User login
Small renal masses: Toward more rational treatment
Opinion about treatment of mall renal masses has changed considerably in the past 2 decades.
Traditionally, the most common treatment was surgical removal of the whole kidney, ie, radical nephrectomy. However, recent studies have shown that many patients who undergo radical nephrectomy develop chronic kidney disease. Furthermore, radical nephrectomy often constitutes over-treatment, as many of these lesions are benign or, if malignant, would follow an indolent course if left alone.
Now that we better understand the biology of small renal masses and are more aware of the morbidity and mortality related to chronic kidney disease, we try to avoid radical nephrectomy whenever possible, favoring nephron-sparing approaches instead.
In this article, we review the current clinical management of small renal masses.
SMALL RENAL MASSES ARE A HETEROGENEOUS GROUP
Small renal masses are defined as solid renal tumors that enhance on computed tomography (CT) and magnetic resonance imaging (MRI) and are suspected of being renal cell carcinomas. They are generally low-stage and relatively small (< 4 cm in diameter) at presentation. Most are now discovered incidentally on CT or MRI done for various abdominal symptoms. From 20,000 to 30,000 new cases are diagnosed each year in the United States, and the rate is increasing by 3% to 4% per year as the use of CT and MRI increases.1,2
With more small renal masses being detected incidentally, renal cell carcinoma has been going through a stage and size migration—ie, more of these tumors are being discovered in clinical stage T1 (ie, confined to the kidney and measuring less than 7 cm) than in the past. Currently, clinical T1 renal tumors account for 48% to 66% of cases.3
This indicates that the disease is being detected and treated earlier in its course than in the past. However, cancer-specific deaths from renal cell carcinoma have not declined, suggesting that for many of these patients, our traditional practice of aggressive surgical management with radical nephrectomy may not be warranted.4
Small renal masses vary in biologic aggressiveness
Recent large surgical series indicate that up to 20% of small renal masses are benign, 55% to 60% are indolent renal cell carcinomas, and only 20% to 25% have potentially aggressive features, defined by high nuclear grade or locally invasive characteristics.5–7
A relatively strong predictor of the aggressiveness of renal tumors is their size, which directly correlates with the risk of malignant pathology. Of lesions smaller than 1.0 cm, 38% to 46% are benign, dramatically decreasing to 6.3% to 7.1% for lesions larger than 7.0 cm.5 Each 1.0-cm increase in tumor diameter correlates with a 16% increase in the risk of malignancy.8
Our knowledge of the natural history of small renal masses is limited, being based on small, retrospective series. In these studies, when small renal masses were followed over time, relatively few progressed (ie, metastasized), and there have been no documented reports of disease progression in the absence of demonstrable tumor growth, suggesting a predominance of nonaggressive phenotypes.9
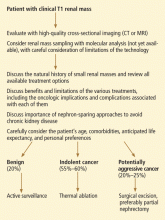
In light of these observations, patients with small renal masses should be carefully evaluated to determine if they are candidates for active surveillance as opposed to more aggressive treatment, ie, surgery or thermal ablation.
CT AND MRI ARE THE PREFERRED DIAGNOSTIC STUDIES
In the past, most patients with renal tumors presented with gross hematuria, flank pain, or a palpable abdominal mass. These presentations are now uncommon, as most cases are asymptomatic and are diagnosed incidentally. In a series of 349 small renal masses, microhematuria was found in only 8 cases.10
Systemic manifestations or paraneoplastic syndromes such as hypercalcemia or hypertension are more common in patients with metastatic renal cell carcinoma than in those with localized tumors. It was because of these varied clinical presentations that renal cell carcinoma was previously known as the “internist’s tumor”; however, small renal masses are better termed the “radiologist’s tumor.”11
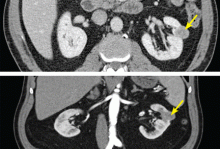
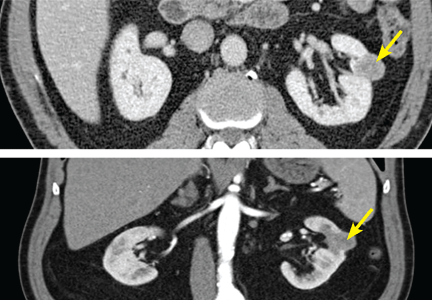
High-quality axial imaging with CT or MRI is preferred for evaluating renal cortical neoplasms. Enhancement on CT or MRI is the characteristic finding of a renal lesion that should be suspected of being renal cell carcinoma (Figure 1). Triple-phase CT is ideal, with images taken before contrast is given, immediately after contrast (the early vascular phase), and later (the delayed phase). Alternatively, MRI can be used in patients who are allergic to intravenous contrast or who have moderate renal dysfunction.
Renal tumors with enhancement of more than 15 Hounsfield units (HU) on CT imaging are considered suggestive of renal cell carcinoma, whereas those with less than 10 HU of enhancement are more likely to be benign. Enhancement in the range of 10 to 15 HU is considered equivocal.
Differential diagnosis
By far, most small renal masses are renal cell carcinomas. However, other possibilities include oncocytoma, atypical or fat-poor angiomyolipoma, metanephric adenoma, urothelial carcinoma, metastatic lesions, lymphoma, renal abscess or infarction, mixed epithelial or stromal tumor, pseudotumor, and vascular malformations.
With rare exceptions, dense fat within a renal mass reliably indicates benign angiomyolipoma, and all renal tumors should be reviewed carefully for this feature. Beyond this, no clinical or radiologic feature ensures that a small renal mass is benign.
Imaging’s inability to accurately classify these enhancing renal lesions has led to a renewed interest in renal mass sampling to aid in the evaluation of small renal masses.
RENAL MASS SAMPLING: SAFER, MORE ACCURATE THAN THOUGHT
Renal mass sampling (ie, biopsy) has traditionally had a restricted role in the management of small renal masses, limited specifically to patients with a clinical history suggesting renal lymphoma, carcinoma that had metastasized to the kidney, or primary renal abscess. However, this may be changing, with more interest in it as a way to subtype and stratify select patients with small renal masses, especially potential candidates for active surveillance.
Our thinking about renal mass sampling has changed substantially over the last 2 decades. Previously, it was not routinely performed, because of concern over high false-negative rates (commonly quoted as being as high as 18%) and its potential associated morbidity. A common perception was that a negative biopsy could not be trusted and, therefore, renal mass sampling would not ultimately change patient management. However, many of these false-negative results were actually “noninformative,” ie, cases in which the renal tumor could not be adequately sampled or the pathologist lacked a sufficient specimen to allow for a definitive diagnosis.
Recent evidence suggests that these concerns were exaggerated and that renal mass sampling is more accurate and safer than previously thought. A meta-analysis of studies done before 2001 found that the diagnostic accuracy of renal mass sampling averaged 82%, whereas contemporary series indicate that its accuracy in differentiating benign from malignant tumors is actually greater than 95%.12 In addition, false-negative rates are now consistently less than 1%.13
Furthermore, serious complications requiring clinical intervention or hospitalization occur in fewer than 1% of cases. Seeding of the needle tract with tumor cells, which was another concern, is also exceedingly rare for these small, well-circumscribed renal masses.12
Overall morbidity is low with renal mass sampling, which is routinely performed as an outpatient procedure using CT or ultrasono-graphic guidance and local anesthesia.
However, 10% of biopsy results are still noninformative. In this situation, biopsy can be repeated, or the mass can be surgically excised, or the patient can undergo conservative management if he or she is unfit or unwilling to undergo surgery.
The encouraging results with renal mass sampling have led to greater use of it at many centers in the evaluation and risk-stratification of patients with small renal masses. It may be especially useful in patients considering several treatment options.
For example, a 75-year-old patient with modest comorbidities and a 2.0-cm enhancing renal mass could be a candidate for partial nephrectomy, thermal ablation, or active surveillance, and a reasonable argument could be made for each of these options. Renal mass sampling in this instance could be instrumental in guiding this decision, as a tissue diagnosis of high-grade renal cell carcinoma would favor partial nephrectomy, whereas a diagnosis of “oncocytoma neoplasm” would support a more conservative approach.
Older, frail patients with significant comorbidities who are unlikely to be candidates for aggressive surgical management would not need renal mass sampling, as they will ultimately be managed with active surveillance or thermal ablation.
Recent studies have also indicated that molecular profiling through gene expression analysis or proteomic analysis can further improve the accuracy of renal mass sampling.14 This will likely be the holy grail for this field, allowing for truly rational management (Figure 2).
TREATMENT OPTIONS
Radical nephrectomy: Still the most common treatment
In the past, complete removal of the kidney was standard for nearly all renal masses suspected of being renal cell carcinomas. Partial nephrectomy was generally reserved for patients who had a solitary kidney, bilateral tumors, or preexisting chronic kidney disease.
Although the two procedures provide equivalent oncologic outcomes for clinical T1 lesions, Miller et al15 reported that, before 2001, only 20% of small renal masses in the United States were managed with partial nephrectomy. That percentage has increased modestly, but radical nephrectomy still predominates.
One explanation for why the radical procedure is done more frequently is that partial nephrectomy is more technically difficult, as it involves renal reconstruction. Furthermore, radical nephrectomy can almost always be performed via a minimally invasive approach, which is inherently appealing to patients and surgeons alike. Laparoscopic radical nephrectomy has been called “the great seductress” because of these considerations.16 However, total removal of the kidney comes at a great price—loss of renal function.
Over the last decade, various studies have highlighted the association between radical nephrectomy and the subsequent clinical onset of chronic kidney disease, and the potential correlations between chronic kidney disease and cardiovascular events and elevated mortality rates.17
In a landmark study, Huang et al18 evaluated the outcomes of 662 patients who had small renal masses, a “normal” serum creatinine concentration (≤ 124 μmol/L [1.4 mg/dL]), and a normal-appearing contralateral kidney who underwent radical or partial nephrectomy. Of these, 26% were found to have preexisting stage 3 chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated using the Modification of Diet in Renal Disease equation). Additionally, 65% of patients treated with radical nephrectomy were found to have stage 3 chronic kidney disease after surgery vs 20% of patients managed with partial nephrectomy.
The misconception remains that the risk of chronic kidney disease after radical nephrectomy is insignificant, since the risk is low in renal transplant donors.19 However, renal transplant donors undergo stringent screening to ensure that their general health is good and that their renal function is robust, both of which are not true in many patients with small renal masses, particularly if they are elderly.
The overuse of radical nephrectomy is worrisome in light of the potential implications of chronic kidney disease, such as increased risk of morbid cardiovascular events and elevated mortality rates. Many experts believe that over-treatment of small renal masses may have contributed to the paradoxical increase in overall mortality rates observed with radical nephrectomy in some studies.4
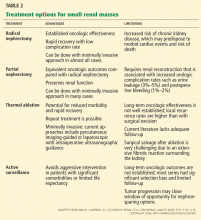
Although radical nephrectomy remains an important treatment for locally advanced renal cell carcinoma, it should be performed for small renal masses only if nephron-sparing surgery is not feasible (Table 2).
Partial nephrectomy: The new gold standard for most patients
Over the last 5 years, greater emphasis has been placed on lessening the risk of chronic kidney disease in the management of all urologic conditions, including small renal masses.
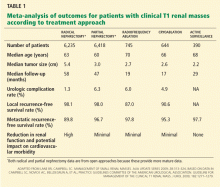
The overuse of radical nephrectomy prompted the American Urological Association to commission a panel to provide guidelines for the management of clinical stage T1 renal masses.17 After an extensive review and rigorous meta-analysis, the panel concluded that partial nephrectomy is the gold standard for most patients (Table 1, Table 2).
Partial nephrectomy involves excision of the tumor with a small margin of normal tissue, preserving as much functional renal parenchyma as possible, followed by closure of the collecting system, suture ligation of any transected vessels, and reapproximation of the capsule. Tumor excision is usually performed during temporary occlusion of the renal vasculature, allowing for a bloodless field. Regional hypothermia (cold ischemia) can also be used to minimize ischemic injury.
Contemporary series have documented that partial and radical nephrectomy have comparable oncologic efficacy for patients with small renal masses.20,21 Local recurrence rates are only 1% to 2% with partial nephrectomy, and 5- and 10-year cancer-specific survival rates of 96% and 90% have been reported.22
Furthermore, some studies have shown that patients undergoing partial nephrectomy have higher overall survival rates than those managed with radical nephrectomy—perhaps in part due to greater preservation of renal function and a lower incidence of subsequent chronic kidney disease.23,24 At Cleveland Clinic, we are now studying the determinants of ultimate renal function after partial nephrectomy in an effort to minimize ischemic injury and optimize this technique.25
Complications. Partial nephrectomy does have a potential downside in that it carries a higher risk of urologic complications such as urine leak and postoperative hemorrhage, which is not surprising because it requires a reconstruction that must heal. In a recent meta-analysis, urologic complications occurred in 6.3% patients who underwent open partial nephrectomy and in 9.0% of patients who underwent laparoscopic partial nephrectomy.17 Fortunately, most complications associated with partial nephrectomy can be managed with conservative measures.
Postoperative bleeding occurs in about 1% to 2% of patients and is the most serious complication. However, it is typically managed with superselective embolization, which has a high success rate and facilitates renal preservation.
Urine leak occurs in about 3% to 5% of cases and almost always resolves with prolonged drainage, occasionally complemented with a ureteral stent to promote antegrade drainage.
A new refinement, robotic-assisted partial nephrectomy promises to reduce the morbidity of this procedure. This approach takes less time to learn than standard laparoscopic surgery and has expanded the indications for minimally invasive partial nephrectomy, although more-difficult cases are still better done through a traditional, open surgical approach.
Thermal ablation: Another minimally invasive option
Cryoablation and radiofrequency ablation (collectively called thermal ablation) have recently emerged as alternate minimally invasive treatments for small renal masses. They are appealing options for patients with small renal tumors (< 3.5 cm) who have significant comorbidities but still prefer a proactive approach. They can also be considered as salvage procedures in patients with local recurrence after partial nephrectomy or in select patients with multifocal disease.
Both procedures can be performed percutaneously or laparoscopically, offering the potential for rapid convalescence and reduced morbidity.26,27 A laparoscopic approach is necessary to mobilize the tumor from adjacent organs if they are juxtaposed, whereas a percutaneous approach is less invasive and is better suited for posterior renal masses.28 Renal mass sampling should be performed in these patients before treatment to define the histology and to guide surveillance and should be repeated postoperatively if there is suspicion of local recurrence based on imaging.
Cryoablation destroys tumor cells through rapid cycles of freezing to less than −20°C (−4°F) and thawing, which can be monitored in real time via thermocoupling (ie, a thermometer microprobe strategically placed outside the tumor to ensure that lethal temperatures are extended beyond the edge of the tumor) or via ultrasonography, or both. Treatment is continued until the “ice ball” extends about 1 cm beyond the edge of the tumor.
Initial series reported local tumor control rates in the range of 90% to 95%; however, follow-up was very limited.29 In a more robust single-institution experience,30 renal cryoablation demonstrated 5-year cancer-specific and recurrence-free survival rates of 93% and 83%, respectively, substantially lower than what would be expected with surgical excision in a similar patient population.
Another concern with cryoablation is that options are limited for surgical salvage if the initial treatment fails. Nguyen and Campbell31 reported that partial nephrectomy and minimally invasive surgery were often precluded in this situation because of the extensive fibrotic reaction caused by the prior treatment. If cryoablation fails, surgical salvage thus often requires open, radical surgery.
Radiofrequency ablation produces tumor coagulation via protein denaturation and disruption of cell membranes after heating tissues to temperatures above 50°C (122°F) for 4 to 6 minutes.32 One of its disadvantages is that one cannot monitor treatment progress in real time, as there is no identifiable change in tissue appearance analagous to the ice ball that is seen with cryoablation.
Although the outcomes of radiofrequency ablation are less robust than those of cryoablation, most studies suggest that local control is achieved in 80% to 90% of cases based on radiographic loss of enhancement after treatment.17,30,33 A recent meta-analysis comparing these treatments found that lesions treated with radiofrequency ablation had a significantly higher rate of local tumor progression than those treated with cryoablation (12.3% vs 4.7%, P < .0001).34 Both of these local recurrence rates are substantially higher than that seen after surgical excision, despite much shorter follow-up after thermal ablation.
Tempered enthusiasm. Because thermal ablation has been developed relatively recently, its long-term outcomes and treatment efficacy have not been well established, and current studies have confirmed higher local recurrence rates with thermal ablation than with surgical excision (Table 1). Furthermore, there are significant deficiencies in the literature about thermal ablation, including limited follow-up, lack of pathologic confirmation, and controversies regarding histologic or radiologic definitions of success (Table 2).
Although current enthusiasm for thermal ablation has been tempered by suboptimal results, further refinement in technique and acknowledgment of its limitations will help to define appropriate candidates for these treatments.
Active surveillance for select patients
In select patients with extensive medical comorbidities or short life expectancy, the risks associated with proactive management may outweigh the benefits, especially considering the indolent nature of many small renal masses. In such patients, active surveillance is reasonable.
A recent meta-analysis found that most small enhancing renal masses grew relatively slowly (median 0.28 cm/year) and posed a low risk of metastasis (1%–2%).17,22 Furthermore, almost all renal lesions that progressed to metastatic disease demonstrated rapid radiographic growth, suggesting that the radiographic growth of a renal mass under active surveillance may serve as an indicator for aggressive behavior.35
Unfortunately, the growth rates of small renal masses do not reliably predict malignancy, and one study reported that 83% of tumors without demonstrable growth were malignant.36
Studies of active surveillance to date have had several other important limitations. Many did not incorporate pathologic confirmation, so that about 20% of the tumors were actually benign, thus artificially reducing the risk of adverse outcomes.5,22,37 Furthermore, the follow-up has been short, with most studies including data for only 2 to 3 years, which is clearly inadequate for this type of malignancy.37,38 Finally, most series had significant selection bias towards small, homogenous masses. In general, small renal masses that appear to be more aggressive are treated and thus excluded from these surveillance populations (Table 2).
Another concern about active surveillance is the small but real risk of tumor progression to metastatic disease, rendering these patients incurable even with new, targeted molecular therapies. Additionally, some patients may lose their window of opportunity for nephron-sparing surgery if significant tumor growth occurs during observation, rendering partial nephrectomy unfeasible. Therefore, active surveillance is not advisable for young, otherwise healthy patients (Table 2).
In the future, advances in renal mass sampling with molecular profiling may help determine which renal lesions are less biologically aggressive and, thereby, help identify appropriate candidates for observation (Figure 2).
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J 2008; 14:288–301.
- Lane BR, Campbell SC. Management of small renal masses. AUA Update Series 2009; 28:313–324.
- Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer 2004; 100:738–745.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98:1331–1334.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003; 170:2217–2220.
- Russo P. Should elective partial nephrectomy be performed for renal cell carcinoma >4 cm in size? Nat Clin Pract Urol 2008; 5:482–483.
- Thomas AA, Aron M, Hernandez AV, Lane BR, Gill IS. Laparoscopic partial nephrectomy in octogenarians. Urology 2009; 74:1042–1046.
- Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol 2009; 181:2033–2036.
- Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol 2010; 20:105–110.
- Patard JJ, Bensalah K, Vincendeau S, Rioux-Leclerq N, Guillé F, Lobel B. [Correlation between the mode of presentation of renal tumors and patient survival]. Prog Urol 2003; 13:23–28.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373:1119–1132.
- Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy—a renaissance? J Urol 2008; 179:20–27.
- Samplaski MK, Zhou M, Lane BR, Herts B, Campbell SC. Renal mass sampling: an enlightened perspective. Int J Urol 2011; 18:5–19.
- Tan MH, Rogers CG, Cooper JT, et al. Gene expression profiling of renal cell carcinoma. Clin Cancer Res 2004; 10:6315S–6321S.
- Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol 2006; 175:853–857.
- Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol 2009; 182:435–444.
- Campbell SC, Novick AC, Belldegrun A, et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182:1271–1279.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7:735–740.
- Boorjian SA, Uzzo RG. The evolving management of small renal masses. Curr Oncol Rep 2009; 11:211–217.
- Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol 1999; 162:1930–1933.
- Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000; 163:730–736.
- Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006; 175:425–431.
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181:55–61.
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008; 179:468–471.
- Thomas AA, Demirjian S, Lane BR, et al. Acute kidney injury: novel biomarkers and potential utility for patient care in urology. Urology 2011; 77:5–11.
- Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FT. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol 2008; 191:1159–1168.
- Sterrett SP, Nakada SY, Wingo MS, Williams SK, Leveillee RJ. Renal thermal ablative therapy. Urol Clin North Am 2008; 35:397–414.
- Hafron J, Kaouk JH. Ablative techniques for the management of kidney cancer. Nat Clin Pract Urol 2007; 4:261–269.
- Matin SF, Ahrar K. Nephron-sparing probe ablative therapy: longterm outcomes. Curr Opin Urol 2008; 18:150–156.
- Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol 2009; 19:138–142.
- Nguyen CT, Campbell SC. Salvage of local recurrence after primary thermal ablation for small renal masses. Expert Rev Anticancer Ther 2008; 8:1899–1905.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174:323–331.
- Carraway WA, Raman JD, Cadeddu JA. Current status of renal radiofrequency ablation. Curr Opin Urol 2009; 19:143–147.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008; 113:2671–2680.
- Kunkle DA, Kutikov A, Uzzo RG. Management of small renal masses. Semin Ultrasound CT MR 2009; 30:352–358.
- Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol 2007; 177:849–853.
- Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 2008; 179:1227–1233.
- Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am 2008; 35:627–634.
Opinion about treatment of mall renal masses has changed considerably in the past 2 decades.
Traditionally, the most common treatment was surgical removal of the whole kidney, ie, radical nephrectomy. However, recent studies have shown that many patients who undergo radical nephrectomy develop chronic kidney disease. Furthermore, radical nephrectomy often constitutes over-treatment, as many of these lesions are benign or, if malignant, would follow an indolent course if left alone.
Now that we better understand the biology of small renal masses and are more aware of the morbidity and mortality related to chronic kidney disease, we try to avoid radical nephrectomy whenever possible, favoring nephron-sparing approaches instead.
In this article, we review the current clinical management of small renal masses.
SMALL RENAL MASSES ARE A HETEROGENEOUS GROUP
Small renal masses are defined as solid renal tumors that enhance on computed tomography (CT) and magnetic resonance imaging (MRI) and are suspected of being renal cell carcinomas. They are generally low-stage and relatively small (< 4 cm in diameter) at presentation. Most are now discovered incidentally on CT or MRI done for various abdominal symptoms. From 20,000 to 30,000 new cases are diagnosed each year in the United States, and the rate is increasing by 3% to 4% per year as the use of CT and MRI increases.1,2
With more small renal masses being detected incidentally, renal cell carcinoma has been going through a stage and size migration—ie, more of these tumors are being discovered in clinical stage T1 (ie, confined to the kidney and measuring less than 7 cm) than in the past. Currently, clinical T1 renal tumors account for 48% to 66% of cases.3
This indicates that the disease is being detected and treated earlier in its course than in the past. However, cancer-specific deaths from renal cell carcinoma have not declined, suggesting that for many of these patients, our traditional practice of aggressive surgical management with radical nephrectomy may not be warranted.4
Small renal masses vary in biologic aggressiveness
Recent large surgical series indicate that up to 20% of small renal masses are benign, 55% to 60% are indolent renal cell carcinomas, and only 20% to 25% have potentially aggressive features, defined by high nuclear grade or locally invasive characteristics.5–7
A relatively strong predictor of the aggressiveness of renal tumors is their size, which directly correlates with the risk of malignant pathology. Of lesions smaller than 1.0 cm, 38% to 46% are benign, dramatically decreasing to 6.3% to 7.1% for lesions larger than 7.0 cm.5 Each 1.0-cm increase in tumor diameter correlates with a 16% increase in the risk of malignancy.8
Our knowledge of the natural history of small renal masses is limited, being based on small, retrospective series. In these studies, when small renal masses were followed over time, relatively few progressed (ie, metastasized), and there have been no documented reports of disease progression in the absence of demonstrable tumor growth, suggesting a predominance of nonaggressive phenotypes.9
In light of these observations, patients with small renal masses should be carefully evaluated to determine if they are candidates for active surveillance as opposed to more aggressive treatment, ie, surgery or thermal ablation.
CT AND MRI ARE THE PREFERRED DIAGNOSTIC STUDIES
In the past, most patients with renal tumors presented with gross hematuria, flank pain, or a palpable abdominal mass. These presentations are now uncommon, as most cases are asymptomatic and are diagnosed incidentally. In a series of 349 small renal masses, microhematuria was found in only 8 cases.10
Systemic manifestations or paraneoplastic syndromes such as hypercalcemia or hypertension are more common in patients with metastatic renal cell carcinoma than in those with localized tumors. It was because of these varied clinical presentations that renal cell carcinoma was previously known as the “internist’s tumor”; however, small renal masses are better termed the “radiologist’s tumor.”11
High-quality axial imaging with CT or MRI is preferred for evaluating renal cortical neoplasms. Enhancement on CT or MRI is the characteristic finding of a renal lesion that should be suspected of being renal cell carcinoma (Figure 1). Triple-phase CT is ideal, with images taken before contrast is given, immediately after contrast (the early vascular phase), and later (the delayed phase). Alternatively, MRI can be used in patients who are allergic to intravenous contrast or who have moderate renal dysfunction.
Renal tumors with enhancement of more than 15 Hounsfield units (HU) on CT imaging are considered suggestive of renal cell carcinoma, whereas those with less than 10 HU of enhancement are more likely to be benign. Enhancement in the range of 10 to 15 HU is considered equivocal.
Differential diagnosis
By far, most small renal masses are renal cell carcinomas. However, other possibilities include oncocytoma, atypical or fat-poor angiomyolipoma, metanephric adenoma, urothelial carcinoma, metastatic lesions, lymphoma, renal abscess or infarction, mixed epithelial or stromal tumor, pseudotumor, and vascular malformations.
With rare exceptions, dense fat within a renal mass reliably indicates benign angiomyolipoma, and all renal tumors should be reviewed carefully for this feature. Beyond this, no clinical or radiologic feature ensures that a small renal mass is benign.
Imaging’s inability to accurately classify these enhancing renal lesions has led to a renewed interest in renal mass sampling to aid in the evaluation of small renal masses.
RENAL MASS SAMPLING: SAFER, MORE ACCURATE THAN THOUGHT
Renal mass sampling (ie, biopsy) has traditionally had a restricted role in the management of small renal masses, limited specifically to patients with a clinical history suggesting renal lymphoma, carcinoma that had metastasized to the kidney, or primary renal abscess. However, this may be changing, with more interest in it as a way to subtype and stratify select patients with small renal masses, especially potential candidates for active surveillance.
Our thinking about renal mass sampling has changed substantially over the last 2 decades. Previously, it was not routinely performed, because of concern over high false-negative rates (commonly quoted as being as high as 18%) and its potential associated morbidity. A common perception was that a negative biopsy could not be trusted and, therefore, renal mass sampling would not ultimately change patient management. However, many of these false-negative results were actually “noninformative,” ie, cases in which the renal tumor could not be adequately sampled or the pathologist lacked a sufficient specimen to allow for a definitive diagnosis.
Recent evidence suggests that these concerns were exaggerated and that renal mass sampling is more accurate and safer than previously thought. A meta-analysis of studies done before 2001 found that the diagnostic accuracy of renal mass sampling averaged 82%, whereas contemporary series indicate that its accuracy in differentiating benign from malignant tumors is actually greater than 95%.12 In addition, false-negative rates are now consistently less than 1%.13
Furthermore, serious complications requiring clinical intervention or hospitalization occur in fewer than 1% of cases. Seeding of the needle tract with tumor cells, which was another concern, is also exceedingly rare for these small, well-circumscribed renal masses.12
Overall morbidity is low with renal mass sampling, which is routinely performed as an outpatient procedure using CT or ultrasono-graphic guidance and local anesthesia.
However, 10% of biopsy results are still noninformative. In this situation, biopsy can be repeated, or the mass can be surgically excised, or the patient can undergo conservative management if he or she is unfit or unwilling to undergo surgery.
The encouraging results with renal mass sampling have led to greater use of it at many centers in the evaluation and risk-stratification of patients with small renal masses. It may be especially useful in patients considering several treatment options.
For example, a 75-year-old patient with modest comorbidities and a 2.0-cm enhancing renal mass could be a candidate for partial nephrectomy, thermal ablation, or active surveillance, and a reasonable argument could be made for each of these options. Renal mass sampling in this instance could be instrumental in guiding this decision, as a tissue diagnosis of high-grade renal cell carcinoma would favor partial nephrectomy, whereas a diagnosis of “oncocytoma neoplasm” would support a more conservative approach.
Older, frail patients with significant comorbidities who are unlikely to be candidates for aggressive surgical management would not need renal mass sampling, as they will ultimately be managed with active surveillance or thermal ablation.
Recent studies have also indicated that molecular profiling through gene expression analysis or proteomic analysis can further improve the accuracy of renal mass sampling.14 This will likely be the holy grail for this field, allowing for truly rational management (Figure 2).
TREATMENT OPTIONS
Radical nephrectomy: Still the most common treatment
In the past, complete removal of the kidney was standard for nearly all renal masses suspected of being renal cell carcinomas. Partial nephrectomy was generally reserved for patients who had a solitary kidney, bilateral tumors, or preexisting chronic kidney disease.
Although the two procedures provide equivalent oncologic outcomes for clinical T1 lesions, Miller et al15 reported that, before 2001, only 20% of small renal masses in the United States were managed with partial nephrectomy. That percentage has increased modestly, but radical nephrectomy still predominates.
One explanation for why the radical procedure is done more frequently is that partial nephrectomy is more technically difficult, as it involves renal reconstruction. Furthermore, radical nephrectomy can almost always be performed via a minimally invasive approach, which is inherently appealing to patients and surgeons alike. Laparoscopic radical nephrectomy has been called “the great seductress” because of these considerations.16 However, total removal of the kidney comes at a great price—loss of renal function.
Over the last decade, various studies have highlighted the association between radical nephrectomy and the subsequent clinical onset of chronic kidney disease, and the potential correlations between chronic kidney disease and cardiovascular events and elevated mortality rates.17
In a landmark study, Huang et al18 evaluated the outcomes of 662 patients who had small renal masses, a “normal” serum creatinine concentration (≤ 124 μmol/L [1.4 mg/dL]), and a normal-appearing contralateral kidney who underwent radical or partial nephrectomy. Of these, 26% were found to have preexisting stage 3 chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated using the Modification of Diet in Renal Disease equation). Additionally, 65% of patients treated with radical nephrectomy were found to have stage 3 chronic kidney disease after surgery vs 20% of patients managed with partial nephrectomy.
The misconception remains that the risk of chronic kidney disease after radical nephrectomy is insignificant, since the risk is low in renal transplant donors.19 However, renal transplant donors undergo stringent screening to ensure that their general health is good and that their renal function is robust, both of which are not true in many patients with small renal masses, particularly if they are elderly.
The overuse of radical nephrectomy is worrisome in light of the potential implications of chronic kidney disease, such as increased risk of morbid cardiovascular events and elevated mortality rates. Many experts believe that over-treatment of small renal masses may have contributed to the paradoxical increase in overall mortality rates observed with radical nephrectomy in some studies.4
Although radical nephrectomy remains an important treatment for locally advanced renal cell carcinoma, it should be performed for small renal masses only if nephron-sparing surgery is not feasible (Table 2).
Partial nephrectomy: The new gold standard for most patients
Over the last 5 years, greater emphasis has been placed on lessening the risk of chronic kidney disease in the management of all urologic conditions, including small renal masses.
The overuse of radical nephrectomy prompted the American Urological Association to commission a panel to provide guidelines for the management of clinical stage T1 renal masses.17 After an extensive review and rigorous meta-analysis, the panel concluded that partial nephrectomy is the gold standard for most patients (Table 1, Table 2).
Partial nephrectomy involves excision of the tumor with a small margin of normal tissue, preserving as much functional renal parenchyma as possible, followed by closure of the collecting system, suture ligation of any transected vessels, and reapproximation of the capsule. Tumor excision is usually performed during temporary occlusion of the renal vasculature, allowing for a bloodless field. Regional hypothermia (cold ischemia) can also be used to minimize ischemic injury.
Contemporary series have documented that partial and radical nephrectomy have comparable oncologic efficacy for patients with small renal masses.20,21 Local recurrence rates are only 1% to 2% with partial nephrectomy, and 5- and 10-year cancer-specific survival rates of 96% and 90% have been reported.22
Furthermore, some studies have shown that patients undergoing partial nephrectomy have higher overall survival rates than those managed with radical nephrectomy—perhaps in part due to greater preservation of renal function and a lower incidence of subsequent chronic kidney disease.23,24 At Cleveland Clinic, we are now studying the determinants of ultimate renal function after partial nephrectomy in an effort to minimize ischemic injury and optimize this technique.25
Complications. Partial nephrectomy does have a potential downside in that it carries a higher risk of urologic complications such as urine leak and postoperative hemorrhage, which is not surprising because it requires a reconstruction that must heal. In a recent meta-analysis, urologic complications occurred in 6.3% patients who underwent open partial nephrectomy and in 9.0% of patients who underwent laparoscopic partial nephrectomy.17 Fortunately, most complications associated with partial nephrectomy can be managed with conservative measures.
Postoperative bleeding occurs in about 1% to 2% of patients and is the most serious complication. However, it is typically managed with superselective embolization, which has a high success rate and facilitates renal preservation.
Urine leak occurs in about 3% to 5% of cases and almost always resolves with prolonged drainage, occasionally complemented with a ureteral stent to promote antegrade drainage.
A new refinement, robotic-assisted partial nephrectomy promises to reduce the morbidity of this procedure. This approach takes less time to learn than standard laparoscopic surgery and has expanded the indications for minimally invasive partial nephrectomy, although more-difficult cases are still better done through a traditional, open surgical approach.
Thermal ablation: Another minimally invasive option
Cryoablation and radiofrequency ablation (collectively called thermal ablation) have recently emerged as alternate minimally invasive treatments for small renal masses. They are appealing options for patients with small renal tumors (< 3.5 cm) who have significant comorbidities but still prefer a proactive approach. They can also be considered as salvage procedures in patients with local recurrence after partial nephrectomy or in select patients with multifocal disease.
Both procedures can be performed percutaneously or laparoscopically, offering the potential for rapid convalescence and reduced morbidity.26,27 A laparoscopic approach is necessary to mobilize the tumor from adjacent organs if they are juxtaposed, whereas a percutaneous approach is less invasive and is better suited for posterior renal masses.28 Renal mass sampling should be performed in these patients before treatment to define the histology and to guide surveillance and should be repeated postoperatively if there is suspicion of local recurrence based on imaging.
Cryoablation destroys tumor cells through rapid cycles of freezing to less than −20°C (−4°F) and thawing, which can be monitored in real time via thermocoupling (ie, a thermometer microprobe strategically placed outside the tumor to ensure that lethal temperatures are extended beyond the edge of the tumor) or via ultrasonography, or both. Treatment is continued until the “ice ball” extends about 1 cm beyond the edge of the tumor.
Initial series reported local tumor control rates in the range of 90% to 95%; however, follow-up was very limited.29 In a more robust single-institution experience,30 renal cryoablation demonstrated 5-year cancer-specific and recurrence-free survival rates of 93% and 83%, respectively, substantially lower than what would be expected with surgical excision in a similar patient population.
Another concern with cryoablation is that options are limited for surgical salvage if the initial treatment fails. Nguyen and Campbell31 reported that partial nephrectomy and minimally invasive surgery were often precluded in this situation because of the extensive fibrotic reaction caused by the prior treatment. If cryoablation fails, surgical salvage thus often requires open, radical surgery.
Radiofrequency ablation produces tumor coagulation via protein denaturation and disruption of cell membranes after heating tissues to temperatures above 50°C (122°F) for 4 to 6 minutes.32 One of its disadvantages is that one cannot monitor treatment progress in real time, as there is no identifiable change in tissue appearance analagous to the ice ball that is seen with cryoablation.
Although the outcomes of radiofrequency ablation are less robust than those of cryoablation, most studies suggest that local control is achieved in 80% to 90% of cases based on radiographic loss of enhancement after treatment.17,30,33 A recent meta-analysis comparing these treatments found that lesions treated with radiofrequency ablation had a significantly higher rate of local tumor progression than those treated with cryoablation (12.3% vs 4.7%, P < .0001).34 Both of these local recurrence rates are substantially higher than that seen after surgical excision, despite much shorter follow-up after thermal ablation.
Tempered enthusiasm. Because thermal ablation has been developed relatively recently, its long-term outcomes and treatment efficacy have not been well established, and current studies have confirmed higher local recurrence rates with thermal ablation than with surgical excision (Table 1). Furthermore, there are significant deficiencies in the literature about thermal ablation, including limited follow-up, lack of pathologic confirmation, and controversies regarding histologic or radiologic definitions of success (Table 2).
Although current enthusiasm for thermal ablation has been tempered by suboptimal results, further refinement in technique and acknowledgment of its limitations will help to define appropriate candidates for these treatments.
Active surveillance for select patients
In select patients with extensive medical comorbidities or short life expectancy, the risks associated with proactive management may outweigh the benefits, especially considering the indolent nature of many small renal masses. In such patients, active surveillance is reasonable.
A recent meta-analysis found that most small enhancing renal masses grew relatively slowly (median 0.28 cm/year) and posed a low risk of metastasis (1%–2%).17,22 Furthermore, almost all renal lesions that progressed to metastatic disease demonstrated rapid radiographic growth, suggesting that the radiographic growth of a renal mass under active surveillance may serve as an indicator for aggressive behavior.35
Unfortunately, the growth rates of small renal masses do not reliably predict malignancy, and one study reported that 83% of tumors without demonstrable growth were malignant.36
Studies of active surveillance to date have had several other important limitations. Many did not incorporate pathologic confirmation, so that about 20% of the tumors were actually benign, thus artificially reducing the risk of adverse outcomes.5,22,37 Furthermore, the follow-up has been short, with most studies including data for only 2 to 3 years, which is clearly inadequate for this type of malignancy.37,38 Finally, most series had significant selection bias towards small, homogenous masses. In general, small renal masses that appear to be more aggressive are treated and thus excluded from these surveillance populations (Table 2).
Another concern about active surveillance is the small but real risk of tumor progression to metastatic disease, rendering these patients incurable even with new, targeted molecular therapies. Additionally, some patients may lose their window of opportunity for nephron-sparing surgery if significant tumor growth occurs during observation, rendering partial nephrectomy unfeasible. Therefore, active surveillance is not advisable for young, otherwise healthy patients (Table 2).
In the future, advances in renal mass sampling with molecular profiling may help determine which renal lesions are less biologically aggressive and, thereby, help identify appropriate candidates for observation (Figure 2).
Opinion about treatment of mall renal masses has changed considerably in the past 2 decades.
Traditionally, the most common treatment was surgical removal of the whole kidney, ie, radical nephrectomy. However, recent studies have shown that many patients who undergo radical nephrectomy develop chronic kidney disease. Furthermore, radical nephrectomy often constitutes over-treatment, as many of these lesions are benign or, if malignant, would follow an indolent course if left alone.
Now that we better understand the biology of small renal masses and are more aware of the morbidity and mortality related to chronic kidney disease, we try to avoid radical nephrectomy whenever possible, favoring nephron-sparing approaches instead.
In this article, we review the current clinical management of small renal masses.
SMALL RENAL MASSES ARE A HETEROGENEOUS GROUP
Small renal masses are defined as solid renal tumors that enhance on computed tomography (CT) and magnetic resonance imaging (MRI) and are suspected of being renal cell carcinomas. They are generally low-stage and relatively small (< 4 cm in diameter) at presentation. Most are now discovered incidentally on CT or MRI done for various abdominal symptoms. From 20,000 to 30,000 new cases are diagnosed each year in the United States, and the rate is increasing by 3% to 4% per year as the use of CT and MRI increases.1,2
With more small renal masses being detected incidentally, renal cell carcinoma has been going through a stage and size migration—ie, more of these tumors are being discovered in clinical stage T1 (ie, confined to the kidney and measuring less than 7 cm) than in the past. Currently, clinical T1 renal tumors account for 48% to 66% of cases.3
This indicates that the disease is being detected and treated earlier in its course than in the past. However, cancer-specific deaths from renal cell carcinoma have not declined, suggesting that for many of these patients, our traditional practice of aggressive surgical management with radical nephrectomy may not be warranted.4
Small renal masses vary in biologic aggressiveness
Recent large surgical series indicate that up to 20% of small renal masses are benign, 55% to 60% are indolent renal cell carcinomas, and only 20% to 25% have potentially aggressive features, defined by high nuclear grade or locally invasive characteristics.5–7
A relatively strong predictor of the aggressiveness of renal tumors is their size, which directly correlates with the risk of malignant pathology. Of lesions smaller than 1.0 cm, 38% to 46% are benign, dramatically decreasing to 6.3% to 7.1% for lesions larger than 7.0 cm.5 Each 1.0-cm increase in tumor diameter correlates with a 16% increase in the risk of malignancy.8
Our knowledge of the natural history of small renal masses is limited, being based on small, retrospective series. In these studies, when small renal masses were followed over time, relatively few progressed (ie, metastasized), and there have been no documented reports of disease progression in the absence of demonstrable tumor growth, suggesting a predominance of nonaggressive phenotypes.9
In light of these observations, patients with small renal masses should be carefully evaluated to determine if they are candidates for active surveillance as opposed to more aggressive treatment, ie, surgery or thermal ablation.
CT AND MRI ARE THE PREFERRED DIAGNOSTIC STUDIES
In the past, most patients with renal tumors presented with gross hematuria, flank pain, or a palpable abdominal mass. These presentations are now uncommon, as most cases are asymptomatic and are diagnosed incidentally. In a series of 349 small renal masses, microhematuria was found in only 8 cases.10
Systemic manifestations or paraneoplastic syndromes such as hypercalcemia or hypertension are more common in patients with metastatic renal cell carcinoma than in those with localized tumors. It was because of these varied clinical presentations that renal cell carcinoma was previously known as the “internist’s tumor”; however, small renal masses are better termed the “radiologist’s tumor.”11
High-quality axial imaging with CT or MRI is preferred for evaluating renal cortical neoplasms. Enhancement on CT or MRI is the characteristic finding of a renal lesion that should be suspected of being renal cell carcinoma (Figure 1). Triple-phase CT is ideal, with images taken before contrast is given, immediately after contrast (the early vascular phase), and later (the delayed phase). Alternatively, MRI can be used in patients who are allergic to intravenous contrast or who have moderate renal dysfunction.
Renal tumors with enhancement of more than 15 Hounsfield units (HU) on CT imaging are considered suggestive of renal cell carcinoma, whereas those with less than 10 HU of enhancement are more likely to be benign. Enhancement in the range of 10 to 15 HU is considered equivocal.
Differential diagnosis
By far, most small renal masses are renal cell carcinomas. However, other possibilities include oncocytoma, atypical or fat-poor angiomyolipoma, metanephric adenoma, urothelial carcinoma, metastatic lesions, lymphoma, renal abscess or infarction, mixed epithelial or stromal tumor, pseudotumor, and vascular malformations.
With rare exceptions, dense fat within a renal mass reliably indicates benign angiomyolipoma, and all renal tumors should be reviewed carefully for this feature. Beyond this, no clinical or radiologic feature ensures that a small renal mass is benign.
Imaging’s inability to accurately classify these enhancing renal lesions has led to a renewed interest in renal mass sampling to aid in the evaluation of small renal masses.
RENAL MASS SAMPLING: SAFER, MORE ACCURATE THAN THOUGHT
Renal mass sampling (ie, biopsy) has traditionally had a restricted role in the management of small renal masses, limited specifically to patients with a clinical history suggesting renal lymphoma, carcinoma that had metastasized to the kidney, or primary renal abscess. However, this may be changing, with more interest in it as a way to subtype and stratify select patients with small renal masses, especially potential candidates for active surveillance.
Our thinking about renal mass sampling has changed substantially over the last 2 decades. Previously, it was not routinely performed, because of concern over high false-negative rates (commonly quoted as being as high as 18%) and its potential associated morbidity. A common perception was that a negative biopsy could not be trusted and, therefore, renal mass sampling would not ultimately change patient management. However, many of these false-negative results were actually “noninformative,” ie, cases in which the renal tumor could not be adequately sampled or the pathologist lacked a sufficient specimen to allow for a definitive diagnosis.
Recent evidence suggests that these concerns were exaggerated and that renal mass sampling is more accurate and safer than previously thought. A meta-analysis of studies done before 2001 found that the diagnostic accuracy of renal mass sampling averaged 82%, whereas contemporary series indicate that its accuracy in differentiating benign from malignant tumors is actually greater than 95%.12 In addition, false-negative rates are now consistently less than 1%.13
Furthermore, serious complications requiring clinical intervention or hospitalization occur in fewer than 1% of cases. Seeding of the needle tract with tumor cells, which was another concern, is also exceedingly rare for these small, well-circumscribed renal masses.12
Overall morbidity is low with renal mass sampling, which is routinely performed as an outpatient procedure using CT or ultrasono-graphic guidance and local anesthesia.
However, 10% of biopsy results are still noninformative. In this situation, biopsy can be repeated, or the mass can be surgically excised, or the patient can undergo conservative management if he or she is unfit or unwilling to undergo surgery.
The encouraging results with renal mass sampling have led to greater use of it at many centers in the evaluation and risk-stratification of patients with small renal masses. It may be especially useful in patients considering several treatment options.
For example, a 75-year-old patient with modest comorbidities and a 2.0-cm enhancing renal mass could be a candidate for partial nephrectomy, thermal ablation, or active surveillance, and a reasonable argument could be made for each of these options. Renal mass sampling in this instance could be instrumental in guiding this decision, as a tissue diagnosis of high-grade renal cell carcinoma would favor partial nephrectomy, whereas a diagnosis of “oncocytoma neoplasm” would support a more conservative approach.
Older, frail patients with significant comorbidities who are unlikely to be candidates for aggressive surgical management would not need renal mass sampling, as they will ultimately be managed with active surveillance or thermal ablation.
Recent studies have also indicated that molecular profiling through gene expression analysis or proteomic analysis can further improve the accuracy of renal mass sampling.14 This will likely be the holy grail for this field, allowing for truly rational management (Figure 2).
TREATMENT OPTIONS
Radical nephrectomy: Still the most common treatment
In the past, complete removal of the kidney was standard for nearly all renal masses suspected of being renal cell carcinomas. Partial nephrectomy was generally reserved for patients who had a solitary kidney, bilateral tumors, or preexisting chronic kidney disease.
Although the two procedures provide equivalent oncologic outcomes for clinical T1 lesions, Miller et al15 reported that, before 2001, only 20% of small renal masses in the United States were managed with partial nephrectomy. That percentage has increased modestly, but radical nephrectomy still predominates.
One explanation for why the radical procedure is done more frequently is that partial nephrectomy is more technically difficult, as it involves renal reconstruction. Furthermore, radical nephrectomy can almost always be performed via a minimally invasive approach, which is inherently appealing to patients and surgeons alike. Laparoscopic radical nephrectomy has been called “the great seductress” because of these considerations.16 However, total removal of the kidney comes at a great price—loss of renal function.
Over the last decade, various studies have highlighted the association between radical nephrectomy and the subsequent clinical onset of chronic kidney disease, and the potential correlations between chronic kidney disease and cardiovascular events and elevated mortality rates.17
In a landmark study, Huang et al18 evaluated the outcomes of 662 patients who had small renal masses, a “normal” serum creatinine concentration (≤ 124 μmol/L [1.4 mg/dL]), and a normal-appearing contralateral kidney who underwent radical or partial nephrectomy. Of these, 26% were found to have preexisting stage 3 chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated using the Modification of Diet in Renal Disease equation). Additionally, 65% of patients treated with radical nephrectomy were found to have stage 3 chronic kidney disease after surgery vs 20% of patients managed with partial nephrectomy.
The misconception remains that the risk of chronic kidney disease after radical nephrectomy is insignificant, since the risk is low in renal transplant donors.19 However, renal transplant donors undergo stringent screening to ensure that their general health is good and that their renal function is robust, both of which are not true in many patients with small renal masses, particularly if they are elderly.
The overuse of radical nephrectomy is worrisome in light of the potential implications of chronic kidney disease, such as increased risk of morbid cardiovascular events and elevated mortality rates. Many experts believe that over-treatment of small renal masses may have contributed to the paradoxical increase in overall mortality rates observed with radical nephrectomy in some studies.4
Although radical nephrectomy remains an important treatment for locally advanced renal cell carcinoma, it should be performed for small renal masses only if nephron-sparing surgery is not feasible (Table 2).
Partial nephrectomy: The new gold standard for most patients
Over the last 5 years, greater emphasis has been placed on lessening the risk of chronic kidney disease in the management of all urologic conditions, including small renal masses.
The overuse of radical nephrectomy prompted the American Urological Association to commission a panel to provide guidelines for the management of clinical stage T1 renal masses.17 After an extensive review and rigorous meta-analysis, the panel concluded that partial nephrectomy is the gold standard for most patients (Table 1, Table 2).
Partial nephrectomy involves excision of the tumor with a small margin of normal tissue, preserving as much functional renal parenchyma as possible, followed by closure of the collecting system, suture ligation of any transected vessels, and reapproximation of the capsule. Tumor excision is usually performed during temporary occlusion of the renal vasculature, allowing for a bloodless field. Regional hypothermia (cold ischemia) can also be used to minimize ischemic injury.
Contemporary series have documented that partial and radical nephrectomy have comparable oncologic efficacy for patients with small renal masses.20,21 Local recurrence rates are only 1% to 2% with partial nephrectomy, and 5- and 10-year cancer-specific survival rates of 96% and 90% have been reported.22
Furthermore, some studies have shown that patients undergoing partial nephrectomy have higher overall survival rates than those managed with radical nephrectomy—perhaps in part due to greater preservation of renal function and a lower incidence of subsequent chronic kidney disease.23,24 At Cleveland Clinic, we are now studying the determinants of ultimate renal function after partial nephrectomy in an effort to minimize ischemic injury and optimize this technique.25
Complications. Partial nephrectomy does have a potential downside in that it carries a higher risk of urologic complications such as urine leak and postoperative hemorrhage, which is not surprising because it requires a reconstruction that must heal. In a recent meta-analysis, urologic complications occurred in 6.3% patients who underwent open partial nephrectomy and in 9.0% of patients who underwent laparoscopic partial nephrectomy.17 Fortunately, most complications associated with partial nephrectomy can be managed with conservative measures.
Postoperative bleeding occurs in about 1% to 2% of patients and is the most serious complication. However, it is typically managed with superselective embolization, which has a high success rate and facilitates renal preservation.
Urine leak occurs in about 3% to 5% of cases and almost always resolves with prolonged drainage, occasionally complemented with a ureteral stent to promote antegrade drainage.
A new refinement, robotic-assisted partial nephrectomy promises to reduce the morbidity of this procedure. This approach takes less time to learn than standard laparoscopic surgery and has expanded the indications for minimally invasive partial nephrectomy, although more-difficult cases are still better done through a traditional, open surgical approach.
Thermal ablation: Another minimally invasive option
Cryoablation and radiofrequency ablation (collectively called thermal ablation) have recently emerged as alternate minimally invasive treatments for small renal masses. They are appealing options for patients with small renal tumors (< 3.5 cm) who have significant comorbidities but still prefer a proactive approach. They can also be considered as salvage procedures in patients with local recurrence after partial nephrectomy or in select patients with multifocal disease.
Both procedures can be performed percutaneously or laparoscopically, offering the potential for rapid convalescence and reduced morbidity.26,27 A laparoscopic approach is necessary to mobilize the tumor from adjacent organs if they are juxtaposed, whereas a percutaneous approach is less invasive and is better suited for posterior renal masses.28 Renal mass sampling should be performed in these patients before treatment to define the histology and to guide surveillance and should be repeated postoperatively if there is suspicion of local recurrence based on imaging.
Cryoablation destroys tumor cells through rapid cycles of freezing to less than −20°C (−4°F) and thawing, which can be monitored in real time via thermocoupling (ie, a thermometer microprobe strategically placed outside the tumor to ensure that lethal temperatures are extended beyond the edge of the tumor) or via ultrasonography, or both. Treatment is continued until the “ice ball” extends about 1 cm beyond the edge of the tumor.
Initial series reported local tumor control rates in the range of 90% to 95%; however, follow-up was very limited.29 In a more robust single-institution experience,30 renal cryoablation demonstrated 5-year cancer-specific and recurrence-free survival rates of 93% and 83%, respectively, substantially lower than what would be expected with surgical excision in a similar patient population.
Another concern with cryoablation is that options are limited for surgical salvage if the initial treatment fails. Nguyen and Campbell31 reported that partial nephrectomy and minimally invasive surgery were often precluded in this situation because of the extensive fibrotic reaction caused by the prior treatment. If cryoablation fails, surgical salvage thus often requires open, radical surgery.
Radiofrequency ablation produces tumor coagulation via protein denaturation and disruption of cell membranes after heating tissues to temperatures above 50°C (122°F) for 4 to 6 minutes.32 One of its disadvantages is that one cannot monitor treatment progress in real time, as there is no identifiable change in tissue appearance analagous to the ice ball that is seen with cryoablation.
Although the outcomes of radiofrequency ablation are less robust than those of cryoablation, most studies suggest that local control is achieved in 80% to 90% of cases based on radiographic loss of enhancement after treatment.17,30,33 A recent meta-analysis comparing these treatments found that lesions treated with radiofrequency ablation had a significantly higher rate of local tumor progression than those treated with cryoablation (12.3% vs 4.7%, P < .0001).34 Both of these local recurrence rates are substantially higher than that seen after surgical excision, despite much shorter follow-up after thermal ablation.
Tempered enthusiasm. Because thermal ablation has been developed relatively recently, its long-term outcomes and treatment efficacy have not been well established, and current studies have confirmed higher local recurrence rates with thermal ablation than with surgical excision (Table 1). Furthermore, there are significant deficiencies in the literature about thermal ablation, including limited follow-up, lack of pathologic confirmation, and controversies regarding histologic or radiologic definitions of success (Table 2).
Although current enthusiasm for thermal ablation has been tempered by suboptimal results, further refinement in technique and acknowledgment of its limitations will help to define appropriate candidates for these treatments.
Active surveillance for select patients
In select patients with extensive medical comorbidities or short life expectancy, the risks associated with proactive management may outweigh the benefits, especially considering the indolent nature of many small renal masses. In such patients, active surveillance is reasonable.
A recent meta-analysis found that most small enhancing renal masses grew relatively slowly (median 0.28 cm/year) and posed a low risk of metastasis (1%–2%).17,22 Furthermore, almost all renal lesions that progressed to metastatic disease demonstrated rapid radiographic growth, suggesting that the radiographic growth of a renal mass under active surveillance may serve as an indicator for aggressive behavior.35
Unfortunately, the growth rates of small renal masses do not reliably predict malignancy, and one study reported that 83% of tumors without demonstrable growth were malignant.36
Studies of active surveillance to date have had several other important limitations. Many did not incorporate pathologic confirmation, so that about 20% of the tumors were actually benign, thus artificially reducing the risk of adverse outcomes.5,22,37 Furthermore, the follow-up has been short, with most studies including data for only 2 to 3 years, which is clearly inadequate for this type of malignancy.37,38 Finally, most series had significant selection bias towards small, homogenous masses. In general, small renal masses that appear to be more aggressive are treated and thus excluded from these surveillance populations (Table 2).
Another concern about active surveillance is the small but real risk of tumor progression to metastatic disease, rendering these patients incurable even with new, targeted molecular therapies. Additionally, some patients may lose their window of opportunity for nephron-sparing surgery if significant tumor growth occurs during observation, rendering partial nephrectomy unfeasible. Therefore, active surveillance is not advisable for young, otherwise healthy patients (Table 2).
In the future, advances in renal mass sampling with molecular profiling may help determine which renal lesions are less biologically aggressive and, thereby, help identify appropriate candidates for observation (Figure 2).
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J 2008; 14:288–301.
- Lane BR, Campbell SC. Management of small renal masses. AUA Update Series 2009; 28:313–324.
- Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer 2004; 100:738–745.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98:1331–1334.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003; 170:2217–2220.
- Russo P. Should elective partial nephrectomy be performed for renal cell carcinoma >4 cm in size? Nat Clin Pract Urol 2008; 5:482–483.
- Thomas AA, Aron M, Hernandez AV, Lane BR, Gill IS. Laparoscopic partial nephrectomy in octogenarians. Urology 2009; 74:1042–1046.
- Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol 2009; 181:2033–2036.
- Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol 2010; 20:105–110.
- Patard JJ, Bensalah K, Vincendeau S, Rioux-Leclerq N, Guillé F, Lobel B. [Correlation between the mode of presentation of renal tumors and patient survival]. Prog Urol 2003; 13:23–28.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373:1119–1132.
- Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy—a renaissance? J Urol 2008; 179:20–27.
- Samplaski MK, Zhou M, Lane BR, Herts B, Campbell SC. Renal mass sampling: an enlightened perspective. Int J Urol 2011; 18:5–19.
- Tan MH, Rogers CG, Cooper JT, et al. Gene expression profiling of renal cell carcinoma. Clin Cancer Res 2004; 10:6315S–6321S.
- Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol 2006; 175:853–857.
- Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol 2009; 182:435–444.
- Campbell SC, Novick AC, Belldegrun A, et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182:1271–1279.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7:735–740.
- Boorjian SA, Uzzo RG. The evolving management of small renal masses. Curr Oncol Rep 2009; 11:211–217.
- Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol 1999; 162:1930–1933.
- Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000; 163:730–736.
- Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006; 175:425–431.
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181:55–61.
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008; 179:468–471.
- Thomas AA, Demirjian S, Lane BR, et al. Acute kidney injury: novel biomarkers and potential utility for patient care in urology. Urology 2011; 77:5–11.
- Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FT. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol 2008; 191:1159–1168.
- Sterrett SP, Nakada SY, Wingo MS, Williams SK, Leveillee RJ. Renal thermal ablative therapy. Urol Clin North Am 2008; 35:397–414.
- Hafron J, Kaouk JH. Ablative techniques for the management of kidney cancer. Nat Clin Pract Urol 2007; 4:261–269.
- Matin SF, Ahrar K. Nephron-sparing probe ablative therapy: longterm outcomes. Curr Opin Urol 2008; 18:150–156.
- Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol 2009; 19:138–142.
- Nguyen CT, Campbell SC. Salvage of local recurrence after primary thermal ablation for small renal masses. Expert Rev Anticancer Ther 2008; 8:1899–1905.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174:323–331.
- Carraway WA, Raman JD, Cadeddu JA. Current status of renal radiofrequency ablation. Curr Opin Urol 2009; 19:143–147.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008; 113:2671–2680.
- Kunkle DA, Kutikov A, Uzzo RG. Management of small renal masses. Semin Ultrasound CT MR 2009; 30:352–358.
- Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol 2007; 177:849–853.
- Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 2008; 179:1227–1233.
- Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am 2008; 35:627–634.
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J 2008; 14:288–301.
- Lane BR, Campbell SC. Management of small renal masses. AUA Update Series 2009; 28:313–324.
- Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer 2004; 100:738–745.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98:1331–1334.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003; 170:2217–2220.
- Russo P. Should elective partial nephrectomy be performed for renal cell carcinoma >4 cm in size? Nat Clin Pract Urol 2008; 5:482–483.
- Thomas AA, Aron M, Hernandez AV, Lane BR, Gill IS. Laparoscopic partial nephrectomy in octogenarians. Urology 2009; 74:1042–1046.
- Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol 2009; 181:2033–2036.
- Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol 2010; 20:105–110.
- Patard JJ, Bensalah K, Vincendeau S, Rioux-Leclerq N, Guillé F, Lobel B. [Correlation between the mode of presentation of renal tumors and patient survival]. Prog Urol 2003; 13:23–28.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373:1119–1132.
- Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy—a renaissance? J Urol 2008; 179:20–27.
- Samplaski MK, Zhou M, Lane BR, Herts B, Campbell SC. Renal mass sampling: an enlightened perspective. Int J Urol 2011; 18:5–19.
- Tan MH, Rogers CG, Cooper JT, et al. Gene expression profiling of renal cell carcinoma. Clin Cancer Res 2004; 10:6315S–6321S.
- Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol 2006; 175:853–857.
- Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assessment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol 2009; 182:435–444.
- Campbell SC, Novick AC, Belldegrun A, et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182:1271–1279.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7:735–740.
- Boorjian SA, Uzzo RG. The evolving management of small renal masses. Curr Oncol Rep 2009; 11:211–217.
- Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol 1999; 162:1930–1933.
- Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000; 163:730–736.
- Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006; 175:425–431.
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181:55–61.
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008; 179:468–471.
- Thomas AA, Demirjian S, Lane BR, et al. Acute kidney injury: novel biomarkers and potential utility for patient care in urology. Urology 2011; 77:5–11.
- Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FT. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol 2008; 191:1159–1168.
- Sterrett SP, Nakada SY, Wingo MS, Williams SK, Leveillee RJ. Renal thermal ablative therapy. Urol Clin North Am 2008; 35:397–414.
- Hafron J, Kaouk JH. Ablative techniques for the management of kidney cancer. Nat Clin Pract Urol 2007; 4:261–269.
- Matin SF, Ahrar K. Nephron-sparing probe ablative therapy: longterm outcomes. Curr Opin Urol 2008; 18:150–156.
- Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol 2009; 19:138–142.
- Nguyen CT, Campbell SC. Salvage of local recurrence after primary thermal ablation for small renal masses. Expert Rev Anticancer Ther 2008; 8:1899–1905.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174:323–331.
- Carraway WA, Raman JD, Cadeddu JA. Current status of renal radiofrequency ablation. Curr Opin Urol 2009; 19:143–147.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008; 113:2671–2680.
- Kunkle DA, Kutikov A, Uzzo RG. Management of small renal masses. Semin Ultrasound CT MR 2009; 30:352–358.
- Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol 2007; 177:849–853.
- Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 2008; 179:1227–1233.
- Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am 2008; 35:627–634.
KEY POINTS
- Small renal masses are a heterogeneous group of tumors, and only 20% are aggressive renal cell carcinoma.
- In general, nephron-sparing treatments are preferred to avoid chronic kidney disease, which often occurs after radical nephrectomy.
- Thermal ablation and active surveillance are valid treatment strategies in select patients who are not optimal surgical candidates or who have limited life expectancy.