User login
Cutaneous melanoma: Detecting it earlier, weighing management options
- Arrange a biopsy of any pigmented lesion that changes significantly on serial examinations.
- Full-thickness excisional biopsy is preferred; for large lesions, incisional or punch biopsy at the deepest point of the tumor may be an option.
- For thin lesions, a surgical margin encompassing 1 cm normal skin is recommended.
- Specimens submitted in formalin for permanent sections are preferred to frozen sections.
A 51-year-old man of northern European descent who works outdoors for the city asks you to look at a “mole” on his face. The lesion does not have the classic appearance of a melanoma that you have seen before, and it is still fairly small. Should you advise a wait-and-see approach or perform a biopsy?
Opt for early detection
Given the patient’s likely genetic predisposition to skin cancer and his regular, lengthy exposure to sunlight (see Risk factors), you would be wise to follow your cutaneous examination with a biopsy.
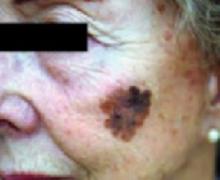
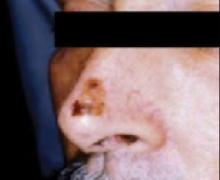
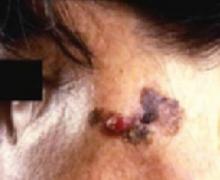
The ABCDs of visual assessment. The classic clinical presentation of melanoma is well known. The most publicized means for identifying potentially atypical pigmented skin lesions is the “ABCD” mnemonic (asymmetry, border irregularity, color variegation, and lesion diameter >6 mm) (FIGURES 1 AND 2).7,8 The ABCDs are primarily an educational tool for patients. This mnemonic was expanded to include E, representing evolution of a pigmented lesion.9 Any pigmented lesion observed to change significantly on serial examinations warrants biopsy to exclude melanoma.
Caveat: not all melanomas are pigmented. Amelanotic melanomas are a diagnostic challenge and may be lethal if left unattended.
Routine screening. Large-scale skin cancer screening has been performed and found to be a statistically ineffective means of detection. Moreover, the US Preventive Services Task Force found insufficient evidence to recommend for or against routine counseling by primary care physicians to prevent skin cancer.10
A large-scale educational and screening campaign was performed in Italy from 1991 to 1996. During this period, 90,000 educational leaflets were distributed to a target population of approximately 243,000. A total of 2050 individuals requested a skin examination, resulting in detection of 13 melanomas.11 However, 92.3% of the melanomas were thin (<1.52 mm deep). Despite the lack of statistical significance for such screenings, many organizations do perform them and find melanomas, which can be life saving for those few individuals.
Anatomic areas to focus on. While melanoma can affect any anatomic region, it is especially common on sun-exposed areas, including the head, neck, and upper extremities. Acral lentiginous melanoma is found on palmar, plantar, and subungual regions. Include the scalp, ocular mucosa, and oral cavity in your examination.
Four primary groups have been traditionally proposed based on a combination of clinical and pathologic features: superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma and acral lentiginous melanoma. Furthermore, additional histopathologic variants including desmoplastic, neurotropic, amelanotic, signet-ring cell, small cell and balloon cell melanoma have been described.
FIGURE 1
Melanoma in situ
Classic presentations of melanoma in situ on the left cheek. Sun-exposed areas are most often affected.
FIGURE 2
Melanoma in situ
Classic presentations of melanoma in situ on the tip of the nose. Sun-exposed areas are most often affected.
Which biopsy technique to choose?
A properly performed biopsy is mandatory to accurately diagnose and microstage the tumor. No other test reliably surpasses routine histologic examination.
When possible, arrange for a full-thickness excisional biopsy with a narrow (2-mm) rim of normal skin, especially for large lesions in which sampling error may be a factor.
Tissue samples are submitted for histologic examination in formalin for permanent sections. Frozen sections are not recommended for the initial diagnosis of melanoma due to artifactual changes from the freezing process.
When lesions are too large for primary excision, or if an optimal cosmetic result is important to the patient, an incisional or punch biopsy may be taken from the area believed to be the deepest portion of the tumor.
For the largest lesions, multiple punch or incisional biopsies may help to reduce the risk of sampling error. Diagnostic and management problems arise when the initial biopsy does not sample the complete skin thickness or when large lesions are not sampled adequately.
Classification of melanoma
The traditional melanoma classification scheme includes several subtypes (see Detailed melanoma classifications). Though these terms have limited clinical utility, we include them for the sake of completeness and because the terminology may arise in consultation with colleagues.
Most important for the prognosis is a determination of the tumor’s depth of invasiveness.
In situ or invasive?
Melanoma in situ lesions are confined to the epidermis and may extend along hair appendages. It often occurs on sun-exposed areas (FIGURES 1 AND 2). Histologically, melanoma in situ is an asymmetric and poorly circumscribed proliferation of melanocytes usually larger than 6 mm in diameter (FIGURE 3).
Melanocytes form irregular nests that are not equidistant and have areas of confluence (FIGURE 3). Single melanocytes predominate over nests in some areas and may entirely replace the basal layer.
Additional histologic features include single melanocytes above the dermal-epidermal junction (Pagetoid spread) and uneven distribution of pigment. In many cases the dermis has inflammatory infiltrates, melanophages, and evidence of solar-damage. Particularly in anatomical areas rich in hair follicles, the neoplastic cells may spread into the epithelium of the hair follicles without extending to the dermis.
Melanoma in situ may be quite large in diameter (horizontal growth phase) without becoming invasive; however, always be concerned about invasion.
Invasive melanoma shares the same histologic features but invades the dermis or subcutaneous fat (vertical growth phase). Aside from the conventional criteria used for the histologic diagnosis of melanoma, acral melanomas may show an increased number of dendritic melanocytes loaded with melanin (FIGURE 4). Desmoplastic melanoma is most commonly found on the head and neck; however, it varies in clinical presentation (FIGURE 5). It is often associated with other types of melanoma, is common in older persons, and has a slight male predilection.
FIGURE 3
Melanocytes
Melanoma in situ stained with routine hematoxylin/eosin on permanent sections. Note confluent nesting of atypical melanocytes at the dermal-epidermal junction (arrow). There are several foci of Pagetoid spread as melanocytes are seen migrating upward toward the surface.
FIGURE 4
Acral melanoma
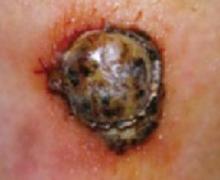
Acral melanoma lesion on the hand.
FIGURE 5
Dermoplastic melanoma
Desmoplastic melanoma lesion, commonly found on the head and neck.
Microstaging: The key to good management
The categories “superficial spreading” and “nodular” are based on the seminal work of Wallace Clark, who described putative growth phases of cutaneous melanoma.12 Clark hypothesized that melanoma initially grows horizontally and only later begins an invasive vertical growth phase.
The horizontal growth phase is common in sun-exposed sites and often occurs over a long period of time. The vertical growth phase is a much more aggressive growth pattern that, if left unchecked, can be lethal. This is often seen in nodular melanoma (FIGURE 6).
Measurement of vertical growth (“microstaging”) is the most important prognostic indicator for localized cutaneous melanoma.13
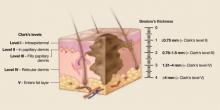
Clark described 5 levels (“Clarks’ levels,” I–V) of invasion (FIGURE 7).
Independently of Clark, Breslow described tumor thickness as an important prognostic factor.14,15 Breslow thickness is measured from the granular layer of the epidermis to the deepest area of invasion (FIGURE 7). Most reports indicate that, overall, Breslow tumor thickness more closely correlates with clinical outcome.16
Special circumstances that affect microstaging. Although Clark and Breslow measurements are both conceptually simple, a number of factors, including hair follicle involvement and ulceration, must be considered when measuring melanoma depth. It is often useful to have a Breslow thickness and a Clark’s level for primary cutaneous melanomas (both methods are used for staging thin primary lesions).
The location of the primary lesion affects interpretation of measurement results. For example, a Breslow thickness of 0.5 mm confers a different meaning on the eyelid (very thin skin, Clark’s level proportionately deeper than tumor thickness might indicate) than the back (thick skin, proportionately more superficial Clark’s level than tumor thickness would indicate).
Measurement of vertical involvement is used to stage the tumor with the TNM classification. This was recently updated as outlined by Balch et al13 in 2001 (TABLE 1).
FIGURE 6
Nodular melanoma
Nodular melanoma often exhibits aggressive vertical growth.
FIGURE 7
Classifying melanoma: Both Clark’s level and Breslow measurement are often used for staging
Clark’s levels are derived from level of tumor invasion compared with layers of the skin. Tumors are divided into 5 levels. Level I: Tumor cells confined to the epidermis (in situ). Level II: Tumor invades the papillary dermis, past basement membrane. level III: Tumor fills papillary dermis, extends to the between the papillary and reticular dermis. Level IV: Tumor invades reticular dermis. Level V: Tumor invasion of subcutaneous tissue. Breslow’s thickness is a measurement of lesion depth in millimeters. Tumors are classified into 4 categories based on the depth vertically from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumor involvement.
TABLE 1
Using the TNM staging system to determine prognosis for melanoma
| T CLASSIFICATION | BRESLOW THICKNESS (MM) | ULCERATION | |||||
| T1 | ≤1.0 | a: no ulceration and Clark’s level II/III | |||||
| b: ulceration or Clark’s level IV/V | |||||||
| T2 | 1.01–2.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| T3 | 2.01–4.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| T4 | >4.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| N CLASSIFICATION | METAST ATIC NODES | NODAL METASTATIC MASS | |||||
| N1 | 1 node | a: micrometastasis* | |||||
| b: macrometastasis† | |||||||
| N2 | 2–3 nodes | a: micrometastasis* | |||||
| b: macrometastasis† | |||||||
| N3 | ≥4 metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic node(s) | ||||||
| M CLASSIFICATION | METASTASES (SITE) | SERUM LDH‡ | |||||
| M1a | Distal skin, subcutaneous/nodal | Normal | |||||
| M1b | Lung | Normal | |||||
| M1c | All other visceral | Normal | |||||
| Any visceral | Elevated | ||||||
| *Micrometastases diagnosed after sentinel or elective lymphadenectomy. | |||||||
| ‡Macrometastases defined as clinically detectable nodal metastases confirmed by therapeutic lymphadenectomy or when nodal metastasis exhibits gross extracapsular extension. | |||||||
| ‡LDH, lactate dehydrogenase. | |||||||
| Once the TNM classification has been determined, the combined findings, as shown below, can help you determine a patient’s relative prognosis. | |||||||
| Clinical Staging | Pathologic Staging | 5-year survival (%) | |||||
| 0 | Tis | N0 | M0 | Tis | N0 | M0 | 96–100 |
| IA | T1a | N0 | M0 | T1a | N0 | M0 | 95 |
| IB | T1b | N0 | M0 | T1b | N0 | M0 | 90 |
| T2a | N0 | M0 | T2a | N0 | M0 | ||
| IIA | T2b | N0 | M0 | T2b | N0 | M0 | 78 |
| T3a | N0 | M0 | T3a | N0 | M0 | ||
| IIB | T3b | N0 | M0 | T3b | N0 | M0 | 65 |
| T4a | N0 | M0 | T4a | N0 | M0 | ||
| IIC | T4b | N0 | M0 | T4b | N0 | M0 | 45 |
| III | Any T | Any N | M0 | ||||
| IIIA | T1-4a | N1a | M0 | 66 | |||
| T1-4a | N2a | M0 | |||||
| IIIB | T1-4b | N1a | M0 | 52 | |||
| T1-4b | N2a | M0 | |||||
| T1-4a | N1b | M0 | |||||
| T1-4a | N2b | M0 | |||||
| T1-4a | N2c | M0 | |||||
| T1-4b | N2c | M0 | |||||
| IIIC | T1-4b | N1b | M0 | 26 | |||
| T1-4b | N2b | M0 | |||||
| Any T | N3 | M0 | |||||
| IV | Any T | Any N | Any M | Any T | Any N | Any M | 7.5–11 |
Preferred management and contingencies
Primary treatment for cutaneous melanoma is wide local excision of the primary site.
How wide should excision margins be? The appropriate margin of normal skin (measured from the biopsy scar or lateral border of residual melanoma) varies with Breslow tumor thickness.17 With thin and intermediate thickness, a surgical margin encompassing 1 cm of normal skin is the consensus.17 For thicker lesions, many authorities recommend a 2-cm margin. A 1-cm margin may be inadequate due to the risk of local recurrence; however, whether a 2 cm or 3 cm margin is optimal remains unclear.18,19TABLE 2 reviews treatment guidelines for surgical excision of primary cutaneous melanoma.20-24
Biopsied tissue submitted as permanent sections. The planned primary excision site should be oriented and submitted to pathology in formalin for permanent section examination. Frozen sections are generally not recommended; when the pathologist assesses surgical margin status, scattered melanocytes in adjacent sun-damaged skin may lead to uncertainty. Moreover, artifacts from the freezing process make interpretation difficult.
2 qualifiers for the above advice: immunostains have increased the utility of frozen sections, and frozen sections were not used in the clinical trials from which current treatment options were derived. These considerations apply particularly to the use of frozen section analysis in Mohs micrographic surgery (see below).
The wide local excision specimen can be examined by multiple surgical pathology orientation methods. In many cases, if the primary lesion was completely excised by the initial biopsy, the pathologist will examine the area of the biopsy scar for residual melanoma and select portions of the lateral margins for examination. Alternatively, the entire lesion, all of the margins, or even the entire specimen may be submitted for examination depending on the clinical circumstances and concerns of the pathologist and surgeon, such as incomplete removal at the time of initial biopsy or close deep or lateral margins. Communication between surgeon and pathologist must be clear and unambiguous.
Examining multiple sections from the tissue block and adding immunohistochemical stains greatly increase sensitivity for metastatic melanoma.37-40 Cochran et al41 originally demonstrated the importance of immunohistochemistry in the pathology examination of lymph nodes. From 2227 lymph nodes removed from 100 patients, they found that 16 additional lymph nodes in 14 patients contained metastatic melanoma when examined with S-100 immunohistostains. Using additional antibodies more recently (see below), these authors reported that up to 12% of metastatic melanoma deposits can be missed by experienced pathologists without the aid of immunohistochemistry.42
Though no antibody is both highly sensitive and specific for malignant melanoma (versus normal melanocytes), the antibodies most commonly used in immunohistochemistry include S-100 (a neuroectodermal tissue maker expressed in nerves, melanocytes, histiocytes and dendritic cells in lymph nodes), HMB 45 (recognizes an oligosaccharide side chain present in immature melanosomes), Melan-A (recognizes the MART-1 protein), and tyrosinase (recognizing the enzyme tyrosinase required for melanin synthesis). S-100, although very sensitive, is not a melanocyte-specific antibody. The remaining antibodies, though melanocyte specific, are non-reactive in 5% to 15% of cutaneous malignant melanoma.42
Using polymerase chain reaction (PCR) and reverse transcriptase polymerase chain reaction (RTPCR) for MRNA for melanocyte-associated proteins further increases sensitivity, detecting positive nodal cells when routine hematoxylin/eosin and immunohistochemical methods do not. The clinical utility of molecular methods for diagnosis and patient management has not been established; these techniques are the subject of ongoing clinical trials.43
In retrospect, it is clear that the standard techniques used to examine lymph nodes taken from regional lymph node dissections underestimate the presence of micrometastatic disease. one could argue, in fact, that the sole advantage of lymphatic mapping and sentinel lymph node biopsy has been to allow the pathologist to perform a focused, extensive examination on 1-3 lymph nodes – an examination that would be impractical (and prohibitively expensive) on a standard regional lymph node dissection specimen. Interestingly, the idea that additional sections from lymph nodes will increase the detection rate of metastatic tumor is not new and was well-known to the surgeons and pathologists involved in the pioneering work on sentinel lymph node biopsy.41
Mohs micrographic surgery increasingly used for melanoma.25-31 This procedure may be performed with frozen or permanent sections, using concomitant immunohistochemical staining with either method of embedding. This has been especially useful in cosmetically sensitive areas such as the face, ear, nose, genitalia, and extremities.30 Mohs micrographic surgery with permanent sections also makes possible the most definitive surgical margin analysis.
TABLE 2
Recommended incision margins
| BRESLOW THICKNESS | EXCISION MARGINS (CM) |
|---|---|
| In situ | 0.5 |
| <1 mm | 1 |
| 1–4 mm | 1–2 |
| >4 mm | 2–3 |
Sentinel lymph node biopsy
Lymphatic mapping and sentinel lymph node biopsy have significantly changed the initial approach to the patient with melanoma and have reinvigorated debate over the importance and management of regional lymph nodes in cutaneous malignant melanoma. The anatomic basis of, and techniques for, lymphatic mapping and sentinel lymph node biopsy are well-described elsewhere and are not reviewed here.32
Risk factors for melanoma include a personal or family history of melanoma and the presence of multiple nevi. Nevi are considered risk factors if an individual has many or if the nevi are unusual in appearance or size. Other risk factors include fair complexion, excessive sun exposure, history of severe sunburns, use of tanning booths, immunosuppression and occupational exposure to certain chemicals.1
Ultraviolet light exposure remains the most well-described risk factor for the development of cutaneous melanoma; however, the pathogenesis remains largely unknown.3,4 This is especially true in patients with increased ultraviolet sensitivity secondary to fair skin type and a tendency to burn. The use of sunscreen is recommended for prevention of all types of skin cancer; however, its use may promote increased ultraviolet exposure from a false sense of protection.5,6 Additionally, proper application (i.e. frequent reapplication) is seldom performed.
Histolopathologic data from regional lymph nodes is the most important prognostic factor for cutaneous melanoma,13 and sentinel lymph node biopsy is proven as a staging procedure—as reflected in the revised AJCC TNM Staging System published in 2001 (TABLES 1 AND 2).13 This staging system differs from the previous one in several ways:
- Thickness and ulceration are the primary predictors of survival with localized melanoma (stages I and II)
- Number of metastatic lymph nodes involved and tumor burden are most important predictors of survival in stage III melanoma
- Anatomic site of metastasis (distant) and presence/absence of elevated lactate dehydrogenase (LDH) are primary predictors of survival with stage IV melanoma
- Ulceration should prompt an overall upstaging for melanoma stages I–III
- Satellite metastases around a primary melanoma and in-transit metastases together indicate a stage III melanoma
- Staging decisions are based on sentinel node biopsy/lymphatic mapping.13
Multiple studies have reviewed the changes between the new and old TNM staging systems for melanoma.13
When to biopsy. Clinical experience supports reserving lymphatic mapping and sentinel lymph node mapping for patients with primary lesions 1 mm or thicker. Some authorities have recommended sentinel lymph node biopsy for lesions <1 mm thick if they are ulcerated or if a discrepancy exists between the Breslow tumor thickness and Clark’s level. For such lesions, biopsy has been recommended for Clark’s level >III.33
Though sentinel lymph node biopsy can be performed successfully after wide local excision, doing it before excision will prevent disruption of lymphatics for node mapping.34 The sentinel lymph node should be submitted to pathology for permanent section analysis. Frozen section analysis is not recommended for melanoma due to its lower sensitivity.35
Though not widely adopted, some surgeons use intraoperative touch prep cytology as part of an initial intraoperative evaluation.36 If the surgeon is prepared to proceed with regional node dissection and a “black” grossly involved node is encountered, intraoperative frozen section may be used to confirm metastastic melanoma. Neither touch preps nor frozen sections are likely to demonstrate the small, isolated metastatic tumor cells commonly identified in small, grossly normal lymph nodes.
What to expect from the pathologist. If blue dye is used for localization, the pathologist should document the presence of that color in the lymph nodes submitted. Outside of a clinical trial, fresh lymph node tissue is not usually preserved for polymerase chain reaction (PCR) or other molecular methods for detecting tumor cells.
Although examination techniques vary in some details among institutions, general principles are well accepted and include submission of the entire lymph node, step or serial sections, and, when initial sections do not reveal metastatic tumor, use of immunohistochemistry for melanocyte-associated antigens (see Immunohistochemical techniques and antibody testing).
Fine points regarding interpretation of sentinel lymph nodes. First, the rationale behind biopsy of sentinel lymph nodes is that the lymphatic system is a potential means of metastasis. However, it is not the only means of spread. Hematogenous and tissue spread are potential mechanisms as well.
Second, the lymphatic system acts as a drain but not a dam for lymphatic flow. Thus a negative lymph node biopsy does not guarantee that a metastasis has not already occurred.
Third, the increased sensitivity of identification of positive sentinel lymph nodes with more specific tests (ie, immunohistochemistry, PCR, rtPCR) calls a negative result into question. A sentinel lymph node that is “negative” with immunohistochemistry may in fact be positive with gene amplification techniques (PCR, rtPCR).
Fourth, surgeons performing sentinel node biopsies vary in thoroughness when identifying which node or nodes contain the dye. Lymphoscintigraphy is more accurate than using dyes; however, many surgeons use both.44 Thus, while sentinel node biopsy has demonstrated a role in staging, its use and the interpretation of results must be performed with these variables in mind.
Regional lymph node dissection: Does it benefit patients?
Patients found to have metastatic melanoma in a sentinel lymph node are advised to undergo regional lymph node dissection. Unfortunately, few data address survival with this therapy. The role of sentinel lymph node biopsy and elective lymph node dissection in the management of cutaneous malignant melanoma generates debate, much of which focuses on alternate interpretations of the data from the era of elective lymph node dissection for intermediate-thickness melanoma.
The debate over elective lymph node dissection has been summarized by others in detail.47
Proponents of an aggressive strategy correctly point out that data from trials are simply not comparable to what is seen in the current era of sentinel lymph node biopsy.
Opponents of an aggressive strategy, who are numerous and vocal,48-50 correctly observe that no survival benefit has been demonstrated for any treatment strategy based on regional lymph node dissection. However, to be balanced, such long-term outcome data are simply not yet available. Certainly questions will continue to arise regarding the theoretical validity and cost-effectiveness of sentinel lymph node biopsy.48,49,51
How good is adjuvant therapy?
Adjuvant therapy for cutaneous melanoma can include regional external beam radiation, systemic cytotoxic chemotherapy, or immunotherapy. Radiation has a limited role, being used postoperatively to treat regional lymph node dissection beds at high risk of recurrence and to treat locally recurrent and in-transit disease.52
Systemic cytotoxin (often dacarbazine) has not been effective as adjuvant therapy.53 When combined chemo- and immunotherapy is used for metastatic disease, it is with the hope of discovering an effective combination that might also be used in the adjuvant setting.54 However, it has been the initial success with immunotherapy that frames the debate regarding adjuvant systemic therapy for cutaneous malignant melanoma.
High-dose interferon alpha 2b. For thick or node-positive cutaneous malignant melanoma, a statistically significant increase in overall survival with high-dose interferon alpha 2b was demonstrated in a landmark Eastern Cooperative Oncology Group trial.55 Though the results have been confirmed in other trials, the clinical benefit is still debated. The expense and toxicity of the trial, as well as the absence of benefit in a second ECOG trial of high-dose systemic therapy, have contributed to the uncertainty.56
In an effort to reduce toxicity, low-dose systemic interferon alpha 2b has been tested in numerous trials and found to be of no benefit.56 High-dose alpha 2b interferon therapy continues to be a focus of active clinical investigation by national and international cooperative groups. In addition to high-dose systemic interferon, a large number of immunotherapeutic approaches for the systemic treatment of melanoma have been devised and are under active investigation, including non-specific immune adjuvants such as Bacile Calmette-Guerin, levaminsole, vaccines prepared from autologous melanoma cells and vaccines designed against chemically defined antigens (gangliocides).57 Because of the controversies surrounding adjuvant systemic therapy, patients are best served by participating in an ongoing clinical trial if the expense and toxicity of high dose systemic interferon alpha 2b are deemed to be unacceptable.
Acknowledgments
We thank Brian C. Brockway, M.S. (Department of Medical Media, Veterans affairs Medical Center, Augusta, Georgia) for illustrations.
CORRESPONDENCE
Joshua E. Lane, MD, 308 Hospital Drive, Suite 200, Macon, GA 31217. E-mail: [email protected]
1. American Cancer Society Cancer Facts and Figures 2006. Atlanta: american Cancer Society; 2006.
2. Anonymous. Melanoma: Clinical Practice Guidelines in Oncology. JNCCN 2004;2:46-60.
3. Elwood JM. Melanoma and sun exposure. Semin Oncol 1996;23:650-666.
4. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer 1997;73:198-203.
5. Whiteman DC, Green AC. Melanoma and sun exposure: where are we now? Int J Dermatol 1999;38:481-489.
6. Katsambas A, Nicolaidou E. Cutaneous malignant melanoma and sun exposure. Recent developments in epidemiology. Arch Dermatol 1996;132:444-450.
7. Polk HC. Surgical progress and understanding in the treatment of the melanoma epidemic. Am J Surg 1999;178:443-448.
8. Baade PD, Balanda KP, Stanton WR, et al. Community perceptions about the important signs of early melanoma. J Am Acad Dermatol 1997;36:199-202.
9. Abbasi NR, Shaw, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004;292:2771-2776.
10. Counseling to prevent skin cancer: recommendations and rationale of the U.S. Preventive Services Task Force. MMWR Recomm Rep 2003;52:13-17.
11. Rossi CR, Vecchiato A, Bezze G, et al. Early detection of melanoma: an educational campaign in Padova, Italy. Melanoma Res 2000;10:181-187.
12. Clark WH, From L, Bernardino EA, et al. Histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 1969;29:705-726.
13. Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635-3648.
14. Breslow A. Tumor thickness, level of invasion and node dissection in Stage 1 cutaneous melanoma. Ann Surg 1976;182:572-575.
15. Breslow A. Thickness, cross sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 1970;172:902-908.
16. Barnhill RL, Fine JA, Roush GC, et al. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer 1996;78:427-432.
17. Lens MD, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma: a systematic review of randomized controlled trials comparing marrow vs wide excision. Arch Surg 2002;137:1101-1105.
18. Brown SE, Chapman PB. Defining adequate surgery for primary melanoma. N Engl J Med 2004;350:823-825.
19. Thomas JM, Newton-Bishop J, A’Hern R, et al. Excision margins in high-risk melanoma. N Engl J Med 2004;350:757-766.
20. Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg 1991;126:438-441.
21. Bono A, Bartoli C, Clemente C, et al. Ambulatory narrow excision for thin melanoma (< or=2 mm): results of a prospective study. Eur J Cancer 1997;33:1330-1332.
22. Cascinelli N. Margin of resection in the management of primary melanoma. Semin Surg Oncol 1998;14:272-275.
23. Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol 2001;8:101-108.
24. Johnson TM, Sondak VK. A centimeter here, a centimeter there: does it matter? J Am Acad Dermatol 1995;33:532-534.
25. Zitelli JA, Brown C, Hanusa BH. Mohs micrographic surgery for the treatment of primary cutaneous melanoma. J Am Acad Dermatol 1997;37:236-245.
26. Zalla MJ, Lim KK, Dicaudo DJ, et al. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg 2000;26:771-884.
27. Kelley LC, Starkus L. Immunohistochemical staining of lentigo maligna during Mohs micrographic surgery using MART-1. J Am Acad Dermatol 2002;46:78-84.
28. Nagi C, O’Grady TC, Izadpanah A. Mohs micrographically controlled surgery and the treatment of malignant melanoma. Semin Oncol 2002;29:336-340.
29. Carucci JA. Mohs’ micrographic surgery for the treatment of melanoma. Dermatol Clin 2002;20:701-708.
30. Bienert TN, Trotter MJ, Arlette JP. Treatment of cutaneous melanoma of the face by Mohs micrographic surgery. J Cutan Med Surg 2003;7:25-30.
31. Cook J. Surgical margins for resection of primary cutaneous melanoma. Clin Dermatol 2004;22:228-233.
32. Jakub JW, Pendas S, Reintgen DS. Current status of sentinel lymph node mapping and biopsy: facts and controversies. Oncologist 2003;8:59-68.
33. Jacobs IA, Chang CK, DasGupta TK, et al. Role of sentinel lymph node biopsy in patients with thin (<1mm) primary cutaneous melanoma. Ann Surg Oncol 2003;10:558-561.
34. Evans HL, Krag DN, Teates D, et al. Lymphoscintigraphy and sentinel node bipsy accurately stage melanoma in patients presenting after wide local excision. Ann Surg Oncol 2003;10:416-425.
35. Tanis PJ, Boom RP, Koops HS, et al. Frozen section investigation of the sentinel node in malignant melanoma and breast cancer. Ann Surg Oncol 2002;8:222-226.
36. Greeger AJ, Shiver SA, Shen P, et al. Intraoperative evaluation of sentinel lymph nodes for metastatic melanoma by imprint cytology. Cancer 2002;94:3016-3022.
37. Gietema HA, Vuylesteke RJ, de Jonge IA, et al. Sentinel node investigation in melanoma: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2004;57:618-620.
38. Torrenga H, Rahusen FD, Meijer S, et al. Sentinel node investigation in breast cancer: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2001;54:550-552.
39. Abrahamsen HN, Hamilton-Dutoit SJ, Larsen J, et al. Sentinel lymph nodes in malignant melanoma: extended histopathologic evaluation improves diagnostic precision. Cancer 2004;100:1683-1691.
40. Ross GL, Shoaib T, Scott J, et al. The impact of immunohistochemistry on sentinel node biopsy for primary cutaneous malignant melanoma. Br J Plast Surg 2003;56:153-155.
41. Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol 1988;12:612-618.
42. Robers A, Cochran AJ. Pathologic analysis of sentinel lymph ndoes in melanoma patients: current and future trends. J Surg Oncol 2004;85:152-161.
43. Reintgen D, Pendas S, Jakub J, et al. National trials involving lymphatic mapping for melanoma: the multicenter selective lymphadenectomy trial, the sunbelt melanoma trial, and the Florida melanoma trial. Sem Oncol 2004;31:363-373.
44. Caprio MG, Carbone G, Bracigliano A, et al. Sentinel lymph node detection by Lymphoscintigraphy in malignant melanoma. Tumori 2002;88:543-545.
45. Maffioli L, Sturm E, Roselli M, et al. State of the art sentinel node biopsy in Oncology. Tumori 2000;86:263-272.
46. Bartolomei M, Testori A, Chinol M, et al. Sentinel node localization in cutaneous melanoma: lymphoscintigraphy with colloids and antibody fragments versus blue dye mapping. Eur J Nucl Med 1998;25:1489-1494.
47. Balch CM. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol 1988;6:163-172.
48. Medalie NS, Ackerman AB. Sentinel lymph node biopsy has no benefit for patients with primary cutaneous melanoma metastatic to a lymph node: An assertion based on comprehensive, critical analysis: Part I. Am J Dermatopathol 2003;25:399-417.
49. Medalie NS, Ackerman AB. Sentinel lymph node biopsy has no benefit for patients with primary cutaneous melanoma metastatic to a lymph node: an assertion based on comprehensive, critical analysis: Part II. Am J Dermatopathol 2003;25:473-484.
50. Eedy DJ. Introducing controversies in dermatology: sentinel lymph node biopsy for cutaneous melanoma—a useful technique or a waste of time? Br J Dermatol 2004;151:267-268.
51. Agnese DM, Abdessalam SF, Burak WE, et al. Cost-effectiveness of sentinel lymph node biopsy in thin melanomas. Surgery 2003;134:542-547.
52. Ang KK, Gera FB, Byers RM, et al. Radiotherapy for melanoma. In: Cutaneous Melanoma, Balch CM, Houghton AN, Sober AJ, Soong S, eds. St. Louis, Mo: Quality Medical Publishing; 1998:389–404.
53. Argawala SS, Neuberg D, Park Y, et al. Mature results of a phase III randomized trial of bacillus calmetteguerin (BCG) versus observation and BCG versus dacarbazine bersus BCG in the adjuvant therapy of American joint committee on cancer State I-III melanoma (E1673). Cancer 2004;100:692-698.
54. Groenewegen G, Bloem A, De Gast GC. Phase I/II study of sequential chemoimmunotherapy (SCIT) for metastatic melanoma: outpatient treatment with dacarbazine, granulocyte-macrophage colony-stimulating factor, low-dose interleukin-2, and interferon-alpha. Cancer Immunol Immunother 2002;51:630-636.
55. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17.
56. Moschos SJ, Kirkwood JM, Konstantinopolis PA. Present status and future prospects for adjuvant therapy of melanoma: time to build upon the foundation of high-dose interferon alfa-2b. J Clin Oncol 2004;22:11-14.
57. Lotze MT, Dalla RM, Kirkwood JM, et al. Cutaneous Melanoma. In: Cancer: Principles and Practice of Oncology, Devita VT, Hellma S, Rosenberg SA, eds. Philadelphia, Pa: lippincott Williams & Wilkins; 2001:2021–2056.
- Arrange a biopsy of any pigmented lesion that changes significantly on serial examinations.
- Full-thickness excisional biopsy is preferred; for large lesions, incisional or punch biopsy at the deepest point of the tumor may be an option.
- For thin lesions, a surgical margin encompassing 1 cm normal skin is recommended.
- Specimens submitted in formalin for permanent sections are preferred to frozen sections.
A 51-year-old man of northern European descent who works outdoors for the city asks you to look at a “mole” on his face. The lesion does not have the classic appearance of a melanoma that you have seen before, and it is still fairly small. Should you advise a wait-and-see approach or perform a biopsy?
Opt for early detection
Given the patient’s likely genetic predisposition to skin cancer and his regular, lengthy exposure to sunlight (see Risk factors), you would be wise to follow your cutaneous examination with a biopsy.
The ABCDs of visual assessment. The classic clinical presentation of melanoma is well known. The most publicized means for identifying potentially atypical pigmented skin lesions is the “ABCD” mnemonic (asymmetry, border irregularity, color variegation, and lesion diameter >6 mm) (FIGURES 1 AND 2).7,8 The ABCDs are primarily an educational tool for patients. This mnemonic was expanded to include E, representing evolution of a pigmented lesion.9 Any pigmented lesion observed to change significantly on serial examinations warrants biopsy to exclude melanoma.
Caveat: not all melanomas are pigmented. Amelanotic melanomas are a diagnostic challenge and may be lethal if left unattended.
Routine screening. Large-scale skin cancer screening has been performed and found to be a statistically ineffective means of detection. Moreover, the US Preventive Services Task Force found insufficient evidence to recommend for or against routine counseling by primary care physicians to prevent skin cancer.10
A large-scale educational and screening campaign was performed in Italy from 1991 to 1996. During this period, 90,000 educational leaflets were distributed to a target population of approximately 243,000. A total of 2050 individuals requested a skin examination, resulting in detection of 13 melanomas.11 However, 92.3% of the melanomas were thin (<1.52 mm deep). Despite the lack of statistical significance for such screenings, many organizations do perform them and find melanomas, which can be life saving for those few individuals.
Anatomic areas to focus on. While melanoma can affect any anatomic region, it is especially common on sun-exposed areas, including the head, neck, and upper extremities. Acral lentiginous melanoma is found on palmar, plantar, and subungual regions. Include the scalp, ocular mucosa, and oral cavity in your examination.
Four primary groups have been traditionally proposed based on a combination of clinical and pathologic features: superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma and acral lentiginous melanoma. Furthermore, additional histopathologic variants including desmoplastic, neurotropic, amelanotic, signet-ring cell, small cell and balloon cell melanoma have been described.
FIGURE 1
Melanoma in situ
Classic presentations of melanoma in situ on the left cheek. Sun-exposed areas are most often affected.
FIGURE 2
Melanoma in situ
Classic presentations of melanoma in situ on the tip of the nose. Sun-exposed areas are most often affected.
Which biopsy technique to choose?
A properly performed biopsy is mandatory to accurately diagnose and microstage the tumor. No other test reliably surpasses routine histologic examination.
When possible, arrange for a full-thickness excisional biopsy with a narrow (2-mm) rim of normal skin, especially for large lesions in which sampling error may be a factor.
Tissue samples are submitted for histologic examination in formalin for permanent sections. Frozen sections are not recommended for the initial diagnosis of melanoma due to artifactual changes from the freezing process.
When lesions are too large for primary excision, or if an optimal cosmetic result is important to the patient, an incisional or punch biopsy may be taken from the area believed to be the deepest portion of the tumor.
For the largest lesions, multiple punch or incisional biopsies may help to reduce the risk of sampling error. Diagnostic and management problems arise when the initial biopsy does not sample the complete skin thickness or when large lesions are not sampled adequately.
Classification of melanoma
The traditional melanoma classification scheme includes several subtypes (see Detailed melanoma classifications). Though these terms have limited clinical utility, we include them for the sake of completeness and because the terminology may arise in consultation with colleagues.
Most important for the prognosis is a determination of the tumor’s depth of invasiveness.
In situ or invasive?
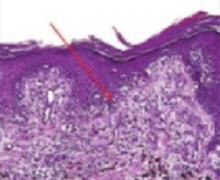
Melanoma in situ lesions are confined to the epidermis and may extend along hair appendages. It often occurs on sun-exposed areas (FIGURES 1 AND 2). Histologically, melanoma in situ is an asymmetric and poorly circumscribed proliferation of melanocytes usually larger than 6 mm in diameter (FIGURE 3).
Melanocytes form irregular nests that are not equidistant and have areas of confluence (FIGURE 3). Single melanocytes predominate over nests in some areas and may entirely replace the basal layer.
Additional histologic features include single melanocytes above the dermal-epidermal junction (Pagetoid spread) and uneven distribution of pigment. In many cases the dermis has inflammatory infiltrates, melanophages, and evidence of solar-damage. Particularly in anatomical areas rich in hair follicles, the neoplastic cells may spread into the epithelium of the hair follicles without extending to the dermis.
Melanoma in situ may be quite large in diameter (horizontal growth phase) without becoming invasive; however, always be concerned about invasion.
Invasive melanoma shares the same histologic features but invades the dermis or subcutaneous fat (vertical growth phase). Aside from the conventional criteria used for the histologic diagnosis of melanoma, acral melanomas may show an increased number of dendritic melanocytes loaded with melanin (FIGURE 4). Desmoplastic melanoma is most commonly found on the head and neck; however, it varies in clinical presentation (FIGURE 5). It is often associated with other types of melanoma, is common in older persons, and has a slight male predilection.
FIGURE 3
Melanocytes
Melanoma in situ stained with routine hematoxylin/eosin on permanent sections. Note confluent nesting of atypical melanocytes at the dermal-epidermal junction (arrow). There are several foci of Pagetoid spread as melanocytes are seen migrating upward toward the surface.
FIGURE 4
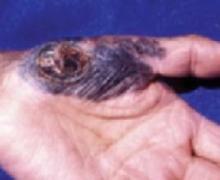
Acral melanoma
Acral melanoma lesion on the hand.
FIGURE 5
Dermoplastic melanoma
Desmoplastic melanoma lesion, commonly found on the head and neck.
Microstaging: The key to good management
The categories “superficial spreading” and “nodular” are based on the seminal work of Wallace Clark, who described putative growth phases of cutaneous melanoma.12 Clark hypothesized that melanoma initially grows horizontally and only later begins an invasive vertical growth phase.
The horizontal growth phase is common in sun-exposed sites and often occurs over a long period of time. The vertical growth phase is a much more aggressive growth pattern that, if left unchecked, can be lethal. This is often seen in nodular melanoma (FIGURE 6).
Measurement of vertical growth (“microstaging”) is the most important prognostic indicator for localized cutaneous melanoma.13
Clark described 5 levels (“Clarks’ levels,” I–V) of invasion (FIGURE 7).
Independently of Clark, Breslow described tumor thickness as an important prognostic factor.14,15 Breslow thickness is measured from the granular layer of the epidermis to the deepest area of invasion (FIGURE 7). Most reports indicate that, overall, Breslow tumor thickness more closely correlates with clinical outcome.16
Special circumstances that affect microstaging. Although Clark and Breslow measurements are both conceptually simple, a number of factors, including hair follicle involvement and ulceration, must be considered when measuring melanoma depth. It is often useful to have a Breslow thickness and a Clark’s level for primary cutaneous melanomas (both methods are used for staging thin primary lesions).
The location of the primary lesion affects interpretation of measurement results. For example, a Breslow thickness of 0.5 mm confers a different meaning on the eyelid (very thin skin, Clark’s level proportionately deeper than tumor thickness might indicate) than the back (thick skin, proportionately more superficial Clark’s level than tumor thickness would indicate).
Measurement of vertical involvement is used to stage the tumor with the TNM classification. This was recently updated as outlined by Balch et al13 in 2001 (TABLE 1).
FIGURE 6
Nodular melanoma
Nodular melanoma often exhibits aggressive vertical growth.
FIGURE 7
Classifying melanoma: Both Clark’s level and Breslow measurement are often used for staging
Clark’s levels are derived from level of tumor invasion compared with layers of the skin. Tumors are divided into 5 levels. Level I: Tumor cells confined to the epidermis (in situ). Level II: Tumor invades the papillary dermis, past basement membrane. level III: Tumor fills papillary dermis, extends to the between the papillary and reticular dermis. Level IV: Tumor invades reticular dermis. Level V: Tumor invasion of subcutaneous tissue. Breslow’s thickness is a measurement of lesion depth in millimeters. Tumors are classified into 4 categories based on the depth vertically from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumor involvement.
TABLE 1
Using the TNM staging system to determine prognosis for melanoma
| T CLASSIFICATION | BRESLOW THICKNESS (MM) | ULCERATION | |||||
| T1 | ≤1.0 | a: no ulceration and Clark’s level II/III | |||||
| b: ulceration or Clark’s level IV/V | |||||||
| T2 | 1.01–2.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| T3 | 2.01–4.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| T4 | >4.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| N CLASSIFICATION | METAST ATIC NODES | NODAL METASTATIC MASS | |||||
| N1 | 1 node | a: micrometastasis* | |||||
| b: macrometastasis† | |||||||
| N2 | 2–3 nodes | a: micrometastasis* | |||||
| b: macrometastasis† | |||||||
| N3 | ≥4 metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic node(s) | ||||||
| M CLASSIFICATION | METASTASES (SITE) | SERUM LDH‡ | |||||
| M1a | Distal skin, subcutaneous/nodal | Normal | |||||
| M1b | Lung | Normal | |||||
| M1c | All other visceral | Normal | |||||
| Any visceral | Elevated | ||||||
| *Micrometastases diagnosed after sentinel or elective lymphadenectomy. | |||||||
| ‡Macrometastases defined as clinically detectable nodal metastases confirmed by therapeutic lymphadenectomy or when nodal metastasis exhibits gross extracapsular extension. | |||||||
| ‡LDH, lactate dehydrogenase. | |||||||
| Once the TNM classification has been determined, the combined findings, as shown below, can help you determine a patient’s relative prognosis. | |||||||
| Clinical Staging | Pathologic Staging | 5-year survival (%) | |||||
| 0 | Tis | N0 | M0 | Tis | N0 | M0 | 96–100 |
| IA | T1a | N0 | M0 | T1a | N0 | M0 | 95 |
| IB | T1b | N0 | M0 | T1b | N0 | M0 | 90 |
| T2a | N0 | M0 | T2a | N0 | M0 | ||
| IIA | T2b | N0 | M0 | T2b | N0 | M0 | 78 |
| T3a | N0 | M0 | T3a | N0 | M0 | ||
| IIB | T3b | N0 | M0 | T3b | N0 | M0 | 65 |
| T4a | N0 | M0 | T4a | N0 | M0 | ||
| IIC | T4b | N0 | M0 | T4b | N0 | M0 | 45 |
| III | Any T | Any N | M0 | ||||
| IIIA | T1-4a | N1a | M0 | 66 | |||
| T1-4a | N2a | M0 | |||||
| IIIB | T1-4b | N1a | M0 | 52 | |||
| T1-4b | N2a | M0 | |||||
| T1-4a | N1b | M0 | |||||
| T1-4a | N2b | M0 | |||||
| T1-4a | N2c | M0 | |||||
| T1-4b | N2c | M0 | |||||
| IIIC | T1-4b | N1b | M0 | 26 | |||
| T1-4b | N2b | M0 | |||||
| Any T | N3 | M0 | |||||
| IV | Any T | Any N | Any M | Any T | Any N | Any M | 7.5–11 |
Preferred management and contingencies
Primary treatment for cutaneous melanoma is wide local excision of the primary site.
How wide should excision margins be? The appropriate margin of normal skin (measured from the biopsy scar or lateral border of residual melanoma) varies with Breslow tumor thickness.17 With thin and intermediate thickness, a surgical margin encompassing 1 cm of normal skin is the consensus.17 For thicker lesions, many authorities recommend a 2-cm margin. A 1-cm margin may be inadequate due to the risk of local recurrence; however, whether a 2 cm or 3 cm margin is optimal remains unclear.18,19TABLE 2 reviews treatment guidelines for surgical excision of primary cutaneous melanoma.20-24
Biopsied tissue submitted as permanent sections. The planned primary excision site should be oriented and submitted to pathology in formalin for permanent section examination. Frozen sections are generally not recommended; when the pathologist assesses surgical margin status, scattered melanocytes in adjacent sun-damaged skin may lead to uncertainty. Moreover, artifacts from the freezing process make interpretation difficult.
2 qualifiers for the above advice: immunostains have increased the utility of frozen sections, and frozen sections were not used in the clinical trials from which current treatment options were derived. These considerations apply particularly to the use of frozen section analysis in Mohs micrographic surgery (see below).
The wide local excision specimen can be examined by multiple surgical pathology orientation methods. In many cases, if the primary lesion was completely excised by the initial biopsy, the pathologist will examine the area of the biopsy scar for residual melanoma and select portions of the lateral margins for examination. Alternatively, the entire lesion, all of the margins, or even the entire specimen may be submitted for examination depending on the clinical circumstances and concerns of the pathologist and surgeon, such as incomplete removal at the time of initial biopsy or close deep or lateral margins. Communication between surgeon and pathologist must be clear and unambiguous.
Examining multiple sections from the tissue block and adding immunohistochemical stains greatly increase sensitivity for metastatic melanoma.37-40 Cochran et al41 originally demonstrated the importance of immunohistochemistry in the pathology examination of lymph nodes. From 2227 lymph nodes removed from 100 patients, they found that 16 additional lymph nodes in 14 patients contained metastatic melanoma when examined with S-100 immunohistostains. Using additional antibodies more recently (see below), these authors reported that up to 12% of metastatic melanoma deposits can be missed by experienced pathologists without the aid of immunohistochemistry.42
Though no antibody is both highly sensitive and specific for malignant melanoma (versus normal melanocytes), the antibodies most commonly used in immunohistochemistry include S-100 (a neuroectodermal tissue maker expressed in nerves, melanocytes, histiocytes and dendritic cells in lymph nodes), HMB 45 (recognizes an oligosaccharide side chain present in immature melanosomes), Melan-A (recognizes the MART-1 protein), and tyrosinase (recognizing the enzyme tyrosinase required for melanin synthesis). S-100, although very sensitive, is not a melanocyte-specific antibody. The remaining antibodies, though melanocyte specific, are non-reactive in 5% to 15% of cutaneous malignant melanoma.42
Using polymerase chain reaction (PCR) and reverse transcriptase polymerase chain reaction (RTPCR) for MRNA for melanocyte-associated proteins further increases sensitivity, detecting positive nodal cells when routine hematoxylin/eosin and immunohistochemical methods do not. The clinical utility of molecular methods for diagnosis and patient management has not been established; these techniques are the subject of ongoing clinical trials.43
In retrospect, it is clear that the standard techniques used to examine lymph nodes taken from regional lymph node dissections underestimate the presence of micrometastatic disease. one could argue, in fact, that the sole advantage of lymphatic mapping and sentinel lymph node biopsy has been to allow the pathologist to perform a focused, extensive examination on 1-3 lymph nodes – an examination that would be impractical (and prohibitively expensive) on a standard regional lymph node dissection specimen. Interestingly, the idea that additional sections from lymph nodes will increase the detection rate of metastatic tumor is not new and was well-known to the surgeons and pathologists involved in the pioneering work on sentinel lymph node biopsy.41
Mohs micrographic surgery increasingly used for melanoma.25-31 This procedure may be performed with frozen or permanent sections, using concomitant immunohistochemical staining with either method of embedding. This has been especially useful in cosmetically sensitive areas such as the face, ear, nose, genitalia, and extremities.30 Mohs micrographic surgery with permanent sections also makes possible the most definitive surgical margin analysis.
TABLE 2
Recommended incision margins
| BRESLOW THICKNESS | EXCISION MARGINS (CM) |
|---|---|
| In situ | 0.5 |
| <1 mm | 1 |
| 1–4 mm | 1–2 |
| >4 mm | 2–3 |
Sentinel lymph node biopsy
Lymphatic mapping and sentinel lymph node biopsy have significantly changed the initial approach to the patient with melanoma and have reinvigorated debate over the importance and management of regional lymph nodes in cutaneous malignant melanoma. The anatomic basis of, and techniques for, lymphatic mapping and sentinel lymph node biopsy are well-described elsewhere and are not reviewed here.32
Risk factors for melanoma include a personal or family history of melanoma and the presence of multiple nevi. Nevi are considered risk factors if an individual has many or if the nevi are unusual in appearance or size. Other risk factors include fair complexion, excessive sun exposure, history of severe sunburns, use of tanning booths, immunosuppression and occupational exposure to certain chemicals.1
Ultraviolet light exposure remains the most well-described risk factor for the development of cutaneous melanoma; however, the pathogenesis remains largely unknown.3,4 This is especially true in patients with increased ultraviolet sensitivity secondary to fair skin type and a tendency to burn. The use of sunscreen is recommended for prevention of all types of skin cancer; however, its use may promote increased ultraviolet exposure from a false sense of protection.5,6 Additionally, proper application (i.e. frequent reapplication) is seldom performed.
Histolopathologic data from regional lymph nodes is the most important prognostic factor for cutaneous melanoma,13 and sentinel lymph node biopsy is proven as a staging procedure—as reflected in the revised AJCC TNM Staging System published in 2001 (TABLES 1 AND 2).13 This staging system differs from the previous one in several ways:
- Thickness and ulceration are the primary predictors of survival with localized melanoma (stages I and II)
- Number of metastatic lymph nodes involved and tumor burden are most important predictors of survival in stage III melanoma
- Anatomic site of metastasis (distant) and presence/absence of elevated lactate dehydrogenase (LDH) are primary predictors of survival with stage IV melanoma
- Ulceration should prompt an overall upstaging for melanoma stages I–III
- Satellite metastases around a primary melanoma and in-transit metastases together indicate a stage III melanoma
- Staging decisions are based on sentinel node biopsy/lymphatic mapping.13
Multiple studies have reviewed the changes between the new and old TNM staging systems for melanoma.13
When to biopsy. Clinical experience supports reserving lymphatic mapping and sentinel lymph node mapping for patients with primary lesions 1 mm or thicker. Some authorities have recommended sentinel lymph node biopsy for lesions <1 mm thick if they are ulcerated or if a discrepancy exists between the Breslow tumor thickness and Clark’s level. For such lesions, biopsy has been recommended for Clark’s level >III.33
Though sentinel lymph node biopsy can be performed successfully after wide local excision, doing it before excision will prevent disruption of lymphatics for node mapping.34 The sentinel lymph node should be submitted to pathology for permanent section analysis. Frozen section analysis is not recommended for melanoma due to its lower sensitivity.35
Though not widely adopted, some surgeons use intraoperative touch prep cytology as part of an initial intraoperative evaluation.36 If the surgeon is prepared to proceed with regional node dissection and a “black” grossly involved node is encountered, intraoperative frozen section may be used to confirm metastastic melanoma. Neither touch preps nor frozen sections are likely to demonstrate the small, isolated metastatic tumor cells commonly identified in small, grossly normal lymph nodes.
What to expect from the pathologist. If blue dye is used for localization, the pathologist should document the presence of that color in the lymph nodes submitted. Outside of a clinical trial, fresh lymph node tissue is not usually preserved for polymerase chain reaction (PCR) or other molecular methods for detecting tumor cells.
Although examination techniques vary in some details among institutions, general principles are well accepted and include submission of the entire lymph node, step or serial sections, and, when initial sections do not reveal metastatic tumor, use of immunohistochemistry for melanocyte-associated antigens (see Immunohistochemical techniques and antibody testing).
Fine points regarding interpretation of sentinel lymph nodes. First, the rationale behind biopsy of sentinel lymph nodes is that the lymphatic system is a potential means of metastasis. However, it is not the only means of spread. Hematogenous and tissue spread are potential mechanisms as well.
Second, the lymphatic system acts as a drain but not a dam for lymphatic flow. Thus a negative lymph node biopsy does not guarantee that a metastasis has not already occurred.
Third, the increased sensitivity of identification of positive sentinel lymph nodes with more specific tests (ie, immunohistochemistry, PCR, rtPCR) calls a negative result into question. A sentinel lymph node that is “negative” with immunohistochemistry may in fact be positive with gene amplification techniques (PCR, rtPCR).
Fourth, surgeons performing sentinel node biopsies vary in thoroughness when identifying which node or nodes contain the dye. Lymphoscintigraphy is more accurate than using dyes; however, many surgeons use both.44 Thus, while sentinel node biopsy has demonstrated a role in staging, its use and the interpretation of results must be performed with these variables in mind.
Regional lymph node dissection: Does it benefit patients?
Patients found to have metastatic melanoma in a sentinel lymph node are advised to undergo regional lymph node dissection. Unfortunately, few data address survival with this therapy. The role of sentinel lymph node biopsy and elective lymph node dissection in the management of cutaneous malignant melanoma generates debate, much of which focuses on alternate interpretations of the data from the era of elective lymph node dissection for intermediate-thickness melanoma.
The debate over elective lymph node dissection has been summarized by others in detail.47
Proponents of an aggressive strategy correctly point out that data from trials are simply not comparable to what is seen in the current era of sentinel lymph node biopsy.
Opponents of an aggressive strategy, who are numerous and vocal,48-50 correctly observe that no survival benefit has been demonstrated for any treatment strategy based on regional lymph node dissection. However, to be balanced, such long-term outcome data are simply not yet available. Certainly questions will continue to arise regarding the theoretical validity and cost-effectiveness of sentinel lymph node biopsy.48,49,51
How good is adjuvant therapy?
Adjuvant therapy for cutaneous melanoma can include regional external beam radiation, systemic cytotoxic chemotherapy, or immunotherapy. Radiation has a limited role, being used postoperatively to treat regional lymph node dissection beds at high risk of recurrence and to treat locally recurrent and in-transit disease.52
Systemic cytotoxin (often dacarbazine) has not been effective as adjuvant therapy.53 When combined chemo- and immunotherapy is used for metastatic disease, it is with the hope of discovering an effective combination that might also be used in the adjuvant setting.54 However, it has been the initial success with immunotherapy that frames the debate regarding adjuvant systemic therapy for cutaneous malignant melanoma.
High-dose interferon alpha 2b. For thick or node-positive cutaneous malignant melanoma, a statistically significant increase in overall survival with high-dose interferon alpha 2b was demonstrated in a landmark Eastern Cooperative Oncology Group trial.55 Though the results have been confirmed in other trials, the clinical benefit is still debated. The expense and toxicity of the trial, as well as the absence of benefit in a second ECOG trial of high-dose systemic therapy, have contributed to the uncertainty.56
In an effort to reduce toxicity, low-dose systemic interferon alpha 2b has been tested in numerous trials and found to be of no benefit.56 High-dose alpha 2b interferon therapy continues to be a focus of active clinical investigation by national and international cooperative groups. In addition to high-dose systemic interferon, a large number of immunotherapeutic approaches for the systemic treatment of melanoma have been devised and are under active investigation, including non-specific immune adjuvants such as Bacile Calmette-Guerin, levaminsole, vaccines prepared from autologous melanoma cells and vaccines designed against chemically defined antigens (gangliocides).57 Because of the controversies surrounding adjuvant systemic therapy, patients are best served by participating in an ongoing clinical trial if the expense and toxicity of high dose systemic interferon alpha 2b are deemed to be unacceptable.
Acknowledgments
We thank Brian C. Brockway, M.S. (Department of Medical Media, Veterans affairs Medical Center, Augusta, Georgia) for illustrations.
CORRESPONDENCE
Joshua E. Lane, MD, 308 Hospital Drive, Suite 200, Macon, GA 31217. E-mail: [email protected]
- Arrange a biopsy of any pigmented lesion that changes significantly on serial examinations.
- Full-thickness excisional biopsy is preferred; for large lesions, incisional or punch biopsy at the deepest point of the tumor may be an option.
- For thin lesions, a surgical margin encompassing 1 cm normal skin is recommended.
- Specimens submitted in formalin for permanent sections are preferred to frozen sections.
A 51-year-old man of northern European descent who works outdoors for the city asks you to look at a “mole” on his face. The lesion does not have the classic appearance of a melanoma that you have seen before, and it is still fairly small. Should you advise a wait-and-see approach or perform a biopsy?
Opt for early detection
Given the patient’s likely genetic predisposition to skin cancer and his regular, lengthy exposure to sunlight (see Risk factors), you would be wise to follow your cutaneous examination with a biopsy.
The ABCDs of visual assessment. The classic clinical presentation of melanoma is well known. The most publicized means for identifying potentially atypical pigmented skin lesions is the “ABCD” mnemonic (asymmetry, border irregularity, color variegation, and lesion diameter >6 mm) (FIGURES 1 AND 2).7,8 The ABCDs are primarily an educational tool for patients. This mnemonic was expanded to include E, representing evolution of a pigmented lesion.9 Any pigmented lesion observed to change significantly on serial examinations warrants biopsy to exclude melanoma.
Caveat: not all melanomas are pigmented. Amelanotic melanomas are a diagnostic challenge and may be lethal if left unattended.
Routine screening. Large-scale skin cancer screening has been performed and found to be a statistically ineffective means of detection. Moreover, the US Preventive Services Task Force found insufficient evidence to recommend for or against routine counseling by primary care physicians to prevent skin cancer.10
A large-scale educational and screening campaign was performed in Italy from 1991 to 1996. During this period, 90,000 educational leaflets were distributed to a target population of approximately 243,000. A total of 2050 individuals requested a skin examination, resulting in detection of 13 melanomas.11 However, 92.3% of the melanomas were thin (<1.52 mm deep). Despite the lack of statistical significance for such screenings, many organizations do perform them and find melanomas, which can be life saving for those few individuals.
Anatomic areas to focus on. While melanoma can affect any anatomic region, it is especially common on sun-exposed areas, including the head, neck, and upper extremities. Acral lentiginous melanoma is found on palmar, plantar, and subungual regions. Include the scalp, ocular mucosa, and oral cavity in your examination.
Four primary groups have been traditionally proposed based on a combination of clinical and pathologic features: superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma and acral lentiginous melanoma. Furthermore, additional histopathologic variants including desmoplastic, neurotropic, amelanotic, signet-ring cell, small cell and balloon cell melanoma have been described.
FIGURE 1
Melanoma in situ
Classic presentations of melanoma in situ on the left cheek. Sun-exposed areas are most often affected.
FIGURE 2
Melanoma in situ
Classic presentations of melanoma in situ on the tip of the nose. Sun-exposed areas are most often affected.
Which biopsy technique to choose?
A properly performed biopsy is mandatory to accurately diagnose and microstage the tumor. No other test reliably surpasses routine histologic examination.
When possible, arrange for a full-thickness excisional biopsy with a narrow (2-mm) rim of normal skin, especially for large lesions in which sampling error may be a factor.
Tissue samples are submitted for histologic examination in formalin for permanent sections. Frozen sections are not recommended for the initial diagnosis of melanoma due to artifactual changes from the freezing process.
When lesions are too large for primary excision, or if an optimal cosmetic result is important to the patient, an incisional or punch biopsy may be taken from the area believed to be the deepest portion of the tumor.
For the largest lesions, multiple punch or incisional biopsies may help to reduce the risk of sampling error. Diagnostic and management problems arise when the initial biopsy does not sample the complete skin thickness or when large lesions are not sampled adequately.
Classification of melanoma
The traditional melanoma classification scheme includes several subtypes (see Detailed melanoma classifications). Though these terms have limited clinical utility, we include them for the sake of completeness and because the terminology may arise in consultation with colleagues.
Most important for the prognosis is a determination of the tumor’s depth of invasiveness.
In situ or invasive?
Melanoma in situ lesions are confined to the epidermis and may extend along hair appendages. It often occurs on sun-exposed areas (FIGURES 1 AND 2). Histologically, melanoma in situ is an asymmetric and poorly circumscribed proliferation of melanocytes usually larger than 6 mm in diameter (FIGURE 3).
Melanocytes form irregular nests that are not equidistant and have areas of confluence (FIGURE 3). Single melanocytes predominate over nests in some areas and may entirely replace the basal layer.
Additional histologic features include single melanocytes above the dermal-epidermal junction (Pagetoid spread) and uneven distribution of pigment. In many cases the dermis has inflammatory infiltrates, melanophages, and evidence of solar-damage. Particularly in anatomical areas rich in hair follicles, the neoplastic cells may spread into the epithelium of the hair follicles without extending to the dermis.
Melanoma in situ may be quite large in diameter (horizontal growth phase) without becoming invasive; however, always be concerned about invasion.
Invasive melanoma shares the same histologic features but invades the dermis or subcutaneous fat (vertical growth phase). Aside from the conventional criteria used for the histologic diagnosis of melanoma, acral melanomas may show an increased number of dendritic melanocytes loaded with melanin (FIGURE 4). Desmoplastic melanoma is most commonly found on the head and neck; however, it varies in clinical presentation (FIGURE 5). It is often associated with other types of melanoma, is common in older persons, and has a slight male predilection.
FIGURE 3
Melanocytes
Melanoma in situ stained with routine hematoxylin/eosin on permanent sections. Note confluent nesting of atypical melanocytes at the dermal-epidermal junction (arrow). There are several foci of Pagetoid spread as melanocytes are seen migrating upward toward the surface.
FIGURE 4
Acral melanoma
Acral melanoma lesion on the hand.
FIGURE 5
Dermoplastic melanoma
Desmoplastic melanoma lesion, commonly found on the head and neck.
Microstaging: The key to good management
The categories “superficial spreading” and “nodular” are based on the seminal work of Wallace Clark, who described putative growth phases of cutaneous melanoma.12 Clark hypothesized that melanoma initially grows horizontally and only later begins an invasive vertical growth phase.
The horizontal growth phase is common in sun-exposed sites and often occurs over a long period of time. The vertical growth phase is a much more aggressive growth pattern that, if left unchecked, can be lethal. This is often seen in nodular melanoma (FIGURE 6).
Measurement of vertical growth (“microstaging”) is the most important prognostic indicator for localized cutaneous melanoma.13
Clark described 5 levels (“Clarks’ levels,” I–V) of invasion (FIGURE 7).
Independently of Clark, Breslow described tumor thickness as an important prognostic factor.14,15 Breslow thickness is measured from the granular layer of the epidermis to the deepest area of invasion (FIGURE 7). Most reports indicate that, overall, Breslow tumor thickness more closely correlates with clinical outcome.16
Special circumstances that affect microstaging. Although Clark and Breslow measurements are both conceptually simple, a number of factors, including hair follicle involvement and ulceration, must be considered when measuring melanoma depth. It is often useful to have a Breslow thickness and a Clark’s level for primary cutaneous melanomas (both methods are used for staging thin primary lesions).
The location of the primary lesion affects interpretation of measurement results. For example, a Breslow thickness of 0.5 mm confers a different meaning on the eyelid (very thin skin, Clark’s level proportionately deeper than tumor thickness might indicate) than the back (thick skin, proportionately more superficial Clark’s level than tumor thickness would indicate).
Measurement of vertical involvement is used to stage the tumor with the TNM classification. This was recently updated as outlined by Balch et al13 in 2001 (TABLE 1).
FIGURE 6
Nodular melanoma
Nodular melanoma often exhibits aggressive vertical growth.
FIGURE 7
Classifying melanoma: Both Clark’s level and Breslow measurement are often used for staging
Clark’s levels are derived from level of tumor invasion compared with layers of the skin. Tumors are divided into 5 levels. Level I: Tumor cells confined to the epidermis (in situ). Level II: Tumor invades the papillary dermis, past basement membrane. level III: Tumor fills papillary dermis, extends to the between the papillary and reticular dermis. Level IV: Tumor invades reticular dermis. Level V: Tumor invasion of subcutaneous tissue. Breslow’s thickness is a measurement of lesion depth in millimeters. Tumors are classified into 4 categories based on the depth vertically from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumor involvement.
TABLE 1
Using the TNM staging system to determine prognosis for melanoma
| T CLASSIFICATION | BRESLOW THICKNESS (MM) | ULCERATION | |||||
| T1 | ≤1.0 | a: no ulceration and Clark’s level II/III | |||||
| b: ulceration or Clark’s level IV/V | |||||||
| T2 | 1.01–2.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| T3 | 2.01–4.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| T4 | >4.0 | a: no ulceration | |||||
| b: with ulceration | |||||||
| N CLASSIFICATION | METAST ATIC NODES | NODAL METASTATIC MASS | |||||
| N1 | 1 node | a: micrometastasis* | |||||
| b: macrometastasis† | |||||||
| N2 | 2–3 nodes | a: micrometastasis* | |||||
| b: macrometastasis† | |||||||
| N3 | ≥4 metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic node(s) | ||||||
| M CLASSIFICATION | METASTASES (SITE) | SERUM LDH‡ | |||||
| M1a | Distal skin, subcutaneous/nodal | Normal | |||||
| M1b | Lung | Normal | |||||
| M1c | All other visceral | Normal | |||||
| Any visceral | Elevated | ||||||
| *Micrometastases diagnosed after sentinel or elective lymphadenectomy. | |||||||
| ‡Macrometastases defined as clinically detectable nodal metastases confirmed by therapeutic lymphadenectomy or when nodal metastasis exhibits gross extracapsular extension. | |||||||
| ‡LDH, lactate dehydrogenase. | |||||||
| Once the TNM classification has been determined, the combined findings, as shown below, can help you determine a patient’s relative prognosis. | |||||||
| Clinical Staging | Pathologic Staging | 5-year survival (%) | |||||
| 0 | Tis | N0 | M0 | Tis | N0 | M0 | 96–100 |
| IA | T1a | N0 | M0 | T1a | N0 | M0 | 95 |
| IB | T1b | N0 | M0 | T1b | N0 | M0 | 90 |
| T2a | N0 | M0 | T2a | N0 | M0 | ||
| IIA | T2b | N0 | M0 | T2b | N0 | M0 | 78 |
| T3a | N0 | M0 | T3a | N0 | M0 | ||
| IIB | T3b | N0 | M0 | T3b | N0 | M0 | 65 |
| T4a | N0 | M0 | T4a | N0 | M0 | ||
| IIC | T4b | N0 | M0 | T4b | N0 | M0 | 45 |
| III | Any T | Any N | M0 | ||||
| IIIA | T1-4a | N1a | M0 | 66 | |||
| T1-4a | N2a | M0 | |||||
| IIIB | T1-4b | N1a | M0 | 52 | |||
| T1-4b | N2a | M0 | |||||
| T1-4a | N1b | M0 | |||||
| T1-4a | N2b | M0 | |||||
| T1-4a | N2c | M0 | |||||
| T1-4b | N2c | M0 | |||||
| IIIC | T1-4b | N1b | M0 | 26 | |||
| T1-4b | N2b | M0 | |||||
| Any T | N3 | M0 | |||||
| IV | Any T | Any N | Any M | Any T | Any N | Any M | 7.5–11 |
Preferred management and contingencies
Primary treatment for cutaneous melanoma is wide local excision of the primary site.
How wide should excision margins be? The appropriate margin of normal skin (measured from the biopsy scar or lateral border of residual melanoma) varies with Breslow tumor thickness.17 With thin and intermediate thickness, a surgical margin encompassing 1 cm of normal skin is the consensus.17 For thicker lesions, many authorities recommend a 2-cm margin. A 1-cm margin may be inadequate due to the risk of local recurrence; however, whether a 2 cm or 3 cm margin is optimal remains unclear.18,19TABLE 2 reviews treatment guidelines for surgical excision of primary cutaneous melanoma.20-24
Biopsied tissue submitted as permanent sections. The planned primary excision site should be oriented and submitted to pathology in formalin for permanent section examination. Frozen sections are generally not recommended; when the pathologist assesses surgical margin status, scattered melanocytes in adjacent sun-damaged skin may lead to uncertainty. Moreover, artifacts from the freezing process make interpretation difficult.
2 qualifiers for the above advice: immunostains have increased the utility of frozen sections, and frozen sections were not used in the clinical trials from which current treatment options were derived. These considerations apply particularly to the use of frozen section analysis in Mohs micrographic surgery (see below).
The wide local excision specimen can be examined by multiple surgical pathology orientation methods. In many cases, if the primary lesion was completely excised by the initial biopsy, the pathologist will examine the area of the biopsy scar for residual melanoma and select portions of the lateral margins for examination. Alternatively, the entire lesion, all of the margins, or even the entire specimen may be submitted for examination depending on the clinical circumstances and concerns of the pathologist and surgeon, such as incomplete removal at the time of initial biopsy or close deep or lateral margins. Communication between surgeon and pathologist must be clear and unambiguous.
Examining multiple sections from the tissue block and adding immunohistochemical stains greatly increase sensitivity for metastatic melanoma.37-40 Cochran et al41 originally demonstrated the importance of immunohistochemistry in the pathology examination of lymph nodes. From 2227 lymph nodes removed from 100 patients, they found that 16 additional lymph nodes in 14 patients contained metastatic melanoma when examined with S-100 immunohistostains. Using additional antibodies more recently (see below), these authors reported that up to 12% of metastatic melanoma deposits can be missed by experienced pathologists without the aid of immunohistochemistry.42
Though no antibody is both highly sensitive and specific for malignant melanoma (versus normal melanocytes), the antibodies most commonly used in immunohistochemistry include S-100 (a neuroectodermal tissue maker expressed in nerves, melanocytes, histiocytes and dendritic cells in lymph nodes), HMB 45 (recognizes an oligosaccharide side chain present in immature melanosomes), Melan-A (recognizes the MART-1 protein), and tyrosinase (recognizing the enzyme tyrosinase required for melanin synthesis). S-100, although very sensitive, is not a melanocyte-specific antibody. The remaining antibodies, though melanocyte specific, are non-reactive in 5% to 15% of cutaneous malignant melanoma.42
Using polymerase chain reaction (PCR) and reverse transcriptase polymerase chain reaction (RTPCR) for MRNA for melanocyte-associated proteins further increases sensitivity, detecting positive nodal cells when routine hematoxylin/eosin and immunohistochemical methods do not. The clinical utility of molecular methods for diagnosis and patient management has not been established; these techniques are the subject of ongoing clinical trials.43
In retrospect, it is clear that the standard techniques used to examine lymph nodes taken from regional lymph node dissections underestimate the presence of micrometastatic disease. one could argue, in fact, that the sole advantage of lymphatic mapping and sentinel lymph node biopsy has been to allow the pathologist to perform a focused, extensive examination on 1-3 lymph nodes – an examination that would be impractical (and prohibitively expensive) on a standard regional lymph node dissection specimen. Interestingly, the idea that additional sections from lymph nodes will increase the detection rate of metastatic tumor is not new and was well-known to the surgeons and pathologists involved in the pioneering work on sentinel lymph node biopsy.41
Mohs micrographic surgery increasingly used for melanoma.25-31 This procedure may be performed with frozen or permanent sections, using concomitant immunohistochemical staining with either method of embedding. This has been especially useful in cosmetically sensitive areas such as the face, ear, nose, genitalia, and extremities.30 Mohs micrographic surgery with permanent sections also makes possible the most definitive surgical margin analysis.
TABLE 2
Recommended incision margins
| BRESLOW THICKNESS | EXCISION MARGINS (CM) |
|---|---|
| In situ | 0.5 |
| <1 mm | 1 |
| 1–4 mm | 1–2 |
| >4 mm | 2–3 |
Sentinel lymph node biopsy
Lymphatic mapping and sentinel lymph node biopsy have significantly changed the initial approach to the patient with melanoma and have reinvigorated debate over the importance and management of regional lymph nodes in cutaneous malignant melanoma. The anatomic basis of, and techniques for, lymphatic mapping and sentinel lymph node biopsy are well-described elsewhere and are not reviewed here.32
Risk factors for melanoma include a personal or family history of melanoma and the presence of multiple nevi. Nevi are considered risk factors if an individual has many or if the nevi are unusual in appearance or size. Other risk factors include fair complexion, excessive sun exposure, history of severe sunburns, use of tanning booths, immunosuppression and occupational exposure to certain chemicals.1
Ultraviolet light exposure remains the most well-described risk factor for the development of cutaneous melanoma; however, the pathogenesis remains largely unknown.3,4 This is especially true in patients with increased ultraviolet sensitivity secondary to fair skin type and a tendency to burn. The use of sunscreen is recommended for prevention of all types of skin cancer; however, its use may promote increased ultraviolet exposure from a false sense of protection.5,6 Additionally, proper application (i.e. frequent reapplication) is seldom performed.
Histolopathologic data from regional lymph nodes is the most important prognostic factor for cutaneous melanoma,13 and sentinel lymph node biopsy is proven as a staging procedure—as reflected in the revised AJCC TNM Staging System published in 2001 (TABLES 1 AND 2).13 This staging system differs from the previous one in several ways:
- Thickness and ulceration are the primary predictors of survival with localized melanoma (stages I and II)
- Number of metastatic lymph nodes involved and tumor burden are most important predictors of survival in stage III melanoma
- Anatomic site of metastasis (distant) and presence/absence of elevated lactate dehydrogenase (LDH) are primary predictors of survival with stage IV melanoma
- Ulceration should prompt an overall upstaging for melanoma stages I–III
- Satellite metastases around a primary melanoma and in-transit metastases together indicate a stage III melanoma
- Staging decisions are based on sentinel node biopsy/lymphatic mapping.13
Multiple studies have reviewed the changes between the new and old TNM staging systems for melanoma.13
When to biopsy. Clinical experience supports reserving lymphatic mapping and sentinel lymph node mapping for patients with primary lesions 1 mm or thicker. Some authorities have recommended sentinel lymph node biopsy for lesions <1 mm thick if they are ulcerated or if a discrepancy exists between the Breslow tumor thickness and Clark’s level. For such lesions, biopsy has been recommended for Clark’s level >III.33
Though sentinel lymph node biopsy can be performed successfully after wide local excision, doing it before excision will prevent disruption of lymphatics for node mapping.34 The sentinel lymph node should be submitted to pathology for permanent section analysis. Frozen section analysis is not recommended for melanoma due to its lower sensitivity.35
Though not widely adopted, some surgeons use intraoperative touch prep cytology as part of an initial intraoperative evaluation.36 If the surgeon is prepared to proceed with regional node dissection and a “black” grossly involved node is encountered, intraoperative frozen section may be used to confirm metastastic melanoma. Neither touch preps nor frozen sections are likely to demonstrate the small, isolated metastatic tumor cells commonly identified in small, grossly normal lymph nodes.
What to expect from the pathologist. If blue dye is used for localization, the pathologist should document the presence of that color in the lymph nodes submitted. Outside of a clinical trial, fresh lymph node tissue is not usually preserved for polymerase chain reaction (PCR) or other molecular methods for detecting tumor cells.
Although examination techniques vary in some details among institutions, general principles are well accepted and include submission of the entire lymph node, step or serial sections, and, when initial sections do not reveal metastatic tumor, use of immunohistochemistry for melanocyte-associated antigens (see Immunohistochemical techniques and antibody testing).
Fine points regarding interpretation of sentinel lymph nodes. First, the rationale behind biopsy of sentinel lymph nodes is that the lymphatic system is a potential means of metastasis. However, it is not the only means of spread. Hematogenous and tissue spread are potential mechanisms as well.
Second, the lymphatic system acts as a drain but not a dam for lymphatic flow. Thus a negative lymph node biopsy does not guarantee that a metastasis has not already occurred.
Third, the increased sensitivity of identification of positive sentinel lymph nodes with more specific tests (ie, immunohistochemistry, PCR, rtPCR) calls a negative result into question. A sentinel lymph node that is “negative” with immunohistochemistry may in fact be positive with gene amplification techniques (PCR, rtPCR).
Fourth, surgeons performing sentinel node biopsies vary in thoroughness when identifying which node or nodes contain the dye. Lymphoscintigraphy is more accurate than using dyes; however, many surgeons use both.44 Thus, while sentinel node biopsy has demonstrated a role in staging, its use and the interpretation of results must be performed with these variables in mind.
Regional lymph node dissection: Does it benefit patients?
Patients found to have metastatic melanoma in a sentinel lymph node are advised to undergo regional lymph node dissection. Unfortunately, few data address survival with this therapy. The role of sentinel lymph node biopsy and elective lymph node dissection in the management of cutaneous malignant melanoma generates debate, much of which focuses on alternate interpretations of the data from the era of elective lymph node dissection for intermediate-thickness melanoma.
The debate over elective lymph node dissection has been summarized by others in detail.47
Proponents of an aggressive strategy correctly point out that data from trials are simply not comparable to what is seen in the current era of sentinel lymph node biopsy.
Opponents of an aggressive strategy, who are numerous and vocal,48-50 correctly observe that no survival benefit has been demonstrated for any treatment strategy based on regional lymph node dissection. However, to be balanced, such long-term outcome data are simply not yet available. Certainly questions will continue to arise regarding the theoretical validity and cost-effectiveness of sentinel lymph node biopsy.48,49,51
How good is adjuvant therapy?
Adjuvant therapy for cutaneous melanoma can include regional external beam radiation, systemic cytotoxic chemotherapy, or immunotherapy. Radiation has a limited role, being used postoperatively to treat regional lymph node dissection beds at high risk of recurrence and to treat locally recurrent and in-transit disease.52
Systemic cytotoxin (often dacarbazine) has not been effective as adjuvant therapy.53 When combined chemo- and immunotherapy is used for metastatic disease, it is with the hope of discovering an effective combination that might also be used in the adjuvant setting.54 However, it has been the initial success with immunotherapy that frames the debate regarding adjuvant systemic therapy for cutaneous malignant melanoma.
High-dose interferon alpha 2b. For thick or node-positive cutaneous malignant melanoma, a statistically significant increase in overall survival with high-dose interferon alpha 2b was demonstrated in a landmark Eastern Cooperative Oncology Group trial.55 Though the results have been confirmed in other trials, the clinical benefit is still debated. The expense and toxicity of the trial, as well as the absence of benefit in a second ECOG trial of high-dose systemic therapy, have contributed to the uncertainty.56
In an effort to reduce toxicity, low-dose systemic interferon alpha 2b has been tested in numerous trials and found to be of no benefit.56 High-dose alpha 2b interferon therapy continues to be a focus of active clinical investigation by national and international cooperative groups. In addition to high-dose systemic interferon, a large number of immunotherapeutic approaches for the systemic treatment of melanoma have been devised and are under active investigation, including non-specific immune adjuvants such as Bacile Calmette-Guerin, levaminsole, vaccines prepared from autologous melanoma cells and vaccines designed against chemically defined antigens (gangliocides).57 Because of the controversies surrounding adjuvant systemic therapy, patients are best served by participating in an ongoing clinical trial if the expense and toxicity of high dose systemic interferon alpha 2b are deemed to be unacceptable.
Acknowledgments
We thank Brian C. Brockway, M.S. (Department of Medical Media, Veterans affairs Medical Center, Augusta, Georgia) for illustrations.
CORRESPONDENCE
Joshua E. Lane, MD, 308 Hospital Drive, Suite 200, Macon, GA 31217. E-mail: [email protected]
1. American Cancer Society Cancer Facts and Figures 2006. Atlanta: american Cancer Society; 2006.
2. Anonymous. Melanoma: Clinical Practice Guidelines in Oncology. JNCCN 2004;2:46-60.
3. Elwood JM. Melanoma and sun exposure. Semin Oncol 1996;23:650-666.
4. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer 1997;73:198-203.
5. Whiteman DC, Green AC. Melanoma and sun exposure: where are we now? Int J Dermatol 1999;38:481-489.
6. Katsambas A, Nicolaidou E. Cutaneous malignant melanoma and sun exposure. Recent developments in epidemiology. Arch Dermatol 1996;132:444-450.
7. Polk HC. Surgical progress and understanding in the treatment of the melanoma epidemic. Am J Surg 1999;178:443-448.
8. Baade PD, Balanda KP, Stanton WR, et al. Community perceptions about the important signs of early melanoma. J Am Acad Dermatol 1997;36:199-202.
9. Abbasi NR, Shaw, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004;292:2771-2776.
10. Counseling to prevent skin cancer: recommendations and rationale of the U.S. Preventive Services Task Force. MMWR Recomm Rep 2003;52:13-17.
11. Rossi CR, Vecchiato A, Bezze G, et al. Early detection of melanoma: an educational campaign in Padova, Italy. Melanoma Res 2000;10:181-187.
12. Clark WH, From L, Bernardino EA, et al. Histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 1969;29:705-726.
13. Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635-3648.
14. Breslow A. Tumor thickness, level of invasion and node dissection in Stage 1 cutaneous melanoma. Ann Surg 1976;182:572-575.
15. Breslow A. Thickness, cross sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 1970;172:902-908.
16. Barnhill RL, Fine JA, Roush GC, et al. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer 1996;78:427-432.
17. Lens MD, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma: a systematic review of randomized controlled trials comparing marrow vs wide excision. Arch Surg 2002;137:1101-1105.
18. Brown SE, Chapman PB. Defining adequate surgery for primary melanoma. N Engl J Med 2004;350:823-825.
19. Thomas JM, Newton-Bishop J, A’Hern R, et al. Excision margins in high-risk melanoma. N Engl J Med 2004;350:757-766.
20. Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg 1991;126:438-441.
21. Bono A, Bartoli C, Clemente C, et al. Ambulatory narrow excision for thin melanoma (< or=2 mm): results of a prospective study. Eur J Cancer 1997;33:1330-1332.
22. Cascinelli N. Margin of resection in the management of primary melanoma. Semin Surg Oncol 1998;14:272-275.
23. Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol 2001;8:101-108.
24. Johnson TM, Sondak VK. A centimeter here, a centimeter there: does it matter? J Am Acad Dermatol 1995;33:532-534.
25. Zitelli JA, Brown C, Hanusa BH. Mohs micrographic surgery for the treatment of primary cutaneous melanoma. J Am Acad Dermatol 1997;37:236-245.
26. Zalla MJ, Lim KK, Dicaudo DJ, et al. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg 2000;26:771-884.
27. Kelley LC, Starkus L. Immunohistochemical staining of lentigo maligna during Mohs micrographic surgery using MART-1. J Am Acad Dermatol 2002;46:78-84.
28. Nagi C, O’Grady TC, Izadpanah A. Mohs micrographically controlled surgery and the treatment of malignant melanoma. Semin Oncol 2002;29:336-340.
29. Carucci JA. Mohs’ micrographic surgery for the treatment of melanoma. Dermatol Clin 2002;20:701-708.
30. Bienert TN, Trotter MJ, Arlette JP. Treatment of cutaneous melanoma of the face by Mohs micrographic surgery. J Cutan Med Surg 2003;7:25-30.
31. Cook J. Surgical margins for resection of primary cutaneous melanoma. Clin Dermatol 2004;22:228-233.
32. Jakub JW, Pendas S, Reintgen DS. Current status of sentinel lymph node mapping and biopsy: facts and controversies. Oncologist 2003;8:59-68.
33. Jacobs IA, Chang CK, DasGupta TK, et al. Role of sentinel lymph node biopsy in patients with thin (<1mm) primary cutaneous melanoma. Ann Surg Oncol 2003;10:558-561.
34. Evans HL, Krag DN, Teates D, et al. Lymphoscintigraphy and sentinel node bipsy accurately stage melanoma in patients presenting after wide local excision. Ann Surg Oncol 2003;10:416-425.
35. Tanis PJ, Boom RP, Koops HS, et al. Frozen section investigation of the sentinel node in malignant melanoma and breast cancer. Ann Surg Oncol 2002;8:222-226.
36. Greeger AJ, Shiver SA, Shen P, et al. Intraoperative evaluation of sentinel lymph nodes for metastatic melanoma by imprint cytology. Cancer 2002;94:3016-3022.
37. Gietema HA, Vuylesteke RJ, de Jonge IA, et al. Sentinel node investigation in melanoma: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2004;57:618-620.
38. Torrenga H, Rahusen FD, Meijer S, et al. Sentinel node investigation in breast cancer: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2001;54:550-552.
39. Abrahamsen HN, Hamilton-Dutoit SJ, Larsen J, et al. Sentinel lymph nodes in malignant melanoma: extended histopathologic evaluation improves diagnostic precision. Cancer 2004;100:1683-1691.
40. Ross GL, Shoaib T, Scott J, et al. The impact of immunohistochemistry on sentinel node biopsy for primary cutaneous malignant melanoma. Br J Plast Surg 2003;56:153-155.
41. Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol 1988;12:612-618.
42. Robers A, Cochran AJ. Pathologic analysis of sentinel lymph ndoes in melanoma patients: current and future trends. J Surg Oncol 2004;85:152-161.
43. Reintgen D, Pendas S, Jakub J, et al. National trials involving lymphatic mapping for melanoma: the multicenter selective lymphadenectomy trial, the sunbelt melanoma trial, and the Florida melanoma trial. Sem Oncol 2004;31:363-373.
44. Caprio MG, Carbone G, Bracigliano A, et al. Sentinel lymph node detection by Lymphoscintigraphy in malignant melanoma. Tumori 2002;88:543-545.
45. Maffioli L, Sturm E, Roselli M, et al. State of the art sentinel node biopsy in Oncology. Tumori 2000;86:263-272.
46. Bartolomei M, Testori A, Chinol M, et al. Sentinel node localization in cutaneous melanoma: lymphoscintigraphy with colloids and antibody fragments versus blue dye mapping. Eur J Nucl Med 1998;25:1489-1494.
47. Balch CM. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol 1988;6:163-172.
48. Medalie NS, Ackerman AB. Sentinel lymph node biopsy has no benefit for patients with primary cutaneous melanoma metastatic to a lymph node: An assertion based on comprehensive, critical analysis: Part I. Am J Dermatopathol 2003;25:399-417.
49. Medalie NS, Ackerman AB. Sentinel lymph node biopsy has no benefit for patients with primary cutaneous melanoma metastatic to a lymph node: an assertion based on comprehensive, critical analysis: Part II. Am J Dermatopathol 2003;25:473-484.
50. Eedy DJ. Introducing controversies in dermatology: sentinel lymph node biopsy for cutaneous melanoma—a useful technique or a waste of time? Br J Dermatol 2004;151:267-268.
51. Agnese DM, Abdessalam SF, Burak WE, et al. Cost-effectiveness of sentinel lymph node biopsy in thin melanomas. Surgery 2003;134:542-547.
52. Ang KK, Gera FB, Byers RM, et al. Radiotherapy for melanoma. In: Cutaneous Melanoma, Balch CM, Houghton AN, Sober AJ, Soong S, eds. St. Louis, Mo: Quality Medical Publishing; 1998:389–404.
53. Argawala SS, Neuberg D, Park Y, et al. Mature results of a phase III randomized trial of bacillus calmetteguerin (BCG) versus observation and BCG versus dacarbazine bersus BCG in the adjuvant therapy of American joint committee on cancer State I-III melanoma (E1673). Cancer 2004;100:692-698.
54. Groenewegen G, Bloem A, De Gast GC. Phase I/II study of sequential chemoimmunotherapy (SCIT) for metastatic melanoma: outpatient treatment with dacarbazine, granulocyte-macrophage colony-stimulating factor, low-dose interleukin-2, and interferon-alpha. Cancer Immunol Immunother 2002;51:630-636.
55. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17.
56. Moschos SJ, Kirkwood JM, Konstantinopolis PA. Present status and future prospects for adjuvant therapy of melanoma: time to build upon the foundation of high-dose interferon alfa-2b. J Clin Oncol 2004;22:11-14.
57. Lotze MT, Dalla RM, Kirkwood JM, et al. Cutaneous Melanoma. In: Cancer: Principles and Practice of Oncology, Devita VT, Hellma S, Rosenberg SA, eds. Philadelphia, Pa: lippincott Williams & Wilkins; 2001:2021–2056.
1. American Cancer Society Cancer Facts and Figures 2006. Atlanta: american Cancer Society; 2006.
2. Anonymous. Melanoma: Clinical Practice Guidelines in Oncology. JNCCN 2004;2:46-60.
3. Elwood JM. Melanoma and sun exposure. Semin Oncol 1996;23:650-666.
4. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer 1997;73:198-203.
5. Whiteman DC, Green AC. Melanoma and sun exposure: where are we now? Int J Dermatol 1999;38:481-489.
6. Katsambas A, Nicolaidou E. Cutaneous malignant melanoma and sun exposure. Recent developments in epidemiology. Arch Dermatol 1996;132:444-450.
7. Polk HC. Surgical progress and understanding in the treatment of the melanoma epidemic. Am J Surg 1999;178:443-448.
8. Baade PD, Balanda KP, Stanton WR, et al. Community perceptions about the important signs of early melanoma. J Am Acad Dermatol 1997;36:199-202.
9. Abbasi NR, Shaw, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004;292:2771-2776.
10. Counseling to prevent skin cancer: recommendations and rationale of the U.S. Preventive Services Task Force. MMWR Recomm Rep 2003;52:13-17.
11. Rossi CR, Vecchiato A, Bezze G, et al. Early detection of melanoma: an educational campaign in Padova, Italy. Melanoma Res 2000;10:181-187.
12. Clark WH, From L, Bernardino EA, et al. Histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 1969;29:705-726.
13. Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635-3648.
14. Breslow A. Tumor thickness, level of invasion and node dissection in Stage 1 cutaneous melanoma. Ann Surg 1976;182:572-575.
15. Breslow A. Thickness, cross sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 1970;172:902-908.
16. Barnhill RL, Fine JA, Roush GC, et al. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer 1996;78:427-432.
17. Lens MD, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma: a systematic review of randomized controlled trials comparing marrow vs wide excision. Arch Surg 2002;137:1101-1105.
18. Brown SE, Chapman PB. Defining adequate surgery for primary melanoma. N Engl J Med 2004;350:823-825.
19. Thomas JM, Newton-Bishop J, A’Hern R, et al. Excision margins in high-risk melanoma. N Engl J Med 2004;350:757-766.
20. Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg 1991;126:438-441.
21. Bono A, Bartoli C, Clemente C, et al. Ambulatory narrow excision for thin melanoma (< or=2 mm): results of a prospective study. Eur J Cancer 1997;33:1330-1332.
22. Cascinelli N. Margin of resection in the management of primary melanoma. Semin Surg Oncol 1998;14:272-275.
23. Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol 2001;8:101-108.
24. Johnson TM, Sondak VK. A centimeter here, a centimeter there: does it matter? J Am Acad Dermatol 1995;33:532-534.
25. Zitelli JA, Brown C, Hanusa BH. Mohs micrographic surgery for the treatment of primary cutaneous melanoma. J Am Acad Dermatol 1997;37:236-245.
26. Zalla MJ, Lim KK, Dicaudo DJ, et al. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg 2000;26:771-884.
27. Kelley LC, Starkus L. Immunohistochemical staining of lentigo maligna during Mohs micrographic surgery using MART-1. J Am Acad Dermatol 2002;46:78-84.
28. Nagi C, O’Grady TC, Izadpanah A. Mohs micrographically controlled surgery and the treatment of malignant melanoma. Semin Oncol 2002;29:336-340.
29. Carucci JA. Mohs’ micrographic surgery for the treatment of melanoma. Dermatol Clin 2002;20:701-708.
30. Bienert TN, Trotter MJ, Arlette JP. Treatment of cutaneous melanoma of the face by Mohs micrographic surgery. J Cutan Med Surg 2003;7:25-30.
31. Cook J. Surgical margins for resection of primary cutaneous melanoma. Clin Dermatol 2004;22:228-233.
32. Jakub JW, Pendas S, Reintgen DS. Current status of sentinel lymph node mapping and biopsy: facts and controversies. Oncologist 2003;8:59-68.
33. Jacobs IA, Chang CK, DasGupta TK, et al. Role of sentinel lymph node biopsy in patients with thin (<1mm) primary cutaneous melanoma. Ann Surg Oncol 2003;10:558-561.
34. Evans HL, Krag DN, Teates D, et al. Lymphoscintigraphy and sentinel node bipsy accurately stage melanoma in patients presenting after wide local excision. Ann Surg Oncol 2003;10:416-425.
35. Tanis PJ, Boom RP, Koops HS, et al. Frozen section investigation of the sentinel node in malignant melanoma and breast cancer. Ann Surg Oncol 2002;8:222-226.
36. Greeger AJ, Shiver SA, Shen P, et al. Intraoperative evaluation of sentinel lymph nodes for metastatic melanoma by imprint cytology. Cancer 2002;94:3016-3022.
37. Gietema HA, Vuylesteke RJ, de Jonge IA, et al. Sentinel node investigation in melanoma: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2004;57:618-620.
38. Torrenga H, Rahusen FD, Meijer S, et al. Sentinel node investigation in breast cancer: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2001;54:550-552.
39. Abrahamsen HN, Hamilton-Dutoit SJ, Larsen J, et al. Sentinel lymph nodes in malignant melanoma: extended histopathologic evaluation improves diagnostic precision. Cancer 2004;100:1683-1691.
40. Ross GL, Shoaib T, Scott J, et al. The impact of immunohistochemistry on sentinel node biopsy for primary cutaneous malignant melanoma. Br J Plast Surg 2003;56:153-155.
41. Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol 1988;12:612-618.
42. Robers A, Cochran AJ. Pathologic analysis of sentinel lymph ndoes in melanoma patients: current and future trends. J Surg Oncol 2004;85:152-161.
43. Reintgen D, Pendas S, Jakub J, et al. National trials involving lymphatic mapping for melanoma: the multicenter selective lymphadenectomy trial, the sunbelt melanoma trial, and the Florida melanoma trial. Sem Oncol 2004;31:363-373.
44. Caprio MG, Carbone G, Bracigliano A, et al. Sentinel lymph node detection by Lymphoscintigraphy in malignant melanoma. Tumori 2002;88:543-545.
45. Maffioli L, Sturm E, Roselli M, et al. State of the art sentinel node biopsy in Oncology. Tumori 2000;86:263-272.
46. Bartolomei M, Testori A, Chinol M, et al. Sentinel node localization in cutaneous melanoma: lymphoscintigraphy with colloids and antibody fragments versus blue dye mapping. Eur J Nucl Med 1998;25:1489-1494.
47. Balch CM. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol 1988;6:163-172.
48. Medalie NS, Ackerman AB. Sentinel lymph node biopsy has no benefit for patients with primary cutaneous melanoma metastatic to a lymph node: An assertion based on comprehensive, critical analysis: Part I. Am J Dermatopathol 2003;25:399-417.
49. Medalie NS, Ackerman AB. Sentinel lymph node biopsy has no benefit for patients with primary cutaneous melanoma metastatic to a lymph node: an assertion based on comprehensive, critical analysis: Part II. Am J Dermatopathol 2003;25:473-484.
50. Eedy DJ. Introducing controversies in dermatology: sentinel lymph node biopsy for cutaneous melanoma—a useful technique or a waste of time? Br J Dermatol 2004;151:267-268.
51. Agnese DM, Abdessalam SF, Burak WE, et al. Cost-effectiveness of sentinel lymph node biopsy in thin melanomas. Surgery 2003;134:542-547.
52. Ang KK, Gera FB, Byers RM, et al. Radiotherapy for melanoma. In: Cutaneous Melanoma, Balch CM, Houghton AN, Sober AJ, Soong S, eds. St. Louis, Mo: Quality Medical Publishing; 1998:389–404.
53. Argawala SS, Neuberg D, Park Y, et al. Mature results of a phase III randomized trial of bacillus calmetteguerin (BCG) versus observation and BCG versus dacarbazine bersus BCG in the adjuvant therapy of American joint committee on cancer State I-III melanoma (E1673). Cancer 2004;100:692-698.
54. Groenewegen G, Bloem A, De Gast GC. Phase I/II study of sequential chemoimmunotherapy (SCIT) for metastatic melanoma: outpatient treatment with dacarbazine, granulocyte-macrophage colony-stimulating factor, low-dose interleukin-2, and interferon-alpha. Cancer Immunol Immunother 2002;51:630-636.
55. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17.
56. Moschos SJ, Kirkwood JM, Konstantinopolis PA. Present status and future prospects for adjuvant therapy of melanoma: time to build upon the foundation of high-dose interferon alfa-2b. J Clin Oncol 2004;22:11-14.
57. Lotze MT, Dalla RM, Kirkwood JM, et al. Cutaneous Melanoma. In: Cancer: Principles and Practice of Oncology, Devita VT, Hellma S, Rosenberg SA, eds. Philadelphia, Pa: lippincott Williams & Wilkins; 2001:2021–2056.