User login
Maximizing lifestyle changes to manage type 2 diabetes
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
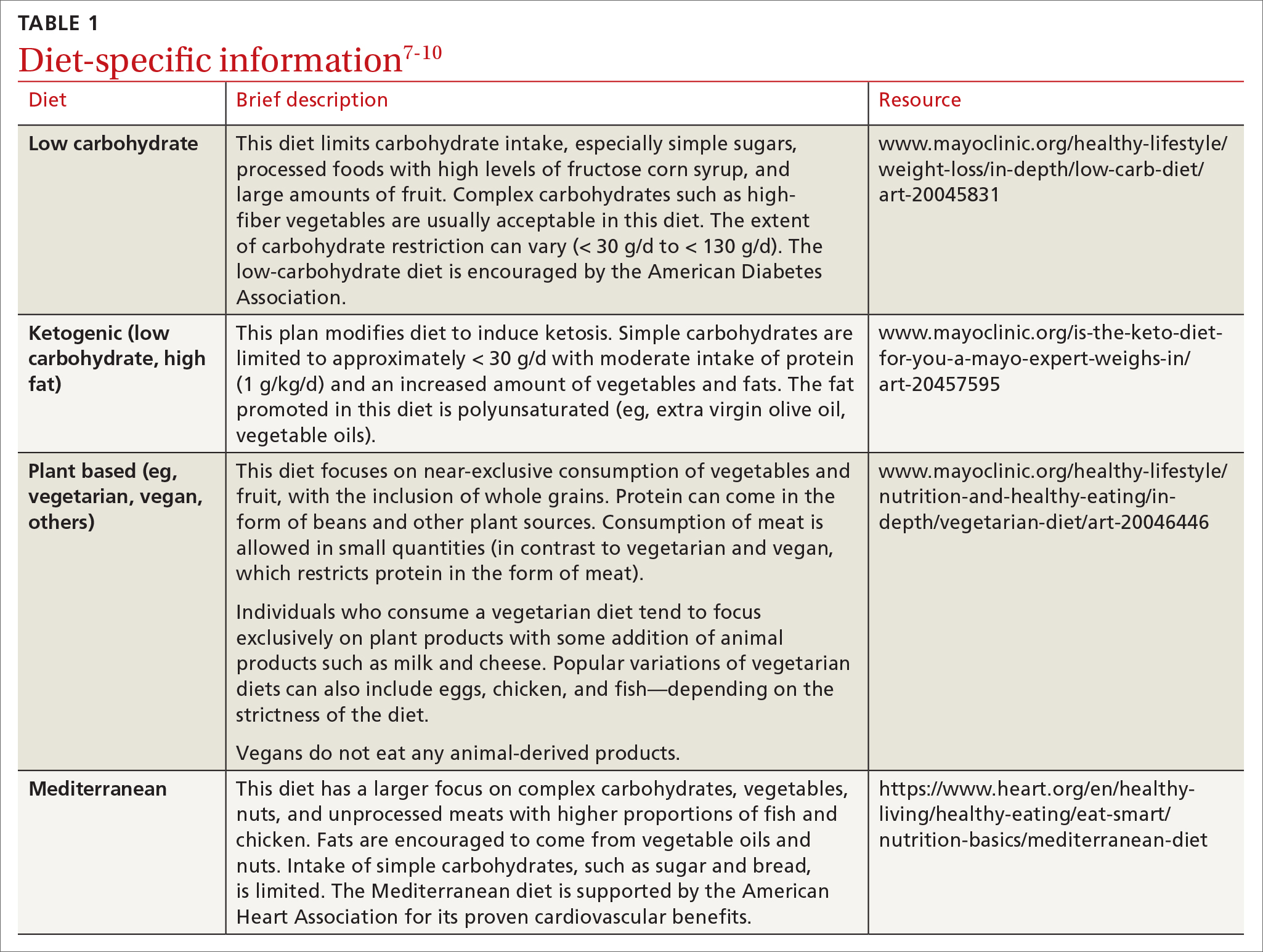
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11
In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
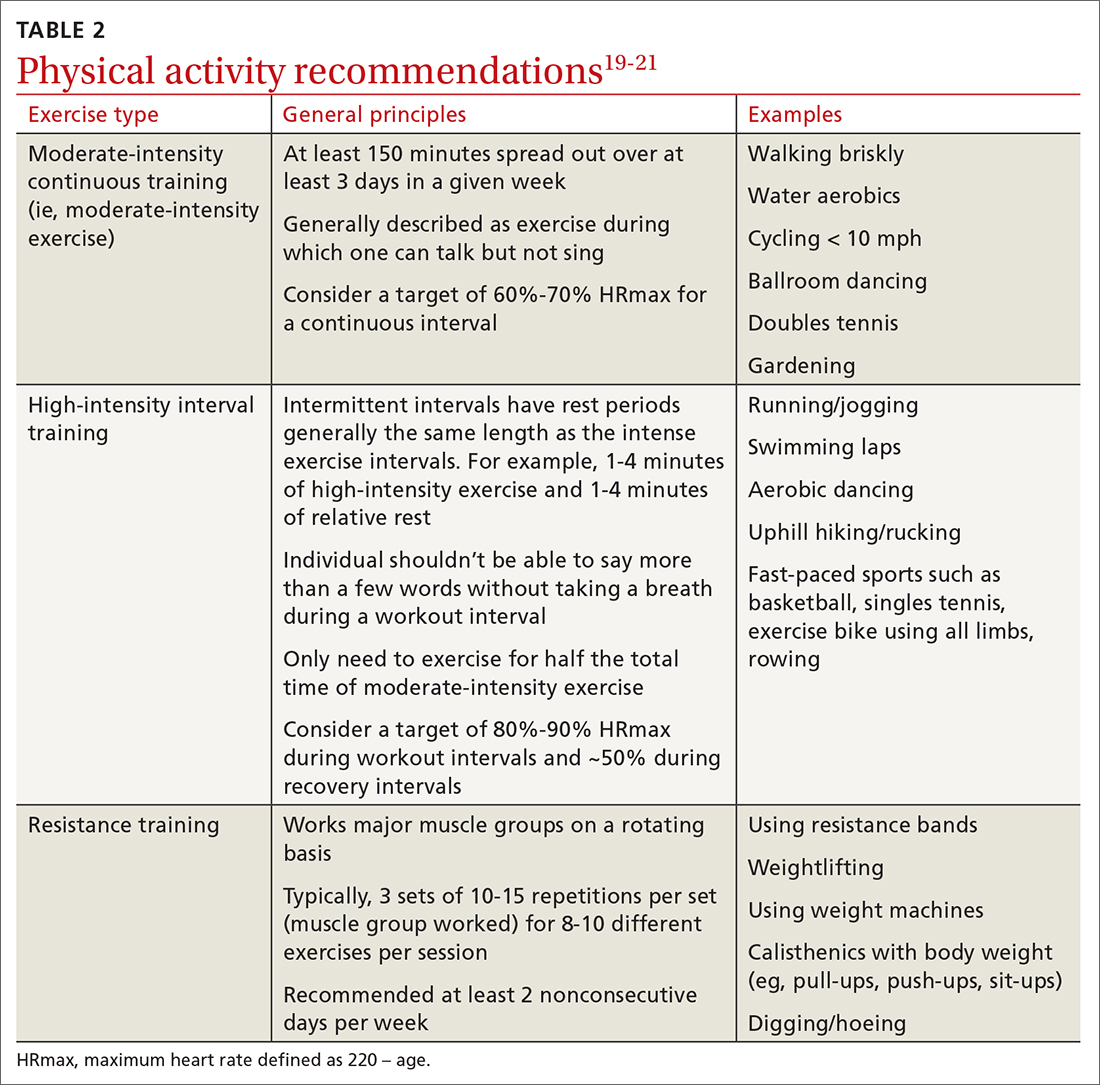
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23
Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
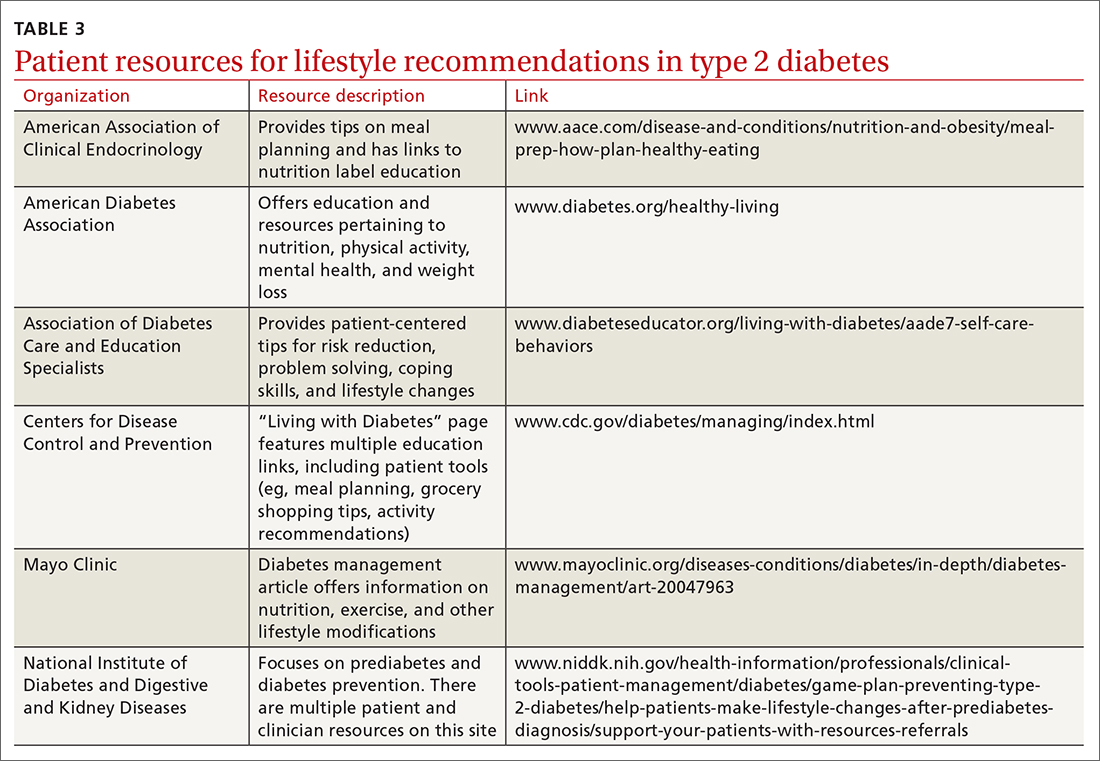
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).
C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11

In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23

Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).

C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11

In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23

Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).

C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
PRACTICE RECOMMENDATIONS
› Recommend a reduced-calorie diet that is generally plant based and low in carbohydrates as part of the treatment plan for type 2 diabetes. B
› Counsel all patients with type 2 diabetes to engage in physical activity for at least 150 minutes per week at moderate intensity and to add resistance training on at least 2 days to improve glycemic control. B
› Teach patients techniques to reduce stress and improve sleep quality. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
