User login
Skin lesions mimicking septic arthritis
A 46-year-old African American woman was admitted to the hospital with complaints of multiple joint pains associated with skin lesions, which had lasted for 3 weeks. The pains were in her right ankle, left wrist, both great toes, and right thumb. Further systems review revealed that the patient had experienced a fever of 102°F, diarrhea (at times bloody), chills, nausea, and decreased appetite for 5 days along with low back pain for 1 week.
Her medical history was significant for hypertension, hypothyroidism, chronic gastrointestinal symptoms attributed to irritable bowel disease for the past 2 years, and intermittent asymmetrical oligoarthritis for 1 year that had responded to episodic treatment with prednisone. She also had 2 episodes of iritis in the previous 4 years responsive to topical corticosteroids.
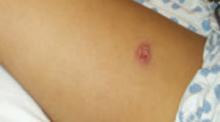
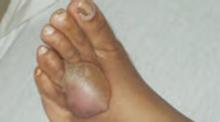
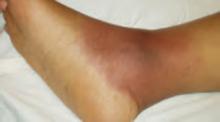
On exam she looked sick, was febrile, and had skin lesions in vaginal, axillary, perianal, foot, and thigh areas (FIGURES 1 AND 2). The lesions were 5 to 40 mm in size, exquisitely tender, bullous, pustular, and formed violaceous ulcers with scanty purulent-looking discharge. Her ankle exam was significant for swelling, redness, warmth, tenderness and severe pain on passive and active movements (FIGURE 3). Results of an examination of her spine was within normal limits. The remainder of her physical exam also showed normal results.
FIGURE 1
Ulcer on thigh
FIGURE 2
Lesion on foot
FIGURE 3
Swollen ankle joint
What is your diagnosis?
Differential diagnosis
The primary differential was focused on joint symptoms, and included disseminated gonococcal, bacterial, atypical infections, Behçet’s disease, rheumatoid arthritis, Reiter’s disease, reactive and inflammatory bowel disease (IBD)-related arthritis.
Differential diagnosis for the skin lesions included pyoderma gangrenosum, Sweets syndrome, sexually transmitted diseases, granulomatous skin lesions of inflammatory bowel disease, Reiter’s, Behçet’s, vasculitis (Wegener’s granulamatosis, polyarteritis nodosa, cryoglobulinemia), cancer (lymphoma cutis, leukemia cutis, Langerhans cell histiocytosis), infectious (sporotrichosis, blastomycosis, local fungal), drug-induced, exogenous tissue injury, vaso-occlusive (antiphospholipid-antibody syndrome).3
Further testing was necessary to determine the diagnosis.
Laboratory results
The following laboratory tests were ordered.
Blood tests. Patient’s complete blood count was remarkable for anemia and thrombocytosis; a comprehensive metabolic panel showed hypoalbuminemia. Erythrocyte sedimentation rate was elevated at 120 mm/h and C-reactive protein was elevated at 24.9 mg/L.
A diagnostic tap of the ankle showed fluid with inflammatory changes, and cultures for aerobic, anaerobic, acid fast bacilli, fungus, gonorrhea, and herpes were negative.
Joints. The patient’s joints were suspected to have septic arthritis, both clinically and on magnetic resonance imaging exam; they demonstrated no improvement of symptoms with intravenous antibiotics. Early on, the orthopedic service recommended joint debridement and open drainage.
Surgical findings included epidermolysis with subcutaneous fluid and purulent-looking material. Progressive necrosis of the debrided margins occurred over the next few days.
Workup for possible rheumatological joint disease included antinuclear antibody, rheumatoid factor, cyclic citrullinated peptide antibody (anti-CCP antibody), and human leukocyte antigens (HLA) B27 and B51. Radiographs of involved joints did not show rheumatic joint disease.
Rheumatoid arthritis was doubtful due to lack of sufficient criteria for diagnosis, and negative rheumatoid factor and anti-CCP antibody.5 HLA B27 is positive in 80% of patients with Reiter’s syndrome, 60% of those with reactive arthritis, and 50% of those with IBD arthritis.6 HLA B51, although not diagnostic, has a higher than baseline prevalence in Behçet’s disease.4 Recurrent oral aphthae, a major criteria for Behçet’s, was absent in our patient.
Gastrointestinal. A preliminary gastrointestinal evaluation included stool culture and tests for clostridium difficile toxin, viral antibodies for cytomegalovirus (CMV), parvovirus, and herpes—all results were negative.
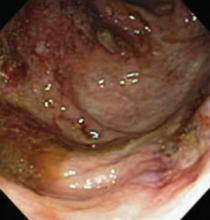
Colonoscopy, however, demonstrated colonic ulcers (mainly of the right colon) with skip pattern associated with polypoid lesions, mild terminal ileitis, and normal-looking left colon and rectum (FIGURE 4). Colonic biopsy showed crypt distortion and abscesses, terminal ileitis, absence of granuloma formation, and negative immunostain for CMV. Colonoscopy findings along with colon biopsy were suggestive of Crohn’s disease.
Conclusions from the lab tests. Blood, urine, and skin lesion cultures remained negative. Urethral and vaginal cultures for gonorrhea, chlamydia, herpes virus, and serum test for syphilis, HIV, and hepatitis B and C were all negative. Biopsy of skin lesions showed dermal mixed inflammatory infiltrate predominantly neutrophilic, and no vasculitis or micropathogens. Absence of micropathogens, vasculitis, vascular thrombosis, and lack of neoplastic cells made infectious, vasculitic, vaso-occlusive and malignant ulcers implausible.
There is no specific laboratory or pathological finding for diagnosis of pyoderma gangrenosum. Diagnosis is based on exclusion of other ulcerative conditions. Negative skin, arthrocentesis, joint fluid and tissue cultures, progressive necrosis of debrided tissue margins (pathergy), and skin biopsy suggested a diagnosis of pyoderma gangrenosum.
Joint lesions were considered to be atypical pyoderma gangrenosum. Investigations to diagnose or exclude a concomitant systemic disorder are required in patients with pyoderma gangrenosum. This patient had Crohn’s disease associated with pyoderma gangrenosum.
FIGURE 4
Colonoscopy findings
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum is a rare, ulcerative, neutrophilic dermatosis of uncertain etiology. It occurs in 1 in 100,000 people in the US per year.1 Fifty percent of patients with pyoderma gangrenosum have an underlying disorder such as inflammatory bowel disease, arthritis, hematological malignancy, infections, sarcoidosis, hypogammaglobulinemia, or HIV.2 Pyoderma gangrenosum occurs in 2% of patients with Crohn’s disease.9
Variants of pyoderma gangrenosum
The 2 primary variants are classical and atypical. Four types of lesions are seen.2
Ulcerative. Ulcerative pyoderma gangrenosum a classical form of disease and occurs usually in lower limb and trunk. Lesions begin as pustules or as a pathergic phenomenon (development of ulcer at site of minimal trauma). This progresses to a large violaceous ulcer with undermined borders and surrounding halo of erythema. The lesions are distinctively painful.
Pyoderma gangrenosum may occur on the genitalia and would need to be differentiated from sexually transmitted disease. Extracutaneous sterile neutrophilic abscesses have been reported in lungs, eyes, liver, spleen, bones, heart, central nervous system, and gastrointestinal tract.
Pustular. Present as painful postulations on extensor surfaces of the limbs. Pyostomatitis vegetans is a variant of this form and occurs in the oral cavity.
Bullous/atypical. Bullae most often occur on hands, forearm, or face. Most commonly associated with hematological malignancy.
Vegetative. Chronic, nonpainful superficial ulcer.
Treatment: Topical vs systemic
To date there are no established guidelines for treatment of pyoderma gangrenosum. Treatment of underlying disease often results in improvement of pyoderma gangrenosum.
Treat localized cases topically
Localized pyoderma gangrenosum may initially be treated with corticosteroids (topical or intralesional) or tacrolimus (SOR: B),7 reserving systemic treatment for refractory cases.
Topical care of the lesions along with systemic therapy should be limited to antiseptic or occlusive wound dressing in an effort to minimize secondary bacterial infection.
Surgery not recommended in active disease
Adjuvant surgery such as excision of ulcer, skin grafting, etc, has not been shown to reduce morbidity even when done in conjunction with systemic immunosuppressive therapy. Re-epithelialization procedures like debridements or allografts may be done during disease remission (SOR: C).7
These procedures should be avoided in active disease due to risk of pathergy and worsening of original lesions of pyoderma gangrenosum. Similarly, elective surgery should be avoided during active disease, and when necessary should be done alongside systemic pyoderma gangrenosum therapy.
Refractory cases
In refractory or idiopathic cases, disseminated pyoderma gangrenosum is treated systemically with corticosteroids or cyclosporine alone or in combination (strength of recommendation [SOR]: B).7 In steroid-resistant pyoderma gangrenosum, thalidomide, mycophenolate mofetil, tacrolimus, dapsone, azathioprine, and infliximab may be tried (SOR: C).7
Immunoglobulin, plasmapheresis, and cyclophosphamide have shown some efficacy in patients without systemic disease (SOR: C).7 Tumor necrosis factor-α inhibitor infliximab (Remicade) is considered first-line therapy for pyoderma gangrenosum associated with Crohn’s disease (SOR: B).7
The patient’s outcome
The patient did not improve after initial treatment with high-dose systemic corticosteroids. Infliximab in combination with methotrexate dramatically improved her skin and joint lesions and induced remission of Crohn’s disease. Early on, during the active phase of the disease, she also had surgical debridement of the lesions due to confounding diagnosis.
The patient has continued with infliximab infusions every 2 months and weekly oral methotrexate to maintain remission. Data show that 56% of patients with pyoderma gangrenosum require long-term therapy to prevent recurrences.8
This case is interesting because the bullous lesions of pyoderma gangrenosum appeared over the joints, mimicking septic arthritis and resulting in preventable surgical debridement. A case report with pyoderma gangrenosum lesions predominantly over the joints has not been described in the published literature. Recognition of lesions over the joints is important because treatment remains nonsurgical and surgery may exacerbate these skin lesions.
CORRESPONDENCE
Shashi Mittal, MD, Faculty Director of Research, Baylor Family Medicine Residency at Garland, Suite 340, Clara Barton Blvd, Garland, TX 75042.
1. Jackson JM, Callen JP. Pyoderma gangrenosum. Available at: Emedicine.com. Accessed on March 21, 2006.
2. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: A Review.Available at www.Medscape.com. Accessed on March 21, 2006.
3. Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med 2002;347:1412-1418.
4. Smith EL. Clinical manifestations and diagnosis of Behcet’s disease. Available at www.uptodate.com. Accessed on April 2, 2006.
5. Venables PJW, Maini RN. Diagnosis and differential diagnosis of Rheumatoid arthritis. Available at www.uptodate.com. Accessed on April 2, 2006.
6. Yu DT, Wiesenhutter CW. Course and treatment of Reiter’s syndrome; reactive arthritis and undifferentiated spondyloarthropathy. Available at www.uptodate.com. Accessed on April 2, 2006.
7. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-283.
8. Driesch VD. Pyoderma gangrenosum: a report of 44 cases with follow up. Br J Dermatol 1997;137:1000-1005.
9. Moschella SL. Neutrophilic dermatosis. Available at www.uptodate.com.
A 46-year-old African American woman was admitted to the hospital with complaints of multiple joint pains associated with skin lesions, which had lasted for 3 weeks. The pains were in her right ankle, left wrist, both great toes, and right thumb. Further systems review revealed that the patient had experienced a fever of 102°F, diarrhea (at times bloody), chills, nausea, and decreased appetite for 5 days along with low back pain for 1 week.
Her medical history was significant for hypertension, hypothyroidism, chronic gastrointestinal symptoms attributed to irritable bowel disease for the past 2 years, and intermittent asymmetrical oligoarthritis for 1 year that had responded to episodic treatment with prednisone. She also had 2 episodes of iritis in the previous 4 years responsive to topical corticosteroids.
On exam she looked sick, was febrile, and had skin lesions in vaginal, axillary, perianal, foot, and thigh areas (FIGURES 1 AND 2). The lesions were 5 to 40 mm in size, exquisitely tender, bullous, pustular, and formed violaceous ulcers with scanty purulent-looking discharge. Her ankle exam was significant for swelling, redness, warmth, tenderness and severe pain on passive and active movements (FIGURE 3). Results of an examination of her spine was within normal limits. The remainder of her physical exam also showed normal results.
FIGURE 1
Ulcer on thigh
FIGURE 2
Lesion on foot
FIGURE 3
Swollen ankle joint
What is your diagnosis?
Differential diagnosis
The primary differential was focused on joint symptoms, and included disseminated gonococcal, bacterial, atypical infections, Behçet’s disease, rheumatoid arthritis, Reiter’s disease, reactive and inflammatory bowel disease (IBD)-related arthritis.
Differential diagnosis for the skin lesions included pyoderma gangrenosum, Sweets syndrome, sexually transmitted diseases, granulomatous skin lesions of inflammatory bowel disease, Reiter’s, Behçet’s, vasculitis (Wegener’s granulamatosis, polyarteritis nodosa, cryoglobulinemia), cancer (lymphoma cutis, leukemia cutis, Langerhans cell histiocytosis), infectious (sporotrichosis, blastomycosis, local fungal), drug-induced, exogenous tissue injury, vaso-occlusive (antiphospholipid-antibody syndrome).3
Further testing was necessary to determine the diagnosis.
Laboratory results
The following laboratory tests were ordered.
Blood tests. Patient’s complete blood count was remarkable for anemia and thrombocytosis; a comprehensive metabolic panel showed hypoalbuminemia. Erythrocyte sedimentation rate was elevated at 120 mm/h and C-reactive protein was elevated at 24.9 mg/L.
A diagnostic tap of the ankle showed fluid with inflammatory changes, and cultures for aerobic, anaerobic, acid fast bacilli, fungus, gonorrhea, and herpes were negative.
Joints. The patient’s joints were suspected to have septic arthritis, both clinically and on magnetic resonance imaging exam; they demonstrated no improvement of symptoms with intravenous antibiotics. Early on, the orthopedic service recommended joint debridement and open drainage.
Surgical findings included epidermolysis with subcutaneous fluid and purulent-looking material. Progressive necrosis of the debrided margins occurred over the next few days.
Workup for possible rheumatological joint disease included antinuclear antibody, rheumatoid factor, cyclic citrullinated peptide antibody (anti-CCP antibody), and human leukocyte antigens (HLA) B27 and B51. Radiographs of involved joints did not show rheumatic joint disease.
Rheumatoid arthritis was doubtful due to lack of sufficient criteria for diagnosis, and negative rheumatoid factor and anti-CCP antibody.5 HLA B27 is positive in 80% of patients with Reiter’s syndrome, 60% of those with reactive arthritis, and 50% of those with IBD arthritis.6 HLA B51, although not diagnostic, has a higher than baseline prevalence in Behçet’s disease.4 Recurrent oral aphthae, a major criteria for Behçet’s, was absent in our patient.
Gastrointestinal. A preliminary gastrointestinal evaluation included stool culture and tests for clostridium difficile toxin, viral antibodies for cytomegalovirus (CMV), parvovirus, and herpes—all results were negative.
Colonoscopy, however, demonstrated colonic ulcers (mainly of the right colon) with skip pattern associated with polypoid lesions, mild terminal ileitis, and normal-looking left colon and rectum (FIGURE 4). Colonic biopsy showed crypt distortion and abscesses, terminal ileitis, absence of granuloma formation, and negative immunostain for CMV. Colonoscopy findings along with colon biopsy were suggestive of Crohn’s disease.
Conclusions from the lab tests. Blood, urine, and skin lesion cultures remained negative. Urethral and vaginal cultures for gonorrhea, chlamydia, herpes virus, and serum test for syphilis, HIV, and hepatitis B and C were all negative. Biopsy of skin lesions showed dermal mixed inflammatory infiltrate predominantly neutrophilic, and no vasculitis or micropathogens. Absence of micropathogens, vasculitis, vascular thrombosis, and lack of neoplastic cells made infectious, vasculitic, vaso-occlusive and malignant ulcers implausible.
There is no specific laboratory or pathological finding for diagnosis of pyoderma gangrenosum. Diagnosis is based on exclusion of other ulcerative conditions. Negative skin, arthrocentesis, joint fluid and tissue cultures, progressive necrosis of debrided tissue margins (pathergy), and skin biopsy suggested a diagnosis of pyoderma gangrenosum.
Joint lesions were considered to be atypical pyoderma gangrenosum. Investigations to diagnose or exclude a concomitant systemic disorder are required in patients with pyoderma gangrenosum. This patient had Crohn’s disease associated with pyoderma gangrenosum.
FIGURE 4
Colonoscopy findings
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum is a rare, ulcerative, neutrophilic dermatosis of uncertain etiology. It occurs in 1 in 100,000 people in the US per year.1 Fifty percent of patients with pyoderma gangrenosum have an underlying disorder such as inflammatory bowel disease, arthritis, hematological malignancy, infections, sarcoidosis, hypogammaglobulinemia, or HIV.2 Pyoderma gangrenosum occurs in 2% of patients with Crohn’s disease.9
Variants of pyoderma gangrenosum
The 2 primary variants are classical and atypical. Four types of lesions are seen.2
Ulcerative. Ulcerative pyoderma gangrenosum a classical form of disease and occurs usually in lower limb and trunk. Lesions begin as pustules or as a pathergic phenomenon (development of ulcer at site of minimal trauma). This progresses to a large violaceous ulcer with undermined borders and surrounding halo of erythema. The lesions are distinctively painful.
Pyoderma gangrenosum may occur on the genitalia and would need to be differentiated from sexually transmitted disease. Extracutaneous sterile neutrophilic abscesses have been reported in lungs, eyes, liver, spleen, bones, heart, central nervous system, and gastrointestinal tract.
Pustular. Present as painful postulations on extensor surfaces of the limbs. Pyostomatitis vegetans is a variant of this form and occurs in the oral cavity.
Bullous/atypical. Bullae most often occur on hands, forearm, or face. Most commonly associated with hematological malignancy.
Vegetative. Chronic, nonpainful superficial ulcer.
Treatment: Topical vs systemic
To date there are no established guidelines for treatment of pyoderma gangrenosum. Treatment of underlying disease often results in improvement of pyoderma gangrenosum.
Treat localized cases topically
Localized pyoderma gangrenosum may initially be treated with corticosteroids (topical or intralesional) or tacrolimus (SOR: B),7 reserving systemic treatment for refractory cases.
Topical care of the lesions along with systemic therapy should be limited to antiseptic or occlusive wound dressing in an effort to minimize secondary bacterial infection.
Surgery not recommended in active disease
Adjuvant surgery such as excision of ulcer, skin grafting, etc, has not been shown to reduce morbidity even when done in conjunction with systemic immunosuppressive therapy. Re-epithelialization procedures like debridements or allografts may be done during disease remission (SOR: C).7
These procedures should be avoided in active disease due to risk of pathergy and worsening of original lesions of pyoderma gangrenosum. Similarly, elective surgery should be avoided during active disease, and when necessary should be done alongside systemic pyoderma gangrenosum therapy.
Refractory cases
In refractory or idiopathic cases, disseminated pyoderma gangrenosum is treated systemically with corticosteroids or cyclosporine alone or in combination (strength of recommendation [SOR]: B).7 In steroid-resistant pyoderma gangrenosum, thalidomide, mycophenolate mofetil, tacrolimus, dapsone, azathioprine, and infliximab may be tried (SOR: C).7
Immunoglobulin, plasmapheresis, and cyclophosphamide have shown some efficacy in patients without systemic disease (SOR: C).7 Tumor necrosis factor-α inhibitor infliximab (Remicade) is considered first-line therapy for pyoderma gangrenosum associated with Crohn’s disease (SOR: B).7
The patient’s outcome
The patient did not improve after initial treatment with high-dose systemic corticosteroids. Infliximab in combination with methotrexate dramatically improved her skin and joint lesions and induced remission of Crohn’s disease. Early on, during the active phase of the disease, she also had surgical debridement of the lesions due to confounding diagnosis.
The patient has continued with infliximab infusions every 2 months and weekly oral methotrexate to maintain remission. Data show that 56% of patients with pyoderma gangrenosum require long-term therapy to prevent recurrences.8
This case is interesting because the bullous lesions of pyoderma gangrenosum appeared over the joints, mimicking septic arthritis and resulting in preventable surgical debridement. A case report with pyoderma gangrenosum lesions predominantly over the joints has not been described in the published literature. Recognition of lesions over the joints is important because treatment remains nonsurgical and surgery may exacerbate these skin lesions.
CORRESPONDENCE
Shashi Mittal, MD, Faculty Director of Research, Baylor Family Medicine Residency at Garland, Suite 340, Clara Barton Blvd, Garland, TX 75042.
A 46-year-old African American woman was admitted to the hospital with complaints of multiple joint pains associated with skin lesions, which had lasted for 3 weeks. The pains were in her right ankle, left wrist, both great toes, and right thumb. Further systems review revealed that the patient had experienced a fever of 102°F, diarrhea (at times bloody), chills, nausea, and decreased appetite for 5 days along with low back pain for 1 week.
Her medical history was significant for hypertension, hypothyroidism, chronic gastrointestinal symptoms attributed to irritable bowel disease for the past 2 years, and intermittent asymmetrical oligoarthritis for 1 year that had responded to episodic treatment with prednisone. She also had 2 episodes of iritis in the previous 4 years responsive to topical corticosteroids.
On exam she looked sick, was febrile, and had skin lesions in vaginal, axillary, perianal, foot, and thigh areas (FIGURES 1 AND 2). The lesions were 5 to 40 mm in size, exquisitely tender, bullous, pustular, and formed violaceous ulcers with scanty purulent-looking discharge. Her ankle exam was significant for swelling, redness, warmth, tenderness and severe pain on passive and active movements (FIGURE 3). Results of an examination of her spine was within normal limits. The remainder of her physical exam also showed normal results.
FIGURE 1
Ulcer on thigh
FIGURE 2
Lesion on foot
FIGURE 3
Swollen ankle joint
What is your diagnosis?
Differential diagnosis
The primary differential was focused on joint symptoms, and included disseminated gonococcal, bacterial, atypical infections, Behçet’s disease, rheumatoid arthritis, Reiter’s disease, reactive and inflammatory bowel disease (IBD)-related arthritis.
Differential diagnosis for the skin lesions included pyoderma gangrenosum, Sweets syndrome, sexually transmitted diseases, granulomatous skin lesions of inflammatory bowel disease, Reiter’s, Behçet’s, vasculitis (Wegener’s granulamatosis, polyarteritis nodosa, cryoglobulinemia), cancer (lymphoma cutis, leukemia cutis, Langerhans cell histiocytosis), infectious (sporotrichosis, blastomycosis, local fungal), drug-induced, exogenous tissue injury, vaso-occlusive (antiphospholipid-antibody syndrome).3
Further testing was necessary to determine the diagnosis.
Laboratory results
The following laboratory tests were ordered.
Blood tests. Patient’s complete blood count was remarkable for anemia and thrombocytosis; a comprehensive metabolic panel showed hypoalbuminemia. Erythrocyte sedimentation rate was elevated at 120 mm/h and C-reactive protein was elevated at 24.9 mg/L.
A diagnostic tap of the ankle showed fluid with inflammatory changes, and cultures for aerobic, anaerobic, acid fast bacilli, fungus, gonorrhea, and herpes were negative.
Joints. The patient’s joints were suspected to have septic arthritis, both clinically and on magnetic resonance imaging exam; they demonstrated no improvement of symptoms with intravenous antibiotics. Early on, the orthopedic service recommended joint debridement and open drainage.
Surgical findings included epidermolysis with subcutaneous fluid and purulent-looking material. Progressive necrosis of the debrided margins occurred over the next few days.
Workup for possible rheumatological joint disease included antinuclear antibody, rheumatoid factor, cyclic citrullinated peptide antibody (anti-CCP antibody), and human leukocyte antigens (HLA) B27 and B51. Radiographs of involved joints did not show rheumatic joint disease.
Rheumatoid arthritis was doubtful due to lack of sufficient criteria for diagnosis, and negative rheumatoid factor and anti-CCP antibody.5 HLA B27 is positive in 80% of patients with Reiter’s syndrome, 60% of those with reactive arthritis, and 50% of those with IBD arthritis.6 HLA B51, although not diagnostic, has a higher than baseline prevalence in Behçet’s disease.4 Recurrent oral aphthae, a major criteria for Behçet’s, was absent in our patient.
Gastrointestinal. A preliminary gastrointestinal evaluation included stool culture and tests for clostridium difficile toxin, viral antibodies for cytomegalovirus (CMV), parvovirus, and herpes—all results were negative.
Colonoscopy, however, demonstrated colonic ulcers (mainly of the right colon) with skip pattern associated with polypoid lesions, mild terminal ileitis, and normal-looking left colon and rectum (FIGURE 4). Colonic biopsy showed crypt distortion and abscesses, terminal ileitis, absence of granuloma formation, and negative immunostain for CMV. Colonoscopy findings along with colon biopsy were suggestive of Crohn’s disease.
Conclusions from the lab tests. Blood, urine, and skin lesion cultures remained negative. Urethral and vaginal cultures for gonorrhea, chlamydia, herpes virus, and serum test for syphilis, HIV, and hepatitis B and C were all negative. Biopsy of skin lesions showed dermal mixed inflammatory infiltrate predominantly neutrophilic, and no vasculitis or micropathogens. Absence of micropathogens, vasculitis, vascular thrombosis, and lack of neoplastic cells made infectious, vasculitic, vaso-occlusive and malignant ulcers implausible.
There is no specific laboratory or pathological finding for diagnosis of pyoderma gangrenosum. Diagnosis is based on exclusion of other ulcerative conditions. Negative skin, arthrocentesis, joint fluid and tissue cultures, progressive necrosis of debrided tissue margins (pathergy), and skin biopsy suggested a diagnosis of pyoderma gangrenosum.
Joint lesions were considered to be atypical pyoderma gangrenosum. Investigations to diagnose or exclude a concomitant systemic disorder are required in patients with pyoderma gangrenosum. This patient had Crohn’s disease associated with pyoderma gangrenosum.
FIGURE 4
Colonoscopy findings
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum is a rare, ulcerative, neutrophilic dermatosis of uncertain etiology. It occurs in 1 in 100,000 people in the US per year.1 Fifty percent of patients with pyoderma gangrenosum have an underlying disorder such as inflammatory bowel disease, arthritis, hematological malignancy, infections, sarcoidosis, hypogammaglobulinemia, or HIV.2 Pyoderma gangrenosum occurs in 2% of patients with Crohn’s disease.9
Variants of pyoderma gangrenosum
The 2 primary variants are classical and atypical. Four types of lesions are seen.2
Ulcerative. Ulcerative pyoderma gangrenosum a classical form of disease and occurs usually in lower limb and trunk. Lesions begin as pustules or as a pathergic phenomenon (development of ulcer at site of minimal trauma). This progresses to a large violaceous ulcer with undermined borders and surrounding halo of erythema. The lesions are distinctively painful.
Pyoderma gangrenosum may occur on the genitalia and would need to be differentiated from sexually transmitted disease. Extracutaneous sterile neutrophilic abscesses have been reported in lungs, eyes, liver, spleen, bones, heart, central nervous system, and gastrointestinal tract.
Pustular. Present as painful postulations on extensor surfaces of the limbs. Pyostomatitis vegetans is a variant of this form and occurs in the oral cavity.
Bullous/atypical. Bullae most often occur on hands, forearm, or face. Most commonly associated with hematological malignancy.
Vegetative. Chronic, nonpainful superficial ulcer.
Treatment: Topical vs systemic
To date there are no established guidelines for treatment of pyoderma gangrenosum. Treatment of underlying disease often results in improvement of pyoderma gangrenosum.
Treat localized cases topically
Localized pyoderma gangrenosum may initially be treated with corticosteroids (topical or intralesional) or tacrolimus (SOR: B),7 reserving systemic treatment for refractory cases.
Topical care of the lesions along with systemic therapy should be limited to antiseptic or occlusive wound dressing in an effort to minimize secondary bacterial infection.
Surgery not recommended in active disease
Adjuvant surgery such as excision of ulcer, skin grafting, etc, has not been shown to reduce morbidity even when done in conjunction with systemic immunosuppressive therapy. Re-epithelialization procedures like debridements or allografts may be done during disease remission (SOR: C).7
These procedures should be avoided in active disease due to risk of pathergy and worsening of original lesions of pyoderma gangrenosum. Similarly, elective surgery should be avoided during active disease, and when necessary should be done alongside systemic pyoderma gangrenosum therapy.
Refractory cases
In refractory or idiopathic cases, disseminated pyoderma gangrenosum is treated systemically with corticosteroids or cyclosporine alone or in combination (strength of recommendation [SOR]: B).7 In steroid-resistant pyoderma gangrenosum, thalidomide, mycophenolate mofetil, tacrolimus, dapsone, azathioprine, and infliximab may be tried (SOR: C).7
Immunoglobulin, plasmapheresis, and cyclophosphamide have shown some efficacy in patients without systemic disease (SOR: C).7 Tumor necrosis factor-α inhibitor infliximab (Remicade) is considered first-line therapy for pyoderma gangrenosum associated with Crohn’s disease (SOR: B).7
The patient’s outcome
The patient did not improve after initial treatment with high-dose systemic corticosteroids. Infliximab in combination with methotrexate dramatically improved her skin and joint lesions and induced remission of Crohn’s disease. Early on, during the active phase of the disease, she also had surgical debridement of the lesions due to confounding diagnosis.
The patient has continued with infliximab infusions every 2 months and weekly oral methotrexate to maintain remission. Data show that 56% of patients with pyoderma gangrenosum require long-term therapy to prevent recurrences.8
This case is interesting because the bullous lesions of pyoderma gangrenosum appeared over the joints, mimicking septic arthritis and resulting in preventable surgical debridement. A case report with pyoderma gangrenosum lesions predominantly over the joints has not been described in the published literature. Recognition of lesions over the joints is important because treatment remains nonsurgical and surgery may exacerbate these skin lesions.
CORRESPONDENCE
Shashi Mittal, MD, Faculty Director of Research, Baylor Family Medicine Residency at Garland, Suite 340, Clara Barton Blvd, Garland, TX 75042.
1. Jackson JM, Callen JP. Pyoderma gangrenosum. Available at: Emedicine.com. Accessed on March 21, 2006.
2. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: A Review.Available at www.Medscape.com. Accessed on March 21, 2006.
3. Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med 2002;347:1412-1418.
4. Smith EL. Clinical manifestations and diagnosis of Behcet’s disease. Available at www.uptodate.com. Accessed on April 2, 2006.
5. Venables PJW, Maini RN. Diagnosis and differential diagnosis of Rheumatoid arthritis. Available at www.uptodate.com. Accessed on April 2, 2006.
6. Yu DT, Wiesenhutter CW. Course and treatment of Reiter’s syndrome; reactive arthritis and undifferentiated spondyloarthropathy. Available at www.uptodate.com. Accessed on April 2, 2006.
7. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-283.
8. Driesch VD. Pyoderma gangrenosum: a report of 44 cases with follow up. Br J Dermatol 1997;137:1000-1005.
9. Moschella SL. Neutrophilic dermatosis. Available at www.uptodate.com.
1. Jackson JM, Callen JP. Pyoderma gangrenosum. Available at: Emedicine.com. Accessed on March 21, 2006.
2. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: A Review.Available at www.Medscape.com. Accessed on March 21, 2006.
3. Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med 2002;347:1412-1418.
4. Smith EL. Clinical manifestations and diagnosis of Behcet’s disease. Available at www.uptodate.com. Accessed on April 2, 2006.
5. Venables PJW, Maini RN. Diagnosis and differential diagnosis of Rheumatoid arthritis. Available at www.uptodate.com. Accessed on April 2, 2006.
6. Yu DT, Wiesenhutter CW. Course and treatment of Reiter’s syndrome; reactive arthritis and undifferentiated spondyloarthropathy. Available at www.uptodate.com. Accessed on April 2, 2006.
7. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-283.
8. Driesch VD. Pyoderma gangrenosum: a report of 44 cases with follow up. Br J Dermatol 1997;137:1000-1005.
9. Moschella SL. Neutrophilic dermatosis. Available at www.uptodate.com.