User login
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
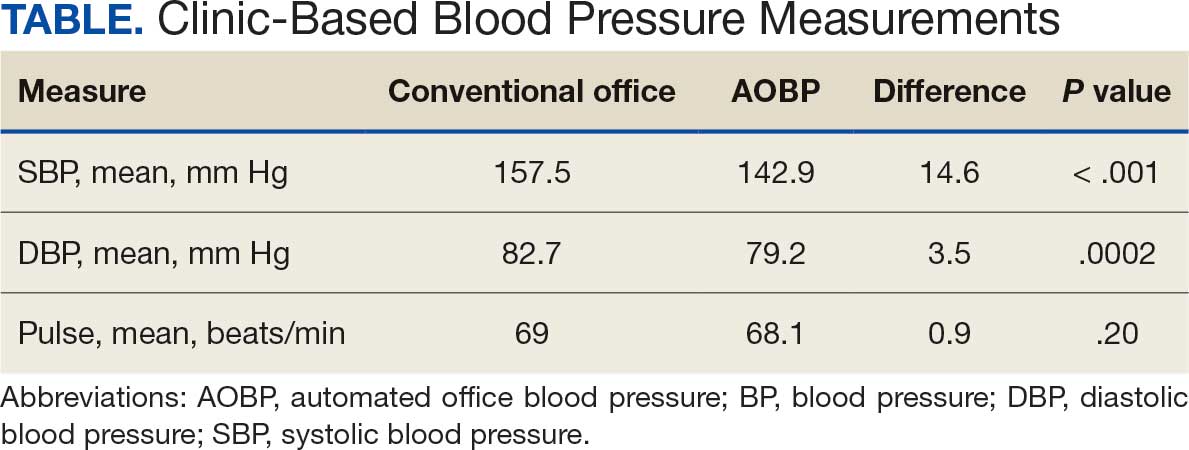
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).
A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).

A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).

A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).
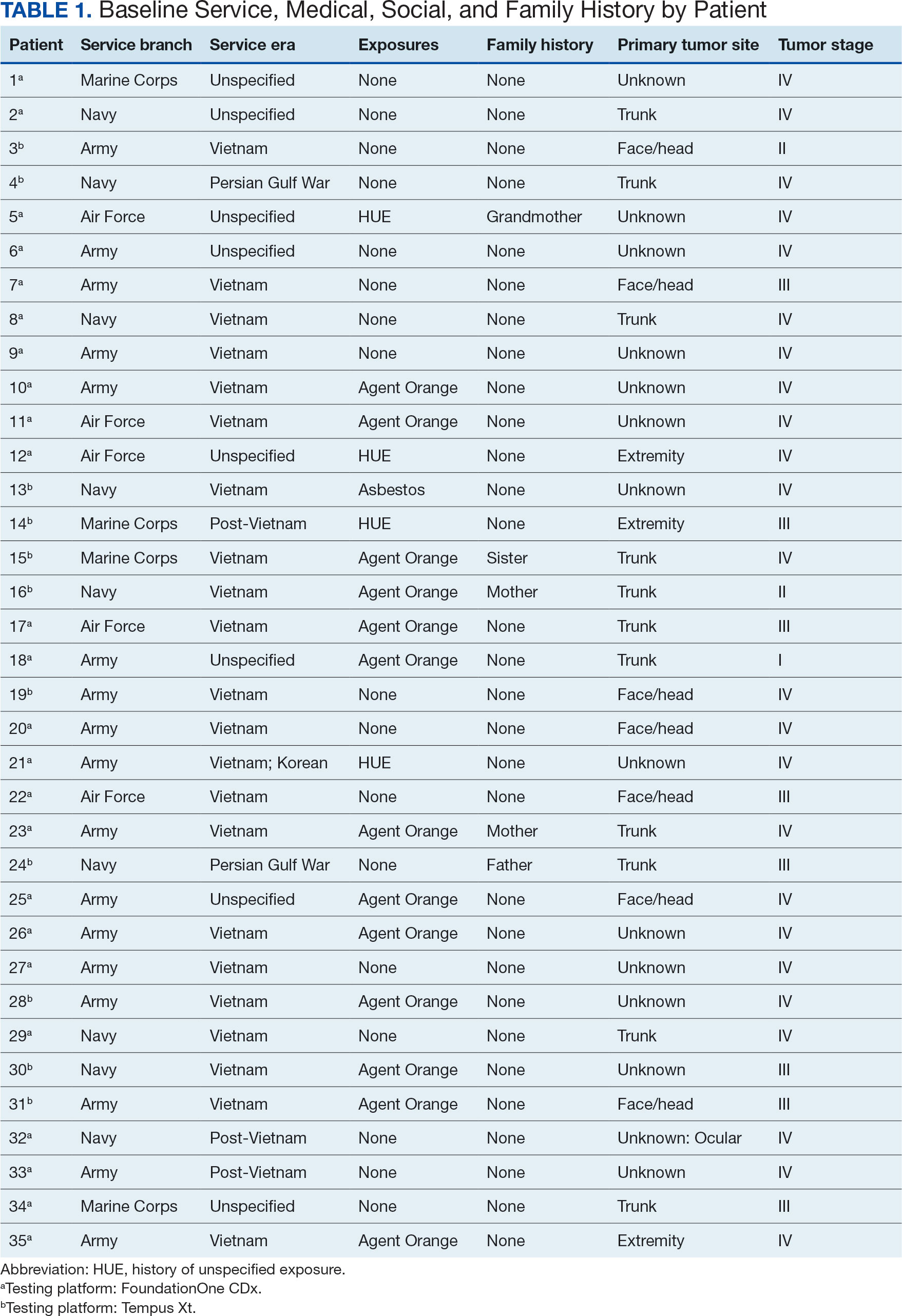
Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.
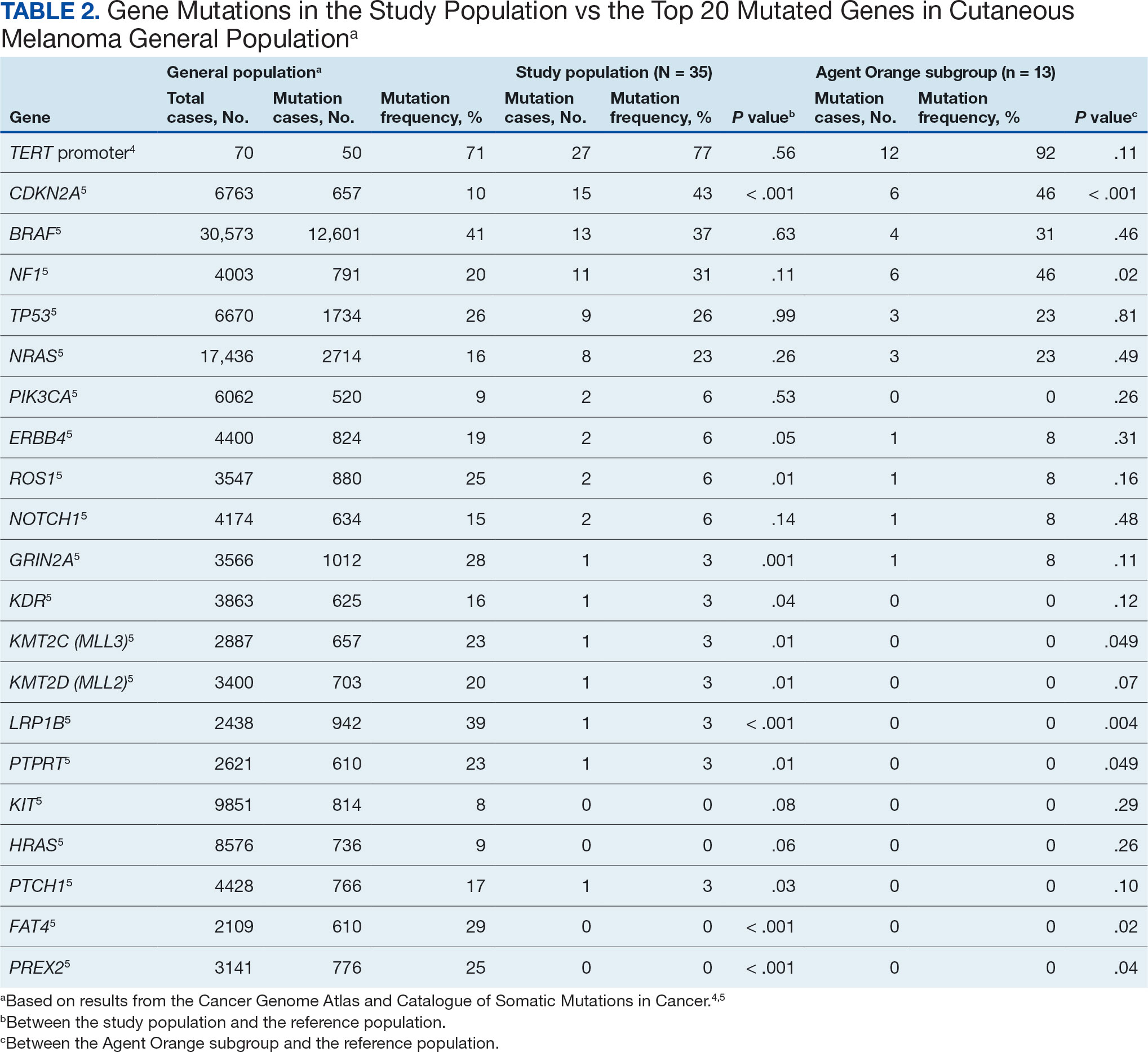
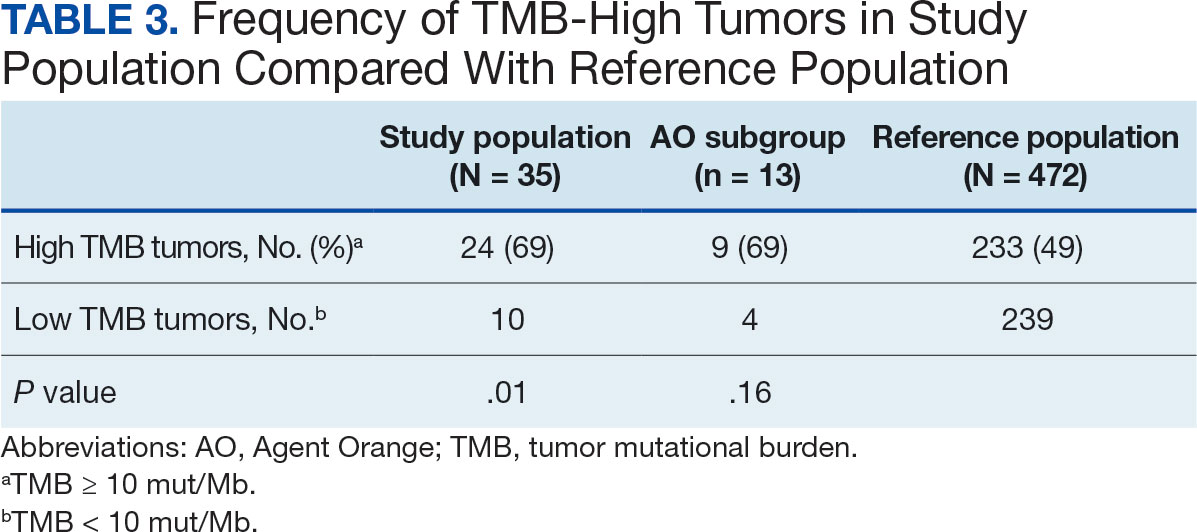
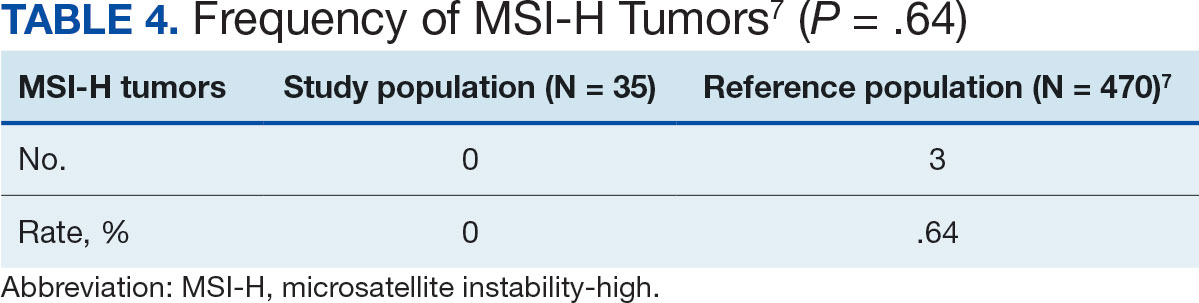
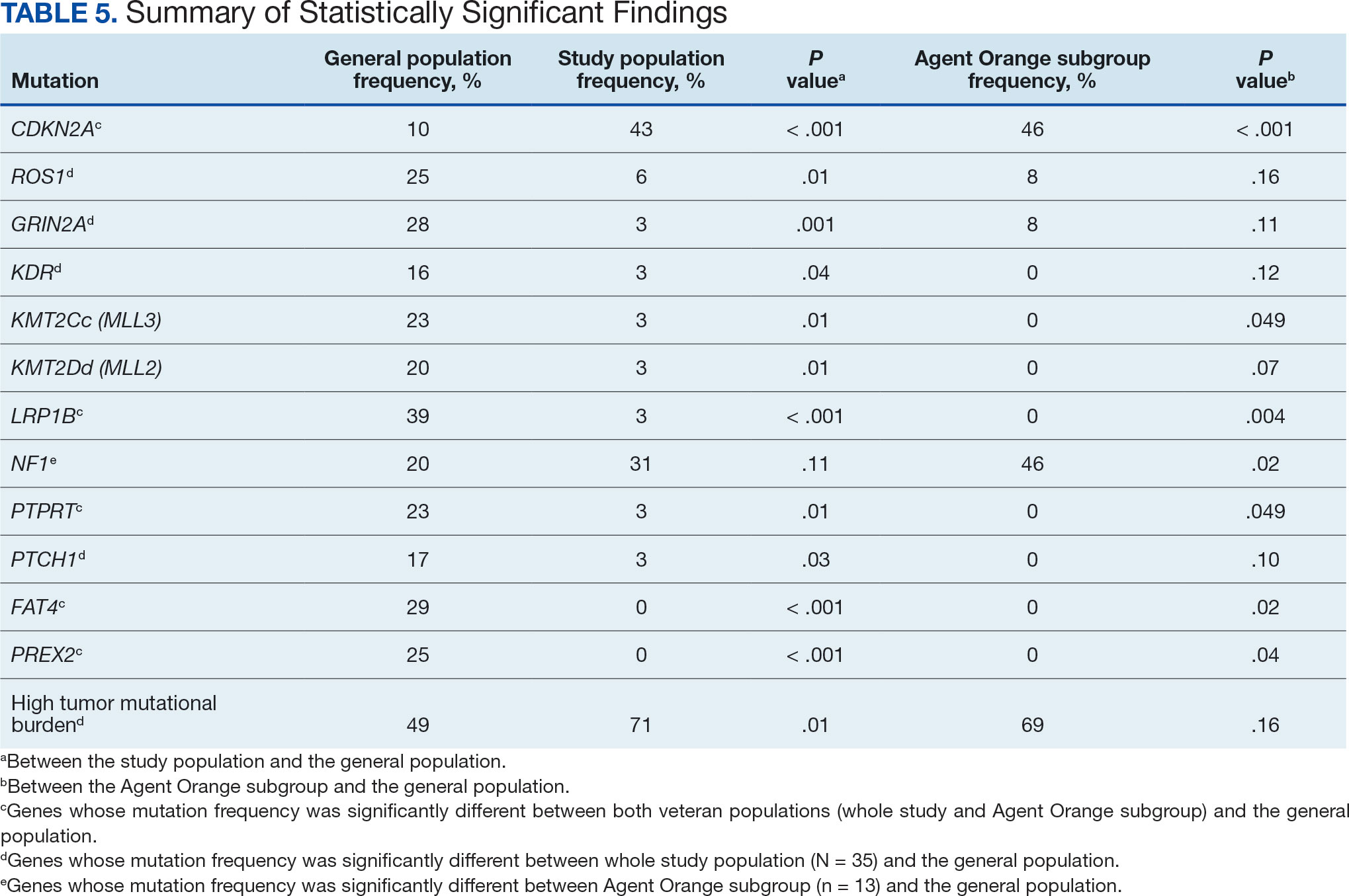
Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
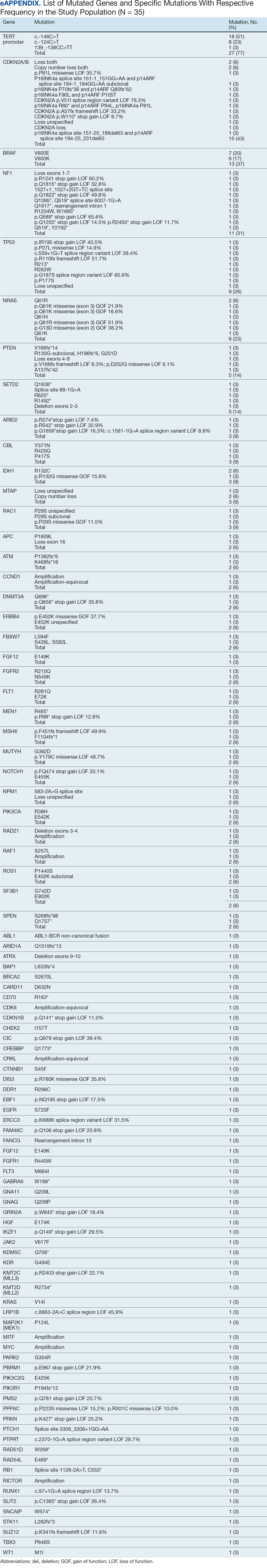
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).
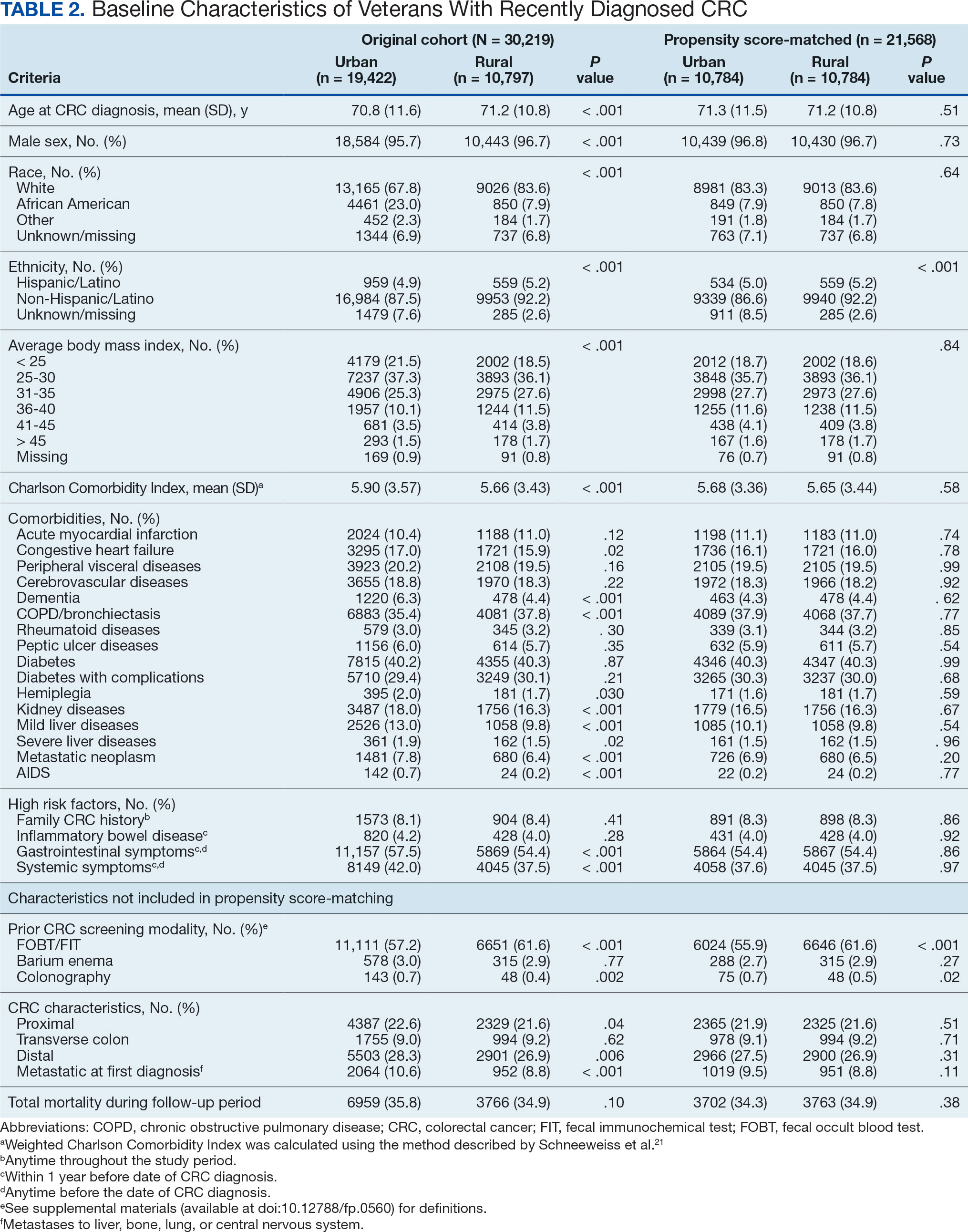
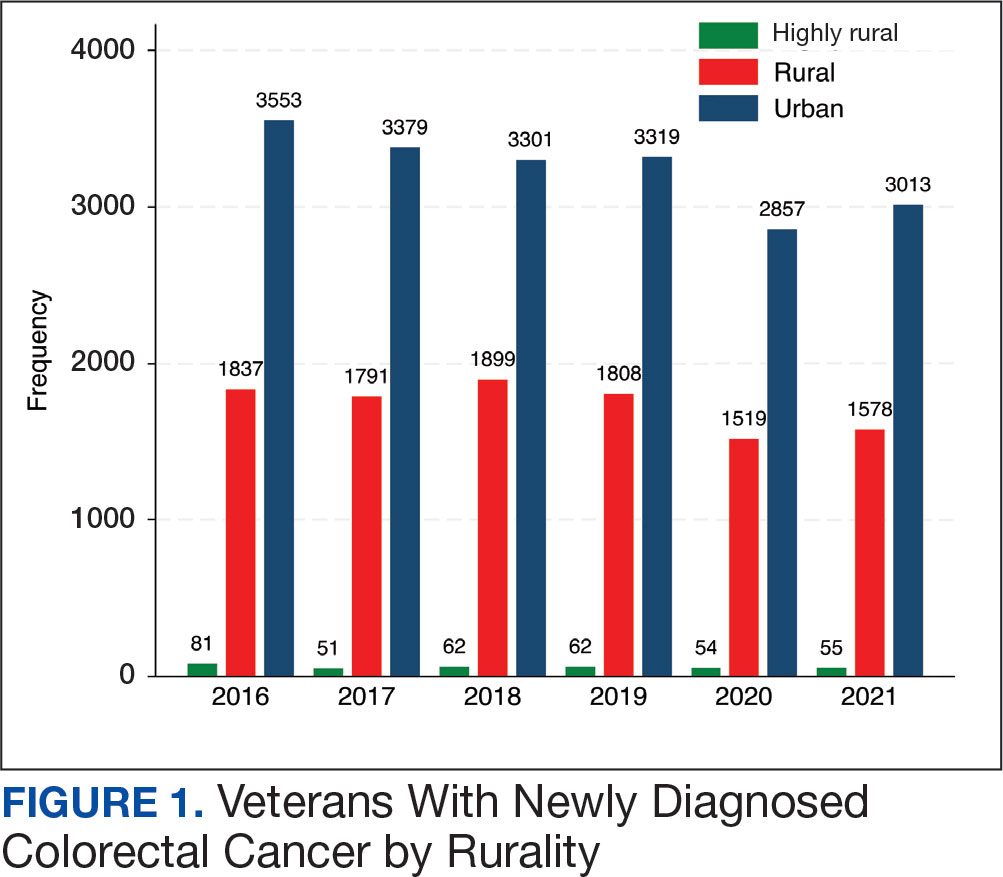
There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).
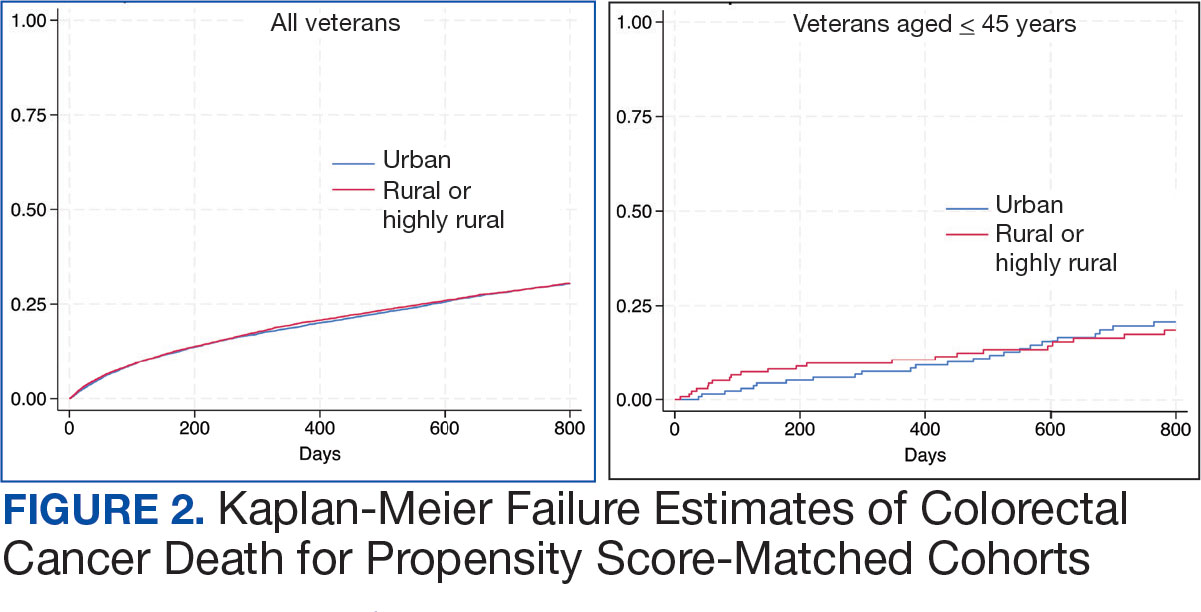
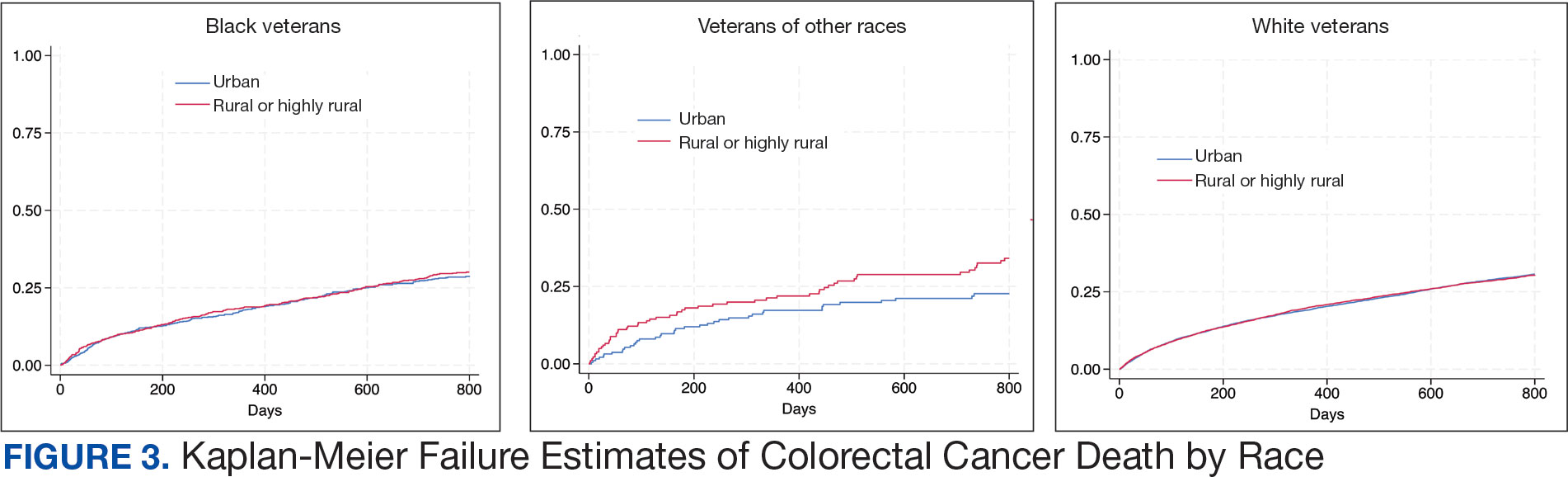
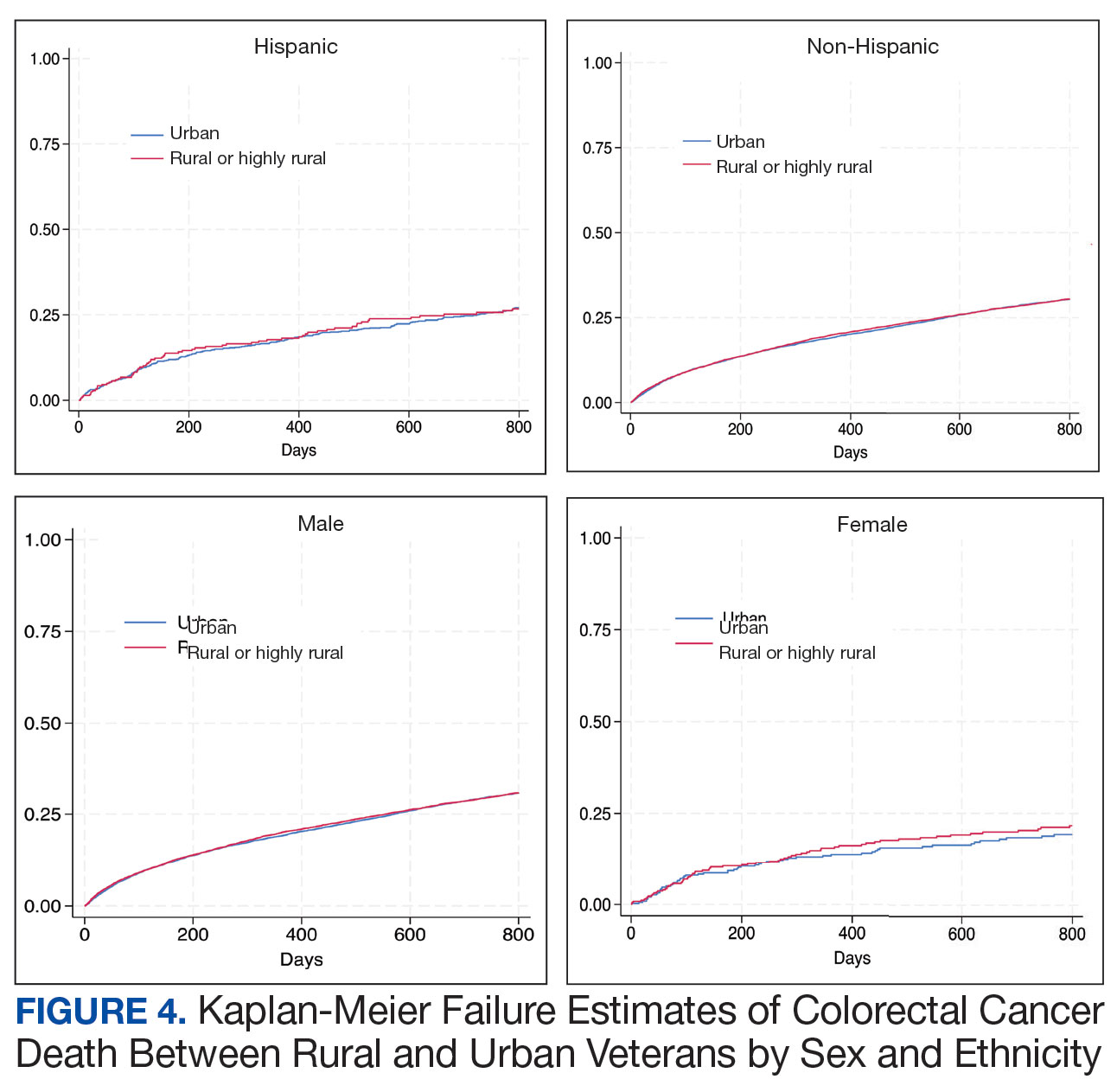
Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).
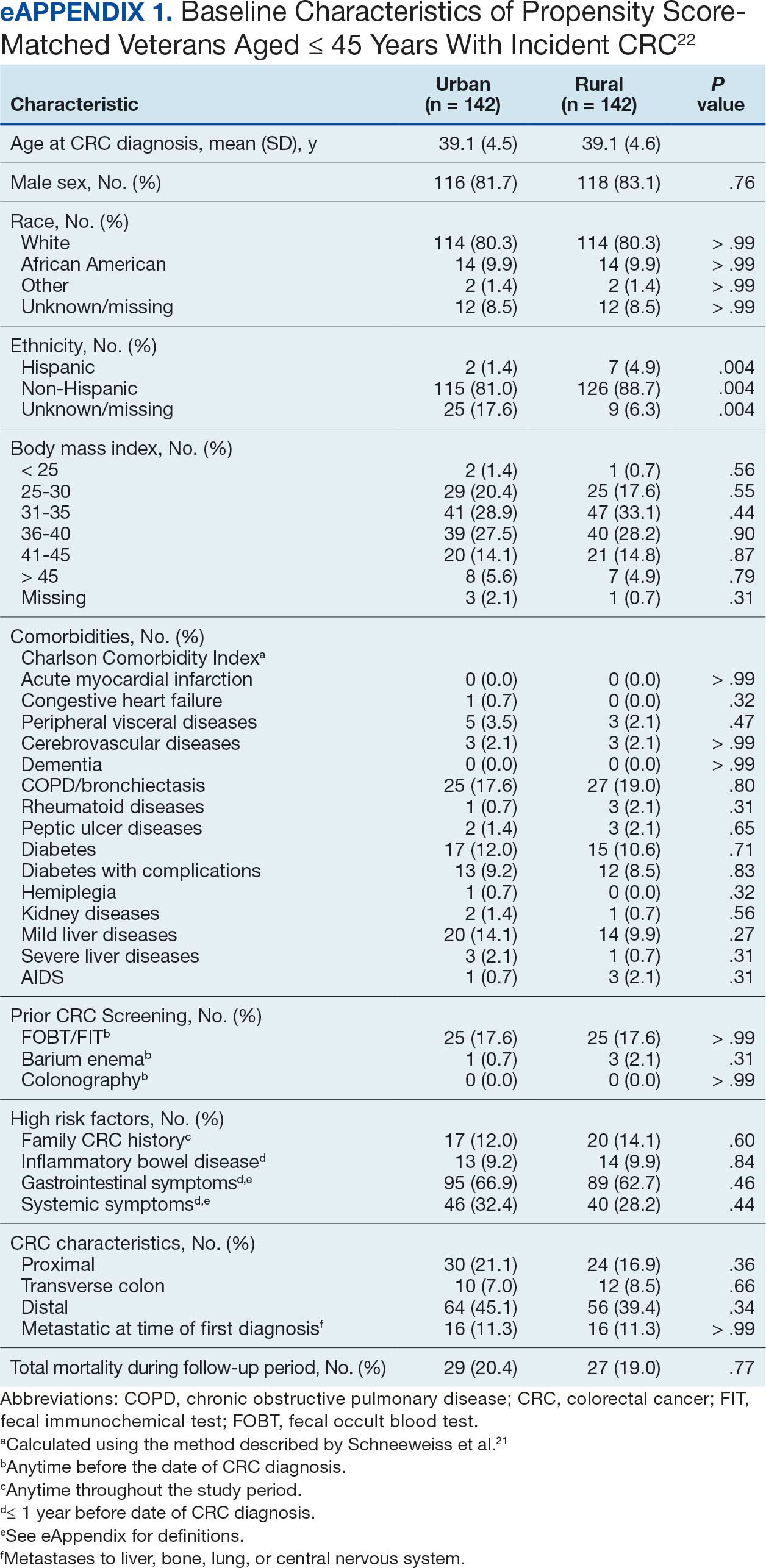
There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).
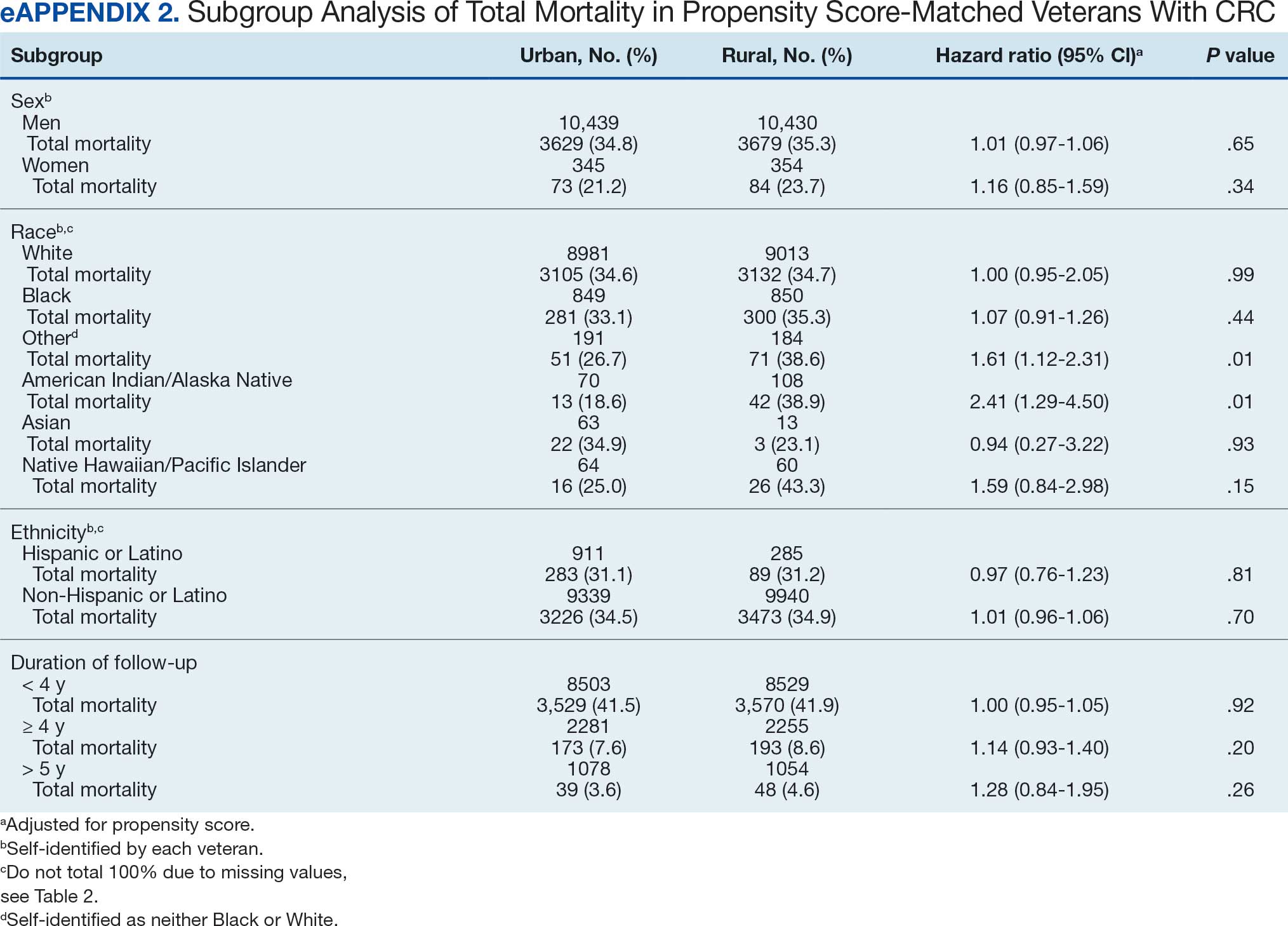
Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Continuous Glucose Monitoring vs Fingerstick Monitoring for Hemoglobin A1c Control in Veterans
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.
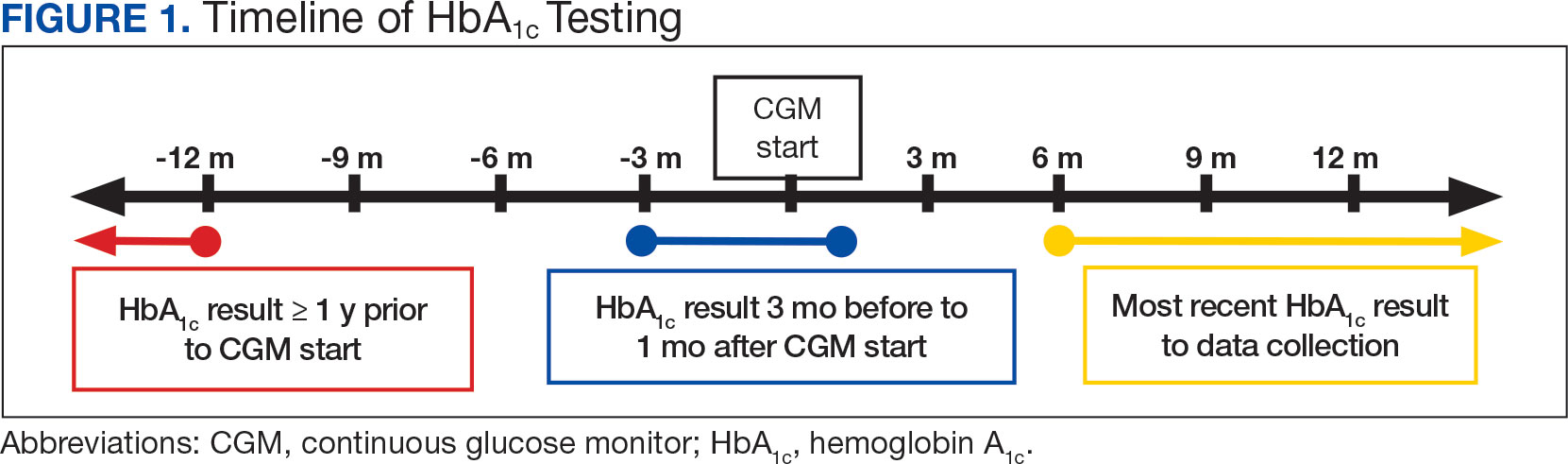
This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.
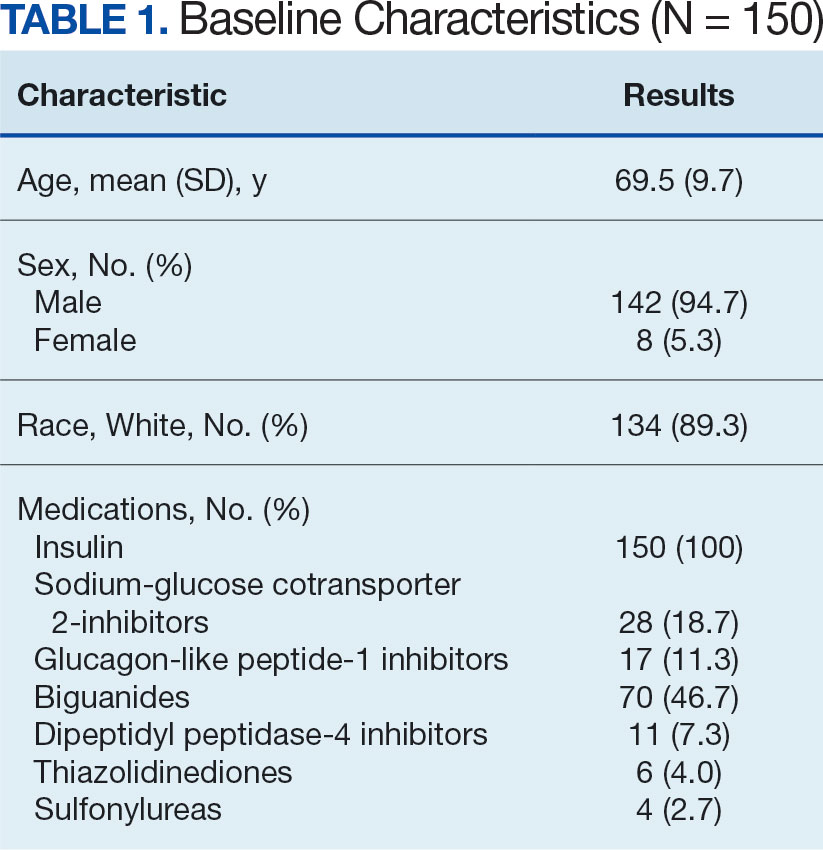
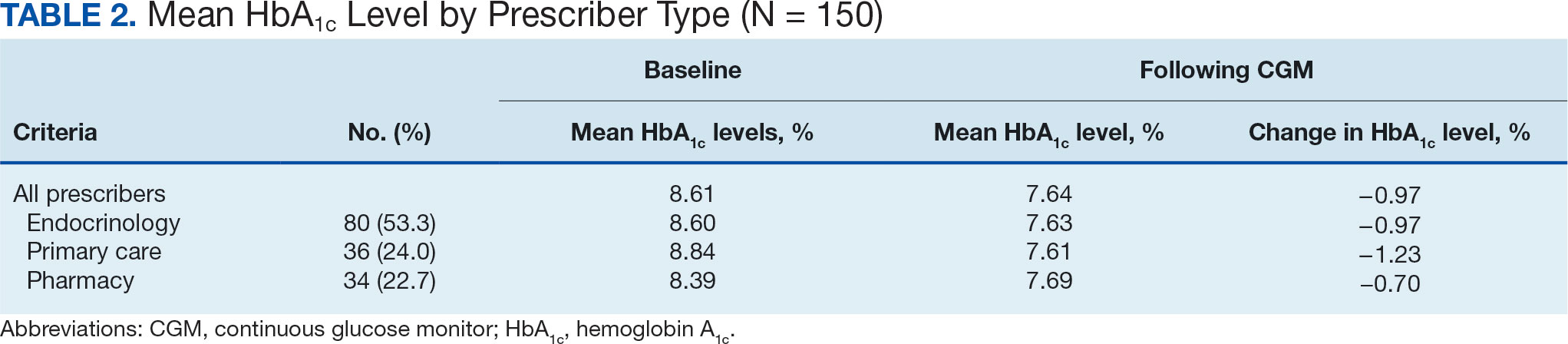
The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.
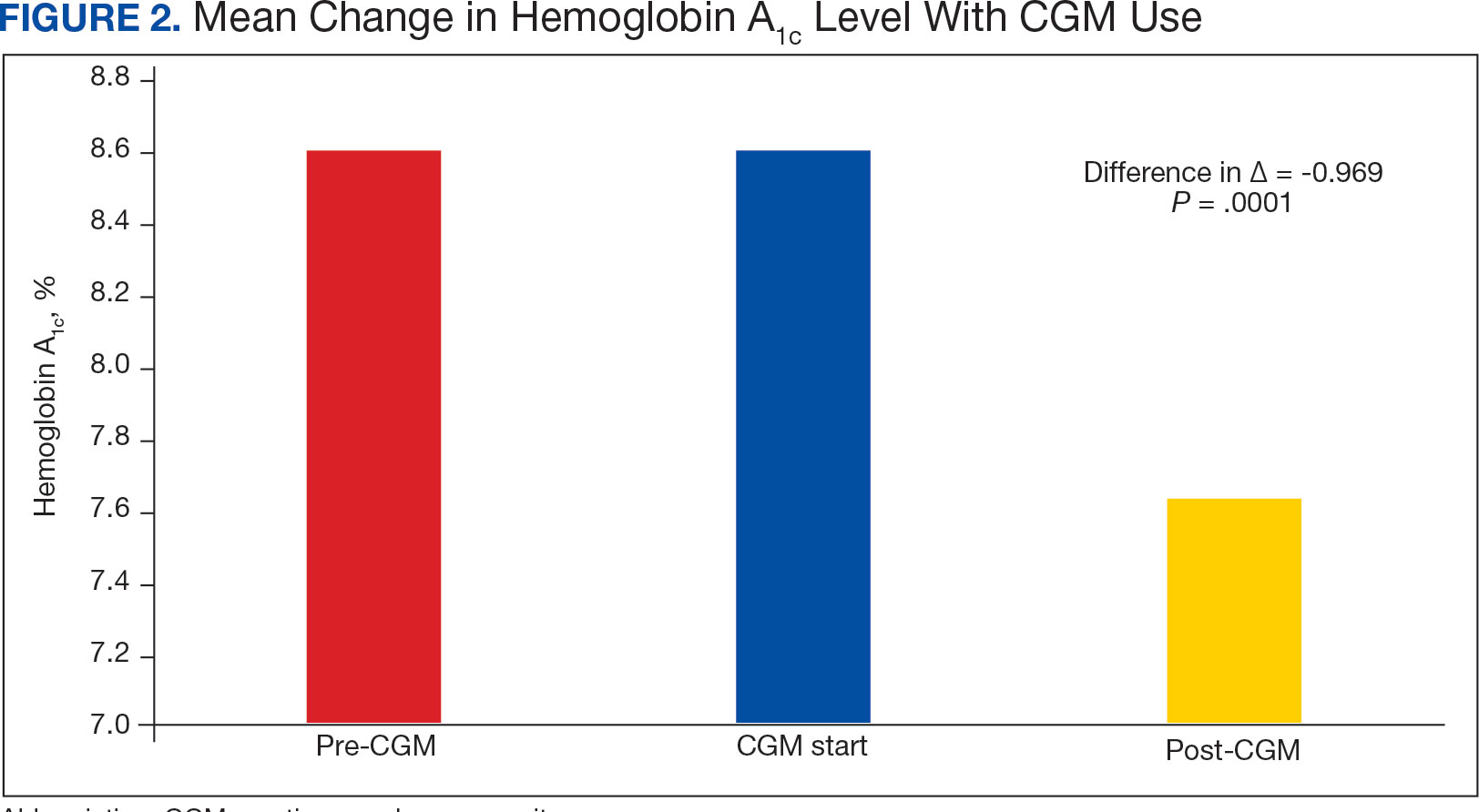
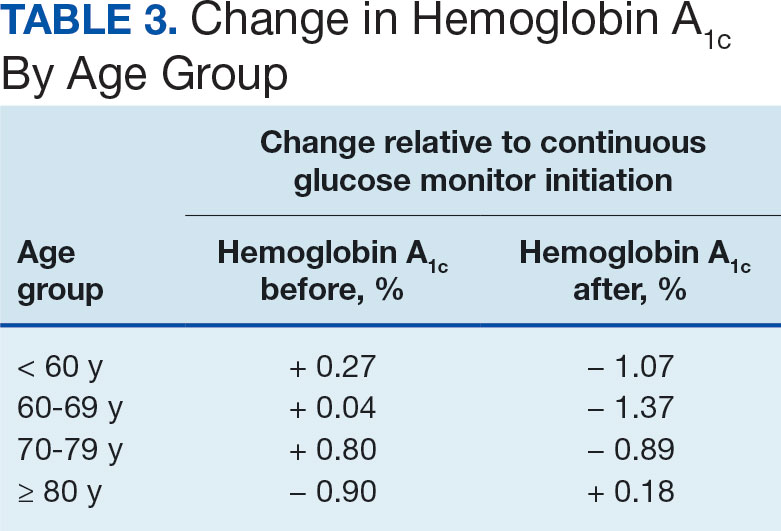
Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
In the United States, 1 in 4 veterans lives with type 2 diabetes mellitus (T2DM), double the rate of the general population.1 Medications are important for the treatment of T2DM and preventing complications that may develop if not properly managed. Common classes of medications for diabetes include biguanides, sodiumglucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. The selection of treatment depends on patient-specific factors including hemoglobin A1c (HbA1c) goal, potential effects on weight, risk of hypoglycemia, and comorbidities such as atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2
HbA1c level reflects the mean blood glucose over the previous 3 months and serves as an indication of diabetes control. In patients with diabetes, it is recommended that HbA1c is checked ≥ 2 times annually for those meeting treatment goals, or more often if the patient needs to adjust medications to reach their HbA1c goal. The goal HbA1c level for most adults with diabetes is < 7%.3 This target can be adjusted based on age, comorbidities, or other patient factors. It is generally recommended that frequent glucose monitoring is not needed for patients with T2DM who are only taking oral agents and/or noninsulin injectables. However, for those on insulin regimens, it is advised to monitor glucose closely, with even more frequent testing for those with an intensive insulin regimen.3
Most patients with diabetes use fingerstick testing to self-monitor their blood glucose. However, continuous glucose monitors (CGMs) are becoming widely available and offer a solution to those who do not have the ability to check their glucose multiple times a day and throughout the night. The American Diabetes Association recommends that the frequency and timing of blood glucose monitoring, or the consideration of CGM use, should be based on the specific needs and goals of each patient.3 Guidelines also encourage those on intensive insulin regimens to check glucose levels when fasting, before and after meals, prior to exercise, and when hypoglycemia or hyperglycemia is suspected. Frequent testing can become a burden for patients, whereas once a CGM sensor is placed, it can be worn for 10 to 14 days. CGMs are also capable of transmitting glucose readings every 1 to 15 minutes to a receiver or mobile phone, allowing for further adaptability to a patient’s lifestyle.3
CGMs work by measuring the interstitial glucose with a small filament sensor and have demonstrated accuracy when compared to blood glucose readings. The ability of a CGM to accurately reflect HbA1c levels is a potential benefit, reducing the need for frequent testing to determine whether patients have achieved glycemic control.4 Another benefit of a CGM is the ease of sharing data; patient accounts can be linked with a health care site, allowing clinicians to access glucose data even if the patient is not able to be seen in clinic. This allows health care practitioners (HCPs) to more efficiently tailor medications and optimize regimens based on patient-specific data that was not available by fingerstick testing alone.
Vigersky and colleagues provided one of the few studies on the long-term effects of CGM in patients managing T2DM through diet and exercise alone, oral medications, or basal insulin and found significant improvement in HbA1c after only 3 months of CGM use.5
An important aspect of CGM use is the ability to alert the patient to low blood glucose readings, which can be dangerous for those unaware of hypoglycemia. Many studies have investigated the association between CGM use and acute metabolic events, demonstrating the potential for CGMs to prevent these emergencies. Karter and colleagues found a reduction in emergency department visits and hospitalizations for hypoglycemia associated with the use of CGMs in patients with type 1 DM (T1DM) and T2DM.6
There have been few studies on the use of CGM in veterans. Langford and colleagues found a reduction of HbA1c among veterans with T2DM using CGMs. However, > 50% of the patients in the study were not receiving insulin therapy, which currently is a US Department of Veterans Affairs (VA) CGM criteria for use.7 While current studies provide evidence that supports improvement in HbA1c levels with the use of CGMs, data are lacking for veterans with T2DM taking insulin. There is also minimal research that indicates which patients should be offered a CGM. The objective of this study was to evaluate glycemic control in veterans with T2DM on insulin using a CGM who were previously monitoring blood glucose with fingerstick testing. Secondary endpoints were explored to identify subgroups that may benefit from a CGM and other potential advantages of CGMs.
Methods
This was a retrospective study of veterans who transitioned from fingerstick testing to CGM for glucose monitoring. Each veteran served as their own control to limit confounding variables when comparing HbA1c levels. Veterans with an active or suspended CGM order were identified by reviewing outpatient prescription data. All data collection and analysis were done within the Veterans Affairs Sioux Falls Health Care System.
The primary objective of this study was to assess glycemic control from the use of a CGM by evaluating the change in HbA1c after transitioning to a CGM compared to the change in HbA1c with standard fingerstick monitoring. Three HbA1c values were collected for each veteran: before starting CGM, at initiation, and following CGM initiation (Figure 1). CGM start date was the date the CGM prescription order was placed. The pre-CGM HbA1c level was ≥ 1 year prior to the CGM start date or the HbA1c closest to 1 year. The start CGM HbA1c level was within 3 months before or 1 month after the CGM start date. The post-CGM HbA1c level was the most recent time of data collection and at least 6 months after CGM initiation. The change in HbA1c from fingerstick glucose monitoring was the difference between the pre-CGM and start CGM values. The change in HbA1c from use of a CGM was the difference between start CGM and post-CGM values, which were compared to determine HbA1c reduction from CGM use.

This study also explored secondary outcomes including changes in HbA1c by prescriber type, differences in HbA1c reduction based on age, and changes in diabetes medications, including total daily insulin doses. For secondary outcomes, diabetes medication information and the total daily dose of insulin were gathered at the start of CGM use and at the time of data collection. The most recent CGM order prescribed was also collected.
Veterans were included if they were aged ≥ 18 years, had an active order for a CGM, T2DM diagnosis, an insulin prescription, and previously used test strips for glucose monitoring. Patients with T1DM, those who accessed CGMs or care in the community, and patients without HbA1c values pre-CGM, were excluded.
Statistical Analysis
The primary endpoint of change in HbA1c level before and after CGM use was compared using a paired t test. A 0.5% change in HbA1c was considered clinically significant, as suggested in other studies.8,9 P < .05 was considered statistically significant. Analysis for continuous baseline characteristics, including age and total daily insulin, were reported as mean values. Nominal characteristics including sex, race, diabetes medications, and prescriber type are reported as percentages.
Results
A total of 402 veterans were identified with an active CGM at the time of initial data collection in January 2024 and 175 met inclusion criteria. Sixty patients were excluded due to diabetes managed through a community HCP, 38 had T1DM, and 129 lacked HbA1c within all specified time periods. The 175 veterans were randomized, and 150 were selected to perform a chart review for data collection. The mean age was 70 years, most were male and identified as White (Table 1). The majority of patients were managed by endocrinology (53.3%), followed by primary care (24.0%), and pharmacy (22.7%) (Table 2). The mean baseline HbA1c was 8.6%.


The difference in HbA1c before and after use of CGM was -0.97% (P = .0001). Prior to use of a CGM the change in HbA1c was minimal, with an increase of 0.003% with the use of selfmonitoring glucose. After use of a CGM, HbA1c decreased by 0.971%. This reduction in HbA1c would also be considered clinically significant as the change was > 0.5%. The mean pre-, at start, and post-CGM HbA1c levels were 8.6%, 8.6%, and 7.6%, respectively (Figure 2). Pharmacy prescribers had a 0.7% reduction in HbA1c post-CGM, the least of all prescribers. While most age groups saw a reduction in HbA1c, those aged ≥ 80 years had an increase of 0.18% (Table 3). There was an overall mean reduction in insulin of 22 units, which was similar between all prescribers.


Discussion
The primary endpoint of difference in change of HbA1c before and after CGM use was found to be statistically and clinically significant, with a nearly 1% reduction in HbA1c, which was similar to the reduction found by Vigersky and colleagues. 5 Across all prescribers, post-CGM HbA1c levels were similar; however, patients with CGM prescribed by pharmacists had the smallest change in HbA1c. VA pharmacists primarily assess veterans taking insulin who have HbA1c levels that are below the goal with the aim of decreasing insulin to reduce the risk of hypoglycemia, which could result in increased HbA1c levels. This may also explain the observed increase in post-CGM HbA1c levels in patients aged ≥ 80 years. Patients under the care of pharmacists also had baseline mean HbA1c levels that were lower than primary care and endocrinology prescribers and were closer to their HbA1c goal at baseline, which likely was reflected in the smaller reduction in post-CGM HbA1c level.
While there was a decrease in HbA1c levels with CGM use, there were also changes to medications during this timeframe that also may have impacted HbA1c levels. The most common diabetes medications started during CGM use were GLP-1 agonists and SGLT2-inhibitors. Additionally, there was a reduction in the total daily dose of insulin in the study population. These results demonstrate the potential benefits of CGMs for prescribers who take advantage of the CGM glucose data available to assist with medication adjustments. Another consideration for differences in changes of HbA1c among prescriber types is the opportunity for more frequent follow- up visits with pharmacy or endocrinology compared with primary care. If veterans are followed more closely, it may be associated with improved HbA1c control. Further research investigating changes in HbA1c levels based on followup frequency may be useful.
Strengths and Limitations
The crossover design was a strength of this study. This design reduced confounding variables by having veterans serve as their own controls. In addition, the collection of multiple secondary outcomes adds to the knowledge base for future studies. This study focused on a unique population of veterans with T2DM who were taking insulin, an area that previously had very little data available to determine the benefits of CGM use.
Although the use of a CGM showed statistical significance in lowering HbA1c, many veterans were started on new diabetes medication during the period of CGM use, which also likely contributed to the reduction in HbA1c and may have confounded the results. The study was limited by its small population size due to time constraints of chart reviews and the limited generalizability of results outside of the VA system. The majority of patients were from a single site, male and identified as White, which may not be reflective of other VA and community health care systems. It was also noted that the time from the initiation of CGM use to the most recent HbA1c level varied from 6 months to several years. Additionally, veterans managed by community-based HCPs with complex diabetes cases were excluded.
Conclusions
This study demonstrated a clinically and statistically significant reduction in HbA1c with the use of a CGM compared to fingerstick monitoring in veterans with T2DM who were being treated with insulin. The change in post-CGM HbA1c levels across prescribers was similar. In the subgroup analysis of change in HbA1c among age groups, there was a lower HbA1c reduction in individuals aged ≥ 80 years. The results from this study support the idea that CGM use may be beneficial for patients who require a reduction in HbA1c by allowing more precise adjustments to medications and optimization of therapy, as well as the potential to reduce insulin requirements, which is especially valuable in the older adult veteran population.
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
- US Department of Veterans Affairs. VA supports veterans who have type 2 diabetes. VA News. Accessed September 30, 2024. https://news.va.gov/107579/va-supports-veterans-who-have-type-2-diabetes/
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140- S157. doi:10.2337/dc23-S009
- ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- Miller E, Gavin JR, Kruger DF, Brunton SA. Continuous glucose monitoring: optimizing diabetes care: executive summary. Clin Diabetes. 2022;40(4):394-398. doi:10.2337/cd22-0043
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. doi:10.2337/dc11-1438
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi:10.1001/JAMA.2021.6530
- Langford SN, Lane M, Karounos D. Continuous blood glucose monitoring outcomes in veterans with type 2 diabetes. Fed Pract. 2021;38(Suppl 4):S14-S17. doi:10.12788/fp.0189
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. doi:10.1007/s11606-013-2595-x.
- Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) steering committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi:10.1373/clinchem.2010.148841
VA Cancer Clinical Trials as a Strategy for Increasing Accrual of Racial and Ethnic Underrepresented Groups
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.

Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).
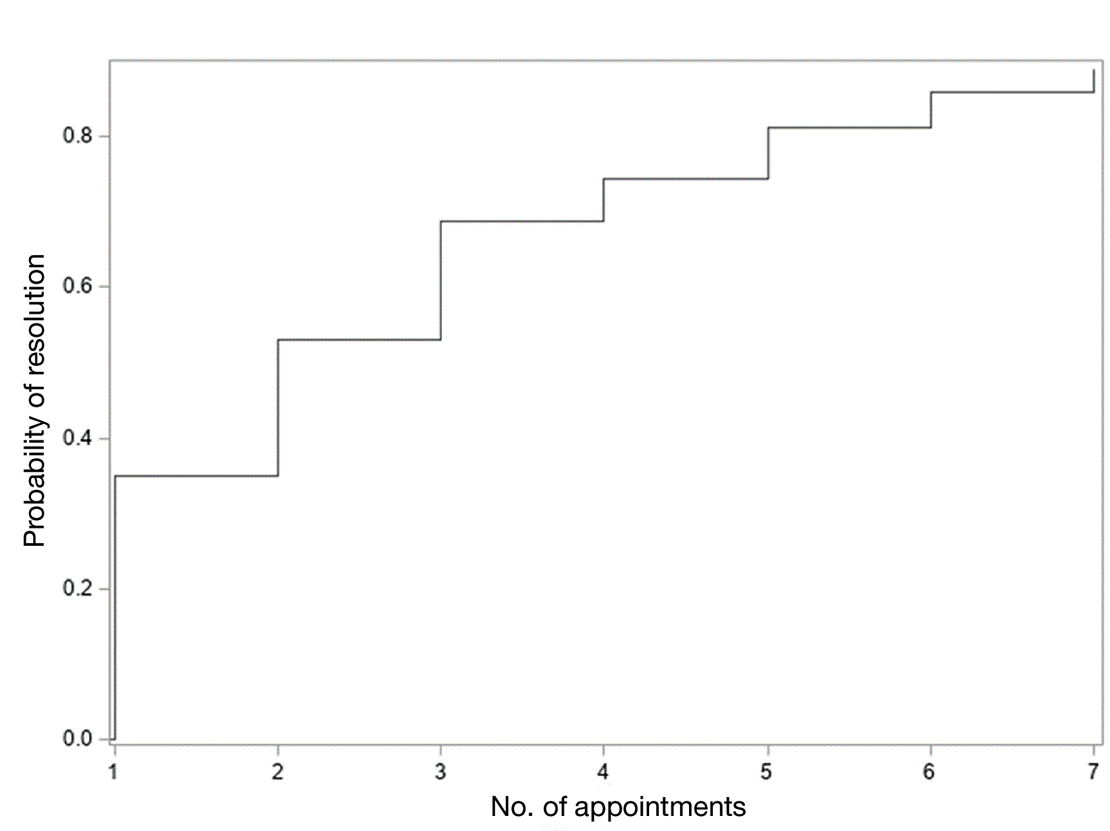
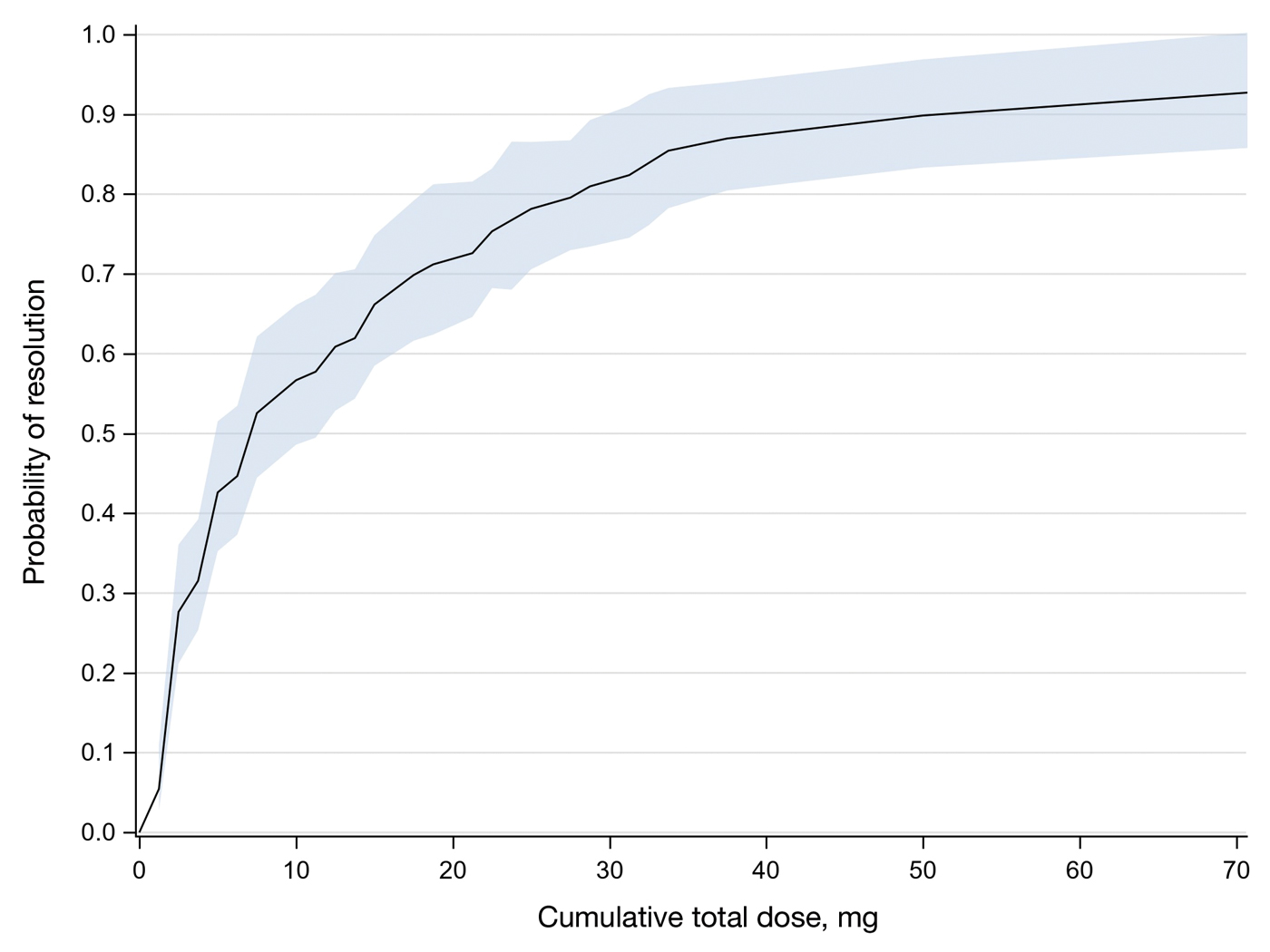
Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
PRACTICE POINTS
- Intralesional methotrexate (IL-MTX) is an efficacious treatment option for cutaneous squamous cell carcinoma lesions in patients who are not good candidates for surgical excision.
- The starting concentration of the initial IL-MTX dose did not substantially impact outcomes; however, a 25 mg/mL concentration is standard for subsequent treatments to maintain efficacy.
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.
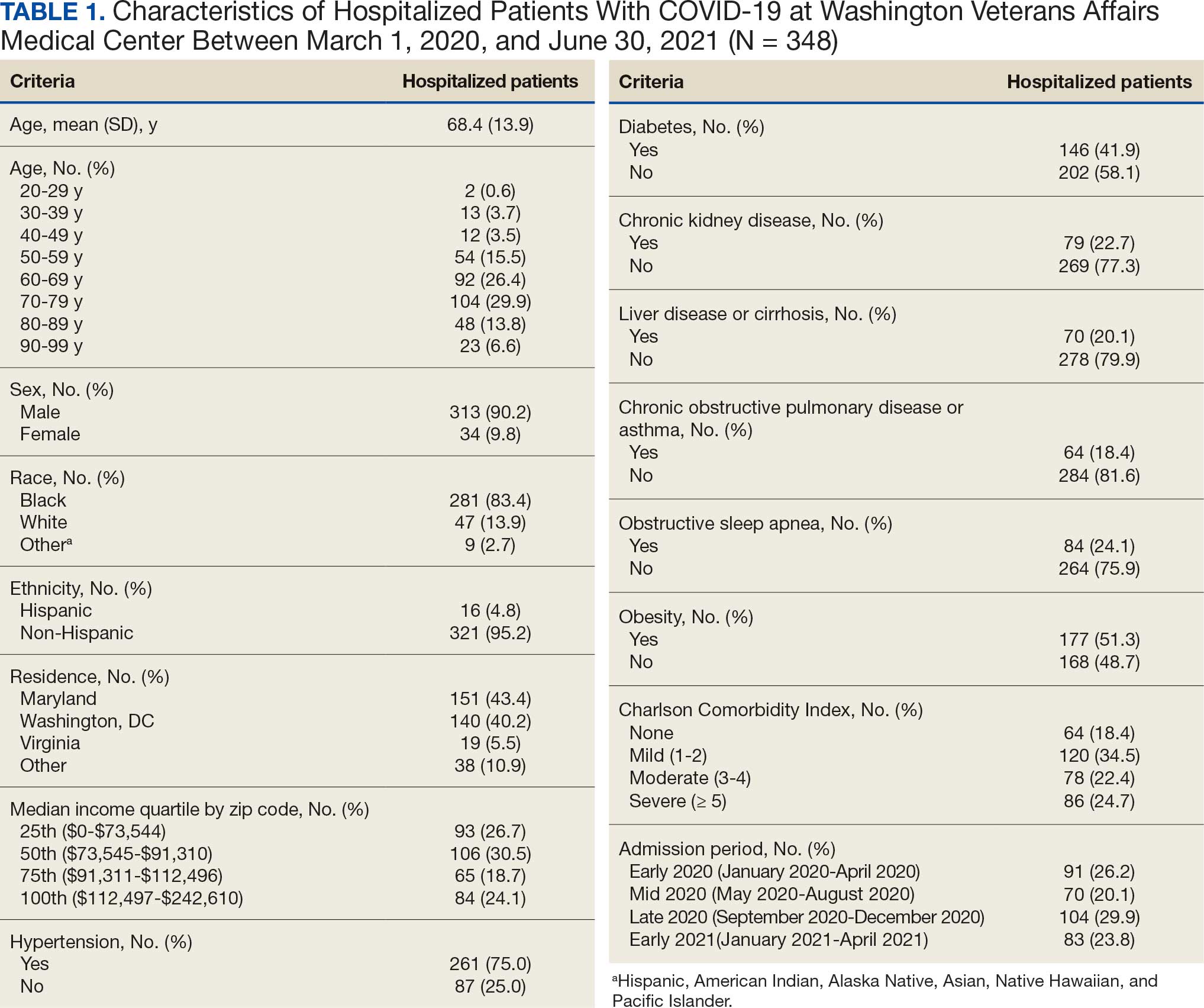
Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).
Code
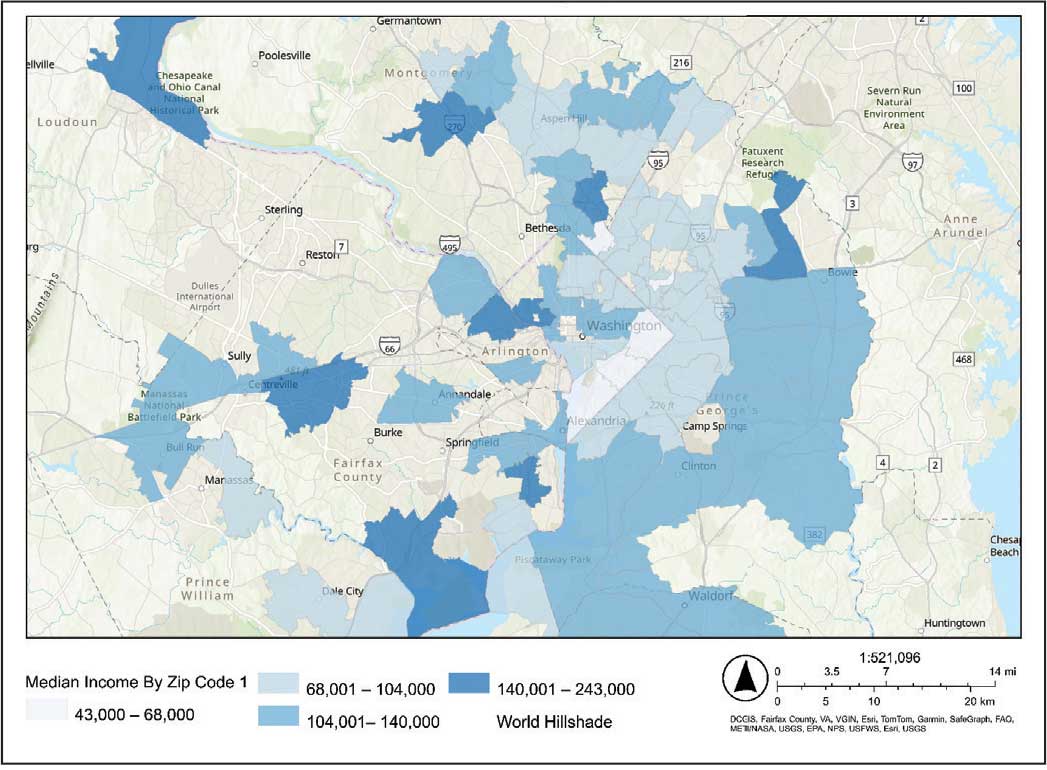
Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.
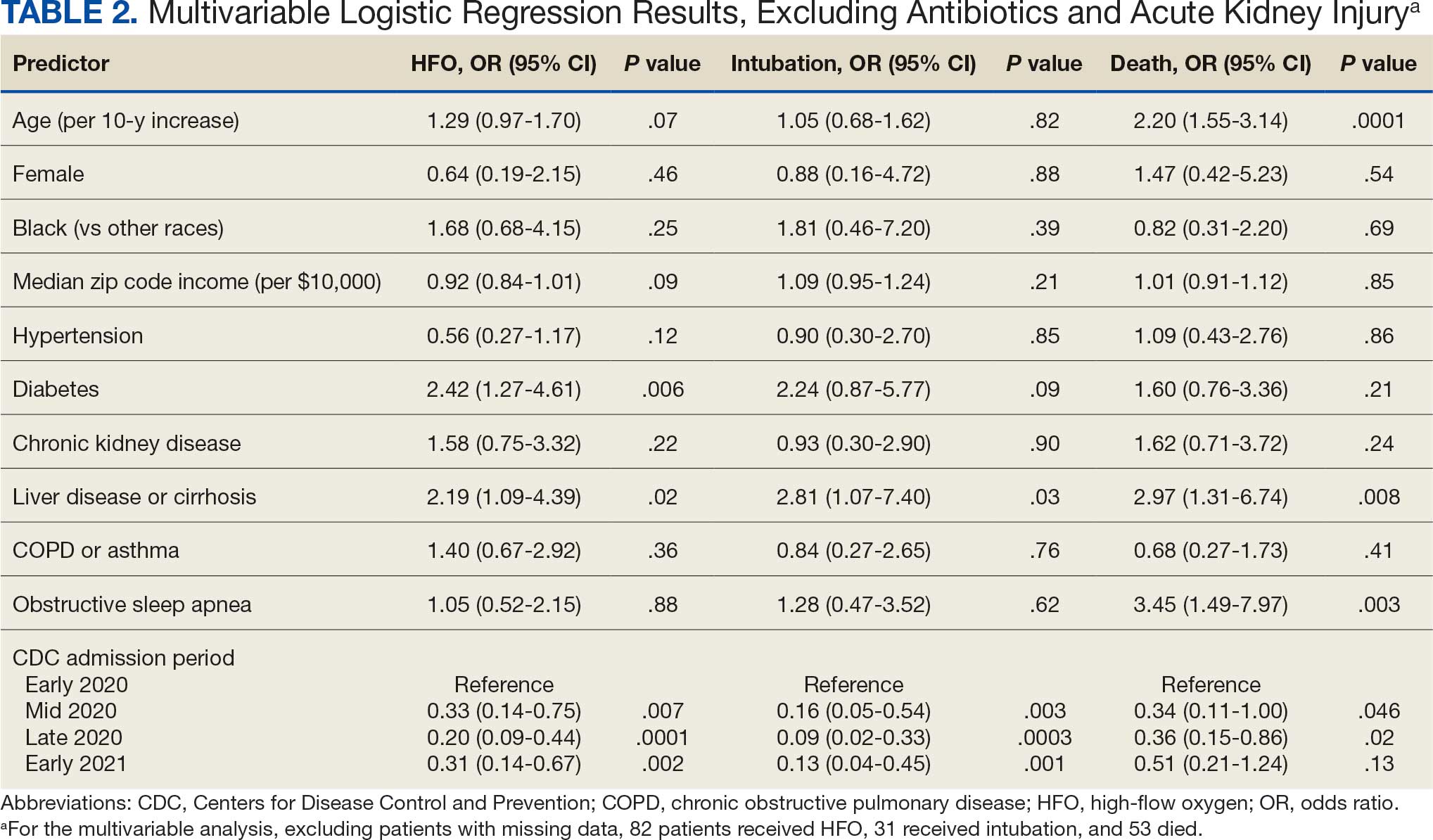
Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.

Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).

Code

Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.

Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.

Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).

Code

Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.

Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).
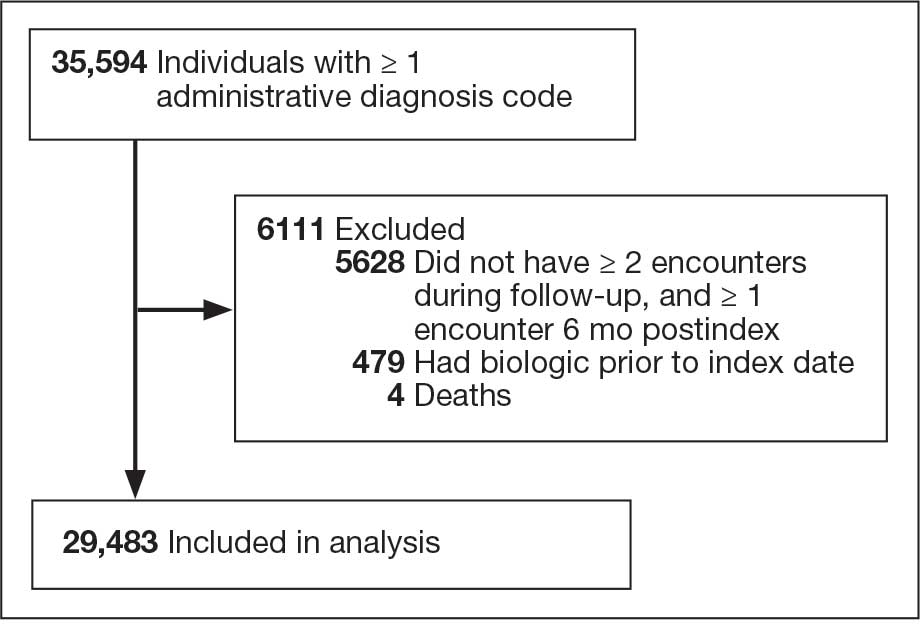
Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7
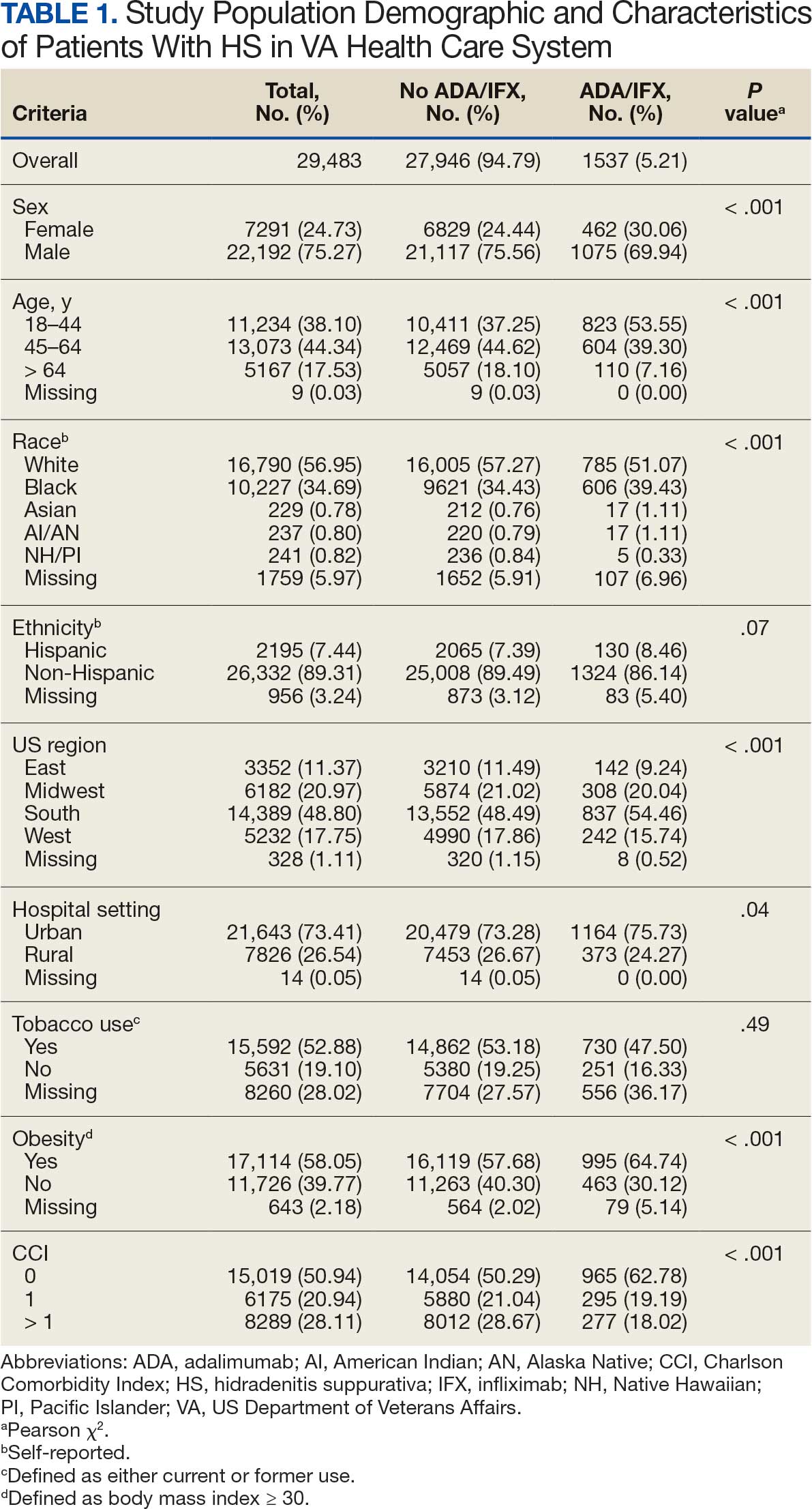
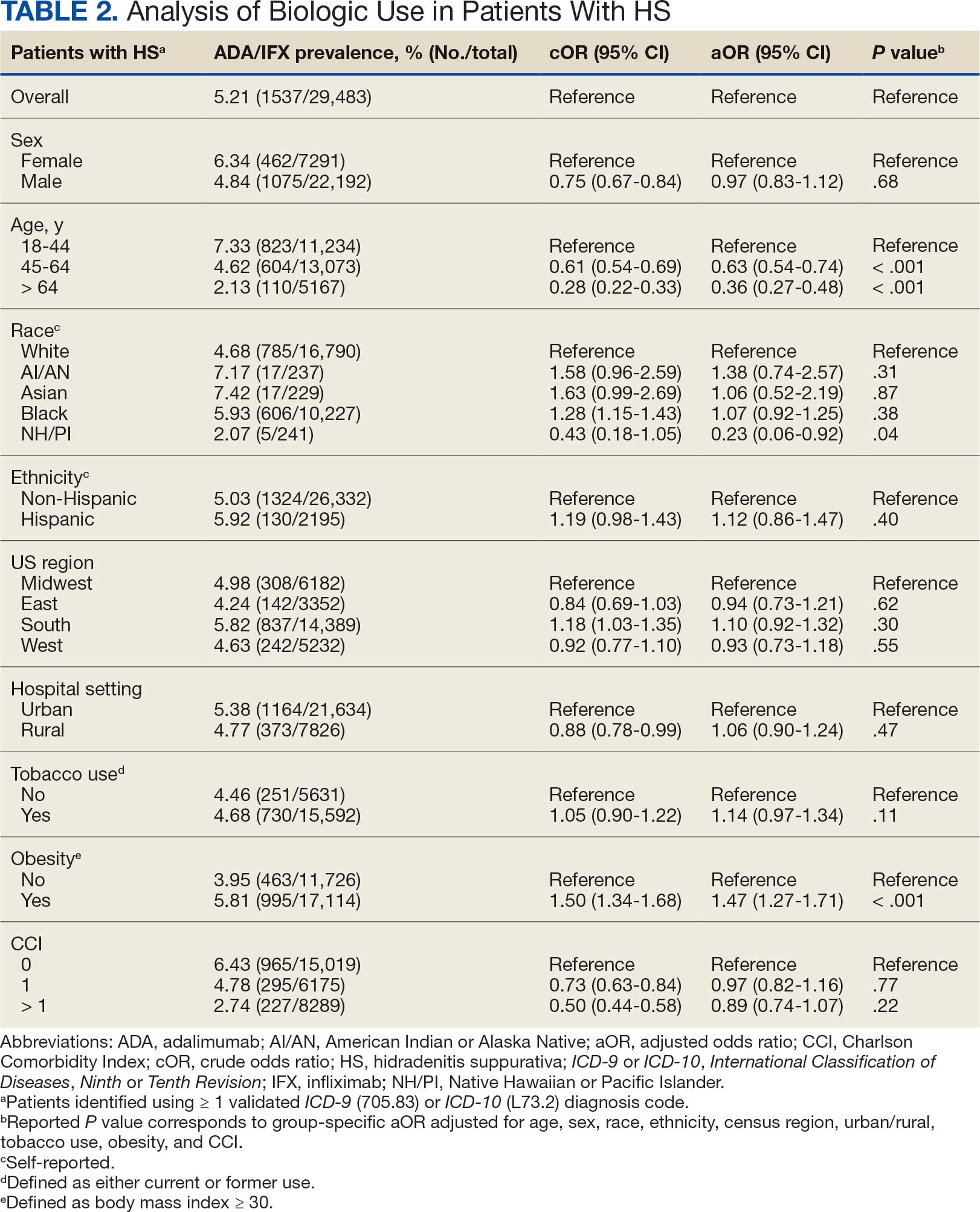
Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2
In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.
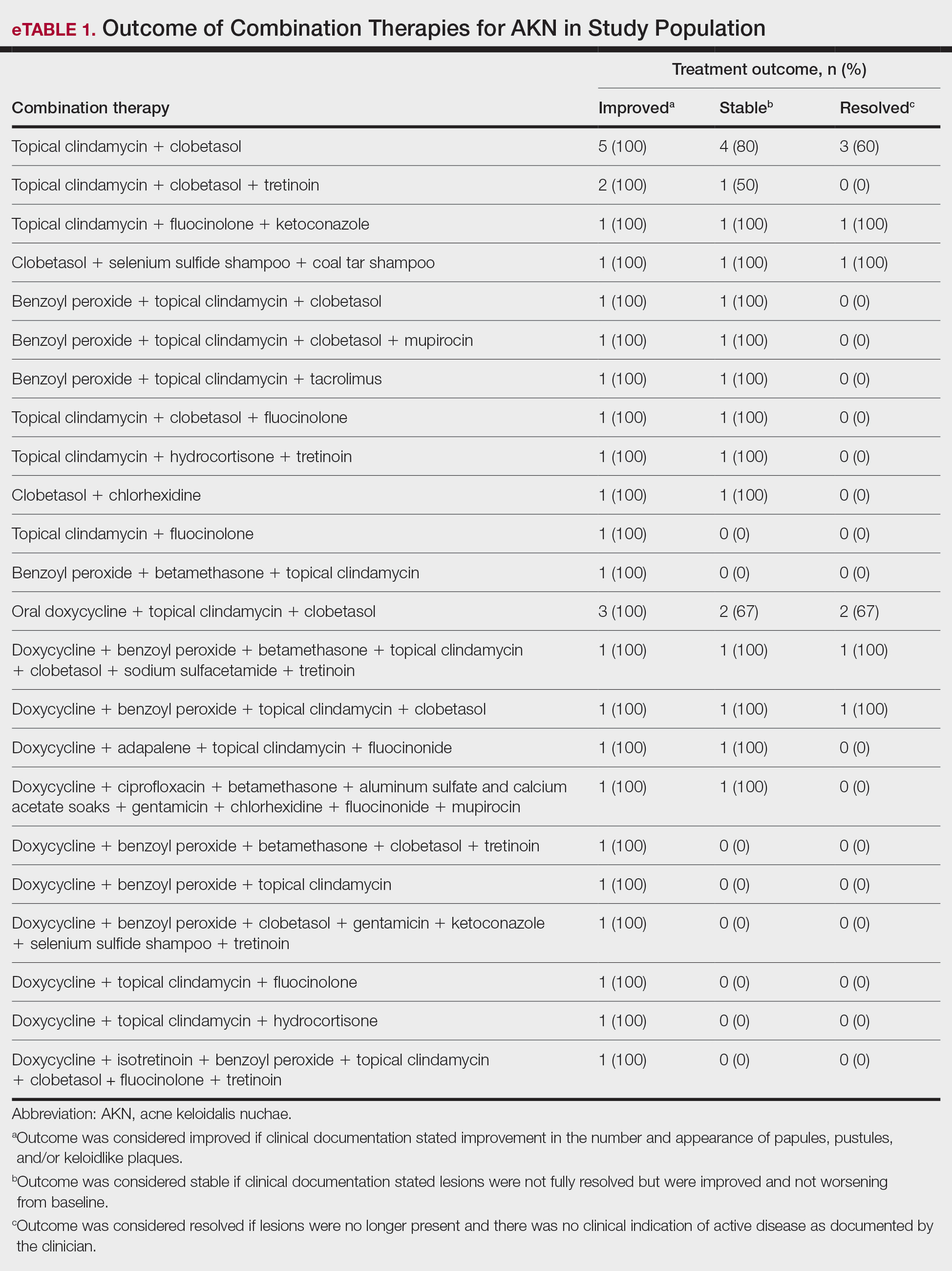
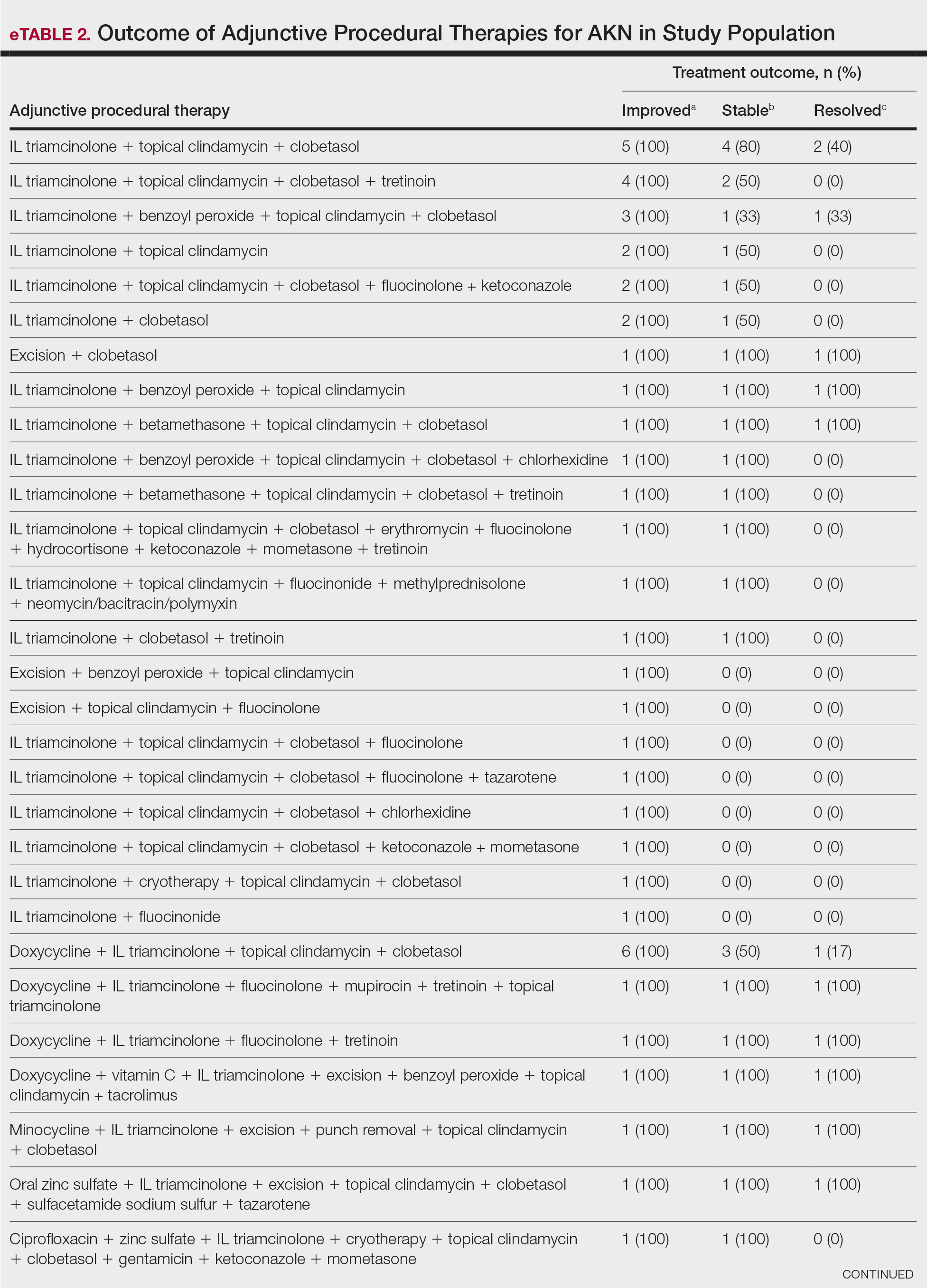
Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2

In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.



Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2

In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.



Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Treatment of Acne Keloidalis Nuchae in a Southern California Population
PRACTICE POINTS
- Acne keloidalis nuchae (AKN) is a rare inflammatory skin disease that manifests with papules, pustules, and plaques on the occipital scalp.
- Initial treatment for patients with mild to moderate AKN disease most commonly is topical clindamycin and clobetasol; patients with moderate to severe AKN disease may require adjunctive treatment with oral doxycycline and/or intralesional triamcinolone.
- Combination therapy that targets the multifactorial pathophysiology of AKN (inflammatory, infectious, and traumatic) is most efficacious overall.
- The majority of patients experience improvement of AKN with treatment, but full resolution is less common.