User login
Treating recurrent vulvovaginal candidiasis

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
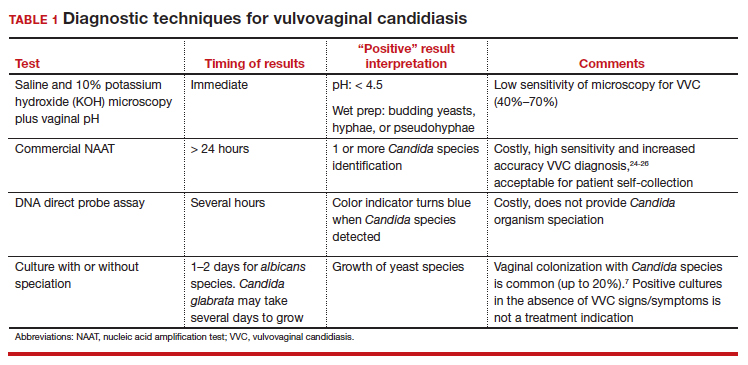
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.
Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592