User login
Essential strategies and tactics for managing sickle cell disease
The group of disorders known as sickle cell disease (SCD) is one of the more common genetic hemoglobinopathies. Homozygous production of the S variant of hemoglobin (Hb) in red blood cells (RBCs) results in profound sickling under conditions of physiologic stress, a condition known as Hb SS disease. People with Hb SS disease are at risk of chronic hemolytic anemia, tissue ischemia that causes vaso-occlusive pain syndrome, and other vaso-occlusive complications.1 They also experience a > 20-year reduction in life expectancy, compared to age-matched controls; onset of risk of early death is usually after age 25 years.
People with heterozygous expression of the Hb S variant—that is, from one parent, and expression of Hb A from the other parent—are said to have sickle cell trait (SCT). They typically do not have symptoms of SCD, although they can experience vaso-occlusive pain under severe physiologic stress and suffer sudden death more often than age-matched controls. People who are heterozygous for Hb S but have another hemoglobinopathy (eg,
Alleviating the harsh burden of illness. All patients with SCD are more likely than age-matched counterparts to experience income loss because of their disability; the same loss is true for their caregivers. Such loss, when combined with time spent in the health care system, can be catastrophic.2,3 But this loss can be mitigated with access to regular, comprehensive health care that includes the steps described here to detect SCD early and reduce the likelihood of complications.4,5
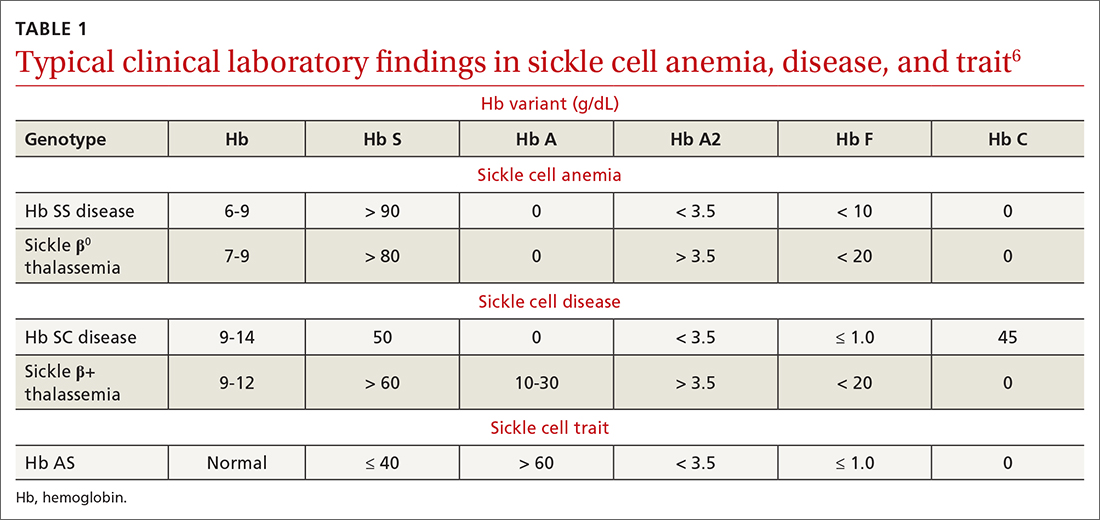
To begin, TABLE 16 lists typical laboratory findings and classifications in patients who are homozygous or heterozygous for Hb S, and therefore experience more severe Hb SS disease or milder SCD, respectively.
Who should be screened for hemoglobinopathy?
Because of the presence of the fetal Hb (Hb F) in newborns and infants, clinical signs of Hb SS before age 2 months are uncommon. Neonatal clinical laboratory testing is necessary for prompt identification of Hb SS; universal screening is now required by all states (although parents can opt out by claiming a religious exemption). A positive test result requires confirmatory testing: most often, Hb electrophoresis or DNA testing.
A confirmed positive homozygous (Hb SS) or heterozygous (Hb S) result is reported to the patient’s identified medical home for subsequent management. Thus, pediatric patients with SCD can be identified, and prophylactic treatment initiated, as early as possible. Later in the patient’s life, repeat screening for SCD and SCT is recommended at the initiation of pregnancy care7 and prior to the start of high-intensity physical training, as occurs in college and professional athletics and in certain branches of the military.8
Putting prevention into practice
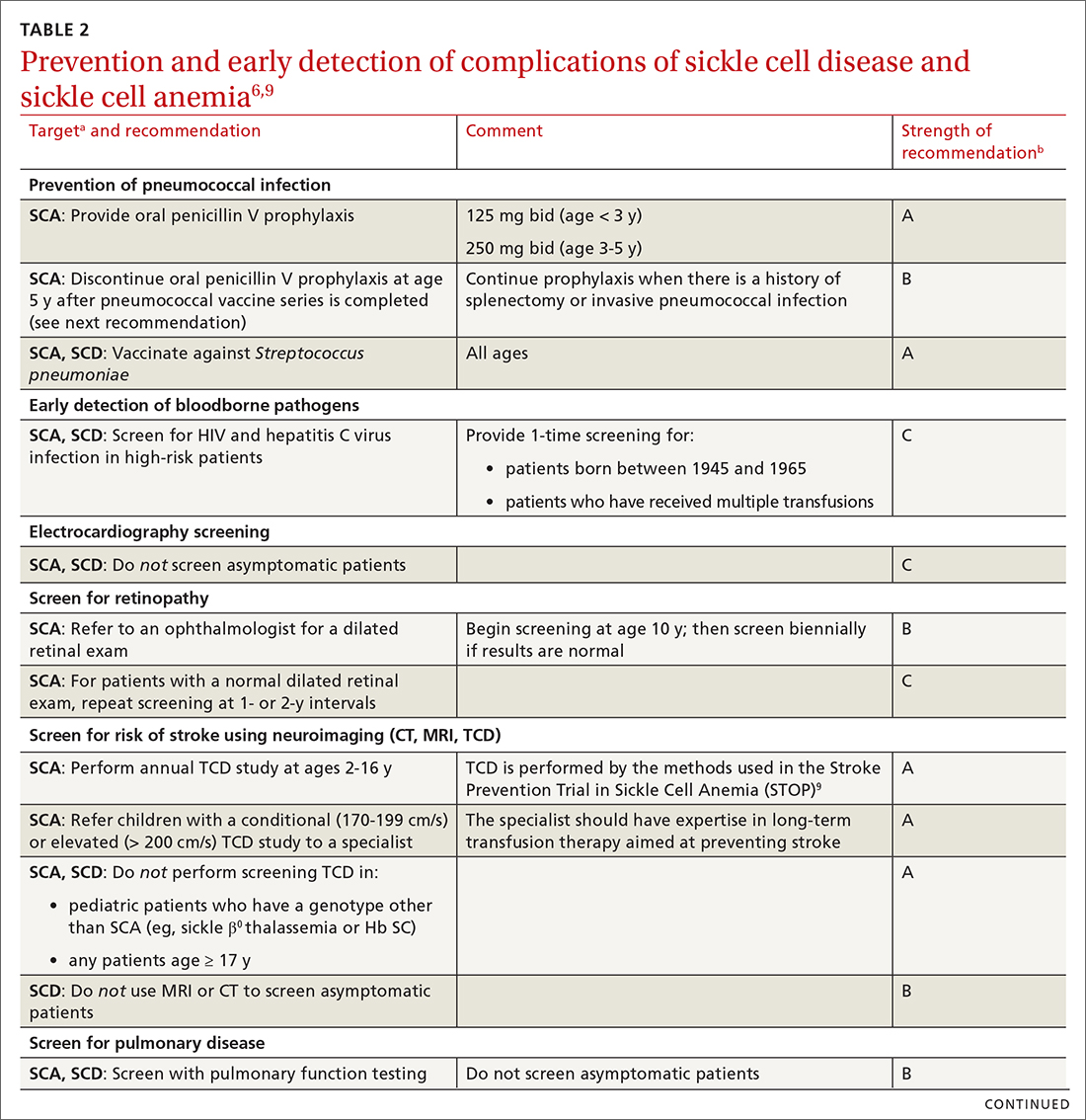
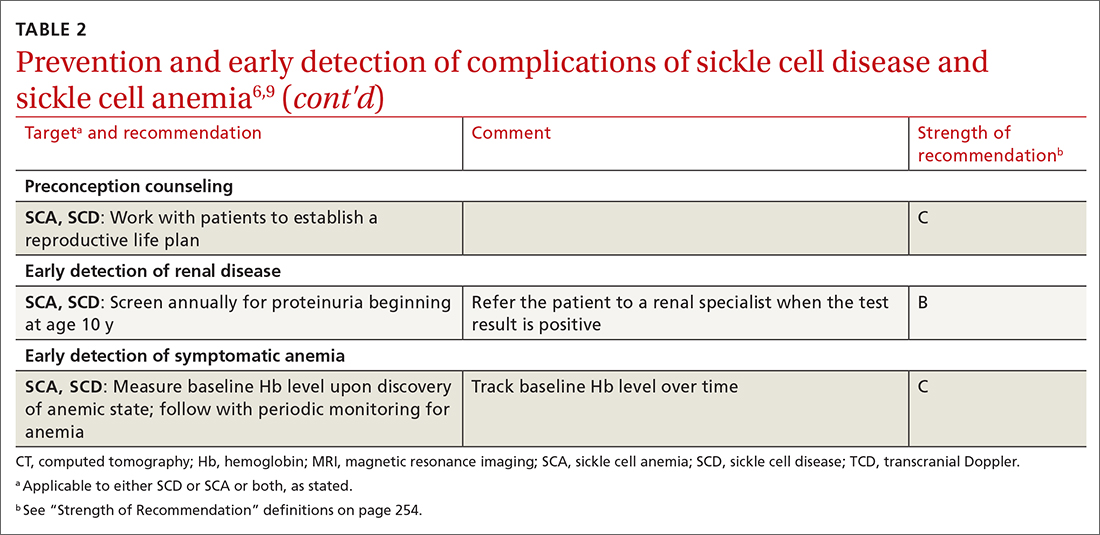
Some of the recommendations we make to prevent complications of SCD are directed only at patients with severe disease—ie, those who have Hb SS SCD or sickle β0 thalassemia (SCA); the rest apply to all patients with SCD (TABLE 26,9). (For patients with SCT, follow guidelines as you would for patients who do not have SCD, unless otherwise noted.)
Continue to: In addition, keep in mind...
In addition, keep in mind that preventive recommendations made by the US Preventive Services Task Force (Exhibit 5 in the Expert Panel Report)6 apply to all patients with SCD and SCT.
Prevention of invasive pneumococcal disease
All patients with SCD are assumed to have lifelong splenic dysfunction that begins in childhood. This is particularly true for those with SCA. In the absence of vaccination, the lifetime incidence of pneumococcal bacteremia resulting in serious complications is as high as 16% in SCD.10 In multiple randomized clinical trials, prophylactic penicillin dosing has proved beneficial in these patients, demonstrating a decrease in the risk of (1) pneumococcal infection and (2) early death during the study period, with minimal adverse effects.5
Prophylactic penicillin dosing should be initiated during infancy in patients with SCA. From ages 3 months to 3 years, the dosage of penicillin V is 125 mg twice daily; from 3 to 5 years, 250 mg twice daily. After age 5 years, the decision to continue penicillin is individualized, with consideration of prior severe pneumococcal infection and general preventive health maintenance. Penicillin-allergic patients can be given erythromycin. All patients with SCD who have had surgical splenectomy should be placed on antibiotic prophylaxis (ie, penicillin as dosed above).5
The polyvalent pneumococcal vaccine has resulted in significant protection against invasive pneumococcal disease; mortality from pneumococcal disease among patients with SCD who are younger than 14 years has decreased dramatically since the vaccine was introduced.6 For all patients with SCD, the standard PCV13 series should be administered beginning at age 6 weeks. A 2-dose series of the PPSV23 vaccine, which includes more Streptococcus pneumoniae serotypes than the PCV13 vaccine, should be administered beginning at age 2 years or 8 weeks after completion of the PCV13 series, whichever comes first.
Prevention of flu, COVID-19, and other vaccine-preventable illness
Influenza. Beginning at age 6 months, all patients with SCD should receive inactivated influenza vaccine annually at the beginning of the influenza season. Avoid using the live attenuated vaccine (Flumist) because of an associated increased risk of severe or complicated infection.11
Continue to: COVID-19, caused by severe...
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is especially problematic in patients with SCD12; infection causes mortality at a rate as high as 7%.5 The SARS-CoV-2 mRNA vaccine series is potentially lifesaving for these patients.12 In addition, at times of high community prevalence, make an effort to minimize patients’ exposure to SARS-CoV-2, by providing telemedicine visits.
Follow the immunization schedule. Patients with SCD should receive all standard recommended vaccinations (ie, those recommended by the Advisory Committee on Immunization Practices.a) Inactivated virus vaccines are preferred in SCD, when available. For patients who are behind on vaccinations, use a standard vaccine catch-up schedule.
Screening and prevention of complications such as stroke
Determining the risk of stroke. Patients with SCA who are not monitored have a 10% to 11% lifetime prevalence of stroke.5,6,10 An abnormal transcranial Doppler (TCD) study (defined as a time-averaged mean maximum velocity ≥ 200 cm/s in the distal internal carotid artery or proximal middle cerebral artery) is predictive of a 40% risk of stroke in patients with SCA. With chronic transfusion therapy, a 92% reduction in the risk of stroke is achievable.10
All patients with SCA should undergo annual screening with TCD ultrasonography from ages 2 to 16 years.6 Those who have an abnormal TCD study should receive chronic transfusion therapy. Screening is not recommended for patients with SCD or SCT.
Other complications. Screen and manage as follows:
- Proteinuria. Left untreated, SCD can affect the kidneys and lead to renal failure. Annual screening for proteinuria is recommended beginning at age 10 years, with referral when the test is positive and reproducible.
- Lung disease and cardiovascular disease. Screening for progression of lung disease and for cardiovascular disease is not recommended in asymptomatic patients with SCD, except through the history.
- Blood pressure screening and management of hypertension are based on Joint National Committee (JNC 8) guidelines.13
- Screening for ocular complications by an eye care provider is recommended beginning at age 10 years.
TABLE 26,9 summarizes recommendations on the prevention and early detection of complications of SCD.
Continue to: Pregnancy planning
Pregnancy planning
The Centers for Disease Control and Prevention recommends that a “reproductive life plan” be part of every person’s health journey (TABLE 2).6,9 The plan is especially relevant for female patients who have a known heritable concern, such as SCD, in which a pregnancy is more likely to be complicated by growth restriction, preterm delivery, and fetal demise. These risks are reduced—but not eliminated—with intensive surveillance of the pregnancy. Pregnancy in patients with SCD is also more likely to be complicated by preeclampsia, venous thromboembolism, infection, and maternal death.
Other recommendations:
- Every patient with SCD should receive genetic counselling before conceiving, when possible.
- Pregnancy should be considered high risk in women who have SCD, and monitored as such.
- Women with SCD can use any method of contraception—none of which put them at increased risk of complications, compared to the general population. Rather, it is pregnancy that puts them at greater risk of morbidity and mortality in every age group.
Ambulatory management of acute complications
Vaso-occlusive pain crisis. The hallmark of SCD is the acute pain crisis. Almost all patients with SCD (and the occasional patient with SCT) will experience a pain crisis. In more affluent countries, management of an acute pain crisis almost always includes opioid analgesia.6
For the most part, pain crises manifest in a predictable pattern. Although patients with SCD might have acute pain, other causes of acute pain, such as an acute intra-abdominal process or (in older patients) a cardiac process, should be considered as well.
For patients having a vaso-occlusive pain crisis, achieving rapid analgesia is key to management. Ready availability of narcotics, at home or under observation, prevents subsequent hospitalization; nonsteroidal anti-inflammatory drugs can be used as adjuvant treatment in patients without contraindications.5,6 An individualized treatment plan, including access to analgesia at an appropriate dosage, should be negotiated, and adhered to, by the patient and the care team.
Continue to: Rapid access to higher-level care...
Rapid access to higher-level care, including parenteral analgesia, is important if outpatient management is desired. In addition, escalation to a higher level of care should occur if there is hypoxia (or another reason to suspect acute chest syndrome [ACS; discussed in a bit]) or dehydration that requires parenteral therapy. Use of nondrug therapy, such as heat, should be encouraged. The care team should work with the patient’s school or employer to negotiate time away through the federal Family Medical Leave Act of 1993, or other means, because a pain crisis is not a planned event.
Fever. Because of the risk of serious infection as a consequence of functional asplenia, fever is particularly worrisome in patients with SCA and problematic in patients with SCD. The increased risk begins as the physiologic level of Hb F declines beginning at age 2 months.
ACS, characterized by a combination of respiratory symptoms or new infiltrates, often manifests initially with fever, and can progress rapidly to death if treatment is delayed. The initial signs and symptoms may be subtle; suspicion should remain high in any patient with respiratory symptoms who is newly hypoxic, even those who do not have fever. Osteomyelitis, another febrile illness, is also potentially life threatening if not treated promptly.
All patients with SCD whose body temperature is > 101.3 °F should be evaluated with appropriate clinical laboratory testing (complete blood count; inflammatory markers, such as C-reactive protein; basic chemistry parameters; and other tests as indicated, including serum lactate and urine culture), blood culture, and chest radiography. Empiric parenteral antibiotics are required until the patient is known to be nonbacteremic, regardless of vaccination status. Outpatient follow-up and even outpatient management with ceftriaxone can be offered in select circumstances (eg, if the patient so desires or is nontoxic, and if close follow-up can be assured).14 If ACS is a possibility based on symptoms or radiographic findings, outpatient management is not an option.
Anemia. Patients with SCA, and some with SCD, have an Hb level that is chronically, sometimes critically, low. A baseline Hb level should be established for a patient with SCD and then monitored periodically. A drop in the Hb level > 2 g/dL from baseline (or an initial Hb level of 6 g/dL if the baseline is unknown) constitutes acute anemia. Patients in whom anemia has been diagnosed should be emergently evaluated for acute splenic sequestration, an aplastic episode, a delayed hemolytic transfusion reaction, ACS, or infection, and should be treated appropriately.
Continue to: Simple transfusion can be used...
Simple transfusion can be used in an acute setting to restore and maintain Hb at a safe level. Iron overload and formation of RBC alloantigen are associated with multiple transfusions; once either of these conditions is established, subsequent transfusion therapy can be harmful. Care must be taken to prescribe transfusion appropriately; leukocyte-depleted RBCs should be used when available.
It is important to define specific goals of transfusion to optimize its use. Patients who have received multiple transfusions should have enhanced monitoring for bloodborne infection, such as hepatitis C virus. Acute aplastic crises are caused by parvovirus B19; when other members of the household who have SCD are present, they should be monitored for this viral infection with serial measurement of Hb and white blood cell count.6
Other acute problems. Should stroke, acute renal failure, priapism, or hepatobiliary complications develop, evaluate the patient rapidly and refer them to the appropriate care team for management.
Management of chronic complications
Chronic pain is a problem for many patients with SCD. The etiology of this symptom should be investigated fully because a vaso-occlusive crisis is characterized by acute pain. Avascular necrosis or ulcers due to chronic vaso-occlusion should be managed definitively when possible. Adjuvant therapy for chronic pain, such as heat or massage, should be encouraged.
In some patients, chronic pain without objective findings develops over time and becomes unresponsive to nonopioid pharmacotherapy. Such patients might require chronic opioid therapy, the need for which is dictated by the ability of the patient to perform their activities of daily living. For patients who require long-term daily narcotic drugs, best practices—obtaining informed consent, using registries and contracts, random drug testing, and providing naloxone [Narcan] for overdose emergency use—should be employed.15
Continue to: Chronic anemia
Chronic anemia can be managed with transfusion when elevating the Hb level is required (eg, preoperatively, to prevent stroke, to manage priapism). For some patients, ongoing transfusion is required; care should be taken to avoid iron overload and hemolysis due to antibody formation. Ongoing surveillance for these complications is required.6
Other chronic problems. Patients with SCD who develop avascular necrosis, vaso-occlusive ulcers, pulmonary hypertension, renal disease, recurrent priapism, or ophthalmologic complications should be co-managed with a care team.6
Pharmacotherapy and SCA
A principal goal in the management of patients with SCA is prevention of vaso-occlusive events, including ACS and acute pain crises.
Hydroxyurea, a key component of SCA treatment, is a ribonucleotide reductase inhibitor that increases the level of Hb F, thus reducing the absolute number of symptomatic vaso-occlusive events and increasing arterial blood flow. It is most useful for patients who have multiple crises. The drug prolongs survival and reduces the need for transfusion and hospitalization.4,5
Hydroxyurea can be started in patients at age 9 months; blood testing should be performed at the start of treatment and the dosage titrated based on blood counts. Initial blood work includes:
- Hb level;
- Hb electrophoresis with the quantitative percentage of Hb F;
- complete blood count with differential and reticulocyte counts;
- chemistry profile (electrolytes, lactate dehydrogenase, total protein, albumin, total bilirubin);
- liver function tests (aspartate aminotransferase, alanine aminotransferase);
- measurement of renal function (blood urea nitrogen, creatinine);
- serum vitamin B12 and folate;
- serum iron, total iron-binding capacity, and ferritin;
- hepatitis B, hepatitis C, and parvovirus B19 antigen; and
- serologic testing for HIV.
Continue to: Testing should also...
Testing should also include a pregnancy test for postmenarchal females because hydroxyurea is in US Food and Drug Administration pregnancy risk category X.
Avoid hydroxyurea in lactating women; dose the drug renally in the setting of renal disease. Because hydroxyurea has a high rate of serious adverse effects and drug-drug interactions, it should be offered in conjunction with an individualized care plan.
Hydroxyurea can also be offered to patients with other forms of SCD who have recurrent vaso-occlusive symptoms.
Two newer medications improve oxygen delivery in patients with SCD. Voxelotor, approved in 2019, works to reduce Hb S polymerization by binding to the alpha chain of Hb S and, subsequently, increasing its oxygen affinity. The drug is generally well tolerated and can be used in patients with SCD who are ≥ 12 years.16 Crizanlizumab is a monoclonal antibody directed against P-selectin, an adhesion molecule located on endothelial cells and activated platelets. The efficacy of crizanlizumab was demonstrated in the SUSTAIN trial, in which it reduced vaso-occlusive pain in patients ≥ 16 years.17
All of these medications have a narrow toxic–therapeutic window. They should therefore be administered with the participation of a multidisciplinary care team.
Continue to: The need to coordinated, comprehensive care
The need for coordinated, comprehensive care
Patients with SCD report how challenging their disease is. All patients with SCD are more likely than age-matched counterparts to experience loss, including workdays for disability, educational potential, workdays for caregivers of affected children, and time spent in the hospital or the emergency department.4,5 These losses, with the concomitant stress associated with chronic illness and the struggle to manage recurrent pain crises and chronic complications, are often overwhelming.
Comprehensive care can, as we have illustrated in this discussion, mitigate these losses. Such care should include extensive education, genetic counseling, infection prevention, pain management, and implementation of evidence-based management guidelines.3,4,6 Patients with SCD report that their illness outlook would be better with
- greater provider knowledge of SCD,
- destigmatization of narcotics for SCD vaso-occlusive pain management,
- optimal coordination among members of the health care team, and
- improved transportation for appointments.
Patients also report that barriers associated with the unique US health care financing system are often insurmountable. As patients with SCD live longer, improved care management should focus on reducing these barriers and enhancing their quality of life.2,18,19
CORRESPONDENCE
Robert Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama College of Medicine, 1601 Center Street, 2N, Mobile, AL 36604; perkins@health. southalabama.edu
1. Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2:e1915374. doi:10.1001/jamanetworkopen.2019.15374
2. Swanson ME, Grosse SD, Kulkarni R. Disability among individuals with sickle cell disease: literature review from a public health perspective. Am J Prev Med. 2011;41(6 suppl 4):S390-S397. doi: 10.1016/j.amepre.2011.09.006
3. Moskowitz JT, Butensky E, Harmatz P, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48:64-71. doi:10.1002/pbc.20792
4. Mehta SR, Afenyi-Annan A, Lottenberg R. Opportunities to improve outcomes in sickle cell disease. Am Fam Phys. 2006;74:303-310.
5. Yawn BP, John-Sowah J. Management of sickle cell disease: recommendations from the 2014 Expert Panel Report. Am Fam Phys. 2015;92:1069-1077.
6. National Heart, Lung, and Blood Institute, National Institutes of Health. Evidence-based management of sickle cell disease. Expert Panel Report, 2014. Accessed June 9, 2022. www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines
7. Committee Opinion No. 690: Carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40. doi: 10.1097/AOG.0000000000001951
8. Jordan LB, Smith-Whitley K, Treadwell MJ, et al. Screening U.S. college athletes for their sickle cell disease carrier status. Am J Prev Med. 2011;41:S406-S412. doi: 10.1016/j.amepre.2011.09.014
9. Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998;19:110-129. doi: 10.1016/s0197-2456(97)00099-8
10. Alzahrani F, Alaidarous K, Alqarni S, et al. Incidence and predictors of bacterial infections in febrile children with sickle cell disease. Int J Pediatr Adolesc Med. 2021;8:236-238 doi: 10.1016/j.ijpam.2020.12.005
11. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2021-2022. Pediatrics. 2021;148: e2021053745. doi: 10.1542/peds.2021-053745
12. Centers for Disease Control and Prevention. Study finds people with sickle cell disease who developed coronavirus disease have high rates of hospitalization, intensive care unit admission, and death. October 20, 2020. Accessed June 9, 2022. www.cdc.gov/ncbddd/sicklecell/features/scd-and-covid-19.html
13. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
14. Baskin MN, Goh XL, Heeney MM, et al. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131:1035-1041. doi: 10.1542/peds.2012-2139
15. Osunkwo I, Veeramreddy P, Arnall J, et al. Use of buprenorphine/naloxone in amelio rating acute care utilization and chronic opioid use in adults with sickle cell disease. Blood. 2019;134 (suppl 1):790. doi: 10.1182/blood-2019-126589
16. Vichinsky E, Hoppe CC, Ataga KI, et al; HOPE Trial Investigators. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509-519. doi: 10.1056/NEJMoa1903212
17. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429-439. doi: 10.1056/NEJMoa1611770
18. Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. 2016;2:CD011130. doi: 10.1002/14651858.CD011130.pub2
19. Brennan-Cook J, Bonnabeau E, Aponte R, et al. Barriers to care for persons with sickle cell disease: the case manager’s opportunity to improve patient outcomes. Prof Case Manag. 2018;23:213-219. doi: 10.1097/NCM.0000000000000260
The group of disorders known as sickle cell disease (SCD) is one of the more common genetic hemoglobinopathies. Homozygous production of the S variant of hemoglobin (Hb) in red blood cells (RBCs) results in profound sickling under conditions of physiologic stress, a condition known as Hb SS disease. People with Hb SS disease are at risk of chronic hemolytic anemia, tissue ischemia that causes vaso-occlusive pain syndrome, and other vaso-occlusive complications.1 They also experience a > 20-year reduction in life expectancy, compared to age-matched controls; onset of risk of early death is usually after age 25 years.
People with heterozygous expression of the Hb S variant—that is, from one parent, and expression of Hb A from the other parent—are said to have sickle cell trait (SCT). They typically do not have symptoms of SCD, although they can experience vaso-occlusive pain under severe physiologic stress and suffer sudden death more often than age-matched controls. People who are heterozygous for Hb S but have another hemoglobinopathy (eg,
Alleviating the harsh burden of illness. All patients with SCD are more likely than age-matched counterparts to experience income loss because of their disability; the same loss is true for their caregivers. Such loss, when combined with time spent in the health care system, can be catastrophic.2,3 But this loss can be mitigated with access to regular, comprehensive health care that includes the steps described here to detect SCD early and reduce the likelihood of complications.4,5
To begin, TABLE 16 lists typical laboratory findings and classifications in patients who are homozygous or heterozygous for Hb S, and therefore experience more severe Hb SS disease or milder SCD, respectively.

Who should be screened for hemoglobinopathy?
Because of the presence of the fetal Hb (Hb F) in newborns and infants, clinical signs of Hb SS before age 2 months are uncommon. Neonatal clinical laboratory testing is necessary for prompt identification of Hb SS; universal screening is now required by all states (although parents can opt out by claiming a religious exemption). A positive test result requires confirmatory testing: most often, Hb electrophoresis or DNA testing.
A confirmed positive homozygous (Hb SS) or heterozygous (Hb S) result is reported to the patient’s identified medical home for subsequent management. Thus, pediatric patients with SCD can be identified, and prophylactic treatment initiated, as early as possible. Later in the patient’s life, repeat screening for SCD and SCT is recommended at the initiation of pregnancy care7 and prior to the start of high-intensity physical training, as occurs in college and professional athletics and in certain branches of the military.8

Putting prevention into practice
Some of the recommendations we make to prevent complications of SCD are directed only at patients with severe disease—ie, those who have Hb SS SCD or sickle β0 thalassemia (SCA); the rest apply to all patients with SCD (TABLE 26,9). (For patients with SCT, follow guidelines as you would for patients who do not have SCD, unless otherwise noted.)

Continue to: In addition, keep in mind...
In addition, keep in mind that preventive recommendations made by the US Preventive Services Task Force (Exhibit 5 in the Expert Panel Report)6 apply to all patients with SCD and SCT.
Prevention of invasive pneumococcal disease
All patients with SCD are assumed to have lifelong splenic dysfunction that begins in childhood. This is particularly true for those with SCA. In the absence of vaccination, the lifetime incidence of pneumococcal bacteremia resulting in serious complications is as high as 16% in SCD.10 In multiple randomized clinical trials, prophylactic penicillin dosing has proved beneficial in these patients, demonstrating a decrease in the risk of (1) pneumococcal infection and (2) early death during the study period, with minimal adverse effects.5
Prophylactic penicillin dosing should be initiated during infancy in patients with SCA. From ages 3 months to 3 years, the dosage of penicillin V is 125 mg twice daily; from 3 to 5 years, 250 mg twice daily. After age 5 years, the decision to continue penicillin is individualized, with consideration of prior severe pneumococcal infection and general preventive health maintenance. Penicillin-allergic patients can be given erythromycin. All patients with SCD who have had surgical splenectomy should be placed on antibiotic prophylaxis (ie, penicillin as dosed above).5
The polyvalent pneumococcal vaccine has resulted in significant protection against invasive pneumococcal disease; mortality from pneumococcal disease among patients with SCD who are younger than 14 years has decreased dramatically since the vaccine was introduced.6 For all patients with SCD, the standard PCV13 series should be administered beginning at age 6 weeks. A 2-dose series of the PPSV23 vaccine, which includes more Streptococcus pneumoniae serotypes than the PCV13 vaccine, should be administered beginning at age 2 years or 8 weeks after completion of the PCV13 series, whichever comes first.
Prevention of flu, COVID-19, and other vaccine-preventable illness
Influenza. Beginning at age 6 months, all patients with SCD should receive inactivated influenza vaccine annually at the beginning of the influenza season. Avoid using the live attenuated vaccine (Flumist) because of an associated increased risk of severe or complicated infection.11
Continue to: COVID-19, caused by severe...
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is especially problematic in patients with SCD12; infection causes mortality at a rate as high as 7%.5 The SARS-CoV-2 mRNA vaccine series is potentially lifesaving for these patients.12 In addition, at times of high community prevalence, make an effort to minimize patients’ exposure to SARS-CoV-2, by providing telemedicine visits.
Follow the immunization schedule. Patients with SCD should receive all standard recommended vaccinations (ie, those recommended by the Advisory Committee on Immunization Practices.a) Inactivated virus vaccines are preferred in SCD, when available. For patients who are behind on vaccinations, use a standard vaccine catch-up schedule.
Screening and prevention of complications such as stroke
Determining the risk of stroke. Patients with SCA who are not monitored have a 10% to 11% lifetime prevalence of stroke.5,6,10 An abnormal transcranial Doppler (TCD) study (defined as a time-averaged mean maximum velocity ≥ 200 cm/s in the distal internal carotid artery or proximal middle cerebral artery) is predictive of a 40% risk of stroke in patients with SCA. With chronic transfusion therapy, a 92% reduction in the risk of stroke is achievable.10
All patients with SCA should undergo annual screening with TCD ultrasonography from ages 2 to 16 years.6 Those who have an abnormal TCD study should receive chronic transfusion therapy. Screening is not recommended for patients with SCD or SCT.
Other complications. Screen and manage as follows:
- Proteinuria. Left untreated, SCD can affect the kidneys and lead to renal failure. Annual screening for proteinuria is recommended beginning at age 10 years, with referral when the test is positive and reproducible.
- Lung disease and cardiovascular disease. Screening for progression of lung disease and for cardiovascular disease is not recommended in asymptomatic patients with SCD, except through the history.
- Blood pressure screening and management of hypertension are based on Joint National Committee (JNC 8) guidelines.13
- Screening for ocular complications by an eye care provider is recommended beginning at age 10 years.
TABLE 26,9 summarizes recommendations on the prevention and early detection of complications of SCD.
Continue to: Pregnancy planning
Pregnancy planning
The Centers for Disease Control and Prevention recommends that a “reproductive life plan” be part of every person’s health journey (TABLE 2).6,9 The plan is especially relevant for female patients who have a known heritable concern, such as SCD, in which a pregnancy is more likely to be complicated by growth restriction, preterm delivery, and fetal demise. These risks are reduced—but not eliminated—with intensive surveillance of the pregnancy. Pregnancy in patients with SCD is also more likely to be complicated by preeclampsia, venous thromboembolism, infection, and maternal death.
Other recommendations:
- Every patient with SCD should receive genetic counselling before conceiving, when possible.
- Pregnancy should be considered high risk in women who have SCD, and monitored as such.
- Women with SCD can use any method of contraception—none of which put them at increased risk of complications, compared to the general population. Rather, it is pregnancy that puts them at greater risk of morbidity and mortality in every age group.
Ambulatory management of acute complications
Vaso-occlusive pain crisis. The hallmark of SCD is the acute pain crisis. Almost all patients with SCD (and the occasional patient with SCT) will experience a pain crisis. In more affluent countries, management of an acute pain crisis almost always includes opioid analgesia.6
For the most part, pain crises manifest in a predictable pattern. Although patients with SCD might have acute pain, other causes of acute pain, such as an acute intra-abdominal process or (in older patients) a cardiac process, should be considered as well.
For patients having a vaso-occlusive pain crisis, achieving rapid analgesia is key to management. Ready availability of narcotics, at home or under observation, prevents subsequent hospitalization; nonsteroidal anti-inflammatory drugs can be used as adjuvant treatment in patients without contraindications.5,6 An individualized treatment plan, including access to analgesia at an appropriate dosage, should be negotiated, and adhered to, by the patient and the care team.
Continue to: Rapid access to higher-level care...
Rapid access to higher-level care, including parenteral analgesia, is important if outpatient management is desired. In addition, escalation to a higher level of care should occur if there is hypoxia (or another reason to suspect acute chest syndrome [ACS; discussed in a bit]) or dehydration that requires parenteral therapy. Use of nondrug therapy, such as heat, should be encouraged. The care team should work with the patient’s school or employer to negotiate time away through the federal Family Medical Leave Act of 1993, or other means, because a pain crisis is not a planned event.
Fever. Because of the risk of serious infection as a consequence of functional asplenia, fever is particularly worrisome in patients with SCA and problematic in patients with SCD. The increased risk begins as the physiologic level of Hb F declines beginning at age 2 months.
ACS, characterized by a combination of respiratory symptoms or new infiltrates, often manifests initially with fever, and can progress rapidly to death if treatment is delayed. The initial signs and symptoms may be subtle; suspicion should remain high in any patient with respiratory symptoms who is newly hypoxic, even those who do not have fever. Osteomyelitis, another febrile illness, is also potentially life threatening if not treated promptly.
All patients with SCD whose body temperature is > 101.3 °F should be evaluated with appropriate clinical laboratory testing (complete blood count; inflammatory markers, such as C-reactive protein; basic chemistry parameters; and other tests as indicated, including serum lactate and urine culture), blood culture, and chest radiography. Empiric parenteral antibiotics are required until the patient is known to be nonbacteremic, regardless of vaccination status. Outpatient follow-up and even outpatient management with ceftriaxone can be offered in select circumstances (eg, if the patient so desires or is nontoxic, and if close follow-up can be assured).14 If ACS is a possibility based on symptoms or radiographic findings, outpatient management is not an option.
Anemia. Patients with SCA, and some with SCD, have an Hb level that is chronically, sometimes critically, low. A baseline Hb level should be established for a patient with SCD and then monitored periodically. A drop in the Hb level > 2 g/dL from baseline (or an initial Hb level of 6 g/dL if the baseline is unknown) constitutes acute anemia. Patients in whom anemia has been diagnosed should be emergently evaluated for acute splenic sequestration, an aplastic episode, a delayed hemolytic transfusion reaction, ACS, or infection, and should be treated appropriately.
Continue to: Simple transfusion can be used...
Simple transfusion can be used in an acute setting to restore and maintain Hb at a safe level. Iron overload and formation of RBC alloantigen are associated with multiple transfusions; once either of these conditions is established, subsequent transfusion therapy can be harmful. Care must be taken to prescribe transfusion appropriately; leukocyte-depleted RBCs should be used when available.
It is important to define specific goals of transfusion to optimize its use. Patients who have received multiple transfusions should have enhanced monitoring for bloodborne infection, such as hepatitis C virus. Acute aplastic crises are caused by parvovirus B19; when other members of the household who have SCD are present, they should be monitored for this viral infection with serial measurement of Hb and white blood cell count.6
Other acute problems. Should stroke, acute renal failure, priapism, or hepatobiliary complications develop, evaluate the patient rapidly and refer them to the appropriate care team for management.
Management of chronic complications
Chronic pain is a problem for many patients with SCD. The etiology of this symptom should be investigated fully because a vaso-occlusive crisis is characterized by acute pain. Avascular necrosis or ulcers due to chronic vaso-occlusion should be managed definitively when possible. Adjuvant therapy for chronic pain, such as heat or massage, should be encouraged.
In some patients, chronic pain without objective findings develops over time and becomes unresponsive to nonopioid pharmacotherapy. Such patients might require chronic opioid therapy, the need for which is dictated by the ability of the patient to perform their activities of daily living. For patients who require long-term daily narcotic drugs, best practices—obtaining informed consent, using registries and contracts, random drug testing, and providing naloxone [Narcan] for overdose emergency use—should be employed.15
Continue to: Chronic anemia
Chronic anemia can be managed with transfusion when elevating the Hb level is required (eg, preoperatively, to prevent stroke, to manage priapism). For some patients, ongoing transfusion is required; care should be taken to avoid iron overload and hemolysis due to antibody formation. Ongoing surveillance for these complications is required.6
Other chronic problems. Patients with SCD who develop avascular necrosis, vaso-occlusive ulcers, pulmonary hypertension, renal disease, recurrent priapism, or ophthalmologic complications should be co-managed with a care team.6
Pharmacotherapy and SCA
A principal goal in the management of patients with SCA is prevention of vaso-occlusive events, including ACS and acute pain crises.
Hydroxyurea, a key component of SCA treatment, is a ribonucleotide reductase inhibitor that increases the level of Hb F, thus reducing the absolute number of symptomatic vaso-occlusive events and increasing arterial blood flow. It is most useful for patients who have multiple crises. The drug prolongs survival and reduces the need for transfusion and hospitalization.4,5
Hydroxyurea can be started in patients at age 9 months; blood testing should be performed at the start of treatment and the dosage titrated based on blood counts. Initial blood work includes:
- Hb level;
- Hb electrophoresis with the quantitative percentage of Hb F;
- complete blood count with differential and reticulocyte counts;
- chemistry profile (electrolytes, lactate dehydrogenase, total protein, albumin, total bilirubin);
- liver function tests (aspartate aminotransferase, alanine aminotransferase);
- measurement of renal function (blood urea nitrogen, creatinine);
- serum vitamin B12 and folate;
- serum iron, total iron-binding capacity, and ferritin;
- hepatitis B, hepatitis C, and parvovirus B19 antigen; and
- serologic testing for HIV.
Continue to: Testing should also...
Testing should also include a pregnancy test for postmenarchal females because hydroxyurea is in US Food and Drug Administration pregnancy risk category X.
Avoid hydroxyurea in lactating women; dose the drug renally in the setting of renal disease. Because hydroxyurea has a high rate of serious adverse effects and drug-drug interactions, it should be offered in conjunction with an individualized care plan.
Hydroxyurea can also be offered to patients with other forms of SCD who have recurrent vaso-occlusive symptoms.
Two newer medications improve oxygen delivery in patients with SCD. Voxelotor, approved in 2019, works to reduce Hb S polymerization by binding to the alpha chain of Hb S and, subsequently, increasing its oxygen affinity. The drug is generally well tolerated and can be used in patients with SCD who are ≥ 12 years.16 Crizanlizumab is a monoclonal antibody directed against P-selectin, an adhesion molecule located on endothelial cells and activated platelets. The efficacy of crizanlizumab was demonstrated in the SUSTAIN trial, in which it reduced vaso-occlusive pain in patients ≥ 16 years.17
All of these medications have a narrow toxic–therapeutic window. They should therefore be administered with the participation of a multidisciplinary care team.
Continue to: The need to coordinated, comprehensive care
The need for coordinated, comprehensive care
Patients with SCD report how challenging their disease is. All patients with SCD are more likely than age-matched counterparts to experience loss, including workdays for disability, educational potential, workdays for caregivers of affected children, and time spent in the hospital or the emergency department.4,5 These losses, with the concomitant stress associated with chronic illness and the struggle to manage recurrent pain crises and chronic complications, are often overwhelming.
Comprehensive care can, as we have illustrated in this discussion, mitigate these losses. Such care should include extensive education, genetic counseling, infection prevention, pain management, and implementation of evidence-based management guidelines.3,4,6 Patients with SCD report that their illness outlook would be better with
- greater provider knowledge of SCD,
- destigmatization of narcotics for SCD vaso-occlusive pain management,
- optimal coordination among members of the health care team, and
- improved transportation for appointments.
Patients also report that barriers associated with the unique US health care financing system are often insurmountable. As patients with SCD live longer, improved care management should focus on reducing these barriers and enhancing their quality of life.2,18,19
CORRESPONDENCE
Robert Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama College of Medicine, 1601 Center Street, 2N, Mobile, AL 36604; perkins@health. southalabama.edu
The group of disorders known as sickle cell disease (SCD) is one of the more common genetic hemoglobinopathies. Homozygous production of the S variant of hemoglobin (Hb) in red blood cells (RBCs) results in profound sickling under conditions of physiologic stress, a condition known as Hb SS disease. People with Hb SS disease are at risk of chronic hemolytic anemia, tissue ischemia that causes vaso-occlusive pain syndrome, and other vaso-occlusive complications.1 They also experience a > 20-year reduction in life expectancy, compared to age-matched controls; onset of risk of early death is usually after age 25 years.
People with heterozygous expression of the Hb S variant—that is, from one parent, and expression of Hb A from the other parent—are said to have sickle cell trait (SCT). They typically do not have symptoms of SCD, although they can experience vaso-occlusive pain under severe physiologic stress and suffer sudden death more often than age-matched controls. People who are heterozygous for Hb S but have another hemoglobinopathy (eg,
Alleviating the harsh burden of illness. All patients with SCD are more likely than age-matched counterparts to experience income loss because of their disability; the same loss is true for their caregivers. Such loss, when combined with time spent in the health care system, can be catastrophic.2,3 But this loss can be mitigated with access to regular, comprehensive health care that includes the steps described here to detect SCD early and reduce the likelihood of complications.4,5
To begin, TABLE 16 lists typical laboratory findings and classifications in patients who are homozygous or heterozygous for Hb S, and therefore experience more severe Hb SS disease or milder SCD, respectively.

Who should be screened for hemoglobinopathy?
Because of the presence of the fetal Hb (Hb F) in newborns and infants, clinical signs of Hb SS before age 2 months are uncommon. Neonatal clinical laboratory testing is necessary for prompt identification of Hb SS; universal screening is now required by all states (although parents can opt out by claiming a religious exemption). A positive test result requires confirmatory testing: most often, Hb electrophoresis or DNA testing.
A confirmed positive homozygous (Hb SS) or heterozygous (Hb S) result is reported to the patient’s identified medical home for subsequent management. Thus, pediatric patients with SCD can be identified, and prophylactic treatment initiated, as early as possible. Later in the patient’s life, repeat screening for SCD and SCT is recommended at the initiation of pregnancy care7 and prior to the start of high-intensity physical training, as occurs in college and professional athletics and in certain branches of the military.8

Putting prevention into practice
Some of the recommendations we make to prevent complications of SCD are directed only at patients with severe disease—ie, those who have Hb SS SCD or sickle β0 thalassemia (SCA); the rest apply to all patients with SCD (TABLE 26,9). (For patients with SCT, follow guidelines as you would for patients who do not have SCD, unless otherwise noted.)

Continue to: In addition, keep in mind...
In addition, keep in mind that preventive recommendations made by the US Preventive Services Task Force (Exhibit 5 in the Expert Panel Report)6 apply to all patients with SCD and SCT.
Prevention of invasive pneumococcal disease
All patients with SCD are assumed to have lifelong splenic dysfunction that begins in childhood. This is particularly true for those with SCA. In the absence of vaccination, the lifetime incidence of pneumococcal bacteremia resulting in serious complications is as high as 16% in SCD.10 In multiple randomized clinical trials, prophylactic penicillin dosing has proved beneficial in these patients, demonstrating a decrease in the risk of (1) pneumococcal infection and (2) early death during the study period, with minimal adverse effects.5
Prophylactic penicillin dosing should be initiated during infancy in patients with SCA. From ages 3 months to 3 years, the dosage of penicillin V is 125 mg twice daily; from 3 to 5 years, 250 mg twice daily. After age 5 years, the decision to continue penicillin is individualized, with consideration of prior severe pneumococcal infection and general preventive health maintenance. Penicillin-allergic patients can be given erythromycin. All patients with SCD who have had surgical splenectomy should be placed on antibiotic prophylaxis (ie, penicillin as dosed above).5
The polyvalent pneumococcal vaccine has resulted in significant protection against invasive pneumococcal disease; mortality from pneumococcal disease among patients with SCD who are younger than 14 years has decreased dramatically since the vaccine was introduced.6 For all patients with SCD, the standard PCV13 series should be administered beginning at age 6 weeks. A 2-dose series of the PPSV23 vaccine, which includes more Streptococcus pneumoniae serotypes than the PCV13 vaccine, should be administered beginning at age 2 years or 8 weeks after completion of the PCV13 series, whichever comes first.
Prevention of flu, COVID-19, and other vaccine-preventable illness
Influenza. Beginning at age 6 months, all patients with SCD should receive inactivated influenza vaccine annually at the beginning of the influenza season. Avoid using the live attenuated vaccine (Flumist) because of an associated increased risk of severe or complicated infection.11
Continue to: COVID-19, caused by severe...
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is especially problematic in patients with SCD12; infection causes mortality at a rate as high as 7%.5 The SARS-CoV-2 mRNA vaccine series is potentially lifesaving for these patients.12 In addition, at times of high community prevalence, make an effort to minimize patients’ exposure to SARS-CoV-2, by providing telemedicine visits.
Follow the immunization schedule. Patients with SCD should receive all standard recommended vaccinations (ie, those recommended by the Advisory Committee on Immunization Practices.a) Inactivated virus vaccines are preferred in SCD, when available. For patients who are behind on vaccinations, use a standard vaccine catch-up schedule.
Screening and prevention of complications such as stroke
Determining the risk of stroke. Patients with SCA who are not monitored have a 10% to 11% lifetime prevalence of stroke.5,6,10 An abnormal transcranial Doppler (TCD) study (defined as a time-averaged mean maximum velocity ≥ 200 cm/s in the distal internal carotid artery or proximal middle cerebral artery) is predictive of a 40% risk of stroke in patients with SCA. With chronic transfusion therapy, a 92% reduction in the risk of stroke is achievable.10
All patients with SCA should undergo annual screening with TCD ultrasonography from ages 2 to 16 years.6 Those who have an abnormal TCD study should receive chronic transfusion therapy. Screening is not recommended for patients with SCD or SCT.
Other complications. Screen and manage as follows:
- Proteinuria. Left untreated, SCD can affect the kidneys and lead to renal failure. Annual screening for proteinuria is recommended beginning at age 10 years, with referral when the test is positive and reproducible.
- Lung disease and cardiovascular disease. Screening for progression of lung disease and for cardiovascular disease is not recommended in asymptomatic patients with SCD, except through the history.
- Blood pressure screening and management of hypertension are based on Joint National Committee (JNC 8) guidelines.13
- Screening for ocular complications by an eye care provider is recommended beginning at age 10 years.
TABLE 26,9 summarizes recommendations on the prevention and early detection of complications of SCD.
Continue to: Pregnancy planning
Pregnancy planning
The Centers for Disease Control and Prevention recommends that a “reproductive life plan” be part of every person’s health journey (TABLE 2).6,9 The plan is especially relevant for female patients who have a known heritable concern, such as SCD, in which a pregnancy is more likely to be complicated by growth restriction, preterm delivery, and fetal demise. These risks are reduced—but not eliminated—with intensive surveillance of the pregnancy. Pregnancy in patients with SCD is also more likely to be complicated by preeclampsia, venous thromboembolism, infection, and maternal death.
Other recommendations:
- Every patient with SCD should receive genetic counselling before conceiving, when possible.
- Pregnancy should be considered high risk in women who have SCD, and monitored as such.
- Women with SCD can use any method of contraception—none of which put them at increased risk of complications, compared to the general population. Rather, it is pregnancy that puts them at greater risk of morbidity and mortality in every age group.
Ambulatory management of acute complications
Vaso-occlusive pain crisis. The hallmark of SCD is the acute pain crisis. Almost all patients with SCD (and the occasional patient with SCT) will experience a pain crisis. In more affluent countries, management of an acute pain crisis almost always includes opioid analgesia.6
For the most part, pain crises manifest in a predictable pattern. Although patients with SCD might have acute pain, other causes of acute pain, such as an acute intra-abdominal process or (in older patients) a cardiac process, should be considered as well.
For patients having a vaso-occlusive pain crisis, achieving rapid analgesia is key to management. Ready availability of narcotics, at home or under observation, prevents subsequent hospitalization; nonsteroidal anti-inflammatory drugs can be used as adjuvant treatment in patients without contraindications.5,6 An individualized treatment plan, including access to analgesia at an appropriate dosage, should be negotiated, and adhered to, by the patient and the care team.
Continue to: Rapid access to higher-level care...
Rapid access to higher-level care, including parenteral analgesia, is important if outpatient management is desired. In addition, escalation to a higher level of care should occur if there is hypoxia (or another reason to suspect acute chest syndrome [ACS; discussed in a bit]) or dehydration that requires parenteral therapy. Use of nondrug therapy, such as heat, should be encouraged. The care team should work with the patient’s school or employer to negotiate time away through the federal Family Medical Leave Act of 1993, or other means, because a pain crisis is not a planned event.
Fever. Because of the risk of serious infection as a consequence of functional asplenia, fever is particularly worrisome in patients with SCA and problematic in patients with SCD. The increased risk begins as the physiologic level of Hb F declines beginning at age 2 months.
ACS, characterized by a combination of respiratory symptoms or new infiltrates, often manifests initially with fever, and can progress rapidly to death if treatment is delayed. The initial signs and symptoms may be subtle; suspicion should remain high in any patient with respiratory symptoms who is newly hypoxic, even those who do not have fever. Osteomyelitis, another febrile illness, is also potentially life threatening if not treated promptly.
All patients with SCD whose body temperature is > 101.3 °F should be evaluated with appropriate clinical laboratory testing (complete blood count; inflammatory markers, such as C-reactive protein; basic chemistry parameters; and other tests as indicated, including serum lactate and urine culture), blood culture, and chest radiography. Empiric parenteral antibiotics are required until the patient is known to be nonbacteremic, regardless of vaccination status. Outpatient follow-up and even outpatient management with ceftriaxone can be offered in select circumstances (eg, if the patient so desires or is nontoxic, and if close follow-up can be assured).14 If ACS is a possibility based on symptoms or radiographic findings, outpatient management is not an option.
Anemia. Patients with SCA, and some with SCD, have an Hb level that is chronically, sometimes critically, low. A baseline Hb level should be established for a patient with SCD and then monitored periodically. A drop in the Hb level > 2 g/dL from baseline (or an initial Hb level of 6 g/dL if the baseline is unknown) constitutes acute anemia. Patients in whom anemia has been diagnosed should be emergently evaluated for acute splenic sequestration, an aplastic episode, a delayed hemolytic transfusion reaction, ACS, or infection, and should be treated appropriately.
Continue to: Simple transfusion can be used...
Simple transfusion can be used in an acute setting to restore and maintain Hb at a safe level. Iron overload and formation of RBC alloantigen are associated with multiple transfusions; once either of these conditions is established, subsequent transfusion therapy can be harmful. Care must be taken to prescribe transfusion appropriately; leukocyte-depleted RBCs should be used when available.
It is important to define specific goals of transfusion to optimize its use. Patients who have received multiple transfusions should have enhanced monitoring for bloodborne infection, such as hepatitis C virus. Acute aplastic crises are caused by parvovirus B19; when other members of the household who have SCD are present, they should be monitored for this viral infection with serial measurement of Hb and white blood cell count.6
Other acute problems. Should stroke, acute renal failure, priapism, or hepatobiliary complications develop, evaluate the patient rapidly and refer them to the appropriate care team for management.
Management of chronic complications
Chronic pain is a problem for many patients with SCD. The etiology of this symptom should be investigated fully because a vaso-occlusive crisis is characterized by acute pain. Avascular necrosis or ulcers due to chronic vaso-occlusion should be managed definitively when possible. Adjuvant therapy for chronic pain, such as heat or massage, should be encouraged.
In some patients, chronic pain without objective findings develops over time and becomes unresponsive to nonopioid pharmacotherapy. Such patients might require chronic opioid therapy, the need for which is dictated by the ability of the patient to perform their activities of daily living. For patients who require long-term daily narcotic drugs, best practices—obtaining informed consent, using registries and contracts, random drug testing, and providing naloxone [Narcan] for overdose emergency use—should be employed.15
Continue to: Chronic anemia
Chronic anemia can be managed with transfusion when elevating the Hb level is required (eg, preoperatively, to prevent stroke, to manage priapism). For some patients, ongoing transfusion is required; care should be taken to avoid iron overload and hemolysis due to antibody formation. Ongoing surveillance for these complications is required.6
Other chronic problems. Patients with SCD who develop avascular necrosis, vaso-occlusive ulcers, pulmonary hypertension, renal disease, recurrent priapism, or ophthalmologic complications should be co-managed with a care team.6
Pharmacotherapy and SCA
A principal goal in the management of patients with SCA is prevention of vaso-occlusive events, including ACS and acute pain crises.
Hydroxyurea, a key component of SCA treatment, is a ribonucleotide reductase inhibitor that increases the level of Hb F, thus reducing the absolute number of symptomatic vaso-occlusive events and increasing arterial blood flow. It is most useful for patients who have multiple crises. The drug prolongs survival and reduces the need for transfusion and hospitalization.4,5
Hydroxyurea can be started in patients at age 9 months; blood testing should be performed at the start of treatment and the dosage titrated based on blood counts. Initial blood work includes:
- Hb level;
- Hb electrophoresis with the quantitative percentage of Hb F;
- complete blood count with differential and reticulocyte counts;
- chemistry profile (electrolytes, lactate dehydrogenase, total protein, albumin, total bilirubin);
- liver function tests (aspartate aminotransferase, alanine aminotransferase);
- measurement of renal function (blood urea nitrogen, creatinine);
- serum vitamin B12 and folate;
- serum iron, total iron-binding capacity, and ferritin;
- hepatitis B, hepatitis C, and parvovirus B19 antigen; and
- serologic testing for HIV.
Continue to: Testing should also...
Testing should also include a pregnancy test for postmenarchal females because hydroxyurea is in US Food and Drug Administration pregnancy risk category X.
Avoid hydroxyurea in lactating women; dose the drug renally in the setting of renal disease. Because hydroxyurea has a high rate of serious adverse effects and drug-drug interactions, it should be offered in conjunction with an individualized care plan.
Hydroxyurea can also be offered to patients with other forms of SCD who have recurrent vaso-occlusive symptoms.
Two newer medications improve oxygen delivery in patients with SCD. Voxelotor, approved in 2019, works to reduce Hb S polymerization by binding to the alpha chain of Hb S and, subsequently, increasing its oxygen affinity. The drug is generally well tolerated and can be used in patients with SCD who are ≥ 12 years.16 Crizanlizumab is a monoclonal antibody directed against P-selectin, an adhesion molecule located on endothelial cells and activated platelets. The efficacy of crizanlizumab was demonstrated in the SUSTAIN trial, in which it reduced vaso-occlusive pain in patients ≥ 16 years.17
All of these medications have a narrow toxic–therapeutic window. They should therefore be administered with the participation of a multidisciplinary care team.
Continue to: The need to coordinated, comprehensive care
The need for coordinated, comprehensive care
Patients with SCD report how challenging their disease is. All patients with SCD are more likely than age-matched counterparts to experience loss, including workdays for disability, educational potential, workdays for caregivers of affected children, and time spent in the hospital or the emergency department.4,5 These losses, with the concomitant stress associated with chronic illness and the struggle to manage recurrent pain crises and chronic complications, are often overwhelming.
Comprehensive care can, as we have illustrated in this discussion, mitigate these losses. Such care should include extensive education, genetic counseling, infection prevention, pain management, and implementation of evidence-based management guidelines.3,4,6 Patients with SCD report that their illness outlook would be better with
- greater provider knowledge of SCD,
- destigmatization of narcotics for SCD vaso-occlusive pain management,
- optimal coordination among members of the health care team, and
- improved transportation for appointments.
Patients also report that barriers associated with the unique US health care financing system are often insurmountable. As patients with SCD live longer, improved care management should focus on reducing these barriers and enhancing their quality of life.2,18,19
CORRESPONDENCE
Robert Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama College of Medicine, 1601 Center Street, 2N, Mobile, AL 36604; perkins@health. southalabama.edu
1. Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2:e1915374. doi:10.1001/jamanetworkopen.2019.15374
2. Swanson ME, Grosse SD, Kulkarni R. Disability among individuals with sickle cell disease: literature review from a public health perspective. Am J Prev Med. 2011;41(6 suppl 4):S390-S397. doi: 10.1016/j.amepre.2011.09.006
3. Moskowitz JT, Butensky E, Harmatz P, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48:64-71. doi:10.1002/pbc.20792
4. Mehta SR, Afenyi-Annan A, Lottenberg R. Opportunities to improve outcomes in sickle cell disease. Am Fam Phys. 2006;74:303-310.
5. Yawn BP, John-Sowah J. Management of sickle cell disease: recommendations from the 2014 Expert Panel Report. Am Fam Phys. 2015;92:1069-1077.
6. National Heart, Lung, and Blood Institute, National Institutes of Health. Evidence-based management of sickle cell disease. Expert Panel Report, 2014. Accessed June 9, 2022. www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines
7. Committee Opinion No. 690: Carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40. doi: 10.1097/AOG.0000000000001951
8. Jordan LB, Smith-Whitley K, Treadwell MJ, et al. Screening U.S. college athletes for their sickle cell disease carrier status. Am J Prev Med. 2011;41:S406-S412. doi: 10.1016/j.amepre.2011.09.014
9. Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998;19:110-129. doi: 10.1016/s0197-2456(97)00099-8
10. Alzahrani F, Alaidarous K, Alqarni S, et al. Incidence and predictors of bacterial infections in febrile children with sickle cell disease. Int J Pediatr Adolesc Med. 2021;8:236-238 doi: 10.1016/j.ijpam.2020.12.005
11. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2021-2022. Pediatrics. 2021;148: e2021053745. doi: 10.1542/peds.2021-053745
12. Centers for Disease Control and Prevention. Study finds people with sickle cell disease who developed coronavirus disease have high rates of hospitalization, intensive care unit admission, and death. October 20, 2020. Accessed June 9, 2022. www.cdc.gov/ncbddd/sicklecell/features/scd-and-covid-19.html
13. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
14. Baskin MN, Goh XL, Heeney MM, et al. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131:1035-1041. doi: 10.1542/peds.2012-2139
15. Osunkwo I, Veeramreddy P, Arnall J, et al. Use of buprenorphine/naloxone in amelio rating acute care utilization and chronic opioid use in adults with sickle cell disease. Blood. 2019;134 (suppl 1):790. doi: 10.1182/blood-2019-126589
16. Vichinsky E, Hoppe CC, Ataga KI, et al; HOPE Trial Investigators. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509-519. doi: 10.1056/NEJMoa1903212
17. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429-439. doi: 10.1056/NEJMoa1611770
18. Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. 2016;2:CD011130. doi: 10.1002/14651858.CD011130.pub2
19. Brennan-Cook J, Bonnabeau E, Aponte R, et al. Barriers to care for persons with sickle cell disease: the case manager’s opportunity to improve patient outcomes. Prof Case Manag. 2018;23:213-219. doi: 10.1097/NCM.0000000000000260
1. Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2:e1915374. doi:10.1001/jamanetworkopen.2019.15374
2. Swanson ME, Grosse SD, Kulkarni R. Disability among individuals with sickle cell disease: literature review from a public health perspective. Am J Prev Med. 2011;41(6 suppl 4):S390-S397. doi: 10.1016/j.amepre.2011.09.006
3. Moskowitz JT, Butensky E, Harmatz P, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48:64-71. doi:10.1002/pbc.20792
4. Mehta SR, Afenyi-Annan A, Lottenberg R. Opportunities to improve outcomes in sickle cell disease. Am Fam Phys. 2006;74:303-310.
5. Yawn BP, John-Sowah J. Management of sickle cell disease: recommendations from the 2014 Expert Panel Report. Am Fam Phys. 2015;92:1069-1077.
6. National Heart, Lung, and Blood Institute, National Institutes of Health. Evidence-based management of sickle cell disease. Expert Panel Report, 2014. Accessed June 9, 2022. www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines
7. Committee Opinion No. 690: Carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40. doi: 10.1097/AOG.0000000000001951
8. Jordan LB, Smith-Whitley K, Treadwell MJ, et al. Screening U.S. college athletes for their sickle cell disease carrier status. Am J Prev Med. 2011;41:S406-S412. doi: 10.1016/j.amepre.2011.09.014
9. Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998;19:110-129. doi: 10.1016/s0197-2456(97)00099-8
10. Alzahrani F, Alaidarous K, Alqarni S, et al. Incidence and predictors of bacterial infections in febrile children with sickle cell disease. Int J Pediatr Adolesc Med. 2021;8:236-238 doi: 10.1016/j.ijpam.2020.12.005
11. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2021-2022. Pediatrics. 2021;148: e2021053745. doi: 10.1542/peds.2021-053745
12. Centers for Disease Control and Prevention. Study finds people with sickle cell disease who developed coronavirus disease have high rates of hospitalization, intensive care unit admission, and death. October 20, 2020. Accessed June 9, 2022. www.cdc.gov/ncbddd/sicklecell/features/scd-and-covid-19.html
13. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
14. Baskin MN, Goh XL, Heeney MM, et al. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131:1035-1041. doi: 10.1542/peds.2012-2139
15. Osunkwo I, Veeramreddy P, Arnall J, et al. Use of buprenorphine/naloxone in amelio rating acute care utilization and chronic opioid use in adults with sickle cell disease. Blood. 2019;134 (suppl 1):790. doi: 10.1182/blood-2019-126589
16. Vichinsky E, Hoppe CC, Ataga KI, et al; HOPE Trial Investigators. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509-519. doi: 10.1056/NEJMoa1903212
17. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429-439. doi: 10.1056/NEJMoa1611770
18. Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. 2016;2:CD011130. doi: 10.1002/14651858.CD011130.pub2
19. Brennan-Cook J, Bonnabeau E, Aponte R, et al. Barriers to care for persons with sickle cell disease: the case manager’s opportunity to improve patient outcomes. Prof Case Manag. 2018;23:213-219. doi: 10.1097/NCM.0000000000000260
PRACTICE RECOMMENDATIONS
› Offer rapid access to narcotic analgesia for patients with sickle cell disease (SCD) who have recurrent vaso-occlusive crises, to prevent unnecessary hospitalization. A
› Provide oral penicillin prophylaxis against pneumococcal disease in patients < 5 years of age who have sickle cell anemia (SCA), but not in children whose SCD is less severe. A
› Screen all patients with SCA annually, beginning at age 2 years until age 16 years, for their risk of stroke, using a transcranial Doppler study. A
› Administer the COVID-19 mRNA vaccine series to all patients with SCD, unless contraindicated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
