User login
Sessile serrated polyps: Cancer risk and appropriate surveillance
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
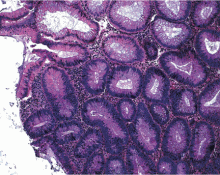
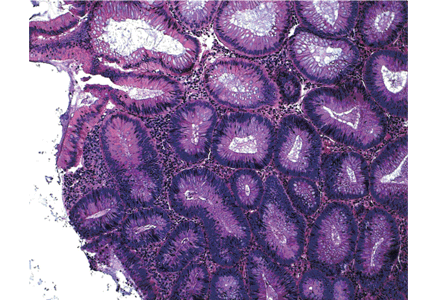
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
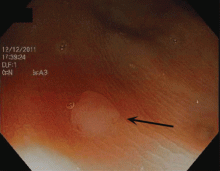
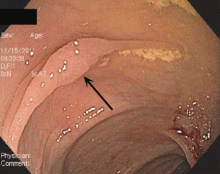
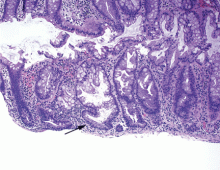
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
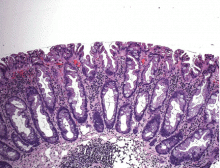
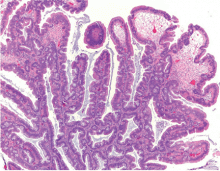
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
KEY POINTS
- From 20% to 30% of colorectal cancers arise through the serrated polyp pathway (the serrated neoplasia pathway.)
- Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium. Types of serrated polyps include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (also known as sessile serrated adenomas).
- Guidelines for surveillance after polypectomy of serrated lesions recommend that patients with a large (≥ 10-mm) or a sessile serrated polyp with cytologic dysplasia or a traditional serrated adenoma be followed more closely than patients with a sessile serrated polyp smaller than 10 mm. Patients with small rectosigmoid hyperplastic polyps should be followed the same as people at average risk.