be approximately twice that saved by reducing the true negatives (eg, a 50% reduction in the rate of contamination would reduce the total number of false‐positive episodes by 246 annually, saving $710,635‐$904,050, whereas reducing the true negatives by 50% would only save approximately $375,000.
There is no independent gold standard for evaluating the operating characteristics of a blood culture.6 Data from a series of repeated blood cultures represent the closest surrogate. Weinstein et al.7 drew at least 3 sets of cultures from 282 bacteremic patients and noted that bacteremia was documented in 91.5% of the first cultures, in 99.3% in 1 of the first 2 cultures, and in 99.6% in 1 of the first 3 cultures. Because 2 blood culture sets are drawn routinely, the difference between those 2 (if negative) and a third (if it represents a true positive) is 0.3% and would represent a false‐negative culture rate. Given that the true‐negative rate of blood cultures is 87%‐90%,1 the potential 0.3% false‐negative rate would not affect our analysis, and as such, we chose to ignore it. Accordingly, all sets of blood cultures with no growth were classified as true negatives.
Although we cannot show a cause‐and‐effect relationship between false‐positive cultures and the charges associated resulting from them, a recent study suggested that much of the excess length of stay of such patients is attributable to the false‐positive culture itself.8
Because health care costs have exceeded increases in general goods and services, adjusting the results of Bates et al.3 using the Consumer Price Index likely underestimated the projected cost of the false‐positive cultures. This limitation likely accounts for the observation that the difference in actual charges for our patients between those who did and those who did not have false‐positive blood cultures was greater than the cost of these false‐positive cultures as estimated by extrapolating from the data of Bates et al.3 Given the magnitude of the financial difference we observed, however, we suggest that this difference is not of sufficient size to alter our conclusion.
Physicians working at Denver Health are directly employed by the hospital, and the cost of physician salaries and benefits is included in the cost‐to‐charge ratio reported in our Medicare Cost Report. For purposes of this study, however, we elected to utilize a cost‐to‐charge ratio that was exclusive of physician salaries and benefits (ie, 0.42 rather than 0.66) because most hospitals in the United States do not employ their physicians. Accordingly, the costs we present underestimate the true cost to our institution by approximately 32% but are more representative of the costs of services provided by most hospitals in the United States.
Recent studies have shown that the rate of false‐positive cultures is higher when blood is drawn from indwelling catheters than when it is obtained by peripheral venipuncture.9, 10 The rates we cite from the literature2 and from our own institution (Table 1) are aggregate data that include samples drawn from both sites. Separating these would not alter our conclusion that a 50% reduction in false positives would save approximately twice as much as a 50% reduction in false negatives. These studies do, however, identify an important method for reducing false positives: sampling by venipuncture whenever possible, and only drawing through a catheter under very limited circumstances.
There are additional factors that favor a strategy of reducing contaminants over one that attempts to reduce the number of true‐negative cultures. First, reducing the total number of true‐negative blood cultures by 50% would require a very ambitious prediction rule that did not reduce the number of true positives to any meaningful extent. Prediction rules to reduce blood culture testing have been developed for patients with community‐acquired pneumonia, but the rules only reduced the number of cultures by 37% and, more importantly, left 11% of true bacteremias undetected.8 Reducing contaminants would have no effect on the detection of true positives, whereas any prediction rule would inevitably increase the risk of missing true bacteremia in at least a fraction of patients. Second, methods aimed at reducing contaminants can be implemented immediately, whereas deriving a prediction rule would take years to develop and test before it could be utilized. Third, implementing prediction rules may be difficult because many physicians prefer to rely on their clinical impressions.11
Reducing contaminants would require improving the technique by which blood cultures are obtained, with the objective of shifting a portion of false positives to true negatives. This might be accomplished in many ways: increasing the time spent on antiseptic scrubbing, improving the ways in which antiseptic devices are used, waiting for the antiseptic to air‐dry completely, choosing the antiseptic that is most effective in trials, drawing blood by venipuncture instead of through an indwelling catheter, limiting the number of venipuncture attempts before requiring a second site to be prepared, requiring all cultures be drawn by trained phlebotomists, and reducing phlebotomist turnover, among others. Denver Health has a 4‐page set of directions for phlebotomists to follow when obtaining blood cultures. Accordingly, there are numerous places the process could break down. Although having 2 phlebotomists involved (ie, one to perform the procedure and the other to observe and guide the first, assuring that all the appropriate steps are followed) might be considered an extraordinary step, our findings suggest the potential saving to the institution could far outweigh the additional personnel expense resulting from such an approach. Other potential solutions we have considered but not tested include providing a monthly salary bonus to the phlebotomist with the lowest contamination rate or giving bonuses to every phlebotomist who achieves a zero contamination rate.
In summary, we have concluded that the resource utilization associated with obtaining blood cultures can best be improved by reducing the small percentage of cultures that represent contaminants rather than by developing rules to reduce the much larger number of true negatives. The magnitude of the potential savings resulting from reducing contaminants is sufficiently large to warrant expending additional resources to accomplish this task.
Because as many as 90% of all blood cultures grow no organisms1 developing rules that predict which patients are at the lowest risk of having bacteremia could improve the utilization of this test and markedly reduce its cost. Of the approximate 10% of cultures that do grow organisms, only about half represent true bacteremia (ie, true positives), whereas the other half are considered contaminants (ie, false positives)2; the latter are known to increase both the cost and duration of care.3 Accordingly, reducing the number of contaminants could also reduce the cost of care. We assessed which of these two strategies would be the most cost effective. Although only 6% of the blood cultures obtained at our hospital represented contaminants, their associated cost was more than twice that associated with the 87% of cultures that were true negatives.
METHODS
We conducted a retrospective review of microbiological results and hospital records of patients for whom blood cultures were obtained in January 2002 at Denver Health Medical Center, a 400‐bed university‐affiliated public safety net hospital. The study was given exempt status by the Colorado Multiple Institutional Review Board. Patients were identified using a preexisting laboratory database.
We adopted the definitions used by Bates et al.3 for the inclusion and exclusion criteria and the definition of a blood culture episode so that we could apply the financial data presented by these authors to our results. Briefly, a blood culture set was defined as a single venipuncture, regardless of the number of bottles sent for culturing, and a blood culture episode was defined as the 48‐hour period beginning when a blood culture was drawn. All sets within the same 48‐hour period were considered part of the same episode. Cultures that grew bacteria were classified as either true positive, representing bacteremia, or false positive, representing contaminants. Determination of whether a patient had a true‐positive culture versus a contaminant was made in a weekly conference attended by the chief of the Infectious Disease Division, an Infectious Disease fellow, and at least one microbiologist, during which the species of organism cultured and the associated clinical data for each patient were considered. Organisms considered to indicate false positives included diphtheroids, Bacillus sp, Propionibacterium sp, coagulase‐negative staphylococci, and micrococci. All other organisms were considered true positives in the setting of appropriate clinical criteria as specified by the CDC guidelines.4 Hospital charges and lengths of stay were obtained from our institutional database.
The cost associated with a true‐negative blood culture was determined by summing the charges for phlebotomy and microbiological testing obtained from the January 2005 Denver Health hospital charge master and applying the cost‐to‐charge ratio reported on the Medicare Cost Report for inpatient services (not including the costs of physician salaries and benefits).
The cost of a false positive was determined two ways: (a) adjusting the data reported by Bates et al.3 for changes in the Consumer Price Index5 and (b) comparing the actual hospital charges of the patients in our sample who had false‐positive cultures with those who did not (adjusting both by the hospital's inpatient cost‐to‐charge ratio, again not including the cost of physician salaries and benefits).
The length of stay and cost of care for patients with true‐ and false‐positive blood cultures were compared by chi‐square analysis. P < .05 was considered statistically significant. The data were not normally distributed and, as such, are presented as medians and interquartile ranges.
RESULTS
Table 1 summarizes the interpretation of the 939 blood cultures drawn in January 2002. Only 6 culture sets (0.6%) could not be classified. The positive predictive value of a positive blood culture was only 53%.
| Blood cultures | Bacteremia | ||
|---|---|---|---|
| Number positive (%) | Number negative (%) | Total | |
| Number positive (%) | 62 (7) | 56 (6) | 118 (13) |
| Number negative (%) | 0 (0) | 815 (87) | 815 (87) |
| Total | 62 (7) | 871 (93) | 933 (100) |
Laboratory charges for patients with true‐negative and false‐positive blood cultures in January 2002 are shown in Table 2. Annualized, the associated charges were $1,781,292, and the costs were $748,143.
| Charge ($) | Tests (N) | Total ($) | |
|---|---|---|---|
| True‐negative cultures | |||
| Phlebotomy | $13.25 | ||
| Microbiology | $147.50 | ||
| Subtotal | $160.75 | 815 | $131,011 |
| False‐positive cultures | |||
| Phlebotomy | $13.25 | ||
| Microbiology | $147.50 | ||
| Identification | $60.75 | ||
| Sensitivity | $89.75 | ||
| Subtotal | $311.25 | 56 | $17,430 |
| $148,441 |
Bates et al.3 found that false‐positive blood cultures increased the length of hospital stay by 4.5 days and increased total charges by $4385 over those for patients with no contaminants. This adjusted to $6878 in 2005 according to the Consumer Price Index.5 After grouping our blood cultures into episodes as defined by Bates et al. (Table 3), we had 41 episodes of contaminated blood cultures that would annualize to charges of $3,383,976 and costs of $1,421,270 after applying the cost‐to‐charge ratio.
| Blood cultures | Bacteremia | ||
|---|---|---|---|
| Number positive (%) | Number negative (%) | Total | |
| |||
| Number positive (%) | 39 (9) | 41 (10) | 80 (19) |
| Number negative (%) | 0 (0) | 335 (81) | 335 (81) |
| Total | 39 (9) | 376 (91) | 415 (100) |
The median length of hospital stay and total charges for the patients with true‐negative and false‐positive blood cultures at Denver Health in January 2002 are summarized in Table 4. Using this approach, patients with false‐positive blood cultures at our institution added 1450‐2200 extra hospital days and accrued additional charges of $4,305,000 and costs of $1,808,100.
| Length of stay (days) | Interquartile range (days) | Total Charges ($) | Interquartile Range ($) | |
|---|---|---|---|---|
| ||||
| True negative | 5 | 2‐12 | $15,158 | $7,007‐$40,270 |
| False positive | 8a | 4‐13.5 | $23,908a | $14,083‐$52,031 |
| Difference | 3 | $8,750 | ||
DISCUSSION
The important finding of this study is that, despite there being nearly 15 times as many true‐negative blood cultures as false‐positive ones, the savings generated by reducing contaminants would be approximately twice that saved by reducing the true negatives (eg, a 50% reduction in the rate of contamination would reduce the total number of false‐positive episodes by 246 annually, saving $710,635‐$904,050, whereas reducing the true negatives by 50% would only save approximately $375,000.
There is no independent gold standard for evaluating the operating characteristics of a blood culture.6 Data from a series of repeated blood cultures represent the closest surrogate. Weinstein et al.7 drew at least 3 sets of cultures from 282 bacteremic patients and noted that bacteremia was documented in 91.5% of the first cultures, in 99.3% in 1 of the first 2 cultures, and in 99.6% in 1 of the first 3 cultures. Because 2 blood culture sets are drawn routinely, the difference between those 2 (if negative) and a third (if it represents a true positive) is 0.3% and would represent a false‐negative culture rate. Given that the true‐negative rate of blood cultures is 87%‐90%,1 the potential 0.3% false‐negative rate would not affect our analysis, and as such, we chose to ignore it. Accordingly, all sets of blood cultures with no growth were classified as true negatives.
Although we cannot show a cause‐and‐effect relationship between false‐positive cultures and the charges associated resulting from them, a recent study suggested that much of the excess length of stay of such patients is attributable to the false‐positive culture itself.8
Because health care costs have exceeded increases in general goods and services, adjusting the results of Bates et al.3 using the Consumer Price Index likely underestimated the projected cost of the false‐positive cultures. This limitation likely accounts for the observation that the difference in actual charges for our patients between those who did and those who did not have false‐positive blood cultures was greater than the cost of these false‐positive cultures as estimated by extrapolating from the data of Bates et al.3 Given the magnitude of the financial difference we observed, however, we suggest that this difference is not of sufficient size to alter our conclusion.
Physicians working at Denver Health are directly employed by the hospital, and the cost of physician salaries and benefits is included in the cost‐to‐charge ratio reported in our Medicare Cost Report. For purposes of this study, however, we elected to utilize a cost‐to‐charge ratio that was exclusive of physician salaries and benefits (ie, 0.42 rather than 0.66) because most hospitals in the United States do not employ their physicians. Accordingly, the costs we present underestimate the true cost to our institution by approximately 32% but are more representative of the costs of services provided by most hospitals in the United States.
Recent studies have shown that the rate of false‐positive cultures is higher when blood is drawn from indwelling catheters than when it is obtained by peripheral venipuncture.9, 10 The rates we cite from the literature2 and from our own institution (Table 1) are aggregate data that include samples drawn from both sites. Separating these would not alter our conclusion that a 50% reduction in false positives would save approximately twice as much as a 50% reduction in false negatives. These studies do, however, identify an important method for reducing false positives: sampling by venipuncture whenever possible, and only drawing through a catheter under very limited circumstances.
There are additional factors that favor a strategy of reducing contaminants over one that attempts to reduce the number of true‐negative cultures. First, reducing the total number of true‐negative blood cultures by 50% would require a very ambitious prediction rule that did not reduce the number of true positives to any meaningful extent. Prediction rules to reduce blood culture testing have been developed for patients with community‐acquired pneumonia, but the rules only reduced the number of cultures by 37% and, more importantly, left 11% of true bacteremias undetected.8 Reducing contaminants would have no effect on the detection of true positives, whereas any prediction rule would inevitably increase the risk of missing true bacteremia in at least a fraction of patients. Second, methods aimed at reducing contaminants can be implemented immediately, whereas deriving a prediction rule would take years to develop and test before it could be utilized. Third, implementing prediction rules may be difficult because many physicians prefer to rely on their clinical impressions.11
Reducing contaminants would require improving the technique by which blood cultures are obtained, with the objective of shifting a portion of false positives to true negatives. This might be accomplished in many ways: increasing the time spent on antiseptic scrubbing, improving the ways in which antiseptic devices are used, waiting for the antiseptic to air‐dry completely, choosing the antiseptic that is most effective in trials, drawing blood by venipuncture instead of through an indwelling catheter, limiting the number of venipuncture attempts before requiring a second site to be prepared, requiring all cultures be drawn by trained phlebotomists, and reducing phlebotomist turnover, among others. Denver Health has a 4‐page set of directions for phlebotomists to follow when obtaining blood cultures. Accordingly, there are numerous places the process could break down. Although having 2 phlebotomists involved (ie, one to perform the procedure and the other to observe and guide the first, assuring that all the appropriate steps are followed) might be considered an extraordinary step, our findings suggest the potential saving to the institution could far outweigh the additional personnel expense resulting from such an approach. Other potential solutions we have considered but not tested include providing a monthly salary bonus to the phlebotomist with the lowest contamination rate or giving bonuses to every phlebotomist who achieves a zero contamination rate.
In summary, we have concluded that the resource utilization associated with obtaining blood cultures can best be improved by reducing the small percentage of cultures that represent contaminants rather than by developing rules to reduce the much larger number of true negatives. The magnitude of the potential savings resulting from reducing contaminants is sufficiently large to warrant expending additional resources to accomplish this task.
- .Clinically relevant, cost‐effective clinical microbiology. Strategies to decrease unnecessary testing.Am J Clin Path.1997;107:154–167.
- ,,, et al.The clinical significance of positive blood cultures in the 1990s: A prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults.Clin Infect Dis.1997;24:584–602.
- ,,.Contaminant blood cultures and resource utilization: the true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,.Surveillance of nosocomial infection. In:Mayhall CG, ed.Hospital Epidemiology and Infection Control.3rd ed.Philadelphia:Lippincott Williams 106:246–253.
- ,,,.The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations.Rev Infect Dis.1983;5:35–70.
- ,,,.Predicting bacteremia in patients with community acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,,.Clinical utility of blood cultures drawn from central venous or arterial catheters in critically ill surgical patients.Crit Care Med.2002;30:7–13.
- ,,.Comparison of contamination rates of catheter‐drawn and peripheral blood cultures.J Hosp Infect.2005;60:118–121.
- ,,,,.Physician response to a prediction rule for the triage of emergency department patients with chest pain.J Gen Intern Med.1994;9:241–247.
- .Clinically relevant, cost‐effective clinical microbiology. Strategies to decrease unnecessary testing.Am J Clin Path.1997;107:154–167.
- ,,, et al.The clinical significance of positive blood cultures in the 1990s: A prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults.Clin Infect Dis.1997;24:584–602.
- ,,.Contaminant blood cultures and resource utilization: the true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,.Surveillance of nosocomial infection. In:Mayhall CG, ed.Hospital Epidemiology and Infection Control.3rd ed.Philadelphia:Lippincott Williams 106:246–253.
- ,,,.The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations.Rev Infect Dis.1983;5:35–70.
- ,,,.Predicting bacteremia in patients with community acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,,.Clinical utility of blood cultures drawn from central venous or arterial catheters in critically ill surgical patients.Crit Care Med.2002;30:7–13.
- ,,.Comparison of contamination rates of catheter‐drawn and peripheral blood cultures.J Hosp Infect.2005;60:118–121.
- ,,,,.Physician response to a prediction rule for the triage of emergency department patients with chest pain.J Gen Intern Med.1994;9:241–247.
Copyright © 2006 Society of Hospital Medicine