User login
Should the 30-minute rule for emergent cesarean delivery be applied universally?
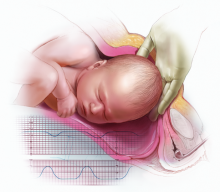
CASE 1: Term delivery: 45 minutes from decision to incision
P. G. is a 27-year-old woman (G2P1) at 38.2 weeks’ gestation who presents to the labor and delivery unit reporting painful contractions after uncomplicated prenatal care. She has a body mass index (BMI) of 40 kg/m2. Upon admission, her fetal heart-rate (FHR) tracing falls into Category 1. An examination reveals a cervix dilated to 4 cm and 70% effaced. Epidural analgesia is administered for pain control.
After 4 hours, the FHR tracing reveals minimal variability with occasional variable decelerations. The obstetrician is informed but issues no specific instructions. After 2 more hours, the FHR tracing lacks variability, with late decelerations and no spontaneous accelerations—a Category 3 tracing, which is predictive of abnormal acid-base status. Contractions occur every 3 to 4 minutes.
When fetal scalp stimulation by the nurse fails to elicit any accelerations, intrauterine resuscitation is attempted with an intravenous fluid bolus, left lateral positioning, and oxygen administration. Despite these measures, the FHR pattern fails to improve.
Although she is apprised of the need for prompt delivery, the patient hopes to avoid cesarean delivery, if possible, and insists on more time before a decision is made to proceed to cesarean. After another 2 hours, the FHR pattern has not improved and cervical dilation remains at 4 cm. The patient gives her consent for cesarean delivery.
Approximately 35 minutes are needed to take the patient to the operating room (OR). About 45 minutes after informed consent, the incision is made. Forty-seven minutes later, a male infant is delivered with Apgar scores of 1, 3, and 4 at 1, 5, and 10 minutes, respectively. Umbilical arterial analysis reveals a pH level of 6.9, with a base excess of –21. The infant has a neonatal seizure within 3 hours and is eventually diagnosed with cerebral palsy.
A claim against the clinicians alleges that deviation from the “standard of care 30-minute rule more than likely caused” hypoxic ische- mic injury and cerebral palsy.
Does the literature support this claim?
Approximately 3% of all births involve cesarean delivery for a nonreassuring FHR tracing.1 Much has been written about the “30-minute rule” for decision to incision time. In this article, we highlight current limitations of this standard in the context of 4 distinct clinical scenarios.
Case 1 highlights several limitations and ambiguities in the obstetric literature. Although a timely delivery is always desirable, it may not always be possible to achieve safely due to intrinsic patient characteristics or situational constraints. Conditions prevailing before the decision to proceed to cesarean delivery also affect overall pregnancy outcomes. Not all cases have the same starting point; fetal status at the time of the cesarean decision also determines the acuity and urgency of the case.
A widely promulgated rule— but is it valid?
Both the American College of Obstetricians and Gynecologists (ACOG) and the Royal College of Obstetricians and Gynaecologists have published guidelines stating that any hospital offering obstetric care should have the capability to perform emergent cesarean delivery within 30 minutes.2,3 This general statement has been touted as the standard by which obstetric services should be evaluated. Regardless of the clinical situation, obstetric providers are expected to abide by this rule.
These guidelines recently have come under scrutiny. For example, a 2014 meta-analysis involving more than 30 studies and 22,000 women revealed that only 36% of all cases with a Category 2 FHR tracing were delivered within 30 minutes.4 Interestingly, investigators reported that infants with a shorter delivery interval had a higher likelihood of having a 5-minute Apgar score below 7 and an umbilical artery pH level below 7.1, with no difference in the rate of admission to a neonatal intensive care unit (NICU) when the time from decision to delivery was examined.4 This finding highlights the questionable nature of the current clinical standard, as well as the conflicting findings currently present in the literature.
In general, patients who have graver clinical findings will be delivered at a shorter interval but may still have worse neonatal outcomes than infants delivered 30 minutes or more after the decision for cesarean is made.
Although Case 1 is complicated by FHR abnormalities, the association between such abnormalities and adverse long-term outcomes in neonates is questionable. Fewer than 1% of cases involving late decelerations or decreased variability during labor lead to cerebral palsy,5 highlighting the weak association between FHR abnormalities and neurologic sequelae. Most adverse neurologic neonatal outcomes are multifactorial in nature and may not be attributable to a single prenatal event.
With such limitations, the application and use of the 30-minute “standard” by hospitals, professional societies, and the medicolegal community may not be appropriate. The literature may not justify using this arbitrary rule as the standard of care. Clearly, there are gaps in our knowledge and understanding of FHR abnormalities and the optimal interval for cesarean delivery. Therefore, it may be unfair and inappropriate to group all cases and clinical situations together.
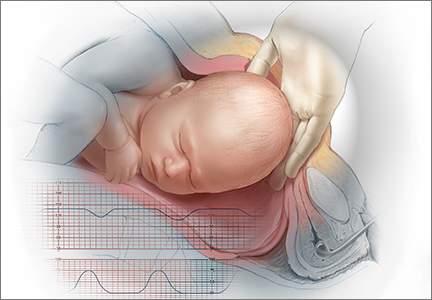
CASE 2: 25 minutes from decision to preterm delivery
J. P. (G2P1) undergoes an ultrasonographic examination at 33.4 weeks’ gestation because of concern about a discrepancy between fetal size and gestational age. The estimated fetal weight is in the 5th percentile. Amniotic fluid level is normal, but the biophysical profile is 6/8, with no breathing for 30 seconds. Umbilical artery Doppler imaging reveals absent end-diastolic flow, and FHR monitoring reveals repetitive late decelerations.
The patient is admitted immediately to the labor and delivery unit and placed on continuous electronic fetal monitoring. Betamethasone is given to enhance fetal lung maturity. FHR monitoring continues to show repetitive late decelerations with every contraction.
After 10 minutes on the labor floor, a decision is made to proceed to emergent cesarean delivery. Within 25 minutes of that decision, a female infant weighing 1,731 g (3rd percentile) is delivered, with Apgar scores of 1, 1, and 4 at 1, 5, and 10 minutes, respectively. The infant is eventually diagnosed with moderate cerebral palsy.
Could this outcome have been prevented?
Published reports on the association between abnormal FHR patterns and adverse perinatal outcomes in preterm infants are even more scarce than they are for infants delivered at term. Case 2 highlights the fact that achievement of a 30-minute interval from decision to delivery doesn’t necessarily eliminate the risk of adverse neonatal outcomes and long-term morbidity.
One of the best evaluations of this association was published by Shy and colleagues in the 1980s.6 In that study, investigators randomly assigned 173 preterm infants to intermittent auscultation or continuous external fetal monitoring. Use of external fetal monitoring did not improve neurologic outcomes at 18 months of age. Nor did the duration of FHR abnormalities predict the development of cerebral palsy.6
A recent secondary analysis from a randomized trial evaluating the use of antenatal magnesium sulfate to prevent cerebral palsy revealed that preterm FHR patterns labeled as “fetal distress” by the treating physician were associated with an increased risk of cerebral palsy in the newborn.7 Although this analysis revealed an association, a causal link could not be established. Damage to a preterm infant’s central nervous system can occur before the mother presents to the ultrasound unit or clinic, and alterations to FHR patterns can reflect previous injury. In such cases, a short decision to incision interval would not prevent damage to the central nervous system of the preterm infant.
CASE 3: 5 minutes from decision to incision after uterine rupture
G. P. is a patient (G2P1) at 38 weeks’ gestation who has had a previous low uterine transverse cesarean delivery. She strongly wishes to attempt vaginal birth after cesarean (VBAC) and has been extensively counseled about the risks and benefits of this approach. This counseling has been appropriately documented in her chart. Her predicted likelihood of success is 54%.
Upon arrival in the triage unit, she reiterates that she hopes to deliver her child vaginally. Upon examination, she is found to be dilated to 4 cm. She is admitted to the labor and delivery unit, with reevaluation planned 2 hours after epidural administration. At that time, her labor is noted to be progressing at an appropriate rate.
After 5 hours of labor, the baseline FHR drops into the 70s. Immediate evaluation reveals significant uterine bleeding, with the fetus no longer engaged in the pelvis. The attending physician immediately suspects uterine rupture.
The patient is rushed to the OR, and delivery is complicated by the presence of extensive adhesions to the uterus and anterior abdominal wall. After 20 minutes, a female infant is delivered, with Apgar scores of 0, 0, and 1 at 1, 5, and 10 minutes, respectively. Medical care is withdrawn after 3 days in the NICU.
In a true obstetric catastrophe such as uterine rupture, should the decision to incision interval be 30 minutes?
Although it is rare, uterine rupture is a known complication of VBAC attempts. The actual rate varies across the literature but appears to be approximately 0.5% to 0.9% in women attempting vaginal birth after a prior lower uterine incision.8
If uterine rupture develops, both mother and fetus are at increased risk of morbidity and mortality. The risk of hypoxic ischemic encephalopathy after uterine rupture is about 6.2% (95% confidence interval [CI], 1.8–10.6), and the risk of neonatal death is about 1.8% (95% CI, 0–4.2).9 Uterine rupture also has been linked to an increase in:
- severe postpartum hemorrhage (odds ratio [OR], 8.51; 95% CI, 4.6–15.1)
- general anesthesia exposure (OR, 14.20; 95% CI, 9.1–22.2)
- hysterectomy (OR, 51.36; 95% CI, 13.6–193.4)
- serious perinatal outcome (OR, 24.51; 95% CI, 11.9–51.9).10
- serious perinatal outcome (OR, 24.51; 95% CI, 11.9–51.9).
Case 3 again highlights the limitations and difficulties of encompassing all cases within a 30-minute timeframe. Although the newborn was delivered within this interval after the initial insult, the intervention was insufficient to prevent severe and long-term damage.
In cases of true obstetric emergency, the catastrophic nature of the event may lead to adverse long-term neonatal outcomes even if the standard of care is met. Immediate delivery still may not allow for the prevention of neurologic morbidity in the fetus. When evaluating such cases retrospectively, all parties involved always should consider these facts before drawing any conclusions on causality and prevention.
CASE 4: Twins delivered 20 minutes after cesarean decision
P. R. (G1P0) presents for routine prenatal care at 36 weeks’ gestation. She is carrying a dichorionic/diamniotic twin gestation that so far has been uncomplicated. She has been experiencing contractions for the past 2 weeks, but they have intensified during the past 2 days. When an examination reveals that she is dilated to 4 cm, she is admitted to the labor and delivery unit.
Both fetuses are evaluated via external FHR monitoring. Initially, both have Category 1 tracings but, approximately 1 hour later, both tracings are noted to have minimal variability with variable decelerations, with a nadir at 80 bpm that lasts 30 to 45 seconds. These abnormalities persist even after intrauterine resuscitation is attempted. The cervix remains dilated at 4 cm.
After a Category 2 tracing persists for 1 hour, the attending physician proceeds to cesarean delivery. Both infants are delivered within 20 minutes after the decision is made. Two female infants of appropriate gestational size are delivered, with Apgar scores of 7 and 8 for Twin A and 8 and 9 for Twin B. The newborns eventually are discharged home with the mother. Twin B is subsequently given a diagnosis of cerebral palsy.
Should the decision to incision rule be applied to twin gestations?
Multifetal gestations carry an increased risk not only of fetal and neonatal death but also of handicap among survivors, compared with singleton pregnancies.11 The literature evaluating the link between abnormal FHR patterns and adverse neonatal outcomes in twin pregnancies is sparse. Adding to the confusion is the fact that signal loss from fetal monitoring during labor occurs more frequently in twins than in singletons, with a reported incidence of 26% to 33% during the 1st stage of labor and 41% to 63% during the 2nd stage.12 Moreover, the FHR pattern of one twin may be recorded twice inadvertently and the same tracing erroneously attributed to both twins.
The decision to incision and delivery time in twin gestations should be evaluated in the context of all the limitations the clinician faces when managing labor in a twin gestation. The 30-minute rule never has been specifically evaluated in the context of multifetal gestations. The pathway and contributing factors that lead to adverse neonatal outcomes in twin gestations may be very different from those related to singleton pregnancies and may be more relevant to antepartum than intrapartum events.
Take-home message
The 4 cases presented here call into question the applicability and generalizability of the 30-minute decision to incision rule. Diverse clinical situations encountered in practice should lead to different interpretations of this standard. No single rule can encompass all possible scenarios; therefore, a single rule should not be touted as universal. All clinical variables should be weighed and interpreted in the retrospective evaluation of a case involving a cesarean delivery performed after a 30-minute decision to incision interval.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337–350.
- American College of Obstetricians and Gynecologists, Committee on Professional Standards. Standards for Obstetric-Gynecologic Hospital Services. 7th ed. Washington, DC: ACOG; 1989.
- National Institute for Health and Care Excellence. Caesarean Section Guideline. London, UK: NICE; 2011.
- Tolcher MC, Johnson RL, El-Nashar SA, West CP. Decision-to-incision time and neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(3):536–548.
- Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain values of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613–618.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Mendez-Figueroa H, Chauhan SP, Pedroza C, Refuerzo JS, Dahlke JD, Rouse DJ. Preterm cesarean delivery for nonreassuring fetal heart rate: neonatal and neurologic morbidity. Obstet Gynecol. 2015;125(3):636–642.
- Macones GA, Cahill AG, Samilio DM, Odibo A, Peipert J, Stevens EJ. Can uterine rupture in patients attempting vaginal birth after cesarean delivery be predicted? Am J Obstet Gynecol. 2006;195(4):1148–1152.
- Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581–2589.
- Al-Zirqi I, Stray-Pedersen B, Forsen L, Daltveit AK, Vangen S. Uterine rupture: trends over 40 years [published online ahead of print April 2, 2015]. BJOG. doi: 10.1111/1471-0528.13394.
- Ramsey PS, Repke JT. Intrapartum management of multifetal pregnancies. Semin Perinatol. 2003;27(1):54–72.
- Bakker PC, Colenbrander GJ, Verstraeten AA, Van Geijn HP. Quality of intrapartum cardiotocography in twin deliveries. Am J Obstet Gynecol. 2004;191(6):2114–2119.
CASE 1: Term delivery: 45 minutes from decision to incision
P. G. is a 27-year-old woman (G2P1) at 38.2 weeks’ gestation who presents to the labor and delivery unit reporting painful contractions after uncomplicated prenatal care. She has a body mass index (BMI) of 40 kg/m2. Upon admission, her fetal heart-rate (FHR) tracing falls into Category 1. An examination reveals a cervix dilated to 4 cm and 70% effaced. Epidural analgesia is administered for pain control.
After 4 hours, the FHR tracing reveals minimal variability with occasional variable decelerations. The obstetrician is informed but issues no specific instructions. After 2 more hours, the FHR tracing lacks variability, with late decelerations and no spontaneous accelerations—a Category 3 tracing, which is predictive of abnormal acid-base status. Contractions occur every 3 to 4 minutes.
When fetal scalp stimulation by the nurse fails to elicit any accelerations, intrauterine resuscitation is attempted with an intravenous fluid bolus, left lateral positioning, and oxygen administration. Despite these measures, the FHR pattern fails to improve.
Although she is apprised of the need for prompt delivery, the patient hopes to avoid cesarean delivery, if possible, and insists on more time before a decision is made to proceed to cesarean. After another 2 hours, the FHR pattern has not improved and cervical dilation remains at 4 cm. The patient gives her consent for cesarean delivery.
Approximately 35 minutes are needed to take the patient to the operating room (OR). About 45 minutes after informed consent, the incision is made. Forty-seven minutes later, a male infant is delivered with Apgar scores of 1, 3, and 4 at 1, 5, and 10 minutes, respectively. Umbilical arterial analysis reveals a pH level of 6.9, with a base excess of –21. The infant has a neonatal seizure within 3 hours and is eventually diagnosed with cerebral palsy.
A claim against the clinicians alleges that deviation from the “standard of care 30-minute rule more than likely caused” hypoxic ische- mic injury and cerebral palsy.
Does the literature support this claim?
Approximately 3% of all births involve cesarean delivery for a nonreassuring FHR tracing.1 Much has been written about the “30-minute rule” for decision to incision time. In this article, we highlight current limitations of this standard in the context of 4 distinct clinical scenarios.
Case 1 highlights several limitations and ambiguities in the obstetric literature. Although a timely delivery is always desirable, it may not always be possible to achieve safely due to intrinsic patient characteristics or situational constraints. Conditions prevailing before the decision to proceed to cesarean delivery also affect overall pregnancy outcomes. Not all cases have the same starting point; fetal status at the time of the cesarean decision also determines the acuity and urgency of the case.
A widely promulgated rule— but is it valid?
Both the American College of Obstetricians and Gynecologists (ACOG) and the Royal College of Obstetricians and Gynaecologists have published guidelines stating that any hospital offering obstetric care should have the capability to perform emergent cesarean delivery within 30 minutes.2,3 This general statement has been touted as the standard by which obstetric services should be evaluated. Regardless of the clinical situation, obstetric providers are expected to abide by this rule.
These guidelines recently have come under scrutiny. For example, a 2014 meta-analysis involving more than 30 studies and 22,000 women revealed that only 36% of all cases with a Category 2 FHR tracing were delivered within 30 minutes.4 Interestingly, investigators reported that infants with a shorter delivery interval had a higher likelihood of having a 5-minute Apgar score below 7 and an umbilical artery pH level below 7.1, with no difference in the rate of admission to a neonatal intensive care unit (NICU) when the time from decision to delivery was examined.4 This finding highlights the questionable nature of the current clinical standard, as well as the conflicting findings currently present in the literature.
In general, patients who have graver clinical findings will be delivered at a shorter interval but may still have worse neonatal outcomes than infants delivered 30 minutes or more after the decision for cesarean is made.
Although Case 1 is complicated by FHR abnormalities, the association between such abnormalities and adverse long-term outcomes in neonates is questionable. Fewer than 1% of cases involving late decelerations or decreased variability during labor lead to cerebral palsy,5 highlighting the weak association between FHR abnormalities and neurologic sequelae. Most adverse neurologic neonatal outcomes are multifactorial in nature and may not be attributable to a single prenatal event.
With such limitations, the application and use of the 30-minute “standard” by hospitals, professional societies, and the medicolegal community may not be appropriate. The literature may not justify using this arbitrary rule as the standard of care. Clearly, there are gaps in our knowledge and understanding of FHR abnormalities and the optimal interval for cesarean delivery. Therefore, it may be unfair and inappropriate to group all cases and clinical situations together.
CASE 2: 25 minutes from decision to preterm delivery
J. P. (G2P1) undergoes an ultrasonographic examination at 33.4 weeks’ gestation because of concern about a discrepancy between fetal size and gestational age. The estimated fetal weight is in the 5th percentile. Amniotic fluid level is normal, but the biophysical profile is 6/8, with no breathing for 30 seconds. Umbilical artery Doppler imaging reveals absent end-diastolic flow, and FHR monitoring reveals repetitive late decelerations.
The patient is admitted immediately to the labor and delivery unit and placed on continuous electronic fetal monitoring. Betamethasone is given to enhance fetal lung maturity. FHR monitoring continues to show repetitive late decelerations with every contraction.
After 10 minutes on the labor floor, a decision is made to proceed to emergent cesarean delivery. Within 25 minutes of that decision, a female infant weighing 1,731 g (3rd percentile) is delivered, with Apgar scores of 1, 1, and 4 at 1, 5, and 10 minutes, respectively. The infant is eventually diagnosed with moderate cerebral palsy.
Could this outcome have been prevented?
Published reports on the association between abnormal FHR patterns and adverse perinatal outcomes in preterm infants are even more scarce than they are for infants delivered at term. Case 2 highlights the fact that achievement of a 30-minute interval from decision to delivery doesn’t necessarily eliminate the risk of adverse neonatal outcomes and long-term morbidity.
One of the best evaluations of this association was published by Shy and colleagues in the 1980s.6 In that study, investigators randomly assigned 173 preterm infants to intermittent auscultation or continuous external fetal monitoring. Use of external fetal monitoring did not improve neurologic outcomes at 18 months of age. Nor did the duration of FHR abnormalities predict the development of cerebral palsy.6
A recent secondary analysis from a randomized trial evaluating the use of antenatal magnesium sulfate to prevent cerebral palsy revealed that preterm FHR patterns labeled as “fetal distress” by the treating physician were associated with an increased risk of cerebral palsy in the newborn.7 Although this analysis revealed an association, a causal link could not be established. Damage to a preterm infant’s central nervous system can occur before the mother presents to the ultrasound unit or clinic, and alterations to FHR patterns can reflect previous injury. In such cases, a short decision to incision interval would not prevent damage to the central nervous system of the preterm infant.
CASE 3: 5 minutes from decision to incision after uterine rupture
G. P. is a patient (G2P1) at 38 weeks’ gestation who has had a previous low uterine transverse cesarean delivery. She strongly wishes to attempt vaginal birth after cesarean (VBAC) and has been extensively counseled about the risks and benefits of this approach. This counseling has been appropriately documented in her chart. Her predicted likelihood of success is 54%.
Upon arrival in the triage unit, she reiterates that she hopes to deliver her child vaginally. Upon examination, she is found to be dilated to 4 cm. She is admitted to the labor and delivery unit, with reevaluation planned 2 hours after epidural administration. At that time, her labor is noted to be progressing at an appropriate rate.
After 5 hours of labor, the baseline FHR drops into the 70s. Immediate evaluation reveals significant uterine bleeding, with the fetus no longer engaged in the pelvis. The attending physician immediately suspects uterine rupture.
The patient is rushed to the OR, and delivery is complicated by the presence of extensive adhesions to the uterus and anterior abdominal wall. After 20 minutes, a female infant is delivered, with Apgar scores of 0, 0, and 1 at 1, 5, and 10 minutes, respectively. Medical care is withdrawn after 3 days in the NICU.
In a true obstetric catastrophe such as uterine rupture, should the decision to incision interval be 30 minutes?
Although it is rare, uterine rupture is a known complication of VBAC attempts. The actual rate varies across the literature but appears to be approximately 0.5% to 0.9% in women attempting vaginal birth after a prior lower uterine incision.8
If uterine rupture develops, both mother and fetus are at increased risk of morbidity and mortality. The risk of hypoxic ischemic encephalopathy after uterine rupture is about 6.2% (95% confidence interval [CI], 1.8–10.6), and the risk of neonatal death is about 1.8% (95% CI, 0–4.2).9 Uterine rupture also has been linked to an increase in:
- severe postpartum hemorrhage (odds ratio [OR], 8.51; 95% CI, 4.6–15.1)
- general anesthesia exposure (OR, 14.20; 95% CI, 9.1–22.2)
- hysterectomy (OR, 51.36; 95% CI, 13.6–193.4)
- serious perinatal outcome (OR, 24.51; 95% CI, 11.9–51.9).10
- serious perinatal outcome (OR, 24.51; 95% CI, 11.9–51.9).
Case 3 again highlights the limitations and difficulties of encompassing all cases within a 30-minute timeframe. Although the newborn was delivered within this interval after the initial insult, the intervention was insufficient to prevent severe and long-term damage.
In cases of true obstetric emergency, the catastrophic nature of the event may lead to adverse long-term neonatal outcomes even if the standard of care is met. Immediate delivery still may not allow for the prevention of neurologic morbidity in the fetus. When evaluating such cases retrospectively, all parties involved always should consider these facts before drawing any conclusions on causality and prevention.
CASE 4: Twins delivered 20 minutes after cesarean decision
P. R. (G1P0) presents for routine prenatal care at 36 weeks’ gestation. She is carrying a dichorionic/diamniotic twin gestation that so far has been uncomplicated. She has been experiencing contractions for the past 2 weeks, but they have intensified during the past 2 days. When an examination reveals that she is dilated to 4 cm, she is admitted to the labor and delivery unit.
Both fetuses are evaluated via external FHR monitoring. Initially, both have Category 1 tracings but, approximately 1 hour later, both tracings are noted to have minimal variability with variable decelerations, with a nadir at 80 bpm that lasts 30 to 45 seconds. These abnormalities persist even after intrauterine resuscitation is attempted. The cervix remains dilated at 4 cm.
After a Category 2 tracing persists for 1 hour, the attending physician proceeds to cesarean delivery. Both infants are delivered within 20 minutes after the decision is made. Two female infants of appropriate gestational size are delivered, with Apgar scores of 7 and 8 for Twin A and 8 and 9 for Twin B. The newborns eventually are discharged home with the mother. Twin B is subsequently given a diagnosis of cerebral palsy.
Should the decision to incision rule be applied to twin gestations?
Multifetal gestations carry an increased risk not only of fetal and neonatal death but also of handicap among survivors, compared with singleton pregnancies.11 The literature evaluating the link between abnormal FHR patterns and adverse neonatal outcomes in twin pregnancies is sparse. Adding to the confusion is the fact that signal loss from fetal monitoring during labor occurs more frequently in twins than in singletons, with a reported incidence of 26% to 33% during the 1st stage of labor and 41% to 63% during the 2nd stage.12 Moreover, the FHR pattern of one twin may be recorded twice inadvertently and the same tracing erroneously attributed to both twins.
The decision to incision and delivery time in twin gestations should be evaluated in the context of all the limitations the clinician faces when managing labor in a twin gestation. The 30-minute rule never has been specifically evaluated in the context of multifetal gestations. The pathway and contributing factors that lead to adverse neonatal outcomes in twin gestations may be very different from those related to singleton pregnancies and may be more relevant to antepartum than intrapartum events.
Take-home message
The 4 cases presented here call into question the applicability and generalizability of the 30-minute decision to incision rule. Diverse clinical situations encountered in practice should lead to different interpretations of this standard. No single rule can encompass all possible scenarios; therefore, a single rule should not be touted as universal. All clinical variables should be weighed and interpreted in the retrospective evaluation of a case involving a cesarean delivery performed after a 30-minute decision to incision interval.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE 1: Term delivery: 45 minutes from decision to incision
P. G. is a 27-year-old woman (G2P1) at 38.2 weeks’ gestation who presents to the labor and delivery unit reporting painful contractions after uncomplicated prenatal care. She has a body mass index (BMI) of 40 kg/m2. Upon admission, her fetal heart-rate (FHR) tracing falls into Category 1. An examination reveals a cervix dilated to 4 cm and 70% effaced. Epidural analgesia is administered for pain control.
After 4 hours, the FHR tracing reveals minimal variability with occasional variable decelerations. The obstetrician is informed but issues no specific instructions. After 2 more hours, the FHR tracing lacks variability, with late decelerations and no spontaneous accelerations—a Category 3 tracing, which is predictive of abnormal acid-base status. Contractions occur every 3 to 4 minutes.
When fetal scalp stimulation by the nurse fails to elicit any accelerations, intrauterine resuscitation is attempted with an intravenous fluid bolus, left lateral positioning, and oxygen administration. Despite these measures, the FHR pattern fails to improve.
Although she is apprised of the need for prompt delivery, the patient hopes to avoid cesarean delivery, if possible, and insists on more time before a decision is made to proceed to cesarean. After another 2 hours, the FHR pattern has not improved and cervical dilation remains at 4 cm. The patient gives her consent for cesarean delivery.
Approximately 35 minutes are needed to take the patient to the operating room (OR). About 45 minutes after informed consent, the incision is made. Forty-seven minutes later, a male infant is delivered with Apgar scores of 1, 3, and 4 at 1, 5, and 10 minutes, respectively. Umbilical arterial analysis reveals a pH level of 6.9, with a base excess of –21. The infant has a neonatal seizure within 3 hours and is eventually diagnosed with cerebral palsy.
A claim against the clinicians alleges that deviation from the “standard of care 30-minute rule more than likely caused” hypoxic ische- mic injury and cerebral palsy.
Does the literature support this claim?
Approximately 3% of all births involve cesarean delivery for a nonreassuring FHR tracing.1 Much has been written about the “30-minute rule” for decision to incision time. In this article, we highlight current limitations of this standard in the context of 4 distinct clinical scenarios.
Case 1 highlights several limitations and ambiguities in the obstetric literature. Although a timely delivery is always desirable, it may not always be possible to achieve safely due to intrinsic patient characteristics or situational constraints. Conditions prevailing before the decision to proceed to cesarean delivery also affect overall pregnancy outcomes. Not all cases have the same starting point; fetal status at the time of the cesarean decision also determines the acuity and urgency of the case.
A widely promulgated rule— but is it valid?
Both the American College of Obstetricians and Gynecologists (ACOG) and the Royal College of Obstetricians and Gynaecologists have published guidelines stating that any hospital offering obstetric care should have the capability to perform emergent cesarean delivery within 30 minutes.2,3 This general statement has been touted as the standard by which obstetric services should be evaluated. Regardless of the clinical situation, obstetric providers are expected to abide by this rule.
These guidelines recently have come under scrutiny. For example, a 2014 meta-analysis involving more than 30 studies and 22,000 women revealed that only 36% of all cases with a Category 2 FHR tracing were delivered within 30 minutes.4 Interestingly, investigators reported that infants with a shorter delivery interval had a higher likelihood of having a 5-minute Apgar score below 7 and an umbilical artery pH level below 7.1, with no difference in the rate of admission to a neonatal intensive care unit (NICU) when the time from decision to delivery was examined.4 This finding highlights the questionable nature of the current clinical standard, as well as the conflicting findings currently present in the literature.
In general, patients who have graver clinical findings will be delivered at a shorter interval but may still have worse neonatal outcomes than infants delivered 30 minutes or more after the decision for cesarean is made.
Although Case 1 is complicated by FHR abnormalities, the association between such abnormalities and adverse long-term outcomes in neonates is questionable. Fewer than 1% of cases involving late decelerations or decreased variability during labor lead to cerebral palsy,5 highlighting the weak association between FHR abnormalities and neurologic sequelae. Most adverse neurologic neonatal outcomes are multifactorial in nature and may not be attributable to a single prenatal event.
With such limitations, the application and use of the 30-minute “standard” by hospitals, professional societies, and the medicolegal community may not be appropriate. The literature may not justify using this arbitrary rule as the standard of care. Clearly, there are gaps in our knowledge and understanding of FHR abnormalities and the optimal interval for cesarean delivery. Therefore, it may be unfair and inappropriate to group all cases and clinical situations together.
CASE 2: 25 minutes from decision to preterm delivery
J. P. (G2P1) undergoes an ultrasonographic examination at 33.4 weeks’ gestation because of concern about a discrepancy between fetal size and gestational age. The estimated fetal weight is in the 5th percentile. Amniotic fluid level is normal, but the biophysical profile is 6/8, with no breathing for 30 seconds. Umbilical artery Doppler imaging reveals absent end-diastolic flow, and FHR monitoring reveals repetitive late decelerations.
The patient is admitted immediately to the labor and delivery unit and placed on continuous electronic fetal monitoring. Betamethasone is given to enhance fetal lung maturity. FHR monitoring continues to show repetitive late decelerations with every contraction.
After 10 minutes on the labor floor, a decision is made to proceed to emergent cesarean delivery. Within 25 minutes of that decision, a female infant weighing 1,731 g (3rd percentile) is delivered, with Apgar scores of 1, 1, and 4 at 1, 5, and 10 minutes, respectively. The infant is eventually diagnosed with moderate cerebral palsy.
Could this outcome have been prevented?
Published reports on the association between abnormal FHR patterns and adverse perinatal outcomes in preterm infants are even more scarce than they are for infants delivered at term. Case 2 highlights the fact that achievement of a 30-minute interval from decision to delivery doesn’t necessarily eliminate the risk of adverse neonatal outcomes and long-term morbidity.
One of the best evaluations of this association was published by Shy and colleagues in the 1980s.6 In that study, investigators randomly assigned 173 preterm infants to intermittent auscultation or continuous external fetal monitoring. Use of external fetal monitoring did not improve neurologic outcomes at 18 months of age. Nor did the duration of FHR abnormalities predict the development of cerebral palsy.6
A recent secondary analysis from a randomized trial evaluating the use of antenatal magnesium sulfate to prevent cerebral palsy revealed that preterm FHR patterns labeled as “fetal distress” by the treating physician were associated with an increased risk of cerebral palsy in the newborn.7 Although this analysis revealed an association, a causal link could not be established. Damage to a preterm infant’s central nervous system can occur before the mother presents to the ultrasound unit or clinic, and alterations to FHR patterns can reflect previous injury. In such cases, a short decision to incision interval would not prevent damage to the central nervous system of the preterm infant.
CASE 3: 5 minutes from decision to incision after uterine rupture
G. P. is a patient (G2P1) at 38 weeks’ gestation who has had a previous low uterine transverse cesarean delivery. She strongly wishes to attempt vaginal birth after cesarean (VBAC) and has been extensively counseled about the risks and benefits of this approach. This counseling has been appropriately documented in her chart. Her predicted likelihood of success is 54%.
Upon arrival in the triage unit, she reiterates that she hopes to deliver her child vaginally. Upon examination, she is found to be dilated to 4 cm. She is admitted to the labor and delivery unit, with reevaluation planned 2 hours after epidural administration. At that time, her labor is noted to be progressing at an appropriate rate.
After 5 hours of labor, the baseline FHR drops into the 70s. Immediate evaluation reveals significant uterine bleeding, with the fetus no longer engaged in the pelvis. The attending physician immediately suspects uterine rupture.
The patient is rushed to the OR, and delivery is complicated by the presence of extensive adhesions to the uterus and anterior abdominal wall. After 20 minutes, a female infant is delivered, with Apgar scores of 0, 0, and 1 at 1, 5, and 10 minutes, respectively. Medical care is withdrawn after 3 days in the NICU.
In a true obstetric catastrophe such as uterine rupture, should the decision to incision interval be 30 minutes?
Although it is rare, uterine rupture is a known complication of VBAC attempts. The actual rate varies across the literature but appears to be approximately 0.5% to 0.9% in women attempting vaginal birth after a prior lower uterine incision.8
If uterine rupture develops, both mother and fetus are at increased risk of morbidity and mortality. The risk of hypoxic ischemic encephalopathy after uterine rupture is about 6.2% (95% confidence interval [CI], 1.8–10.6), and the risk of neonatal death is about 1.8% (95% CI, 0–4.2).9 Uterine rupture also has been linked to an increase in:
- severe postpartum hemorrhage (odds ratio [OR], 8.51; 95% CI, 4.6–15.1)
- general anesthesia exposure (OR, 14.20; 95% CI, 9.1–22.2)
- hysterectomy (OR, 51.36; 95% CI, 13.6–193.4)
- serious perinatal outcome (OR, 24.51; 95% CI, 11.9–51.9).10
- serious perinatal outcome (OR, 24.51; 95% CI, 11.9–51.9).
Case 3 again highlights the limitations and difficulties of encompassing all cases within a 30-minute timeframe. Although the newborn was delivered within this interval after the initial insult, the intervention was insufficient to prevent severe and long-term damage.
In cases of true obstetric emergency, the catastrophic nature of the event may lead to adverse long-term neonatal outcomes even if the standard of care is met. Immediate delivery still may not allow for the prevention of neurologic morbidity in the fetus. When evaluating such cases retrospectively, all parties involved always should consider these facts before drawing any conclusions on causality and prevention.
CASE 4: Twins delivered 20 minutes after cesarean decision
P. R. (G1P0) presents for routine prenatal care at 36 weeks’ gestation. She is carrying a dichorionic/diamniotic twin gestation that so far has been uncomplicated. She has been experiencing contractions for the past 2 weeks, but they have intensified during the past 2 days. When an examination reveals that she is dilated to 4 cm, she is admitted to the labor and delivery unit.
Both fetuses are evaluated via external FHR monitoring. Initially, both have Category 1 tracings but, approximately 1 hour later, both tracings are noted to have minimal variability with variable decelerations, with a nadir at 80 bpm that lasts 30 to 45 seconds. These abnormalities persist even after intrauterine resuscitation is attempted. The cervix remains dilated at 4 cm.
After a Category 2 tracing persists for 1 hour, the attending physician proceeds to cesarean delivery. Both infants are delivered within 20 minutes after the decision is made. Two female infants of appropriate gestational size are delivered, with Apgar scores of 7 and 8 for Twin A and 8 and 9 for Twin B. The newborns eventually are discharged home with the mother. Twin B is subsequently given a diagnosis of cerebral palsy.
Should the decision to incision rule be applied to twin gestations?
Multifetal gestations carry an increased risk not only of fetal and neonatal death but also of handicap among survivors, compared with singleton pregnancies.11 The literature evaluating the link between abnormal FHR patterns and adverse neonatal outcomes in twin pregnancies is sparse. Adding to the confusion is the fact that signal loss from fetal monitoring during labor occurs more frequently in twins than in singletons, with a reported incidence of 26% to 33% during the 1st stage of labor and 41% to 63% during the 2nd stage.12 Moreover, the FHR pattern of one twin may be recorded twice inadvertently and the same tracing erroneously attributed to both twins.
The decision to incision and delivery time in twin gestations should be evaluated in the context of all the limitations the clinician faces when managing labor in a twin gestation. The 30-minute rule never has been specifically evaluated in the context of multifetal gestations. The pathway and contributing factors that lead to adverse neonatal outcomes in twin gestations may be very different from those related to singleton pregnancies and may be more relevant to antepartum than intrapartum events.
Take-home message
The 4 cases presented here call into question the applicability and generalizability of the 30-minute decision to incision rule. Diverse clinical situations encountered in practice should lead to different interpretations of this standard. No single rule can encompass all possible scenarios; therefore, a single rule should not be touted as universal. All clinical variables should be weighed and interpreted in the retrospective evaluation of a case involving a cesarean delivery performed after a 30-minute decision to incision interval.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337–350.
- American College of Obstetricians and Gynecologists, Committee on Professional Standards. Standards for Obstetric-Gynecologic Hospital Services. 7th ed. Washington, DC: ACOG; 1989.
- National Institute for Health and Care Excellence. Caesarean Section Guideline. London, UK: NICE; 2011.
- Tolcher MC, Johnson RL, El-Nashar SA, West CP. Decision-to-incision time and neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(3):536–548.
- Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain values of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613–618.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Mendez-Figueroa H, Chauhan SP, Pedroza C, Refuerzo JS, Dahlke JD, Rouse DJ. Preterm cesarean delivery for nonreassuring fetal heart rate: neonatal and neurologic morbidity. Obstet Gynecol. 2015;125(3):636–642.
- Macones GA, Cahill AG, Samilio DM, Odibo A, Peipert J, Stevens EJ. Can uterine rupture in patients attempting vaginal birth after cesarean delivery be predicted? Am J Obstet Gynecol. 2006;195(4):1148–1152.
- Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581–2589.
- Al-Zirqi I, Stray-Pedersen B, Forsen L, Daltveit AK, Vangen S. Uterine rupture: trends over 40 years [published online ahead of print April 2, 2015]. BJOG. doi: 10.1111/1471-0528.13394.
- Ramsey PS, Repke JT. Intrapartum management of multifetal pregnancies. Semin Perinatol. 2003;27(1):54–72.
- Bakker PC, Colenbrander GJ, Verstraeten AA, Van Geijn HP. Quality of intrapartum cardiotocography in twin deliveries. Am J Obstet Gynecol. 2004;191(6):2114–2119.
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337–350.
- American College of Obstetricians and Gynecologists, Committee on Professional Standards. Standards for Obstetric-Gynecologic Hospital Services. 7th ed. Washington, DC: ACOG; 1989.
- National Institute for Health and Care Excellence. Caesarean Section Guideline. London, UK: NICE; 2011.
- Tolcher MC, Johnson RL, El-Nashar SA, West CP. Decision-to-incision time and neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(3):536–548.
- Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain values of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613–618.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Mendez-Figueroa H, Chauhan SP, Pedroza C, Refuerzo JS, Dahlke JD, Rouse DJ. Preterm cesarean delivery for nonreassuring fetal heart rate: neonatal and neurologic morbidity. Obstet Gynecol. 2015;125(3):636–642.
- Macones GA, Cahill AG, Samilio DM, Odibo A, Peipert J, Stevens EJ. Can uterine rupture in patients attempting vaginal birth after cesarean delivery be predicted? Am J Obstet Gynecol. 2006;195(4):1148–1152.
- Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581–2589.
- Al-Zirqi I, Stray-Pedersen B, Forsen L, Daltveit AK, Vangen S. Uterine rupture: trends over 40 years [published online ahead of print April 2, 2015]. BJOG. doi: 10.1111/1471-0528.13394.
- Ramsey PS, Repke JT. Intrapartum management of multifetal pregnancies. Semin Perinatol. 2003;27(1):54–72.
- Bakker PC, Colenbrander GJ, Verstraeten AA, Van Geijn HP. Quality of intrapartum cardiotocography in twin deliveries. Am J Obstet Gynecol. 2004;191(6):2114–2119.
In this Article
- Is the 30-minute rule valid?
- A case of uterine rupture
- Take-home message
Is neonatal injury more likely outside of a 30-minute decision-to-incision time interval for cesarean delivery?
Cesarean section is one of the most common surgical procedures worldwide. In a review of more than 13 million deliveries, cesarean delivery for nonreassuring fetal heart rate tracing occurred in about 3% of cases.1 Most of these urgent deliveries occur without known predisposing factors.1 A source of consternation for clinicians related to labor and delivery is the decision-to-incision time (DIT) interval for cesarean delivery for nonreassuring fetal heart rate tracing.
Previously, The American College of Obstetricians and Gynecologists (ACOG) suggested the DIT interval should be 30 minutes or less, for prolonged DIT increased the likelihood of neonatal injury.2 A DIT interval of more than 30 minutes became the sine qua non for poor neonatal outcomes and the linchpin for obstetric litigation.3 Starting in the 1990s, publications indicated that neonatal morbidity is not related to DIT and adverse neonatal outcomes may occur with a DIT interval of only a few minutes.4 Most studies, however, were hampered by small sample size.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai, MD (March 2012)
In an attempt to clarify whether neonatal outcomes differed among cesarean deliveries performed before or after 30 minutes lapsed, Tolcher and colleagues recently published a systematic review and meta-analysis evaluating all published reports that assessed adherence to a DIT policy for cesarean deliveries to be performed within 30 minutes of a nonreassuring fetal heart rate tracing. They reported on the number of emergent (Category 1) and urgent (Category 2) cesarean deliveries accomplished within 30 minutes and compared neonatal outcomes for cesarean deliveries before and after the 30-minute DIT.
Some important observations:
- First, all the studies were observational; only one paper focused exclusively on preterm infants, and only five of the identified 34 publications, involving 22,936 women, were determined to be “high quality.”
- Second, one of five neonates (21%) requiring emergent cesarean delivery were not delivered within 30 minutes. And 64% of urgent deliveries were not performed within 30 minutes.
- Third, and most surprisingly, in the 13 studies that included neonatal outcomes, 5-minute Apgar scores less than 7 and cord pH values less than 7.10 were significantly more common among neonates delivered within 30 minutes than among neonates delivered outside of 30 minutes. When the authors limited analysis to infants requiring emergent versus urgent delivery, however, the difference in Apgar scores and pH values was nonsignificant.
Several strengths of this analysis should be mentioned. The careful study design—meticulous and systematic evaluation of all publications and adherence to established publication evaluation and meta-analysis reporting protocols—strengthen the validity of these results. This report is clinically useful because the authors not only evaluated time frames from decision-to-incision but also reported and correlated neonatal outcomes.
Despite the multiple strengths, some weaknesses are worth mentioning. No maternal outcomes were reported. Mothers who require emergent cesarean delivery are at increased risk for adverse outcomes due to the requirement for general anesthesia and urgency with which the surgery is performed. The report only focused on 5-minute Apgar scores less than 7, neonatal intensive care admissions, and cord pH values less than 7.10 as adverse neonatal outcomes. The absence of additional adverse outcomes, as well as long-term neonatal and infant outcomes, hampers our ability to present the patient with all the facts. Lastly, the authors promulgated the classification of degree of urgency for cesarean delivery proposed by Lucas and colleagues5 without providing evidence that this classification is linked with clinically meaningful outcomes.
While a randomized trial would be unethical, a fact acknowledged by the authors, prospective cohort studies with long-term neonatal and infant follow-up could provide us with much needed information that would help us counsel our patients. The frequency with which cesarean deliveries are performed requires us to offer our patients the best and most comprehensive information available.
Related article:
• Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence; December 2013)
• Mother-, baby-, and family- centered cesarean delivery: It is possible. William Camann, MD, and Robert L. Barbieri, MD (Editorial; March 2013)
What this evidence means for practice
The ideal decision-to-incision time is probably best determined individually and may not encompass a “one-size-fits-all” approach. More studies are needed to elucidate this critical clinical question. In the meantime, we suggest: 1) consulting colleagues if interpretation of the tracing is uncertain, especially with preterm parturients, 2) intrauterine resuscitation, including tocolytics and amnioinfusion when appropriate, 3) scalp or vibroacoustic stimulation to elicit acceleration, 4) administering ephedrine if hypotensive, 5) expeditious delivery considering the clinical situation and logistics, 6) documenting decision-to-incision time in operative notes, and 7) sending umbilical arterial and venous blood for acid-base analysis and the placenta to pathology for evaluation.
Suneet P. Chauhan, MD, and Hector Mendez-Figueroa, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: Rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337−350.
- American Academy of Pediatrics; American College of Obstetrics and Gynecology. Guidelines for perinatal care. 2nd ed. Washington, DC: American College of Obstetrics and Gynecology; 1988:71.
- Chauhan SP, Chauhan VB, Cowan BD, Hendrix NW, Magann EF, Morrison JC. Professional liability claims and Central Association of Obstetricians and Gynecologists members: Myth versus reality. Am J Obstet Gynecol. 2005;192(6):1820−1826.
- American College of Obstetricians and Gynecologists and American Academy of Pediatrics Task Force. Neonatal encephalopathy and neurologic outcomes. 2nd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014.
- Lucas DN, Yentis SM, Kinsella SM, et al. Urgency of caesarean section: A new classification. J R Soc Med. 2000;93(7):346–350.
Cesarean section is one of the most common surgical procedures worldwide. In a review of more than 13 million deliveries, cesarean delivery for nonreassuring fetal heart rate tracing occurred in about 3% of cases.1 Most of these urgent deliveries occur without known predisposing factors.1 A source of consternation for clinicians related to labor and delivery is the decision-to-incision time (DIT) interval for cesarean delivery for nonreassuring fetal heart rate tracing.
Previously, The American College of Obstetricians and Gynecologists (ACOG) suggested the DIT interval should be 30 minutes or less, for prolonged DIT increased the likelihood of neonatal injury.2 A DIT interval of more than 30 minutes became the sine qua non for poor neonatal outcomes and the linchpin for obstetric litigation.3 Starting in the 1990s, publications indicated that neonatal morbidity is not related to DIT and adverse neonatal outcomes may occur with a DIT interval of only a few minutes.4 Most studies, however, were hampered by small sample size.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai, MD (March 2012)
In an attempt to clarify whether neonatal outcomes differed among cesarean deliveries performed before or after 30 minutes lapsed, Tolcher and colleagues recently published a systematic review and meta-analysis evaluating all published reports that assessed adherence to a DIT policy for cesarean deliveries to be performed within 30 minutes of a nonreassuring fetal heart rate tracing. They reported on the number of emergent (Category 1) and urgent (Category 2) cesarean deliveries accomplished within 30 minutes and compared neonatal outcomes for cesarean deliveries before and after the 30-minute DIT.
Some important observations:
- First, all the studies were observational; only one paper focused exclusively on preterm infants, and only five of the identified 34 publications, involving 22,936 women, were determined to be “high quality.”
- Second, one of five neonates (21%) requiring emergent cesarean delivery were not delivered within 30 minutes. And 64% of urgent deliveries were not performed within 30 minutes.
- Third, and most surprisingly, in the 13 studies that included neonatal outcomes, 5-minute Apgar scores less than 7 and cord pH values less than 7.10 were significantly more common among neonates delivered within 30 minutes than among neonates delivered outside of 30 minutes. When the authors limited analysis to infants requiring emergent versus urgent delivery, however, the difference in Apgar scores and pH values was nonsignificant.
Several strengths of this analysis should be mentioned. The careful study design—meticulous and systematic evaluation of all publications and adherence to established publication evaluation and meta-analysis reporting protocols—strengthen the validity of these results. This report is clinically useful because the authors not only evaluated time frames from decision-to-incision but also reported and correlated neonatal outcomes.
Despite the multiple strengths, some weaknesses are worth mentioning. No maternal outcomes were reported. Mothers who require emergent cesarean delivery are at increased risk for adverse outcomes due to the requirement for general anesthesia and urgency with which the surgery is performed. The report only focused on 5-minute Apgar scores less than 7, neonatal intensive care admissions, and cord pH values less than 7.10 as adverse neonatal outcomes. The absence of additional adverse outcomes, as well as long-term neonatal and infant outcomes, hampers our ability to present the patient with all the facts. Lastly, the authors promulgated the classification of degree of urgency for cesarean delivery proposed by Lucas and colleagues5 without providing evidence that this classification is linked with clinically meaningful outcomes.
While a randomized trial would be unethical, a fact acknowledged by the authors, prospective cohort studies with long-term neonatal and infant follow-up could provide us with much needed information that would help us counsel our patients. The frequency with which cesarean deliveries are performed requires us to offer our patients the best and most comprehensive information available.
Related article:
• Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence; December 2013)
• Mother-, baby-, and family- centered cesarean delivery: It is possible. William Camann, MD, and Robert L. Barbieri, MD (Editorial; March 2013)
What this evidence means for practice
The ideal decision-to-incision time is probably best determined individually and may not encompass a “one-size-fits-all” approach. More studies are needed to elucidate this critical clinical question. In the meantime, we suggest: 1) consulting colleagues if interpretation of the tracing is uncertain, especially with preterm parturients, 2) intrauterine resuscitation, including tocolytics and amnioinfusion when appropriate, 3) scalp or vibroacoustic stimulation to elicit acceleration, 4) administering ephedrine if hypotensive, 5) expeditious delivery considering the clinical situation and logistics, 6) documenting decision-to-incision time in operative notes, and 7) sending umbilical arterial and venous blood for acid-base analysis and the placenta to pathology for evaluation.
Suneet P. Chauhan, MD, and Hector Mendez-Figueroa, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Cesarean section is one of the most common surgical procedures worldwide. In a review of more than 13 million deliveries, cesarean delivery for nonreassuring fetal heart rate tracing occurred in about 3% of cases.1 Most of these urgent deliveries occur without known predisposing factors.1 A source of consternation for clinicians related to labor and delivery is the decision-to-incision time (DIT) interval for cesarean delivery for nonreassuring fetal heart rate tracing.
Previously, The American College of Obstetricians and Gynecologists (ACOG) suggested the DIT interval should be 30 minutes or less, for prolonged DIT increased the likelihood of neonatal injury.2 A DIT interval of more than 30 minutes became the sine qua non for poor neonatal outcomes and the linchpin for obstetric litigation.3 Starting in the 1990s, publications indicated that neonatal morbidity is not related to DIT and adverse neonatal outcomes may occur with a DIT interval of only a few minutes.4 Most studies, however, were hampered by small sample size.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai, MD (March 2012)
In an attempt to clarify whether neonatal outcomes differed among cesarean deliveries performed before or after 30 minutes lapsed, Tolcher and colleagues recently published a systematic review and meta-analysis evaluating all published reports that assessed adherence to a DIT policy for cesarean deliveries to be performed within 30 minutes of a nonreassuring fetal heart rate tracing. They reported on the number of emergent (Category 1) and urgent (Category 2) cesarean deliveries accomplished within 30 minutes and compared neonatal outcomes for cesarean deliveries before and after the 30-minute DIT.
Some important observations:
- First, all the studies were observational; only one paper focused exclusively on preterm infants, and only five of the identified 34 publications, involving 22,936 women, were determined to be “high quality.”
- Second, one of five neonates (21%) requiring emergent cesarean delivery were not delivered within 30 minutes. And 64% of urgent deliveries were not performed within 30 minutes.
- Third, and most surprisingly, in the 13 studies that included neonatal outcomes, 5-minute Apgar scores less than 7 and cord pH values less than 7.10 were significantly more common among neonates delivered within 30 minutes than among neonates delivered outside of 30 minutes. When the authors limited analysis to infants requiring emergent versus urgent delivery, however, the difference in Apgar scores and pH values was nonsignificant.
Several strengths of this analysis should be mentioned. The careful study design—meticulous and systematic evaluation of all publications and adherence to established publication evaluation and meta-analysis reporting protocols—strengthen the validity of these results. This report is clinically useful because the authors not only evaluated time frames from decision-to-incision but also reported and correlated neonatal outcomes.
Despite the multiple strengths, some weaknesses are worth mentioning. No maternal outcomes were reported. Mothers who require emergent cesarean delivery are at increased risk for adverse outcomes due to the requirement for general anesthesia and urgency with which the surgery is performed. The report only focused on 5-minute Apgar scores less than 7, neonatal intensive care admissions, and cord pH values less than 7.10 as adverse neonatal outcomes. The absence of additional adverse outcomes, as well as long-term neonatal and infant outcomes, hampers our ability to present the patient with all the facts. Lastly, the authors promulgated the classification of degree of urgency for cesarean delivery proposed by Lucas and colleagues5 without providing evidence that this classification is linked with clinically meaningful outcomes.
While a randomized trial would be unethical, a fact acknowledged by the authors, prospective cohort studies with long-term neonatal and infant follow-up could provide us with much needed information that would help us counsel our patients. The frequency with which cesarean deliveries are performed requires us to offer our patients the best and most comprehensive information available.
Related article:
• Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence; December 2013)
• Mother-, baby-, and family- centered cesarean delivery: It is possible. William Camann, MD, and Robert L. Barbieri, MD (Editorial; March 2013)
What this evidence means for practice
The ideal decision-to-incision time is probably best determined individually and may not encompass a “one-size-fits-all” approach. More studies are needed to elucidate this critical clinical question. In the meantime, we suggest: 1) consulting colleagues if interpretation of the tracing is uncertain, especially with preterm parturients, 2) intrauterine resuscitation, including tocolytics and amnioinfusion when appropriate, 3) scalp or vibroacoustic stimulation to elicit acceleration, 4) administering ephedrine if hypotensive, 5) expeditious delivery considering the clinical situation and logistics, 6) documenting decision-to-incision time in operative notes, and 7) sending umbilical arterial and venous blood for acid-base analysis and the placenta to pathology for evaluation.
Suneet P. Chauhan, MD, and Hector Mendez-Figueroa, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: Rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337−350.
- American Academy of Pediatrics; American College of Obstetrics and Gynecology. Guidelines for perinatal care. 2nd ed. Washington, DC: American College of Obstetrics and Gynecology; 1988:71.
- Chauhan SP, Chauhan VB, Cowan BD, Hendrix NW, Magann EF, Morrison JC. Professional liability claims and Central Association of Obstetricians and Gynecologists members: Myth versus reality. Am J Obstet Gynecol. 2005;192(6):1820−1826.
- American College of Obstetricians and Gynecologists and American Academy of Pediatrics Task Force. Neonatal encephalopathy and neurologic outcomes. 2nd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014.
- Lucas DN, Yentis SM, Kinsella SM, et al. Urgency of caesarean section: A new classification. J R Soc Med. 2000;93(7):346–350.
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: Rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337−350.
- American Academy of Pediatrics; American College of Obstetrics and Gynecology. Guidelines for perinatal care. 2nd ed. Washington, DC: American College of Obstetrics and Gynecology; 1988:71.
- Chauhan SP, Chauhan VB, Cowan BD, Hendrix NW, Magann EF, Morrison JC. Professional liability claims and Central Association of Obstetricians and Gynecologists members: Myth versus reality. Am J Obstet Gynecol. 2005;192(6):1820−1826.
- American College of Obstetricians and Gynecologists and American Academy of Pediatrics Task Force. Neonatal encephalopathy and neurologic outcomes. 2nd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014.
- Lucas DN, Yentis SM, Kinsella SM, et al. Urgency of caesarean section: A new classification. J R Soc Med. 2000;93(7):346–350.
Is expectant management superior to elective induction of labor in nulliparous women who have an unfavorable cervix?
Over the past 12 years, several studies have demonstrated a higher rate of cesarean delivery among nulliparous women with an unfavorable cervix who undergo induction of labor. However, these studies typically have compared induction of labor with spontaneous labor rather than with its appropriate counterpart—expectant management. In addition, in some cases, the increased rate of cesarean delivery among women who undergo induction of labor may be related to a comorbidity rather than elective induction.
In this retrospective cohort study, Osmundson and colleagues compared elective induction of labor at 39-0/7 to 40-5/7 weeks’ gestation with expectant management beyond 39 weeks. All women in the study were nulliparous, free of comorbidity, and carrying a singleton gestation; they also had an unfavorable cervix, as demonstrated by a modified Bishop score of less than 5.
(According to ACOG, the goal of induction of labor is to achieve vaginal delivery by stimulating uterine contractions before the onset of spontaneous labor.1 Induction is elective when it is not associated with obstetric or medical complications.)
Although the rate of early term (37-0/7 to 38-6/7 weeks) induction increased significantly between 1991 and 2006, especially among non-Hispanic white women,2 there is now strong evidence that early term delivery is associated with significantly higher neonatal, postneonatal, and infant mortality,3 compared with late term delivery (39 to 41 weeks). Therefore, elective induction should not be performed before 39 weeks’ gestation—and it wasn’t in the study by Osmundson and colleagues.
Strengths and weaknesses of the study
This study has a number of strengths:
- the a priori power calculation
- a review of each chart to ensure that no comorbidity was present
- availability of the Bishop score for each case
- documentation of the duration of labor and the time of delivery (i.e., whether it occurred during daytime hours or at night).
However, some weaknesses are also present:
- the retrospective design, with its inherent limitations
- lack of explanation as to why only 102 women met inclusion criteria when the study period was 2 years at a tertiary center (a flow diagram of total deliveries and the reasons for exclusion would have been useful)
- the fact that all inductions were performed using a Foley catheter balloon and oxytocin, thereby limiting appropriate assessment of resource utilization for other techniques, such as prostaglandin administration
- the small sample size, which prevents determination of whether expectant management is linked to uncommon complications such as macrosomia, shoulder dystocia, or meconium-aspiration syndrome.
Until a randomized, controlled trial provides definitive data on the relative outcomes of induction of labor and expectant management among nulliparous women with an unfavorable cervix, these patients may be informed that induction of labor is not associated with an increased rate of cesarean delivery. However, they also should be apprised that they are likely to spend more time in labor and delivery with induction than if they await spontaneous onset of labor.—SUNEET P. CHAUHAN, MD, AND SHILPA BABBAR, MD
We want to hear from you! Tell us what you think.
1. ACOG Practice Bulletin#107: Induction of labor. Obstet Gynecol. 2009;114:386-397.
2. Murthy K, Grobman WA, Lee TA, Holl JL. Trends in induction of labor at early-term gestation. Am J Obstet Gynecol. 2011 Feb 21.
3. Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117:1279-1287.
Over the past 12 years, several studies have demonstrated a higher rate of cesarean delivery among nulliparous women with an unfavorable cervix who undergo induction of labor. However, these studies typically have compared induction of labor with spontaneous labor rather than with its appropriate counterpart—expectant management. In addition, in some cases, the increased rate of cesarean delivery among women who undergo induction of labor may be related to a comorbidity rather than elective induction.
In this retrospective cohort study, Osmundson and colleagues compared elective induction of labor at 39-0/7 to 40-5/7 weeks’ gestation with expectant management beyond 39 weeks. All women in the study were nulliparous, free of comorbidity, and carrying a singleton gestation; they also had an unfavorable cervix, as demonstrated by a modified Bishop score of less than 5.
(According to ACOG, the goal of induction of labor is to achieve vaginal delivery by stimulating uterine contractions before the onset of spontaneous labor.1 Induction is elective when it is not associated with obstetric or medical complications.)
Although the rate of early term (37-0/7 to 38-6/7 weeks) induction increased significantly between 1991 and 2006, especially among non-Hispanic white women,2 there is now strong evidence that early term delivery is associated with significantly higher neonatal, postneonatal, and infant mortality,3 compared with late term delivery (39 to 41 weeks). Therefore, elective induction should not be performed before 39 weeks’ gestation—and it wasn’t in the study by Osmundson and colleagues.
Strengths and weaknesses of the study
This study has a number of strengths:
- the a priori power calculation
- a review of each chart to ensure that no comorbidity was present
- availability of the Bishop score for each case
- documentation of the duration of labor and the time of delivery (i.e., whether it occurred during daytime hours or at night).
However, some weaknesses are also present:
- the retrospective design, with its inherent limitations
- lack of explanation as to why only 102 women met inclusion criteria when the study period was 2 years at a tertiary center (a flow diagram of total deliveries and the reasons for exclusion would have been useful)
- the fact that all inductions were performed using a Foley catheter balloon and oxytocin, thereby limiting appropriate assessment of resource utilization for other techniques, such as prostaglandin administration
- the small sample size, which prevents determination of whether expectant management is linked to uncommon complications such as macrosomia, shoulder dystocia, or meconium-aspiration syndrome.
Until a randomized, controlled trial provides definitive data on the relative outcomes of induction of labor and expectant management among nulliparous women with an unfavorable cervix, these patients may be informed that induction of labor is not associated with an increased rate of cesarean delivery. However, they also should be apprised that they are likely to spend more time in labor and delivery with induction than if they await spontaneous onset of labor.—SUNEET P. CHAUHAN, MD, AND SHILPA BABBAR, MD
We want to hear from you! Tell us what you think.
Over the past 12 years, several studies have demonstrated a higher rate of cesarean delivery among nulliparous women with an unfavorable cervix who undergo induction of labor. However, these studies typically have compared induction of labor with spontaneous labor rather than with its appropriate counterpart—expectant management. In addition, in some cases, the increased rate of cesarean delivery among women who undergo induction of labor may be related to a comorbidity rather than elective induction.
In this retrospective cohort study, Osmundson and colleagues compared elective induction of labor at 39-0/7 to 40-5/7 weeks’ gestation with expectant management beyond 39 weeks. All women in the study were nulliparous, free of comorbidity, and carrying a singleton gestation; they also had an unfavorable cervix, as demonstrated by a modified Bishop score of less than 5.
(According to ACOG, the goal of induction of labor is to achieve vaginal delivery by stimulating uterine contractions before the onset of spontaneous labor.1 Induction is elective when it is not associated with obstetric or medical complications.)
Although the rate of early term (37-0/7 to 38-6/7 weeks) induction increased significantly between 1991 and 2006, especially among non-Hispanic white women,2 there is now strong evidence that early term delivery is associated with significantly higher neonatal, postneonatal, and infant mortality,3 compared with late term delivery (39 to 41 weeks). Therefore, elective induction should not be performed before 39 weeks’ gestation—and it wasn’t in the study by Osmundson and colleagues.
Strengths and weaknesses of the study
This study has a number of strengths:
- the a priori power calculation
- a review of each chart to ensure that no comorbidity was present
- availability of the Bishop score for each case
- documentation of the duration of labor and the time of delivery (i.e., whether it occurred during daytime hours or at night).
However, some weaknesses are also present:
- the retrospective design, with its inherent limitations
- lack of explanation as to why only 102 women met inclusion criteria when the study period was 2 years at a tertiary center (a flow diagram of total deliveries and the reasons for exclusion would have been useful)
- the fact that all inductions were performed using a Foley catheter balloon and oxytocin, thereby limiting appropriate assessment of resource utilization for other techniques, such as prostaglandin administration
- the small sample size, which prevents determination of whether expectant management is linked to uncommon complications such as macrosomia, shoulder dystocia, or meconium-aspiration syndrome.
Until a randomized, controlled trial provides definitive data on the relative outcomes of induction of labor and expectant management among nulliparous women with an unfavorable cervix, these patients may be informed that induction of labor is not associated with an increased rate of cesarean delivery. However, they also should be apprised that they are likely to spend more time in labor and delivery with induction than if they await spontaneous onset of labor.—SUNEET P. CHAUHAN, MD, AND SHILPA BABBAR, MD
We want to hear from you! Tell us what you think.
1. ACOG Practice Bulletin#107: Induction of labor. Obstet Gynecol. 2009;114:386-397.
2. Murthy K, Grobman WA, Lee TA, Holl JL. Trends in induction of labor at early-term gestation. Am J Obstet Gynecol. 2011 Feb 21.
3. Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117:1279-1287.
1. ACOG Practice Bulletin#107: Induction of labor. Obstet Gynecol. 2009;114:386-397.
2. Murthy K, Grobman WA, Lee TA, Holl JL. Trends in induction of labor at early-term gestation. Am J Obstet Gynecol. 2011 Feb 21.
3. Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117:1279-1287.
Preterm labor: Diagnostic and therapeutic options are not all alike
- Consider progesterone to reduce the risk of recurrent preterm delivery (A).
- Consider calcium channel blockers (CCBs) as initial therapy for preterm labor; though these are Class C agents in pregnancy, CCBs are at least as effective as other agents and cause no known fetal or neonatal side effects (A).
- Consider tocolytic therapy for preterm labor, to prolong pregnancy for 2 to 7 days and thereby permit administration of antenatal corticosteroids and transfer to a tertiary center, if needed (A). Maintenance and recurrent use of tocolytics has not proven beneficial and should be avoided (A).
Despite diagnostic and therapeutic advances in premature labor, the rate of preterm delivery has increased in the United States. Preterm delivery, defined as birth before 37 weeks gestation, occurs in about 12% of pregnancies,1 and it is the leading cause of mortality among non-anomalous fetuses.2 Among premature newborns who survive, 10% to 15% have significant handicaps.3 Long-term sequelae include visual or hearing impairment, chronic lung disease, developmental delay, and cerebral palsy.4 The annual cost of premature birth in the United States exceeds $5 billion, and the rate of preterm delivery has increased in recent years.5,6 Specific maternal attributes increase risk of preterm delivery, though these factors need not be present for premature delivery to occur.
Standard techniques of physical diagnosis may not accurately detect preterm labor, either missing the diagnosis or prompting unnecessary hospitalization and treatment. Select imaging studies can more reliably indicate a true need for intervention.
This article reviews the risk factors for preterm birth, the tests available to accurately diagnose preterm labor, and the medications available to prevent or postpone premature delivery.
Risk factors
Preterm delivery is categorized as spontaneous or indicated. Almost 75% of preterm births occur after spontaneous preterm labor or preterm premature rupture of the membranes (PPROM). The remaining 25% of early births are elective due to conditions potentially harmful to either the mother or the fetus.7
Maternal risk factors for spontaneous preterm births are listed in Table 1.7-13 The risk factors for preterm labor and PPROM are similar, with the exception that those whose membranes rupture prematurely are more likely to be indigent, to smoke cigarettes, and to have bleeding in the current pregnancy.
Not all preterm births carry the same risks. Early preterm birth—delivery before 32 weeks— is associated with clinical or subclinical infection, short cervical length, and the presence of fetal fibronectin in the cervicovaginal secretions. It tends to recur and leads to long-term fetal morbidity. Late preterm birth—delivery after 32 weeks but before 37 weeks—is associated with increased uterine contractions, tends to mimic normal labor, and is less likely to cause peripartum morbidity.7 Despite these known risk factors, women can experience preterm labor without exhibiting any of them.
TABLE 1
Maternal risk factors for spontaneous preterm birth
| Risk factor | Odds ratio |
|---|---|
| Bacterial vaginosis at <16 weeks | 7 |
| Periodontal infection | 4 |
| Prior preterm birth | 4 |
| Prepregnancy BMI <20 | 3 |
| Interpregnancy interval <6 mo | 2 |
| Bleeding in 2nd trimester | 2 |
| African-American race | 2 |
| Psychiatric disorder | 1.6 |
| Cigarette smoking | 1.5 |
| Gestational diabetes | 1.2 |
| Sources: Iams 20027 ; Leitich et al 20038 ; Jeffcoat et al 20019 ; Moutquin 200310 ; Smith et al 200311 ; Kelly et al 200212 ; Hedderson et al 2003.13 | |
Increasing the accuracy of diagnosis
Uterine contractions before 37 weeks and concomitant change in cervical dilation or effacement as detected by digital examination comprise the standard definition of preterm labor.
Caveats with clinical evaluation
The problem with the definition above is that women may not perceive contractions, and that contractions are at times difficult to differentiate from benign Braxton-Hicks contractions of normal pregnancy. Moreover, digital assessment of the cervix lacks reproducibility when the dilation is <3 cm or effacement is < 80%.7 Clinical evaluation alone can easily suggest prematurity when it is absent and miss it when it is present.7
Imaging and lab evaluation more telling
The ability to diagnose preterm labor improves with the use of transvaginal cervical sonography or measurement of fetal fibronectin.
Lesser cervical length means greater risk. Likelihood of preterm delivery is inversely proportional to cervical length measured at 18 to 28 weeks. A length of ≤2.5 cm is the threshold for abnormal condition (level of evidence [LOE]: 2).6
Fibronectin detected late may mean delivery early. Fibronectin, an extracellular glycoprotein described as the glue that attaches the fetal membrane to the underlying uterine decidua, is normally absent in cervicovaginal secretions between weeks 22 and 37 of pregnancy. Its presence (50 ng/mL) between 22 to 24 weeks is a predictor of spontaneous preterm birth; its absence makes premature delivery unlikely (LOE: 2).
Imaging and test results justify management decisions. The predictive accuracies of abnormal cervical length and of the presence of fibronectin are depicted in the Figure .7 The major benefit of these two tests is when they are normal (cervical length >2.5 cm or negative fetal fibronectin), unnecessary intervention is avoided.14 If, for example, a patient is having contractions and the cervical length is greater than 2.5 cm, or fetal fibronectin is absent, tocolytics can be discontinued and hospitalization avoided.
FIGURE
Value of transvaginal cervical imaging and fetal fibronectin measurement in predicting spontaneous preterm birth
Preventing preterm labor
Progestational agents
Many authors have advocated the use of progestational agents to inhibit premature labor. Progesterone’s presumed mechanisms are inhibition of the oxytocin effect of prostaglandin F2a and stimulation of α-adrenergic receptors, thereby increasing the α-adrenergic tocolytic response.15,16 Natural progesterone appears to be free of any untoward teratogenic, metabolic, or hemodynamic effects.17
Studies favoring progesterone. Two double-blind, placebo-controlled studies assessed the use of 17 α-hydroxyprogesterone caproate in preventing premature labor in high-risk populations. These patients had histories of preterm deliveries or spontaneous abortions.
Weekly intramuscular (IM) injections of 250 mg were started at 12 to 16 weeks gestation and given until 37 weeks or delivery, whichever came first. In both studies, the rate of premature deliveries was significantly lower in the treated group than the control group (number needed to treat [NNT]=2.4 and 4.6). In addition, neither mothers nor infants experienced adverse effects attributable to 17 α-hydroxyprogesterone caproate (LOE: 2).18,19 However, these studies were small—43 and 79 patients.
A large double-blind, placebo-controlled, randomized study established the effectiveness of 17 α-hydroxyprogesterone caproate in preventing preterm delivery.23 Four hundred fifty-nine pregnant women with a history of preterm delivery were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo beginning at 16 to 20 weeks gestation and continuing to 36 weeks. Significantly fewer women in the treated group than the control group gave birth before 37 weeks, 36.3% v 54.9%, respectively (NNT=6; LOE: 1). Perhaps more importantly, treatment resulted in significant reductions in birth weight <2500 g (NNT=7), necrotizing enterocolitis (NNT=38), need for supplemental oxygen (NNT=11), and intraventricular hemorrhage (NNT=26). Swelling, bruising, or rash at the injection site were the most common adverse effects of 17 α-hydroxyprogesterone caproate administration.
Vaginal progesterone suppositories have also been shown to decrease the rate of preterm birth in patients at increased risk.17 da Fonseca et al noted that among 142 women who had 1 prior preterm birth, prophylactic cerclage, or uterine malformation, daily use of a 100-mg vaginal progesterone suppository compared with placebo significantly decreased the likelihood of delivery prior to 37 weeks, 14% v 28% (NNT=7; LOE: 1). Adverse effects of progesterone suppositories were not mentioned.
Studies lacking clear benefit. Another investigator enrolled 168 active-duty military women into a randomized double-blind study that evaluated weekly IM injections of 1000 mg 17 α-hydroxyprogesterone caproate or placebo beginning between 16 and 20 weeks gestation.20 The study population was chosen based on a report that active-duty pregnant military personnel had an increased number of pregnancy complications including low birth-weight infants and increased premature delivery.21 In contrast to the previous studies, no significant difference in premature labor or perinatal mortality was seen between the 2 groups (LOE: 2). Perhaps although active-duty personnel are at a higher risk for preterm labor, the risk is not as high as it is with previous preterm birth, prior spontaneous abortion, and so forth.
An additional small study of 77 women with twin pregnancies investigated the use of 17 α-hydroxyprogesterone caproate to prevent preterm delivery.22 Patients were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo from the 28th gestational week until the 37th week or delivery. The mean duration of pregnancy did not differ between the two groups of women, nor did perinatal mortality (LOE: 2).
Evidence generally supports use of progesterone. Based on available data, consider administering progesterone to women at high risk for preterm delivery to prevent recurrent preterm birth (SOR: A). These data are summarized in Table 2 .
Hydroxyprogesterone caproate in oil is commercially available in the United States in 125 mg/mL and 250 mg/mL strengths. It is not, however, approved by the Food and Drug Administration (FDA) for the prevention of preterm labor.
Progesterone suppositories are also not FDA approved, and they are commercially unavailable in the US. Therefore, they have to be extemporaneously compounded.
TABLE 2
Evidence generally supports progesterone in prevention of preterm labor
| Study | Treatment | Comment | NNT | LOE |
|---|---|---|---|---|
| Johnson18 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 2.4 | 2 |
| Yemeni19 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 4.6 | 2 |
| Hauth20 | Weekly IM 1000 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Hartikainen-Sorri22 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Meis23 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm Delivery | 6 | 1 |
| da Fonseca17 | Daily 100 mg progesterone vaginal suppositories | Decreased preterm delivery | 7 | 1 |
| NNT, number needed to treat; LOE, level of evidence; IM, intramuscular; N/A, not applicable. | ||||
Treating bacterial vaginosis may not prevent preterm labor
Bacterial vaginosis (BV) during pregnancy has been associated with preterm birth, and antibiotics have been thought perhaps to reduce this risk. A systematic review of 10 trials including 4249 pregnant women with BV showed that, although antibiotics eradicated BV, they did not significantly reduce the risk of birth before 32, 34, or 37 weeks (LOE: 1).24 This was true even for women with a history of preterm delivery. However, antibiotics did decrease the risk of PPROM. The authors concluded that evidence does not support screening all pregnant women for asymptomatic BV to prevent preterm birth.
Treating asymptomatic bacteriuria may prevent preterm labor
Asymptomatic bacteriuria in pregnant women has also been associated with preterm birth. One review of 14 trials comparing antibiotic treatment with placebo in this patient population—though not of high quality—demonstrated decreased incidences of pyelonephritis and preterm birth or low birth weight (LOE: 1).25
Treating preterm labor
The primary goal of using tocolytics in preterm labor is to prolong pregnancy for 2 to 7 days, permitting administration of corticosteroids and transfer to a tertiary care center when necessary. Recommendations for the treatment of preterm labor are summarized in Table 3 .
TABLE 3
Evaluation of proposed treatments for preterm labor
| Treatment | Comment | NNT | LOE | References |
|---|---|---|---|---|
| Calcium channel blockers | Reduce preterm delivery, decrease neonatal morbidity, well-tolerated | 11 | 1 | 26, 27 |
| Beta-agonists | Prolong pregnancy but no beneficial effect on neonatal morbidity; maternal side effects often lead to discontinuation | N/A | 1 | 33, 34 |
| Magnesium sulfate | Does not prolong pregnancy; maternal side effects often lead to discontinuation | N/A | 1 | 33, 40 |
| Prostaglandin inhibitors | Prolong pregnancy but no beneficial effect on neonatal morbidity; increase risk of postpartum hemorrhage | N/A | 1 | 33 |
| 2 | 43 | |||
| Antibiotics | No evidence to support use | N/A | 1 | 44 |
| Corticosteroids | Strongly recommended; decrease neonatal morbidity, well-tolerated | U | 1 | 46 |
| NNT, number needed to treat; LOE, level of evidence; N/A, not applicable; U, unavailable data. | ||||
Calcium channel blockers safe, effective
Calcium channel blockers (CCBs) are nonspecific smooth muscle relaxants. They prevent the influx of extracellular calcium ions into the myometrial cell, thereby exerting their tocolytic effect. Nifedipine is the most widely studied CCB in the management of preterm labor.
A systematic review of 12 randomized controlled trials including 1029 women demonstrated that CCBs significantly reduced the rate of preterm delivery within 7 days of the start of treatment and before 34 weeks gestation (LOE: 1).26 Compared with betamimetics, CCBs caused fewer maternal adverse effects, and decreased the frequency of neonatal respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice. The authors concluded that CCBs are preferable to betamimetics for tocolysis.
Calcium channel blockers have also been compared with magnesium. Eighty women at 20 to 34 weeks gestation with documented cervical change were randomized to receive nifedipine or magnesium.27 No difference in tocolytic efficacy was observed between the treatments, but there were significantly fewer maternal side effects among the nifedipine-treated patients (LOE: 2).
CCBs appear not to adversely affect the human fetus.28,29 There was no significant increase in congenital anomalies among 586 mothers who had been exposed to CCBs compared with 907 controls: 2.6% vs 2.4%, respectively (LOE: 2).30 CCBs are effective at reducing the rate of preterm delivery, decreasing neonatal morbidity and mortality, and are well tolerated by both mother and baby. Although some other tocolytics (such as beta-agonists and prostaglandin inhibitors) prolong pregnancy, they have not demonstrated a beneficial effect on neonatal morbidity.
Dosing recommendations for nifedipine are a 30 mg loading dose, followed by 10 to 20 mg every 4 to 6 hours.31 Maternal side effects may include flushing, headache, nausea, dizziness, and transient hypotension. Neonatal side effects have not been documented. Nifedipine should not be used in conjunction with magnesium sulfate because the combination has resulted in cardiac collapse (LOE: 3).32
Beta-agonists marginally useful
Beta-agonists increase cyclic AMP, thereby causing smooth muscle relaxation. Betamimetics are widely used as tocolytic agents. Although they have been shown to prolong pregnancy by 24 to 48 hours, they have not decreased adverse perinatal or neonatal outcomes (LOE: 1).33,34 In addition, they have been associated with maternal side effects of palpitations, arrhythmias, nausea, tremor, chorioamnionitis, hyperglycemia, hypokalemia, and pulmonary edema. The risk of pulmonary edema increases if beta-agonists are used with magnesium sulfate.33,35 Fetal and neonatal side effects may include tachycardia, hyperinsulinemia, and hyperglycemia.14,35
Terbutaline is the preferred beta-agonist for preterm labor due to low cost and multiple dose forms. It may be administered subcutaneously, 0.25 mg every 30 minutes up to 1 mg in 4 hours, or by intravenous infusion, 2.5 to 10 μg per minute up to an effective maximum dose of no more than 30 μg per minute. Once stabilized, the patient is often maintained on oral terbutaline, 2.5 to 5 μg by mouth every 4 to 6 hours until term. This practice, however, has not been shown to significantly prolong pregnancy (LOE: 1).36
The major benefit of terbutaline may be in the management of preterm uterine contractions without cervical changes. Among patients with premature contractions at 20 to 34 weeks, who were randomized to hydration or observation or a single subcutaneous dose of 0.25 mg of terbutaline, the shortest length of stay in triage was for the women who received the terbutaline (LOE: 1).37
Magnesium sulfate: No good rationale for use
Magnesium sulfate in the management of preterm labor is controversial. Although widely used in North America, it is generally avoided in Europe, Australia, and the United Kingdom38,39 due to the absence of clear evidence showing efficacy in the face of its adverse effect profile. Data from systematic reviews indicate it does not delay or prevent preterm birth (LOE: 1).33,40 In addition, maternal side effects may require discontinuation of treatment. Side effects include flushing, nausea, headache, ileus, and hypocalcemia. Pulmonary edema is a serious maternal side effect and is increased when magnesium is co-administered with other tocolytic agents. Cardiac arrest is rare.14,33 Not only does evidence suggest magnesium sulfate does not delay or prevent preterm birth, but one study suggests its use may increase infant mortality (LOE: 2).41
In one trial of 1062 women, magnesium sulfate or placebo was given at 30 weeks’ gestation. Two years following birth, children of mothers who received magnesium sulfate exhibited less gross motor dysfunction than those born to mothers who received placebo, 18% vs 34% respectively (LOE: 1).42 However, the rate of pediatric mortality and cerebral palsy, the primary endpoints of the study, did not differ between the groups. Further studies are necessary before magnesium sulfate can be recommended for neuroprotection.
Prostaglandin inhibitors may be preferred for hydramnios
Indomethacin is the prostaglandin synthetase inhibitor most studied in preterm labor, though the numbers of patients in these studies was generally small.33 Indomethacin reduced the rate of preterm delivery within 7 days of treatment and before 37 weeks gestation. However, it did not decrease the rate of neonatal morbidity. Importantly, indomethacin was associated with an increased risk of postpartum hemorrhage (LOE: 1).33 A retrospective review demonstrated an increase in incidence and severity of postnatal patent ductus arteriosus in neonates whose mothers were treated with indomethacin (LOE: 2).43 Most prospective studies, however, have not found this complication. Prostaglandin inhibitors may be the preferred choice of tocolytics if hydramnios is suspected in conjunction with preterm labor.
Antibiotics: one indication only
It is hypothesized that women who experience preterm labor have infections of the upper genital tract, and that infection or inflammation leads to contractions. A meta-analysis of 11 randomized trials with 7428 women in preterm labor and intact membranes demonstrated that, although prophylactic antibiotics decreased the incidence of maternal infection, there was no benefit in neonatal outcomes (LOE: 1).44 Although it did not reach statistical significance, there was a trend towards an increase in neonatal deaths in the antibiotic group, raising concerns about their use. Therefore, administration of prophylactic antibiotics in this patient population is not recommended. Conversely, antibiotic prophylaxis is recommended for all women colonized with group B streptococcus, unless a cesarean delivery is planned, to prevent perinatal disease.45
Corticosteroids beneficial for preterm infants
Antenatal corticosteroids reduce mortality, incidence and severity of respiratory distress syndrome, and intraventricular hemorrhage in preterm infants (LOE: 1).46 Women at risk for preterm delivery between 24 and 34 weeks of gestation should be given betamethasone 12 mg IM, two doses 24 hours apart, or dexamethasone 6 mg IM, two doses 12 hours apart. There are no significant maternal or neonatal adverse effects with these regimens. Administration of tocolytic drugs may be necessary to prolong gestation and provide time for steroids to act.
Corresponding author
Adrienne Z. Ables, PharmD, Spartanburg Family Medicine Residency Program, 853 N Church St Suite 510, Spartanburg, SC 29303. E-mail: [email protected].
1. Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: final data for 1999. Natl Vital Stat Rep 2001;49:1-100.
2. Rush RW, Davey DA, Segal ML. The effect of preterm delivery on perinatal mortality. Br J Obstet Gynaecol 1978;85:806-811.
3. Ehrenhaft PM, Wagner JL, Herdman RC. Changing prognosis for very low birth weight infants. Obstet Gynecol 1989;74:528.-
4. Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Munich N. School-age outcomes in children with birth weight under 750 g. N Engl J Med 1994;331:753-759.
5. Rogowski JA. The economics of preterm delivery. Prenat Neonat Med 1998;3:16-20.
6. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996;334:567-572.
7. Iams JD. Preterm birth. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies 4th ed. New York, NY: Churchill Livingstone; 2002.
8. Leitich H, Bodner-Adler B, Brunbauer M, Egarter C, Husslei P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol 2003;189:139-147.
9. Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of prospective study. J Am Dent Assoc. 2001;132:875-880.
10. Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. Br J Obstet Gynaecol 2003;110:56-60.
11. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: Retrospective cohort study. BMJ 2003;327:313-319.
12. Kelly RH, Russo J, Holt VL, Danielsen BH, Zatzick DF, Walker E, Kato W. Psychiatric and substance use disorders as risk factors for low birth weight and preterm delivery. Obstet Gynecol 2002;100:297-304.
13. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-856.
14. American College of Obstetricians and Gynecologists Management of preterm labor. ACOG practical bulletin no. 43. Washington, DC: ACOG, 2003.
15. Lockwood CJ, Senyei AE, Dischie MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-674.
16. Fuchs AR, Fuchs R. Endocrinology of human parturition: a review. Br J Obstet Gynaecol 1984;91:948-67.
17. da Fonseca EB, Bittar R, Carvalho MHB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double blind study. Am J Obstet Gynecol 2003;188:419-424.
18. Johnson JW, Austin KL, Jones GS, et al. Efficacy of 17 a-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med 1975;293:675-689.
19. Yemeni M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 a-hydroxyprogesterone caproate. Am J Obstet Gynecol 1985;151:574-577.
20. Hauth JC, Gilstrap LC, Brekken AL, et al. The effect of 17 a-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol 1983;146:187-190.
21. Fox ME, Harris RE, Brekken AL. The active duty military pregnancy: a new high-risk category. Am J Obstet Gynecol 1977;129:705-707.
22. Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 a-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol 1980;56:692-695.
23. Meis PJ. 17 Alpha hydroxyprogesterone caproate prevents recurrent preterm birth. N Engl J Med 2003;348:2379-2385.
24. McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
25. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
26. King JF, Flenady VJ, Papatsonis DNM, et al. Calcium channel blockers for inhibiting preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
27. Glock, JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol 1993;169:960-964.
28. Sorensen HT, Steffensen FH, Olesen C, et al. Pregnancy outcome in women exposed to calcium channel blockers. Reprod Toxicol 1998;12:383-384.
29. Magee LA, Schick B, Donnefeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol 1996;174:823-828.
30. Sorensen HT, Czeizel AE, Rockenbauer M, et al. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand 2001;80:397-401.
31. Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol 2000;43:787-801.
32. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol 1994;101:262-263.
33. Gyetvai K, Hannah M E, Hodnett E D, et al. Tocolytics for preterm labor: a systematic review. Obstet Gynecol 1999;94:869-877.
34. King JF, Grant A, Keirse MJNC, et al. Beta-mimetics in preterm labor: an overview of the randomized controlled trials. Br J Obstet Gynaecol 1988;95:211-222.
35. Jeyabalan A, Caritis SN. Pharmacologic inhibition of preterm labor. Clin Obstset Gynecol 2002;45:99-113.
36. Macones GA, Berlin M, Berlin J. Efficacy of oral beta-ago-nist maintenance therapy in preterm labor: a meta-analysis. Obstet Gynecol 1995;85:313-317.
37. Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: A randomized clinical trial. Am J Obstet Gynecol 1997;177:814-818.
38. Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med 1999;341:660-666.
39. Bennett P, Edwards D. Use of magnesium sulphate in obstetrics. Lancet 1997;350:1491.-
40. Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
41. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet 1997;350:1517-1518.
42. Crowther CA, Hiller JE, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth. A randomized controlled trial. JAMA 2003;290:2669-2676.
43. Hammerman C, Glaser J, Kaplan M, et al. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics 1998;102:E56.-
44. King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
45. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002;51(RR-11):1-22.Available at:www.cdc.gov/mmwr/PDF/RR/RR5111.pdf. Accessed January 21, 2005.
46. Crowley P. Prophylactic corticosteroids for preterm birth (Cochrane Review).In: The Cochrane Library, Issue 4, 2003. Oxford: Update Software.
- Consider progesterone to reduce the risk of recurrent preterm delivery (A).
- Consider calcium channel blockers (CCBs) as initial therapy for preterm labor; though these are Class C agents in pregnancy, CCBs are at least as effective as other agents and cause no known fetal or neonatal side effects (A).
- Consider tocolytic therapy for preterm labor, to prolong pregnancy for 2 to 7 days and thereby permit administration of antenatal corticosteroids and transfer to a tertiary center, if needed (A). Maintenance and recurrent use of tocolytics has not proven beneficial and should be avoided (A).
Despite diagnostic and therapeutic advances in premature labor, the rate of preterm delivery has increased in the United States. Preterm delivery, defined as birth before 37 weeks gestation, occurs in about 12% of pregnancies,1 and it is the leading cause of mortality among non-anomalous fetuses.2 Among premature newborns who survive, 10% to 15% have significant handicaps.3 Long-term sequelae include visual or hearing impairment, chronic lung disease, developmental delay, and cerebral palsy.4 The annual cost of premature birth in the United States exceeds $5 billion, and the rate of preterm delivery has increased in recent years.5,6 Specific maternal attributes increase risk of preterm delivery, though these factors need not be present for premature delivery to occur.
Standard techniques of physical diagnosis may not accurately detect preterm labor, either missing the diagnosis or prompting unnecessary hospitalization and treatment. Select imaging studies can more reliably indicate a true need for intervention.
This article reviews the risk factors for preterm birth, the tests available to accurately diagnose preterm labor, and the medications available to prevent or postpone premature delivery.
Risk factors
Preterm delivery is categorized as spontaneous or indicated. Almost 75% of preterm births occur after spontaneous preterm labor or preterm premature rupture of the membranes (PPROM). The remaining 25% of early births are elective due to conditions potentially harmful to either the mother or the fetus.7
Maternal risk factors for spontaneous preterm births are listed in Table 1.7-13 The risk factors for preterm labor and PPROM are similar, with the exception that those whose membranes rupture prematurely are more likely to be indigent, to smoke cigarettes, and to have bleeding in the current pregnancy.
Not all preterm births carry the same risks. Early preterm birth—delivery before 32 weeks— is associated with clinical or subclinical infection, short cervical length, and the presence of fetal fibronectin in the cervicovaginal secretions. It tends to recur and leads to long-term fetal morbidity. Late preterm birth—delivery after 32 weeks but before 37 weeks—is associated with increased uterine contractions, tends to mimic normal labor, and is less likely to cause peripartum morbidity.7 Despite these known risk factors, women can experience preterm labor without exhibiting any of them.
TABLE 1
Maternal risk factors for spontaneous preterm birth
| Risk factor | Odds ratio |
|---|---|
| Bacterial vaginosis at <16 weeks | 7 |
| Periodontal infection | 4 |
| Prior preterm birth | 4 |
| Prepregnancy BMI <20 | 3 |
| Interpregnancy interval <6 mo | 2 |
| Bleeding in 2nd trimester | 2 |
| African-American race | 2 |
| Psychiatric disorder | 1.6 |
| Cigarette smoking | 1.5 |
| Gestational diabetes | 1.2 |
| Sources: Iams 20027 ; Leitich et al 20038 ; Jeffcoat et al 20019 ; Moutquin 200310 ; Smith et al 200311 ; Kelly et al 200212 ; Hedderson et al 2003.13 | |
Increasing the accuracy of diagnosis
Uterine contractions before 37 weeks and concomitant change in cervical dilation or effacement as detected by digital examination comprise the standard definition of preterm labor.
Caveats with clinical evaluation
The problem with the definition above is that women may not perceive contractions, and that contractions are at times difficult to differentiate from benign Braxton-Hicks contractions of normal pregnancy. Moreover, digital assessment of the cervix lacks reproducibility when the dilation is <3 cm or effacement is < 80%.7 Clinical evaluation alone can easily suggest prematurity when it is absent and miss it when it is present.7
Imaging and lab evaluation more telling
The ability to diagnose preterm labor improves with the use of transvaginal cervical sonography or measurement of fetal fibronectin.
Lesser cervical length means greater risk. Likelihood of preterm delivery is inversely proportional to cervical length measured at 18 to 28 weeks. A length of ≤2.5 cm is the threshold for abnormal condition (level of evidence [LOE]: 2).6
Fibronectin detected late may mean delivery early. Fibronectin, an extracellular glycoprotein described as the glue that attaches the fetal membrane to the underlying uterine decidua, is normally absent in cervicovaginal secretions between weeks 22 and 37 of pregnancy. Its presence (50 ng/mL) between 22 to 24 weeks is a predictor of spontaneous preterm birth; its absence makes premature delivery unlikely (LOE: 2).
Imaging and test results justify management decisions. The predictive accuracies of abnormal cervical length and of the presence of fibronectin are depicted in the Figure .7 The major benefit of these two tests is when they are normal (cervical length >2.5 cm or negative fetal fibronectin), unnecessary intervention is avoided.14 If, for example, a patient is having contractions and the cervical length is greater than 2.5 cm, or fetal fibronectin is absent, tocolytics can be discontinued and hospitalization avoided.
FIGURE
Value of transvaginal cervical imaging and fetal fibronectin measurement in predicting spontaneous preterm birth
Preventing preterm labor
Progestational agents
Many authors have advocated the use of progestational agents to inhibit premature labor. Progesterone’s presumed mechanisms are inhibition of the oxytocin effect of prostaglandin F2a and stimulation of α-adrenergic receptors, thereby increasing the α-adrenergic tocolytic response.15,16 Natural progesterone appears to be free of any untoward teratogenic, metabolic, or hemodynamic effects.17
Studies favoring progesterone. Two double-blind, placebo-controlled studies assessed the use of 17 α-hydroxyprogesterone caproate in preventing premature labor in high-risk populations. These patients had histories of preterm deliveries or spontaneous abortions.
Weekly intramuscular (IM) injections of 250 mg were started at 12 to 16 weeks gestation and given until 37 weeks or delivery, whichever came first. In both studies, the rate of premature deliveries was significantly lower in the treated group than the control group (number needed to treat [NNT]=2.4 and 4.6). In addition, neither mothers nor infants experienced adverse effects attributable to 17 α-hydroxyprogesterone caproate (LOE: 2).18,19 However, these studies were small—43 and 79 patients.
A large double-blind, placebo-controlled, randomized study established the effectiveness of 17 α-hydroxyprogesterone caproate in preventing preterm delivery.23 Four hundred fifty-nine pregnant women with a history of preterm delivery were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo beginning at 16 to 20 weeks gestation and continuing to 36 weeks. Significantly fewer women in the treated group than the control group gave birth before 37 weeks, 36.3% v 54.9%, respectively (NNT=6; LOE: 1). Perhaps more importantly, treatment resulted in significant reductions in birth weight <2500 g (NNT=7), necrotizing enterocolitis (NNT=38), need for supplemental oxygen (NNT=11), and intraventricular hemorrhage (NNT=26). Swelling, bruising, or rash at the injection site were the most common adverse effects of 17 α-hydroxyprogesterone caproate administration.
Vaginal progesterone suppositories have also been shown to decrease the rate of preterm birth in patients at increased risk.17 da Fonseca et al noted that among 142 women who had 1 prior preterm birth, prophylactic cerclage, or uterine malformation, daily use of a 100-mg vaginal progesterone suppository compared with placebo significantly decreased the likelihood of delivery prior to 37 weeks, 14% v 28% (NNT=7; LOE: 1). Adverse effects of progesterone suppositories were not mentioned.
Studies lacking clear benefit. Another investigator enrolled 168 active-duty military women into a randomized double-blind study that evaluated weekly IM injections of 1000 mg 17 α-hydroxyprogesterone caproate or placebo beginning between 16 and 20 weeks gestation.20 The study population was chosen based on a report that active-duty pregnant military personnel had an increased number of pregnancy complications including low birth-weight infants and increased premature delivery.21 In contrast to the previous studies, no significant difference in premature labor or perinatal mortality was seen between the 2 groups (LOE: 2). Perhaps although active-duty personnel are at a higher risk for preterm labor, the risk is not as high as it is with previous preterm birth, prior spontaneous abortion, and so forth.
An additional small study of 77 women with twin pregnancies investigated the use of 17 α-hydroxyprogesterone caproate to prevent preterm delivery.22 Patients were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo from the 28th gestational week until the 37th week or delivery. The mean duration of pregnancy did not differ between the two groups of women, nor did perinatal mortality (LOE: 2).
Evidence generally supports use of progesterone. Based on available data, consider administering progesterone to women at high risk for preterm delivery to prevent recurrent preterm birth (SOR: A). These data are summarized in Table 2 .
Hydroxyprogesterone caproate in oil is commercially available in the United States in 125 mg/mL and 250 mg/mL strengths. It is not, however, approved by the Food and Drug Administration (FDA) for the prevention of preterm labor.
Progesterone suppositories are also not FDA approved, and they are commercially unavailable in the US. Therefore, they have to be extemporaneously compounded.
TABLE 2
Evidence generally supports progesterone in prevention of preterm labor
| Study | Treatment | Comment | NNT | LOE |
|---|---|---|---|---|
| Johnson18 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 2.4 | 2 |
| Yemeni19 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 4.6 | 2 |
| Hauth20 | Weekly IM 1000 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Hartikainen-Sorri22 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Meis23 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm Delivery | 6 | 1 |
| da Fonseca17 | Daily 100 mg progesterone vaginal suppositories | Decreased preterm delivery | 7 | 1 |
| NNT, number needed to treat; LOE, level of evidence; IM, intramuscular; N/A, not applicable. | ||||
Treating bacterial vaginosis may not prevent preterm labor
Bacterial vaginosis (BV) during pregnancy has been associated with preterm birth, and antibiotics have been thought perhaps to reduce this risk. A systematic review of 10 trials including 4249 pregnant women with BV showed that, although antibiotics eradicated BV, they did not significantly reduce the risk of birth before 32, 34, or 37 weeks (LOE: 1).24 This was true even for women with a history of preterm delivery. However, antibiotics did decrease the risk of PPROM. The authors concluded that evidence does not support screening all pregnant women for asymptomatic BV to prevent preterm birth.
Treating asymptomatic bacteriuria may prevent preterm labor
Asymptomatic bacteriuria in pregnant women has also been associated with preterm birth. One review of 14 trials comparing antibiotic treatment with placebo in this patient population—though not of high quality—demonstrated decreased incidences of pyelonephritis and preterm birth or low birth weight (LOE: 1).25
Treating preterm labor
The primary goal of using tocolytics in preterm labor is to prolong pregnancy for 2 to 7 days, permitting administration of corticosteroids and transfer to a tertiary care center when necessary. Recommendations for the treatment of preterm labor are summarized in Table 3 .
TABLE 3
Evaluation of proposed treatments for preterm labor
| Treatment | Comment | NNT | LOE | References |
|---|---|---|---|---|
| Calcium channel blockers | Reduce preterm delivery, decrease neonatal morbidity, well-tolerated | 11 | 1 | 26, 27 |
| Beta-agonists | Prolong pregnancy but no beneficial effect on neonatal morbidity; maternal side effects often lead to discontinuation | N/A | 1 | 33, 34 |
| Magnesium sulfate | Does not prolong pregnancy; maternal side effects often lead to discontinuation | N/A | 1 | 33, 40 |
| Prostaglandin inhibitors | Prolong pregnancy but no beneficial effect on neonatal morbidity; increase risk of postpartum hemorrhage | N/A | 1 | 33 |
| 2 | 43 | |||
| Antibiotics | No evidence to support use | N/A | 1 | 44 |
| Corticosteroids | Strongly recommended; decrease neonatal morbidity, well-tolerated | U | 1 | 46 |
| NNT, number needed to treat; LOE, level of evidence; N/A, not applicable; U, unavailable data. | ||||
Calcium channel blockers safe, effective
Calcium channel blockers (CCBs) are nonspecific smooth muscle relaxants. They prevent the influx of extracellular calcium ions into the myometrial cell, thereby exerting their tocolytic effect. Nifedipine is the most widely studied CCB in the management of preterm labor.
A systematic review of 12 randomized controlled trials including 1029 women demonstrated that CCBs significantly reduced the rate of preterm delivery within 7 days of the start of treatment and before 34 weeks gestation (LOE: 1).26 Compared with betamimetics, CCBs caused fewer maternal adverse effects, and decreased the frequency of neonatal respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice. The authors concluded that CCBs are preferable to betamimetics for tocolysis.
Calcium channel blockers have also been compared with magnesium. Eighty women at 20 to 34 weeks gestation with documented cervical change were randomized to receive nifedipine or magnesium.27 No difference in tocolytic efficacy was observed between the treatments, but there were significantly fewer maternal side effects among the nifedipine-treated patients (LOE: 2).
CCBs appear not to adversely affect the human fetus.28,29 There was no significant increase in congenital anomalies among 586 mothers who had been exposed to CCBs compared with 907 controls: 2.6% vs 2.4%, respectively (LOE: 2).30 CCBs are effective at reducing the rate of preterm delivery, decreasing neonatal morbidity and mortality, and are well tolerated by both mother and baby. Although some other tocolytics (such as beta-agonists and prostaglandin inhibitors) prolong pregnancy, they have not demonstrated a beneficial effect on neonatal morbidity.
Dosing recommendations for nifedipine are a 30 mg loading dose, followed by 10 to 20 mg every 4 to 6 hours.31 Maternal side effects may include flushing, headache, nausea, dizziness, and transient hypotension. Neonatal side effects have not been documented. Nifedipine should not be used in conjunction with magnesium sulfate because the combination has resulted in cardiac collapse (LOE: 3).32
Beta-agonists marginally useful
Beta-agonists increase cyclic AMP, thereby causing smooth muscle relaxation. Betamimetics are widely used as tocolytic agents. Although they have been shown to prolong pregnancy by 24 to 48 hours, they have not decreased adverse perinatal or neonatal outcomes (LOE: 1).33,34 In addition, they have been associated with maternal side effects of palpitations, arrhythmias, nausea, tremor, chorioamnionitis, hyperglycemia, hypokalemia, and pulmonary edema. The risk of pulmonary edema increases if beta-agonists are used with magnesium sulfate.33,35 Fetal and neonatal side effects may include tachycardia, hyperinsulinemia, and hyperglycemia.14,35
Terbutaline is the preferred beta-agonist for preterm labor due to low cost and multiple dose forms. It may be administered subcutaneously, 0.25 mg every 30 minutes up to 1 mg in 4 hours, or by intravenous infusion, 2.5 to 10 μg per minute up to an effective maximum dose of no more than 30 μg per minute. Once stabilized, the patient is often maintained on oral terbutaline, 2.5 to 5 μg by mouth every 4 to 6 hours until term. This practice, however, has not been shown to significantly prolong pregnancy (LOE: 1).36
The major benefit of terbutaline may be in the management of preterm uterine contractions without cervical changes. Among patients with premature contractions at 20 to 34 weeks, who were randomized to hydration or observation or a single subcutaneous dose of 0.25 mg of terbutaline, the shortest length of stay in triage was for the women who received the terbutaline (LOE: 1).37
Magnesium sulfate: No good rationale for use
Magnesium sulfate in the management of preterm labor is controversial. Although widely used in North America, it is generally avoided in Europe, Australia, and the United Kingdom38,39 due to the absence of clear evidence showing efficacy in the face of its adverse effect profile. Data from systematic reviews indicate it does not delay or prevent preterm birth (LOE: 1).33,40 In addition, maternal side effects may require discontinuation of treatment. Side effects include flushing, nausea, headache, ileus, and hypocalcemia. Pulmonary edema is a serious maternal side effect and is increased when magnesium is co-administered with other tocolytic agents. Cardiac arrest is rare.14,33 Not only does evidence suggest magnesium sulfate does not delay or prevent preterm birth, but one study suggests its use may increase infant mortality (LOE: 2).41
In one trial of 1062 women, magnesium sulfate or placebo was given at 30 weeks’ gestation. Two years following birth, children of mothers who received magnesium sulfate exhibited less gross motor dysfunction than those born to mothers who received placebo, 18% vs 34% respectively (LOE: 1).42 However, the rate of pediatric mortality and cerebral palsy, the primary endpoints of the study, did not differ between the groups. Further studies are necessary before magnesium sulfate can be recommended for neuroprotection.
Prostaglandin inhibitors may be preferred for hydramnios
Indomethacin is the prostaglandin synthetase inhibitor most studied in preterm labor, though the numbers of patients in these studies was generally small.33 Indomethacin reduced the rate of preterm delivery within 7 days of treatment and before 37 weeks gestation. However, it did not decrease the rate of neonatal morbidity. Importantly, indomethacin was associated with an increased risk of postpartum hemorrhage (LOE: 1).33 A retrospective review demonstrated an increase in incidence and severity of postnatal patent ductus arteriosus in neonates whose mothers were treated with indomethacin (LOE: 2).43 Most prospective studies, however, have not found this complication. Prostaglandin inhibitors may be the preferred choice of tocolytics if hydramnios is suspected in conjunction with preterm labor.
Antibiotics: one indication only
It is hypothesized that women who experience preterm labor have infections of the upper genital tract, and that infection or inflammation leads to contractions. A meta-analysis of 11 randomized trials with 7428 women in preterm labor and intact membranes demonstrated that, although prophylactic antibiotics decreased the incidence of maternal infection, there was no benefit in neonatal outcomes (LOE: 1).44 Although it did not reach statistical significance, there was a trend towards an increase in neonatal deaths in the antibiotic group, raising concerns about their use. Therefore, administration of prophylactic antibiotics in this patient population is not recommended. Conversely, antibiotic prophylaxis is recommended for all women colonized with group B streptococcus, unless a cesarean delivery is planned, to prevent perinatal disease.45
Corticosteroids beneficial for preterm infants
Antenatal corticosteroids reduce mortality, incidence and severity of respiratory distress syndrome, and intraventricular hemorrhage in preterm infants (LOE: 1).46 Women at risk for preterm delivery between 24 and 34 weeks of gestation should be given betamethasone 12 mg IM, two doses 24 hours apart, or dexamethasone 6 mg IM, two doses 12 hours apart. There are no significant maternal or neonatal adverse effects with these regimens. Administration of tocolytic drugs may be necessary to prolong gestation and provide time for steroids to act.
Corresponding author
Adrienne Z. Ables, PharmD, Spartanburg Family Medicine Residency Program, 853 N Church St Suite 510, Spartanburg, SC 29303. E-mail: [email protected].
- Consider progesterone to reduce the risk of recurrent preterm delivery (A).
- Consider calcium channel blockers (CCBs) as initial therapy for preterm labor; though these are Class C agents in pregnancy, CCBs are at least as effective as other agents and cause no known fetal or neonatal side effects (A).
- Consider tocolytic therapy for preterm labor, to prolong pregnancy for 2 to 7 days and thereby permit administration of antenatal corticosteroids and transfer to a tertiary center, if needed (A). Maintenance and recurrent use of tocolytics has not proven beneficial and should be avoided (A).
Despite diagnostic and therapeutic advances in premature labor, the rate of preterm delivery has increased in the United States. Preterm delivery, defined as birth before 37 weeks gestation, occurs in about 12% of pregnancies,1 and it is the leading cause of mortality among non-anomalous fetuses.2 Among premature newborns who survive, 10% to 15% have significant handicaps.3 Long-term sequelae include visual or hearing impairment, chronic lung disease, developmental delay, and cerebral palsy.4 The annual cost of premature birth in the United States exceeds $5 billion, and the rate of preterm delivery has increased in recent years.5,6 Specific maternal attributes increase risk of preterm delivery, though these factors need not be present for premature delivery to occur.
Standard techniques of physical diagnosis may not accurately detect preterm labor, either missing the diagnosis or prompting unnecessary hospitalization and treatment. Select imaging studies can more reliably indicate a true need for intervention.
This article reviews the risk factors for preterm birth, the tests available to accurately diagnose preterm labor, and the medications available to prevent or postpone premature delivery.
Risk factors
Preterm delivery is categorized as spontaneous or indicated. Almost 75% of preterm births occur after spontaneous preterm labor or preterm premature rupture of the membranes (PPROM). The remaining 25% of early births are elective due to conditions potentially harmful to either the mother or the fetus.7
Maternal risk factors for spontaneous preterm births are listed in Table 1.7-13 The risk factors for preterm labor and PPROM are similar, with the exception that those whose membranes rupture prematurely are more likely to be indigent, to smoke cigarettes, and to have bleeding in the current pregnancy.
Not all preterm births carry the same risks. Early preterm birth—delivery before 32 weeks— is associated with clinical or subclinical infection, short cervical length, and the presence of fetal fibronectin in the cervicovaginal secretions. It tends to recur and leads to long-term fetal morbidity. Late preterm birth—delivery after 32 weeks but before 37 weeks—is associated with increased uterine contractions, tends to mimic normal labor, and is less likely to cause peripartum morbidity.7 Despite these known risk factors, women can experience preterm labor without exhibiting any of them.
TABLE 1
Maternal risk factors for spontaneous preterm birth
| Risk factor | Odds ratio |
|---|---|
| Bacterial vaginosis at <16 weeks | 7 |
| Periodontal infection | 4 |
| Prior preterm birth | 4 |
| Prepregnancy BMI <20 | 3 |
| Interpregnancy interval <6 mo | 2 |
| Bleeding in 2nd trimester | 2 |
| African-American race | 2 |
| Psychiatric disorder | 1.6 |
| Cigarette smoking | 1.5 |
| Gestational diabetes | 1.2 |
| Sources: Iams 20027 ; Leitich et al 20038 ; Jeffcoat et al 20019 ; Moutquin 200310 ; Smith et al 200311 ; Kelly et al 200212 ; Hedderson et al 2003.13 | |
Increasing the accuracy of diagnosis
Uterine contractions before 37 weeks and concomitant change in cervical dilation or effacement as detected by digital examination comprise the standard definition of preterm labor.
Caveats with clinical evaluation
The problem with the definition above is that women may not perceive contractions, and that contractions are at times difficult to differentiate from benign Braxton-Hicks contractions of normal pregnancy. Moreover, digital assessment of the cervix lacks reproducibility when the dilation is <3 cm or effacement is < 80%.7 Clinical evaluation alone can easily suggest prematurity when it is absent and miss it when it is present.7
Imaging and lab evaluation more telling
The ability to diagnose preterm labor improves with the use of transvaginal cervical sonography or measurement of fetal fibronectin.
Lesser cervical length means greater risk. Likelihood of preterm delivery is inversely proportional to cervical length measured at 18 to 28 weeks. A length of ≤2.5 cm is the threshold for abnormal condition (level of evidence [LOE]: 2).6
Fibronectin detected late may mean delivery early. Fibronectin, an extracellular glycoprotein described as the glue that attaches the fetal membrane to the underlying uterine decidua, is normally absent in cervicovaginal secretions between weeks 22 and 37 of pregnancy. Its presence (50 ng/mL) between 22 to 24 weeks is a predictor of spontaneous preterm birth; its absence makes premature delivery unlikely (LOE: 2).
Imaging and test results justify management decisions. The predictive accuracies of abnormal cervical length and of the presence of fibronectin are depicted in the Figure .7 The major benefit of these two tests is when they are normal (cervical length >2.5 cm or negative fetal fibronectin), unnecessary intervention is avoided.14 If, for example, a patient is having contractions and the cervical length is greater than 2.5 cm, or fetal fibronectin is absent, tocolytics can be discontinued and hospitalization avoided.
FIGURE
Value of transvaginal cervical imaging and fetal fibronectin measurement in predicting spontaneous preterm birth
Preventing preterm labor
Progestational agents
Many authors have advocated the use of progestational agents to inhibit premature labor. Progesterone’s presumed mechanisms are inhibition of the oxytocin effect of prostaglandin F2a and stimulation of α-adrenergic receptors, thereby increasing the α-adrenergic tocolytic response.15,16 Natural progesterone appears to be free of any untoward teratogenic, metabolic, or hemodynamic effects.17
Studies favoring progesterone. Two double-blind, placebo-controlled studies assessed the use of 17 α-hydroxyprogesterone caproate in preventing premature labor in high-risk populations. These patients had histories of preterm deliveries or spontaneous abortions.
Weekly intramuscular (IM) injections of 250 mg were started at 12 to 16 weeks gestation and given until 37 weeks or delivery, whichever came first. In both studies, the rate of premature deliveries was significantly lower in the treated group than the control group (number needed to treat [NNT]=2.4 and 4.6). In addition, neither mothers nor infants experienced adverse effects attributable to 17 α-hydroxyprogesterone caproate (LOE: 2).18,19 However, these studies were small—43 and 79 patients.
A large double-blind, placebo-controlled, randomized study established the effectiveness of 17 α-hydroxyprogesterone caproate in preventing preterm delivery.23 Four hundred fifty-nine pregnant women with a history of preterm delivery were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo beginning at 16 to 20 weeks gestation and continuing to 36 weeks. Significantly fewer women in the treated group than the control group gave birth before 37 weeks, 36.3% v 54.9%, respectively (NNT=6; LOE: 1). Perhaps more importantly, treatment resulted in significant reductions in birth weight <2500 g (NNT=7), necrotizing enterocolitis (NNT=38), need for supplemental oxygen (NNT=11), and intraventricular hemorrhage (NNT=26). Swelling, bruising, or rash at the injection site were the most common adverse effects of 17 α-hydroxyprogesterone caproate administration.
Vaginal progesterone suppositories have also been shown to decrease the rate of preterm birth in patients at increased risk.17 da Fonseca et al noted that among 142 women who had 1 prior preterm birth, prophylactic cerclage, or uterine malformation, daily use of a 100-mg vaginal progesterone suppository compared with placebo significantly decreased the likelihood of delivery prior to 37 weeks, 14% v 28% (NNT=7; LOE: 1). Adverse effects of progesterone suppositories were not mentioned.
Studies lacking clear benefit. Another investigator enrolled 168 active-duty military women into a randomized double-blind study that evaluated weekly IM injections of 1000 mg 17 α-hydroxyprogesterone caproate or placebo beginning between 16 and 20 weeks gestation.20 The study population was chosen based on a report that active-duty pregnant military personnel had an increased number of pregnancy complications including low birth-weight infants and increased premature delivery.21 In contrast to the previous studies, no significant difference in premature labor or perinatal mortality was seen between the 2 groups (LOE: 2). Perhaps although active-duty personnel are at a higher risk for preterm labor, the risk is not as high as it is with previous preterm birth, prior spontaneous abortion, and so forth.
An additional small study of 77 women with twin pregnancies investigated the use of 17 α-hydroxyprogesterone caproate to prevent preterm delivery.22 Patients were randomized to receive weekly injections of 17 α-hydroxyprogesterone caproate 250 mg or placebo from the 28th gestational week until the 37th week or delivery. The mean duration of pregnancy did not differ between the two groups of women, nor did perinatal mortality (LOE: 2).
Evidence generally supports use of progesterone. Based on available data, consider administering progesterone to women at high risk for preterm delivery to prevent recurrent preterm birth (SOR: A). These data are summarized in Table 2 .
Hydroxyprogesterone caproate in oil is commercially available in the United States in 125 mg/mL and 250 mg/mL strengths. It is not, however, approved by the Food and Drug Administration (FDA) for the prevention of preterm labor.
Progesterone suppositories are also not FDA approved, and they are commercially unavailable in the US. Therefore, they have to be extemporaneously compounded.
TABLE 2
Evidence generally supports progesterone in prevention of preterm labor
| Study | Treatment | Comment | NNT | LOE |
|---|---|---|---|---|
| Johnson18 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 2.4 | 2 |
| Yemeni19 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm delivery | 4.6 | 2 |
| Hauth20 | Weekly IM 1000 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Hartikainen-Sorri22 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Did not decrease preterm delivery | N/A | 2 |
| Meis23 | Weekly IM 250 mg injections of 17 α-hydroxyprogesterone caproate | Decreased preterm Delivery | 6 | 1 |
| da Fonseca17 | Daily 100 mg progesterone vaginal suppositories | Decreased preterm delivery | 7 | 1 |
| NNT, number needed to treat; LOE, level of evidence; IM, intramuscular; N/A, not applicable. | ||||
Treating bacterial vaginosis may not prevent preterm labor
Bacterial vaginosis (BV) during pregnancy has been associated with preterm birth, and antibiotics have been thought perhaps to reduce this risk. A systematic review of 10 trials including 4249 pregnant women with BV showed that, although antibiotics eradicated BV, they did not significantly reduce the risk of birth before 32, 34, or 37 weeks (LOE: 1).24 This was true even for women with a history of preterm delivery. However, antibiotics did decrease the risk of PPROM. The authors concluded that evidence does not support screening all pregnant women for asymptomatic BV to prevent preterm birth.
Treating asymptomatic bacteriuria may prevent preterm labor
Asymptomatic bacteriuria in pregnant women has also been associated with preterm birth. One review of 14 trials comparing antibiotic treatment with placebo in this patient population—though not of high quality—demonstrated decreased incidences of pyelonephritis and preterm birth or low birth weight (LOE: 1).25
Treating preterm labor
The primary goal of using tocolytics in preterm labor is to prolong pregnancy for 2 to 7 days, permitting administration of corticosteroids and transfer to a tertiary care center when necessary. Recommendations for the treatment of preterm labor are summarized in Table 3 .
TABLE 3
Evaluation of proposed treatments for preterm labor
| Treatment | Comment | NNT | LOE | References |
|---|---|---|---|---|
| Calcium channel blockers | Reduce preterm delivery, decrease neonatal morbidity, well-tolerated | 11 | 1 | 26, 27 |
| Beta-agonists | Prolong pregnancy but no beneficial effect on neonatal morbidity; maternal side effects often lead to discontinuation | N/A | 1 | 33, 34 |
| Magnesium sulfate | Does not prolong pregnancy; maternal side effects often lead to discontinuation | N/A | 1 | 33, 40 |
| Prostaglandin inhibitors | Prolong pregnancy but no beneficial effect on neonatal morbidity; increase risk of postpartum hemorrhage | N/A | 1 | 33 |
| 2 | 43 | |||
| Antibiotics | No evidence to support use | N/A | 1 | 44 |
| Corticosteroids | Strongly recommended; decrease neonatal morbidity, well-tolerated | U | 1 | 46 |
| NNT, number needed to treat; LOE, level of evidence; N/A, not applicable; U, unavailable data. | ||||
Calcium channel blockers safe, effective
Calcium channel blockers (CCBs) are nonspecific smooth muscle relaxants. They prevent the influx of extracellular calcium ions into the myometrial cell, thereby exerting their tocolytic effect. Nifedipine is the most widely studied CCB in the management of preterm labor.
A systematic review of 12 randomized controlled trials including 1029 women demonstrated that CCBs significantly reduced the rate of preterm delivery within 7 days of the start of treatment and before 34 weeks gestation (LOE: 1).26 Compared with betamimetics, CCBs caused fewer maternal adverse effects, and decreased the frequency of neonatal respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice. The authors concluded that CCBs are preferable to betamimetics for tocolysis.
Calcium channel blockers have also been compared with magnesium. Eighty women at 20 to 34 weeks gestation with documented cervical change were randomized to receive nifedipine or magnesium.27 No difference in tocolytic efficacy was observed between the treatments, but there were significantly fewer maternal side effects among the nifedipine-treated patients (LOE: 2).
CCBs appear not to adversely affect the human fetus.28,29 There was no significant increase in congenital anomalies among 586 mothers who had been exposed to CCBs compared with 907 controls: 2.6% vs 2.4%, respectively (LOE: 2).30 CCBs are effective at reducing the rate of preterm delivery, decreasing neonatal morbidity and mortality, and are well tolerated by both mother and baby. Although some other tocolytics (such as beta-agonists and prostaglandin inhibitors) prolong pregnancy, they have not demonstrated a beneficial effect on neonatal morbidity.
Dosing recommendations for nifedipine are a 30 mg loading dose, followed by 10 to 20 mg every 4 to 6 hours.31 Maternal side effects may include flushing, headache, nausea, dizziness, and transient hypotension. Neonatal side effects have not been documented. Nifedipine should not be used in conjunction with magnesium sulfate because the combination has resulted in cardiac collapse (LOE: 3).32
Beta-agonists marginally useful
Beta-agonists increase cyclic AMP, thereby causing smooth muscle relaxation. Betamimetics are widely used as tocolytic agents. Although they have been shown to prolong pregnancy by 24 to 48 hours, they have not decreased adverse perinatal or neonatal outcomes (LOE: 1).33,34 In addition, they have been associated with maternal side effects of palpitations, arrhythmias, nausea, tremor, chorioamnionitis, hyperglycemia, hypokalemia, and pulmonary edema. The risk of pulmonary edema increases if beta-agonists are used with magnesium sulfate.33,35 Fetal and neonatal side effects may include tachycardia, hyperinsulinemia, and hyperglycemia.14,35
Terbutaline is the preferred beta-agonist for preterm labor due to low cost and multiple dose forms. It may be administered subcutaneously, 0.25 mg every 30 minutes up to 1 mg in 4 hours, or by intravenous infusion, 2.5 to 10 μg per minute up to an effective maximum dose of no more than 30 μg per minute. Once stabilized, the patient is often maintained on oral terbutaline, 2.5 to 5 μg by mouth every 4 to 6 hours until term. This practice, however, has not been shown to significantly prolong pregnancy (LOE: 1).36
The major benefit of terbutaline may be in the management of preterm uterine contractions without cervical changes. Among patients with premature contractions at 20 to 34 weeks, who were randomized to hydration or observation or a single subcutaneous dose of 0.25 mg of terbutaline, the shortest length of stay in triage was for the women who received the terbutaline (LOE: 1).37
Magnesium sulfate: No good rationale for use
Magnesium sulfate in the management of preterm labor is controversial. Although widely used in North America, it is generally avoided in Europe, Australia, and the United Kingdom38,39 due to the absence of clear evidence showing efficacy in the face of its adverse effect profile. Data from systematic reviews indicate it does not delay or prevent preterm birth (LOE: 1).33,40 In addition, maternal side effects may require discontinuation of treatment. Side effects include flushing, nausea, headache, ileus, and hypocalcemia. Pulmonary edema is a serious maternal side effect and is increased when magnesium is co-administered with other tocolytic agents. Cardiac arrest is rare.14,33 Not only does evidence suggest magnesium sulfate does not delay or prevent preterm birth, but one study suggests its use may increase infant mortality (LOE: 2).41
In one trial of 1062 women, magnesium sulfate or placebo was given at 30 weeks’ gestation. Two years following birth, children of mothers who received magnesium sulfate exhibited less gross motor dysfunction than those born to mothers who received placebo, 18% vs 34% respectively (LOE: 1).42 However, the rate of pediatric mortality and cerebral palsy, the primary endpoints of the study, did not differ between the groups. Further studies are necessary before magnesium sulfate can be recommended for neuroprotection.
Prostaglandin inhibitors may be preferred for hydramnios
Indomethacin is the prostaglandin synthetase inhibitor most studied in preterm labor, though the numbers of patients in these studies was generally small.33 Indomethacin reduced the rate of preterm delivery within 7 days of treatment and before 37 weeks gestation. However, it did not decrease the rate of neonatal morbidity. Importantly, indomethacin was associated with an increased risk of postpartum hemorrhage (LOE: 1).33 A retrospective review demonstrated an increase in incidence and severity of postnatal patent ductus arteriosus in neonates whose mothers were treated with indomethacin (LOE: 2).43 Most prospective studies, however, have not found this complication. Prostaglandin inhibitors may be the preferred choice of tocolytics if hydramnios is suspected in conjunction with preterm labor.
Antibiotics: one indication only
It is hypothesized that women who experience preterm labor have infections of the upper genital tract, and that infection or inflammation leads to contractions. A meta-analysis of 11 randomized trials with 7428 women in preterm labor and intact membranes demonstrated that, although prophylactic antibiotics decreased the incidence of maternal infection, there was no benefit in neonatal outcomes (LOE: 1).44 Although it did not reach statistical significance, there was a trend towards an increase in neonatal deaths in the antibiotic group, raising concerns about their use. Therefore, administration of prophylactic antibiotics in this patient population is not recommended. Conversely, antibiotic prophylaxis is recommended for all women colonized with group B streptococcus, unless a cesarean delivery is planned, to prevent perinatal disease.45
Corticosteroids beneficial for preterm infants
Antenatal corticosteroids reduce mortality, incidence and severity of respiratory distress syndrome, and intraventricular hemorrhage in preterm infants (LOE: 1).46 Women at risk for preterm delivery between 24 and 34 weeks of gestation should be given betamethasone 12 mg IM, two doses 24 hours apart, or dexamethasone 6 mg IM, two doses 12 hours apart. There are no significant maternal or neonatal adverse effects with these regimens. Administration of tocolytic drugs may be necessary to prolong gestation and provide time for steroids to act.
Corresponding author
Adrienne Z. Ables, PharmD, Spartanburg Family Medicine Residency Program, 853 N Church St Suite 510, Spartanburg, SC 29303. E-mail: [email protected].
1. Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: final data for 1999. Natl Vital Stat Rep 2001;49:1-100.
2. Rush RW, Davey DA, Segal ML. The effect of preterm delivery on perinatal mortality. Br J Obstet Gynaecol 1978;85:806-811.
3. Ehrenhaft PM, Wagner JL, Herdman RC. Changing prognosis for very low birth weight infants. Obstet Gynecol 1989;74:528.-
4. Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Munich N. School-age outcomes in children with birth weight under 750 g. N Engl J Med 1994;331:753-759.
5. Rogowski JA. The economics of preterm delivery. Prenat Neonat Med 1998;3:16-20.
6. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996;334:567-572.
7. Iams JD. Preterm birth. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies 4th ed. New York, NY: Churchill Livingstone; 2002.
8. Leitich H, Bodner-Adler B, Brunbauer M, Egarter C, Husslei P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol 2003;189:139-147.
9. Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of prospective study. J Am Dent Assoc. 2001;132:875-880.
10. Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. Br J Obstet Gynaecol 2003;110:56-60.
11. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: Retrospective cohort study. BMJ 2003;327:313-319.
12. Kelly RH, Russo J, Holt VL, Danielsen BH, Zatzick DF, Walker E, Kato W. Psychiatric and substance use disorders as risk factors for low birth weight and preterm delivery. Obstet Gynecol 2002;100:297-304.
13. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-856.
14. American College of Obstetricians and Gynecologists Management of preterm labor. ACOG practical bulletin no. 43. Washington, DC: ACOG, 2003.
15. Lockwood CJ, Senyei AE, Dischie MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-674.
16. Fuchs AR, Fuchs R. Endocrinology of human parturition: a review. Br J Obstet Gynaecol 1984;91:948-67.
17. da Fonseca EB, Bittar R, Carvalho MHB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double blind study. Am J Obstet Gynecol 2003;188:419-424.
18. Johnson JW, Austin KL, Jones GS, et al. Efficacy of 17 a-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med 1975;293:675-689.
19. Yemeni M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 a-hydroxyprogesterone caproate. Am J Obstet Gynecol 1985;151:574-577.
20. Hauth JC, Gilstrap LC, Brekken AL, et al. The effect of 17 a-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol 1983;146:187-190.
21. Fox ME, Harris RE, Brekken AL. The active duty military pregnancy: a new high-risk category. Am J Obstet Gynecol 1977;129:705-707.
22. Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 a-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol 1980;56:692-695.
23. Meis PJ. 17 Alpha hydroxyprogesterone caproate prevents recurrent preterm birth. N Engl J Med 2003;348:2379-2385.
24. McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
25. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
26. King JF, Flenady VJ, Papatsonis DNM, et al. Calcium channel blockers for inhibiting preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
27. Glock, JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol 1993;169:960-964.
28. Sorensen HT, Steffensen FH, Olesen C, et al. Pregnancy outcome in women exposed to calcium channel blockers. Reprod Toxicol 1998;12:383-384.
29. Magee LA, Schick B, Donnefeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol 1996;174:823-828.
30. Sorensen HT, Czeizel AE, Rockenbauer M, et al. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand 2001;80:397-401.
31. Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol 2000;43:787-801.
32. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol 1994;101:262-263.
33. Gyetvai K, Hannah M E, Hodnett E D, et al. Tocolytics for preterm labor: a systematic review. Obstet Gynecol 1999;94:869-877.
34. King JF, Grant A, Keirse MJNC, et al. Beta-mimetics in preterm labor: an overview of the randomized controlled trials. Br J Obstet Gynaecol 1988;95:211-222.
35. Jeyabalan A, Caritis SN. Pharmacologic inhibition of preterm labor. Clin Obstset Gynecol 2002;45:99-113.
36. Macones GA, Berlin M, Berlin J. Efficacy of oral beta-ago-nist maintenance therapy in preterm labor: a meta-analysis. Obstet Gynecol 1995;85:313-317.
37. Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: A randomized clinical trial. Am J Obstet Gynecol 1997;177:814-818.
38. Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med 1999;341:660-666.
39. Bennett P, Edwards D. Use of magnesium sulphate in obstetrics. Lancet 1997;350:1491.-
40. Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
41. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet 1997;350:1517-1518.
42. Crowther CA, Hiller JE, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth. A randomized controlled trial. JAMA 2003;290:2669-2676.
43. Hammerman C, Glaser J, Kaplan M, et al. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics 1998;102:E56.-
44. King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
45. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002;51(RR-11):1-22.Available at:www.cdc.gov/mmwr/PDF/RR/RR5111.pdf. Accessed January 21, 2005.
46. Crowley P. Prophylactic corticosteroids for preterm birth (Cochrane Review).In: The Cochrane Library, Issue 4, 2003. Oxford: Update Software.
1. Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: final data for 1999. Natl Vital Stat Rep 2001;49:1-100.
2. Rush RW, Davey DA, Segal ML. The effect of preterm delivery on perinatal mortality. Br J Obstet Gynaecol 1978;85:806-811.
3. Ehrenhaft PM, Wagner JL, Herdman RC. Changing prognosis for very low birth weight infants. Obstet Gynecol 1989;74:528.-
4. Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Munich N. School-age outcomes in children with birth weight under 750 g. N Engl J Med 1994;331:753-759.
5. Rogowski JA. The economics of preterm delivery. Prenat Neonat Med 1998;3:16-20.
6. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996;334:567-572.
7. Iams JD. Preterm birth. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies 4th ed. New York, NY: Churchill Livingstone; 2002.
8. Leitich H, Bodner-Adler B, Brunbauer M, Egarter C, Husslei P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol 2003;189:139-147.
9. Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of prospective study. J Am Dent Assoc. 2001;132:875-880.
10. Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. Br J Obstet Gynaecol 2003;110:56-60.
11. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: Retrospective cohort study. BMJ 2003;327:313-319.
12. Kelly RH, Russo J, Holt VL, Danielsen BH, Zatzick DF, Walker E, Kato W. Psychiatric and substance use disorders as risk factors for low birth weight and preterm delivery. Obstet Gynecol 2002;100:297-304.
13. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-856.
14. American College of Obstetricians and Gynecologists Management of preterm labor. ACOG practical bulletin no. 43. Washington, DC: ACOG, 2003.
15. Lockwood CJ, Senyei AE, Dischie MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-674.
16. Fuchs AR, Fuchs R. Endocrinology of human parturition: a review. Br J Obstet Gynaecol 1984;91:948-67.
17. da Fonseca EB, Bittar R, Carvalho MHB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double blind study. Am J Obstet Gynecol 2003;188:419-424.
18. Johnson JW, Austin KL, Jones GS, et al. Efficacy of 17 a-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med 1975;293:675-689.
19. Yemeni M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 a-hydroxyprogesterone caproate. Am J Obstet Gynecol 1985;151:574-577.
20. Hauth JC, Gilstrap LC, Brekken AL, et al. The effect of 17 a-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol 1983;146:187-190.
21. Fox ME, Harris RE, Brekken AL. The active duty military pregnancy: a new high-risk category. Am J Obstet Gynecol 1977;129:705-707.
22. Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 a-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol 1980;56:692-695.
23. Meis PJ. 17 Alpha hydroxyprogesterone caproate prevents recurrent preterm birth. N Engl J Med 2003;348:2379-2385.
24. McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
25. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy (Cochrane Review).In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
26. King JF, Flenady VJ, Papatsonis DNM, et al. Calcium channel blockers for inhibiting preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
27. Glock, JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol 1993;169:960-964.
28. Sorensen HT, Steffensen FH, Olesen C, et al. Pregnancy outcome in women exposed to calcium channel blockers. Reprod Toxicol 1998;12:383-384.
29. Magee LA, Schick B, Donnefeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol 1996;174:823-828.
30. Sorensen HT, Czeizel AE, Rockenbauer M, et al. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand 2001;80:397-401.
31. Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol 2000;43:787-801.
32. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol 1994;101:262-263.
33. Gyetvai K, Hannah M E, Hodnett E D, et al. Tocolytics for preterm labor: a systematic review. Obstet Gynecol 1999;94:869-877.
34. King JF, Grant A, Keirse MJNC, et al. Beta-mimetics in preterm labor: an overview of the randomized controlled trials. Br J Obstet Gynaecol 1988;95:211-222.
35. Jeyabalan A, Caritis SN. Pharmacologic inhibition of preterm labor. Clin Obstset Gynecol 2002;45:99-113.
36. Macones GA, Berlin M, Berlin J. Efficacy of oral beta-ago-nist maintenance therapy in preterm labor: a meta-analysis. Obstet Gynecol 1995;85:313-317.
37. Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: A randomized clinical trial. Am J Obstet Gynecol 1997;177:814-818.
38. Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med 1999;341:660-666.
39. Bennett P, Edwards D. Use of magnesium sulphate in obstetrics. Lancet 1997;350:1491.-
40. Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
41. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet 1997;350:1517-1518.
42. Crowther CA, Hiller JE, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth. A randomized controlled trial. JAMA 2003;290:2669-2676.
43. Hammerman C, Glaser J, Kaplan M, et al. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics 1998;102:E56.-
44. King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes (Cochrane Review).In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software.
45. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002;51(RR-11):1-22.Available at:www.cdc.gov/mmwr/PDF/RR/RR5111.pdf. Accessed January 21, 2005.
46. Crowley P. Prophylactic corticosteroids for preterm birth (Cochrane Review).In: The Cochrane Library, Issue 4, 2003. Oxford: Update Software.
Obesity in pregnancy: Risks and interventions by gestational stage
- All obese patients have an increased risk of gestational diabetes and preeclampsia.
- Deep venous thrombosis and its complications—which include maternal mortality—are seen more frequently in the obese patient.
- Obesity is associated with an increased likelihood of induction of labor and cesarean delivery.
- Obesity is a specific risk factor for several operative complications, including hemorrhage during surgery, postoperative wound infections, aspiration, and pulmonary embolism.
Since one third of American women of childbearing age are overweight, obesity clearly has a major impact on the health of pregnant patients. And, as in the general population, the prevalence of this condition is escalating among gravidas. A 2001 study cited a 20% increase in mean maternal weights between 1980 and 1999.1
In the United States, the prevalence of obesity leaped from 12% to 17.9% between 1991 and 1998.2 Even more alarmingly, each year in this country, 280,000 adult deaths are attributable to obesity.
As health-care providers, it is imperative that we understand the impact this epidemic has on pregnancy and delivery so that we can work to minimize related complications.
What is obesity?
There is no single definition of obesity. In obstetric literature, it has been defined as a maternal weight of more than 90 kg (200 lb), more than 114 kg (250 lb), more than 135 kg (300 lb), and anywhere from 50% to 120% above ideal body weight.
In recent years, clinicians have usually determined obesity according to the body mass index (BMI), a simple mathematical formula (weight in kilograms divided by height in square meters) that correlates height and weight with body fat. This method offers several advantages over a basic weight measurement. For one, weight alone does not correlate well with body fat content; BMI, on the other hand, has a 0.7 to 0.8 correlation. In addition, this definition of obesity correlates with morbidity and mortality.3
Using BMI, the Institute of Medicine developed 4 body-type categories4:
- under 19.8: lean
- 19.8 to 26.0: normal
- 26.1 to 29: overweight
- over 29: obese
- lean women: 28 lb to 40 lb
- normal-weight women: 25 lb to 35 lb
- overweight women: 15 lb to 25 lb
- obese women: 15 lb or more
Preconception: Control hypertension and diabetes
The negative impact that excess weight has on pregnancy begins even before conception (TABLE 1). For example, obese women are more likely to have chronic hypertension and diabetes. In 1 study, researchers reported the incidence of chronic hypertension among obese patients (defined as those weighing 300 lb or more) to be 33%, compared with 5% among controls, while diabetes occurred in 15% of obese patients and 3% of controls.9
Through preconception counseling and management, practitioners can improve pregnancy outcomes among patients with these medical complications. Strict glucose control of pregestational diabetes, for example, decreases the risk of congenital malformations. The 4-fold increase in malformations related to poor glucose control during embryogenesis is diminished if preconceptional glycosylated hemoglobin levels are in the normal range.10
Note that hypertension may be falsely diagnosed in an obese woman if an inappropriately small cuff is used. When taking the blood pressure (BP) of these patients, therefore, clinicians should make sure the length of the cuff is 1.5 times the upper arm circumference or that the inflatable bladder of the cuff encircles at least 80% of the arm.11 For women with an arm circumference of more than 41 cm, use a thigh cuff to ensure an accurate measurement.
In general, any hypertensive woman of childbearing age should take only agents with documented fetal safety. Drugs such as angiotensin-converting enzyme inhibitors should not be used due to their association with oligohydramnios, fetal hypocalvaria, and neonatal renal failure.
TABLE 1
Obstetric concerns among obese patients
| Preconception | Pregestational diabetes mellitus |
| Chronic hypertension | |
| Antepartum period | Gestational diabetes |
| Preeclampsia | |
| Deep venous thrombosis | |
| Stillbirth | |
| Intrapartum period | Induction |
| Cesarean delivery | |
| Poor VBAC success | |
| Macrosomia | |
| Postpartum period | Prolonged hospitalization |
| Cesarean complications | |
| Wound infection | |
| VBAC = vaginal birth after cesarean | |
Antepartum
Gestational diabetes and preeclampsia. During pregnancy, all obese patients—even those without a history of hypertension or diabetes—have an increased risk of gestational diabetes and preeclampsia. Baeten et al12 recently reported the odds ratios for gestational diabetes, preeclampsia, and eclampsia in the obese nulliparous patient as 5.2, 3.3, and 3.0, respectively.
What are the reasons for this? For one, obesity and pregnancy are both associated with increased insulin resistance. The combination of these 2 conditions can overwhelm the pancreas and unmask any small abnormality in its ability to secrete insulin.
The pathophysiology of preeclampsia is less clearly understood and, therefore, so is its link with obesity. However, Stone et al13 theorized that the relationship between obesity and hyperlipidemia is what leads to preeclampsia. Hyperlipidemia damages endothelial cells through lipid peroxidases. This damage leads to increased vasoconstriction and platelet aggregation.
The obese gravida should undergo early glucose screening along with regular blood pressure measurements.
For the obese patient, clinicians should place increased emphasis on preeclampsia and gestational diabetes screening and prevention. The obese gravida should undergo early glucose screening along with regular BP measurements. Several studies have investigated possible interventions for women at high risk for pregnancy-induced hypertension. In 1 systematic review of 41 randomized controlled trials, aspirin was associated with a 15% reduction in the relative risk of preeclampsia (95% confidence interval, 0.78 to 0.92), with no increase in adverse outcomes.14 Another systematic review found that calcium supplementation (at least 1 g per day) can reduce the risk of preeclampsia by 30%.15 Still, no trials have examined aspirin or calcium supplementation among obese patients; the clinician must therefore weigh the benefits of these prophylactic measures.
Deep venous thrombosis. Along with preeclampsia and gestational diabetes, deep venous thrombosis and its complications—which include maternal mortality—are seen more frequently in the obese patient. One 10-year review in Minnesota looked at weight distributions for mothers who died. Researchers found that 12% of this population, compared with 2% of the control group, had prepregnancy weights greater than 200 lb.16 The leading cause of death among the obese group was pulmonary embolus.
Fetal death. A large, population-based cohort study reported a relationship between maternal obesity and fetal death.17 Among nulliparous women in this study, the risk of late fetal death (stillbirth occurring at 28 weeks’ gestation or later) increased as the BMI rose. The obese woman was 4 times as likely to have a late fetal death as the lean woman. In parous women, the risk was only increased in the obese BMI category—rather than in all classifications of BMI. After excluding women with hypertensive diseases and diabetes, the association persisted. Huang et al18 supported these findings by identifying maternal prepregnancy weight greater than 68 kg as a risk factor for unexplained fetal deaths, even after controlling for maternal diabetes and hypertensive disease.
Intrapartum
Labor induction. Obesity is associated with an increased likelihood of labor induction. Gross et al19 reported that 15% of obese women (over 90 kg) had labor induced, compared with 8% of controls (P.0001 ekblad and grenman>20 also showed a significantly higher induction rate in obese patients and those with excessive weight gain during pregnancy.
Cesarean delivery. The effect of obesity on cesarean delivery rates has been debated, but most studies indicate a direct correlation (TABLE 2). Kaiser and Kirby21 showed that even among low-risk patients in a nurse-midwifery service, a BMI above 29 was associated with a 3-fold to 4-fold increase in cesarean delivery. A study by Cnattingius et al17 demonstrated that the effect of BMI on cesarean rates also was influenced by maternal height: Short obese women had the highest cesarean rate (36%), followed by (in decreasing order) short, lean women; tall, obese women; and, finally, tall, lean women.
VBAC. These findings raise a natural follow-up question: What is the success rate of vaginal birth after cesarean (VBAC) among obese parturients? Among 30 women weighing more than 300 lb at conception, Chauhan et al22 noted a VBAC success rate of less than 15%.This is much lower than the general success rate of 60% to 80% quoted in the ACOG practice bulletin on VBAC.23 Grobman et al24 reported that VBAC is cost-effective among women with 1 prior cesarean delivery only if the success rate is above 40%; it is therefore worth pondering whether VBAC should be attempted in overweight patients.
TABLE 2
Obesity and cesarean delivery rates
| AUTHORS | NUMBER OF SUBJECTS | OBESITY DEFINED AS | RATE OF CESAREAN DELIVERY | COMMENTS |
|---|---|---|---|---|
| Baeten et al, 200112 | 9,817 | BMI≥30 | Increased | — |
| Kaiser and Kirby, 200121 | 452 | BMI≥29 | Increased* | Population was low risk without prior cesarean. |
| Kumari, 200137 | 188 | BMI >40 | Increased* | Elective* and emergency cesareans examined. |
| Steinfeld et al, 200038 | 168 | BMI≥29 | Increased* | Excluded elective cesareans and those performed due to fetal malpresentation and previa. |
| Jensen et al, 199939 | 163 | BMI≥30 | Increased | Excluded patients with prior cesarean. |
| Ranta et al, 199540 | 53 | BMI≥30 | Increased | — |
| Issacs et al, 19949 | 117 | >300 lb | Increased* | Primary and repeat cesareans examined. |
| Hood and Dewan, 199325 | 117 | >300 lb | Increased* | Elective and emergency* cesareans examined. |
| Ekblad and Grenman, 199220 | 77 | ≥20%† | Increased | Emergency cesareans examined. |
| Perlow et al, 199235 | 111 | >300 lb | Increased* | Primary* cesareans and those performed due to fetal distress examined. |
| Pongthai, 199041 | 741 | ≥80 kg | Increased* | Primary and repeat* cesareans examined. |
| Johnson et al, 198730 | 588 | >113.6 kg | Increased* | Primary cesareans examined only. |
| Garbaciak et al, 198536 | 1,889 | >120%† | Increased* | Primary cesareans examined only. |
| Gross et al, 198019 | 279 | ≥90 kg | Increased | Repeat cesareans omitted. |
| Edwards et al, 197827 | 208 | >50%‡ | Increased | — |
| BMI = body mass index | ||||
| *Significant increase | ||||
| †Over ideal body weight for height | ||||
| ‡Above standard weight for height on the Metropolitan Life Insurance tables | ||||
Postpartum: Longer hospitalization
Although they did not provide the reasons, Hood and Dewan25 linked obesity with prolonged postpartum hospitalization. They found obese patients to have significantly longer hospital stays, regardless of the type of delivery:
- Following vaginal delivery, postpartum hospitalization was 3.8±2.4 days among overweight patients and 2.9±2.1 days among controls.
- After cesarean delivery, obese patients were in the hospital for 7.3±5.0 days; nonobese, for 5.4±3.1 days.
Cesarean complications
Obesity is a specific risk factor for several operative complications, including hemorrhage during surgery, postoperative wound infections, aspiration, and pulmonary embolism. A case-control study by Naef et al26 revealed that a weight of more than 250 lb has an odds ratio of 13.1 (95% confidence interval, 1.7 to 102.7) for hemorrhage (decrease in hematocrit of 10% or greater, estimated blood loss greater than 1,500 mL, or packed red blood cell administration) during abdominal delivery.
Multiple studies have shown obesity to be a risk factor for postoperative wound infections.27-30 For example, Johnson et al30 reported a wound infection rate of 37.6% for the obese parturient and 10.2% for those of normal weight (P.001>
The link between excess weight and infectious morbidity may be secondary to the increased subcutaneous tissue layer and accumulation of loculated fluid. In 2000, Vermillion et al31 published a study that looked at 140 women who had cesarean deliveries. Initially, a univariate analysis identified the risk factors for wound infection as maternal weight (a mean of 82.8 kg±18.6 kg in the uninfected population versus 99.4 kg±33.3 kg in the infected population), BMI (44.5±2.1 for uninfected versus 49.7±6.3 for infected), and thickness of subcutaneous tissue (2.3 cm±1.2 cm for uninfected versus 4.1 cm±1.8 cm for infected). After a multiple logistic regression analysis, however, subcutaneous tissue thickness was the only significant risk factor confirmed. A potential explanation for this finding is that the blood supply to subcutaneous fat is relatively poor.
Reducing infection. By modifying surgical techniques, physicians may be able to decrease the rate of wound infection among overweight parturients. Naumann et al32 randomized closure versus nonclosure of the subcutaneous tissue in 245 patients with at least 2 cm of adipose tissue. There was a significant difference in the incidence of overall wound disruptions (14.5% versus 26.6%)—specifically, seroma formation (5.1% versus 17.2%)—between the closure and nonclosure groups, respectively, but no significant difference in wound infections (6% versus 7.8%).
There is no consistent evidence that obesity alone is associated with poor perinatal outcome.
Allaire et al33 showed that the use of a subcutaneous drain or subcutaneous suture decreased the rates of wound infection or separation among obese women undergoing cesarean delivery. The incidence dropped from 30.8% when neither was used to 15.4% with suture and 4.2% with a drain.
While several investigators have noted the increased rate of postoperative complications among obese parturients, few have systematically analyzed their etiology. Wolfe et al34 reviewed the antepartum and intrapartum variables among 107 consecutive obese parturients (all at least 200 lb) who had cesarean deliveries. Using multivariate analysis, the investigators noted that various degrees of obesity, preexisting medical conditions, the type of skin incision, and the type of anesthesia were not risk factors for postpartum infectious sequelae. Only 2 factors—both of which were under the control of physicians—contributed to morbidity: duration of cesarean delivery and operative blood loss. According to their regression equation, if surgical time was decreased from 1.5 hours to 1 hour, the postoperative stay would decrease by 1 day. These authors did not comment on the estimated blood loss or drop in hematocrit threshold that would minimize postoperative complications.
What about the neonate?
Interestingly, there is no consistent evidence that obesity alone is associated with poor perinatal outcome. A case-control study by Perlow et al35 reported the outcomes of 111 neonates born to obese mothers. These infants were more likely to weigh less than 2,500 g or more than 4,000 g, to have intrauterine growth restriction, and to require admission to a neonatal intensive care unit. However, when patients with prepregnancy diagnoses of chronic hypertension or insulin-requiring diabetes mellitus were excluded, perinatal outcome was similar for obese and nonobese mothers. Garbaciak et al36 reported similar results: They showed that only obese patients with antepartum complications had an increase in perinatal mortality. Two other studies showed no increase in perinatal morbidity or mortality among obese subjects.19,27 It seems, therefore, that the risk for adverse perinatal outcomes may be related to underlying medical diseases rather than excessive weight.
Research has also linked infant birth weight to maternal weight. Studies have shown the incidence of macrosomic infants (birth weight of at least 4,000 g) to be higher in obese women, even in the absence of antenatal complications.19,25,36 Specifically, Gross et al19 concluded that the increase in macrosomic and large-for-gestational-age infants (defined as over 90% of weight for gestational age) born to obese mothers cannot be explained by the presence of maternal diabetes. They noted that the frequency of macrosomia was 15.1% and large-for-gestational-age was 31% among obese patients, while the incidence of diabetes mellitus was only 9%. However, Perlow et al35 demonstrated that the increased rate of macrosomia disappeared if patients with preexisting medical conditions were excluded.
Studies also have shown that newborns of obese patients have weight problems as they age. Edwards et al27 noted that at 1 year, infants of obese mothers were significantly more overweight than those of controls. Specifically, 47% of the infants of obese mothers were above the 75th percentile for weight-length, compared to 22% in the control group.
Counsel weight reduction
Obesity is a major health risk for both the general and obstetric populations. Fortunately, this risk can be addressed through lifestyle modification. Though we lack studies demonstrating improved peripartum outcomes with weight reduction, there is no reason to doubt that weight loss will decrease the rate of adverse events. Ob/Gyns caring for obese patients should inform these women of the unfavorable pregnancy outcomes secondary to excessive weight and encourage preconception weight control.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Lu GC, Rouse DJ, DeBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Obstet Gynecol. 2001;185:845-849.
2. Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States 1991-1998. JAMA. 1999;282:1519-1522.
3. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, Md: National Heart, Lung, and Blood Institute; June 1998;1-226.
4. American College of Obstetricians and Gynecologists. ACOG Education Bulletin #229: nutrition and women. Washington, DC: ACOG; 1996.
5. Institute of Medicine subcommittee on nutritional status and weight gain during pregnancy. Nutrition During Pregnancy. Washington, DC: National Academy Press; 1990.
6. Cogswell ME, Serdula MK, Hungerford DW, Yip R. Gestational weight gain among average-weight and overweight women—what is excessive? Am J Obstet Gynecol. 1995;172:705-712.
7. Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal weight women: effects of gestational weight change. Obstet Gynecol. 1996;87:389-394.
8. Parker JD, Abrams B. Prenatal weight gain advice: an examination of the recent prenatal weight gain recommendations of the Institute of Medicine. Obstet Gynecol. 1992;79:664-669.
9. Isaacs JD, Magann EF, Martin RW, Chauhan SP, Morrison JC. Obstetric challenges of massive obesity complicating pregnancy. J Perinatol. 1994;14:10-14.
10. American College of Obstetricians and Gynecologists. ACOG Technical Bulletin #200: diabetes and pregnancy. Washington, DC: ACOG; 2000.
11. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
12. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91:436-440.
13. Stone JL, Lockwood CJ, Berkowitz GS, Alvarez M, Lapinski R, Berkowitz RL. Risk factors for preeclampsia. Obstet Gynecol. 1994;83:357-361.
14. Knight M, Duley L, Henderson-Smart D, et al. The effectiveness and safety of antiplatelet agents for the prevention and treatment of preeclampsia. In: The Cochrane Library, Issue 4, 2000. Oxford: Update Software. Search date 1999. Primary sources: Cochrane Pregnancy and Childbirth Group Trials Register, conference proceedings.
15. Atallah AN, Hofmeyr GJ, Duley L. Calcium supplementation during pregnancy to prevent hypertensive disorders and related adverse outcomes. In: The Cochrane Library, Issue 4, 2000. Oxford: Updated Software. Search date 2000. Primary sources: Cochrane Pregnancy and Childbirth Group Trials Register.
16. Maeder EC, Barno A, Mecklenburg F. Obesity: a maternal high-risk factor. Obstet Gynecol. 1975;45:669-671.
17. Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147-152.
18. Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts RC. Determinants of unexplained antepartum fetal death. Obstet Gynecol. 2000;95:215-221.
19. Gross T, Sokol RJ, King KC. Obesity in pregnancy: risks and outcome. Obstet Gynecol. 1980;56:446-450.
20. Ekblad U, Grenman S. Maternal weight, weight gain during pregnancy and pregnancy outcome. Int J Gynecol Obstet. 1992;39:277-283.
21. Kaiser PS, Kirby RS. Obesity as a risk factor for cesarean in a low risk population. Obstet Gynecol. 2001;97:39-43.
22. Chauhan SP, Magann EF, Carroll CS, Barrilleaux PS, Scardo JA, Martin JN. Mode of delivery for the morbidly obese with prior cesarean delivery: vaginal versus repeat cesarean section. Am J Obstet Gynecol. 2001;185:349-354.
23. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #5: vaginal birth after previous cesarean delivery. Washington, DC: ACOG; 2000.
24. Grobman WA, Peaceman AM, Socol ML. Cost-effectiveness of elective cesarean delivery after one prior low transverse cesarean. Obstet Gynecol. 2000;95:745-751.
25. Hood DD, Dewan DM. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210-1218.
26. Naef RW, Chauhan SP, Chevalier SP, Roberts WE, Meydrech EF, Morrison JC. Prediction of hemorrhage at cesarean delivery. Obstet Gynecol. 1994;83:923-926.
27. Edwards LE, Dickes WF, Alton IR, Hakanson EY. Pregnancy in the massively obese: course, outcome, and obesity prognosis of the infant. Am J Obstet Gynecol. 1978;131:479-483.
28. Pelle H, Jepsen OB, Larsen SO, et al. Wound infection after cesarean section. Infect Control. 1986;7:456-461.
29. Nielsen TF, Hokegard K. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
30. Johnson SR, Kolberg BH, Varner MW, Railsback LD. Maternal obesity and pregnancy. Surg Gynecol Obstet. 1987;164:431-437.
31. Vermillion ST, Lamoutte C, Soper DE, Verdeja A. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
32. Naumann RW, Hauth JC, Owen J, Hodgkins PM, Lincoln T. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
33. Allaire AD, Fisch J, McMahon MJ. Subcutaneous drain vs suture in obese women undergoing cesarean delivery. J Reprod Med. 2000;45:327-331.
34. Wolfe HM, Gross TL, Sokol RJ, Bottoms SF, Thompson KL. Determinants of morbidity in obese women delivered by cesarean. Obstet Gynecol. 1998;71:691-696.
35. Perlow JH, Morgan MA, Montgomery D, Towers CV, Porto M. Perinatal outcome in pregnancy complicated by massive obesity. Am J Obstet Gynecol. 1992;167:958-962.
36. Garbaciak JA, Jr, Richter M, Miller S, Barton JJ. Maternal weight and pregnancy complications. Obstet Gynecol. 1985;152:238-245.
37. Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynecol Obstet. 2001;73:101-107.
38. Steinfeld JD, Valentine S, Lerer T, Ingardia CJ, Wax JR, Curry SL. Obesity-related complications of pregnancy vary by race. J Matern Fetal Med. 2000;9:238-241.
39. Jensen H, Agger AO, Rasmussen KL. The influence of prepregnancy body mass index on labor complications. Acta Obstet Gynecol Scand. 1999;78:799-802.
40. Ranta P, Jouppila P, Spalding M, Jouppila R. The effect of maternal obesity on labour and labour pain. Anaesthesia. 1995;50:322-326.
41. Pongthai S. Labour and delivery of obese parturients. J Med Assoc Thai. 1990;73:52-56.
- All obese patients have an increased risk of gestational diabetes and preeclampsia.
- Deep venous thrombosis and its complications—which include maternal mortality—are seen more frequently in the obese patient.
- Obesity is associated with an increased likelihood of induction of labor and cesarean delivery.
- Obesity is a specific risk factor for several operative complications, including hemorrhage during surgery, postoperative wound infections, aspiration, and pulmonary embolism.
Since one third of American women of childbearing age are overweight, obesity clearly has a major impact on the health of pregnant patients. And, as in the general population, the prevalence of this condition is escalating among gravidas. A 2001 study cited a 20% increase in mean maternal weights between 1980 and 1999.1
In the United States, the prevalence of obesity leaped from 12% to 17.9% between 1991 and 1998.2 Even more alarmingly, each year in this country, 280,000 adult deaths are attributable to obesity.
As health-care providers, it is imperative that we understand the impact this epidemic has on pregnancy and delivery so that we can work to minimize related complications.
What is obesity?
There is no single definition of obesity. In obstetric literature, it has been defined as a maternal weight of more than 90 kg (200 lb), more than 114 kg (250 lb), more than 135 kg (300 lb), and anywhere from 50% to 120% above ideal body weight.
In recent years, clinicians have usually determined obesity according to the body mass index (BMI), a simple mathematical formula (weight in kilograms divided by height in square meters) that correlates height and weight with body fat. This method offers several advantages over a basic weight measurement. For one, weight alone does not correlate well with body fat content; BMI, on the other hand, has a 0.7 to 0.8 correlation. In addition, this definition of obesity correlates with morbidity and mortality.3
Using BMI, the Institute of Medicine developed 4 body-type categories4:
- under 19.8: lean
- 19.8 to 26.0: normal
- 26.1 to 29: overweight
- over 29: obese
- lean women: 28 lb to 40 lb
- normal-weight women: 25 lb to 35 lb
- overweight women: 15 lb to 25 lb
- obese women: 15 lb or more
Preconception: Control hypertension and diabetes
The negative impact that excess weight has on pregnancy begins even before conception (TABLE 1). For example, obese women are more likely to have chronic hypertension and diabetes. In 1 study, researchers reported the incidence of chronic hypertension among obese patients (defined as those weighing 300 lb or more) to be 33%, compared with 5% among controls, while diabetes occurred in 15% of obese patients and 3% of controls.9
Through preconception counseling and management, practitioners can improve pregnancy outcomes among patients with these medical complications. Strict glucose control of pregestational diabetes, for example, decreases the risk of congenital malformations. The 4-fold increase in malformations related to poor glucose control during embryogenesis is diminished if preconceptional glycosylated hemoglobin levels are in the normal range.10
Note that hypertension may be falsely diagnosed in an obese woman if an inappropriately small cuff is used. When taking the blood pressure (BP) of these patients, therefore, clinicians should make sure the length of the cuff is 1.5 times the upper arm circumference or that the inflatable bladder of the cuff encircles at least 80% of the arm.11 For women with an arm circumference of more than 41 cm, use a thigh cuff to ensure an accurate measurement.
In general, any hypertensive woman of childbearing age should take only agents with documented fetal safety. Drugs such as angiotensin-converting enzyme inhibitors should not be used due to their association with oligohydramnios, fetal hypocalvaria, and neonatal renal failure.
TABLE 1
Obstetric concerns among obese patients
| Preconception | Pregestational diabetes mellitus |
| Chronic hypertension | |
| Antepartum period | Gestational diabetes |
| Preeclampsia | |
| Deep venous thrombosis | |
| Stillbirth | |
| Intrapartum period | Induction |
| Cesarean delivery | |
| Poor VBAC success | |
| Macrosomia | |
| Postpartum period | Prolonged hospitalization |
| Cesarean complications | |
| Wound infection | |
| VBAC = vaginal birth after cesarean | |
Antepartum
Gestational diabetes and preeclampsia. During pregnancy, all obese patients—even those without a history of hypertension or diabetes—have an increased risk of gestational diabetes and preeclampsia. Baeten et al12 recently reported the odds ratios for gestational diabetes, preeclampsia, and eclampsia in the obese nulliparous patient as 5.2, 3.3, and 3.0, respectively.
What are the reasons for this? For one, obesity and pregnancy are both associated with increased insulin resistance. The combination of these 2 conditions can overwhelm the pancreas and unmask any small abnormality in its ability to secrete insulin.
The pathophysiology of preeclampsia is less clearly understood and, therefore, so is its link with obesity. However, Stone et al13 theorized that the relationship between obesity and hyperlipidemia is what leads to preeclampsia. Hyperlipidemia damages endothelial cells through lipid peroxidases. This damage leads to increased vasoconstriction and platelet aggregation.
The obese gravida should undergo early glucose screening along with regular blood pressure measurements.
For the obese patient, clinicians should place increased emphasis on preeclampsia and gestational diabetes screening and prevention. The obese gravida should undergo early glucose screening along with regular BP measurements. Several studies have investigated possible interventions for women at high risk for pregnancy-induced hypertension. In 1 systematic review of 41 randomized controlled trials, aspirin was associated with a 15% reduction in the relative risk of preeclampsia (95% confidence interval, 0.78 to 0.92), with no increase in adverse outcomes.14 Another systematic review found that calcium supplementation (at least 1 g per day) can reduce the risk of preeclampsia by 30%.15 Still, no trials have examined aspirin or calcium supplementation among obese patients; the clinician must therefore weigh the benefits of these prophylactic measures.
Deep venous thrombosis. Along with preeclampsia and gestational diabetes, deep venous thrombosis and its complications—which include maternal mortality—are seen more frequently in the obese patient. One 10-year review in Minnesota looked at weight distributions for mothers who died. Researchers found that 12% of this population, compared with 2% of the control group, had prepregnancy weights greater than 200 lb.16 The leading cause of death among the obese group was pulmonary embolus.
Fetal death. A large, population-based cohort study reported a relationship between maternal obesity and fetal death.17 Among nulliparous women in this study, the risk of late fetal death (stillbirth occurring at 28 weeks’ gestation or later) increased as the BMI rose. The obese woman was 4 times as likely to have a late fetal death as the lean woman. In parous women, the risk was only increased in the obese BMI category—rather than in all classifications of BMI. After excluding women with hypertensive diseases and diabetes, the association persisted. Huang et al18 supported these findings by identifying maternal prepregnancy weight greater than 68 kg as a risk factor for unexplained fetal deaths, even after controlling for maternal diabetes and hypertensive disease.
Intrapartum
Labor induction. Obesity is associated with an increased likelihood of labor induction. Gross et al19 reported that 15% of obese women (over 90 kg) had labor induced, compared with 8% of controls (P.0001 ekblad and grenman>20 also showed a significantly higher induction rate in obese patients and those with excessive weight gain during pregnancy.
Cesarean delivery. The effect of obesity on cesarean delivery rates has been debated, but most studies indicate a direct correlation (TABLE 2). Kaiser and Kirby21 showed that even among low-risk patients in a nurse-midwifery service, a BMI above 29 was associated with a 3-fold to 4-fold increase in cesarean delivery. A study by Cnattingius et al17 demonstrated that the effect of BMI on cesarean rates also was influenced by maternal height: Short obese women had the highest cesarean rate (36%), followed by (in decreasing order) short, lean women; tall, obese women; and, finally, tall, lean women.
VBAC. These findings raise a natural follow-up question: What is the success rate of vaginal birth after cesarean (VBAC) among obese parturients? Among 30 women weighing more than 300 lb at conception, Chauhan et al22 noted a VBAC success rate of less than 15%.This is much lower than the general success rate of 60% to 80% quoted in the ACOG practice bulletin on VBAC.23 Grobman et al24 reported that VBAC is cost-effective among women with 1 prior cesarean delivery only if the success rate is above 40%; it is therefore worth pondering whether VBAC should be attempted in overweight patients.
TABLE 2
Obesity and cesarean delivery rates
| AUTHORS | NUMBER OF SUBJECTS | OBESITY DEFINED AS | RATE OF CESAREAN DELIVERY | COMMENTS |
|---|---|---|---|---|
| Baeten et al, 200112 | 9,817 | BMI≥30 | Increased | — |
| Kaiser and Kirby, 200121 | 452 | BMI≥29 | Increased* | Population was low risk without prior cesarean. |
| Kumari, 200137 | 188 | BMI >40 | Increased* | Elective* and emergency cesareans examined. |
| Steinfeld et al, 200038 | 168 | BMI≥29 | Increased* | Excluded elective cesareans and those performed due to fetal malpresentation and previa. |
| Jensen et al, 199939 | 163 | BMI≥30 | Increased | Excluded patients with prior cesarean. |
| Ranta et al, 199540 | 53 | BMI≥30 | Increased | — |
| Issacs et al, 19949 | 117 | >300 lb | Increased* | Primary and repeat cesareans examined. |
| Hood and Dewan, 199325 | 117 | >300 lb | Increased* | Elective and emergency* cesareans examined. |
| Ekblad and Grenman, 199220 | 77 | ≥20%† | Increased | Emergency cesareans examined. |
| Perlow et al, 199235 | 111 | >300 lb | Increased* | Primary* cesareans and those performed due to fetal distress examined. |
| Pongthai, 199041 | 741 | ≥80 kg | Increased* | Primary and repeat* cesareans examined. |
| Johnson et al, 198730 | 588 | >113.6 kg | Increased* | Primary cesareans examined only. |
| Garbaciak et al, 198536 | 1,889 | >120%† | Increased* | Primary cesareans examined only. |
| Gross et al, 198019 | 279 | ≥90 kg | Increased | Repeat cesareans omitted. |
| Edwards et al, 197827 | 208 | >50%‡ | Increased | — |
| BMI = body mass index | ||||
| *Significant increase | ||||
| †Over ideal body weight for height | ||||
| ‡Above standard weight for height on the Metropolitan Life Insurance tables | ||||
Postpartum: Longer hospitalization
Although they did not provide the reasons, Hood and Dewan25 linked obesity with prolonged postpartum hospitalization. They found obese patients to have significantly longer hospital stays, regardless of the type of delivery:
- Following vaginal delivery, postpartum hospitalization was 3.8±2.4 days among overweight patients and 2.9±2.1 days among controls.
- After cesarean delivery, obese patients were in the hospital for 7.3±5.0 days; nonobese, for 5.4±3.1 days.
Cesarean complications
Obesity is a specific risk factor for several operative complications, including hemorrhage during surgery, postoperative wound infections, aspiration, and pulmonary embolism. A case-control study by Naef et al26 revealed that a weight of more than 250 lb has an odds ratio of 13.1 (95% confidence interval, 1.7 to 102.7) for hemorrhage (decrease in hematocrit of 10% or greater, estimated blood loss greater than 1,500 mL, or packed red blood cell administration) during abdominal delivery.
Multiple studies have shown obesity to be a risk factor for postoperative wound infections.27-30 For example, Johnson et al30 reported a wound infection rate of 37.6% for the obese parturient and 10.2% for those of normal weight (P.001>
The link between excess weight and infectious morbidity may be secondary to the increased subcutaneous tissue layer and accumulation of loculated fluid. In 2000, Vermillion et al31 published a study that looked at 140 women who had cesarean deliveries. Initially, a univariate analysis identified the risk factors for wound infection as maternal weight (a mean of 82.8 kg±18.6 kg in the uninfected population versus 99.4 kg±33.3 kg in the infected population), BMI (44.5±2.1 for uninfected versus 49.7±6.3 for infected), and thickness of subcutaneous tissue (2.3 cm±1.2 cm for uninfected versus 4.1 cm±1.8 cm for infected). After a multiple logistic regression analysis, however, subcutaneous tissue thickness was the only significant risk factor confirmed. A potential explanation for this finding is that the blood supply to subcutaneous fat is relatively poor.
Reducing infection. By modifying surgical techniques, physicians may be able to decrease the rate of wound infection among overweight parturients. Naumann et al32 randomized closure versus nonclosure of the subcutaneous tissue in 245 patients with at least 2 cm of adipose tissue. There was a significant difference in the incidence of overall wound disruptions (14.5% versus 26.6%)—specifically, seroma formation (5.1% versus 17.2%)—between the closure and nonclosure groups, respectively, but no significant difference in wound infections (6% versus 7.8%).
There is no consistent evidence that obesity alone is associated with poor perinatal outcome.
Allaire et al33 showed that the use of a subcutaneous drain or subcutaneous suture decreased the rates of wound infection or separation among obese women undergoing cesarean delivery. The incidence dropped from 30.8% when neither was used to 15.4% with suture and 4.2% with a drain.
While several investigators have noted the increased rate of postoperative complications among obese parturients, few have systematically analyzed their etiology. Wolfe et al34 reviewed the antepartum and intrapartum variables among 107 consecutive obese parturients (all at least 200 lb) who had cesarean deliveries. Using multivariate analysis, the investigators noted that various degrees of obesity, preexisting medical conditions, the type of skin incision, and the type of anesthesia were not risk factors for postpartum infectious sequelae. Only 2 factors—both of which were under the control of physicians—contributed to morbidity: duration of cesarean delivery and operative blood loss. According to their regression equation, if surgical time was decreased from 1.5 hours to 1 hour, the postoperative stay would decrease by 1 day. These authors did not comment on the estimated blood loss or drop in hematocrit threshold that would minimize postoperative complications.
What about the neonate?
Interestingly, there is no consistent evidence that obesity alone is associated with poor perinatal outcome. A case-control study by Perlow et al35 reported the outcomes of 111 neonates born to obese mothers. These infants were more likely to weigh less than 2,500 g or more than 4,000 g, to have intrauterine growth restriction, and to require admission to a neonatal intensive care unit. However, when patients with prepregnancy diagnoses of chronic hypertension or insulin-requiring diabetes mellitus were excluded, perinatal outcome was similar for obese and nonobese mothers. Garbaciak et al36 reported similar results: They showed that only obese patients with antepartum complications had an increase in perinatal mortality. Two other studies showed no increase in perinatal morbidity or mortality among obese subjects.19,27 It seems, therefore, that the risk for adverse perinatal outcomes may be related to underlying medical diseases rather than excessive weight.
Research has also linked infant birth weight to maternal weight. Studies have shown the incidence of macrosomic infants (birth weight of at least 4,000 g) to be higher in obese women, even in the absence of antenatal complications.19,25,36 Specifically, Gross et al19 concluded that the increase in macrosomic and large-for-gestational-age infants (defined as over 90% of weight for gestational age) born to obese mothers cannot be explained by the presence of maternal diabetes. They noted that the frequency of macrosomia was 15.1% and large-for-gestational-age was 31% among obese patients, while the incidence of diabetes mellitus was only 9%. However, Perlow et al35 demonstrated that the increased rate of macrosomia disappeared if patients with preexisting medical conditions were excluded.
Studies also have shown that newborns of obese patients have weight problems as they age. Edwards et al27 noted that at 1 year, infants of obese mothers were significantly more overweight than those of controls. Specifically, 47% of the infants of obese mothers were above the 75th percentile for weight-length, compared to 22% in the control group.
Counsel weight reduction
Obesity is a major health risk for both the general and obstetric populations. Fortunately, this risk can be addressed through lifestyle modification. Though we lack studies demonstrating improved peripartum outcomes with weight reduction, there is no reason to doubt that weight loss will decrease the rate of adverse events. Ob/Gyns caring for obese patients should inform these women of the unfavorable pregnancy outcomes secondary to excessive weight and encourage preconception weight control.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- All obese patients have an increased risk of gestational diabetes and preeclampsia.
- Deep venous thrombosis and its complications—which include maternal mortality—are seen more frequently in the obese patient.
- Obesity is associated with an increased likelihood of induction of labor and cesarean delivery.
- Obesity is a specific risk factor for several operative complications, including hemorrhage during surgery, postoperative wound infections, aspiration, and pulmonary embolism.
Since one third of American women of childbearing age are overweight, obesity clearly has a major impact on the health of pregnant patients. And, as in the general population, the prevalence of this condition is escalating among gravidas. A 2001 study cited a 20% increase in mean maternal weights between 1980 and 1999.1
In the United States, the prevalence of obesity leaped from 12% to 17.9% between 1991 and 1998.2 Even more alarmingly, each year in this country, 280,000 adult deaths are attributable to obesity.
As health-care providers, it is imperative that we understand the impact this epidemic has on pregnancy and delivery so that we can work to minimize related complications.
What is obesity?
There is no single definition of obesity. In obstetric literature, it has been defined as a maternal weight of more than 90 kg (200 lb), more than 114 kg (250 lb), more than 135 kg (300 lb), and anywhere from 50% to 120% above ideal body weight.
In recent years, clinicians have usually determined obesity according to the body mass index (BMI), a simple mathematical formula (weight in kilograms divided by height in square meters) that correlates height and weight with body fat. This method offers several advantages over a basic weight measurement. For one, weight alone does not correlate well with body fat content; BMI, on the other hand, has a 0.7 to 0.8 correlation. In addition, this definition of obesity correlates with morbidity and mortality.3
Using BMI, the Institute of Medicine developed 4 body-type categories4:
- under 19.8: lean
- 19.8 to 26.0: normal
- 26.1 to 29: overweight
- over 29: obese
- lean women: 28 lb to 40 lb
- normal-weight women: 25 lb to 35 lb
- overweight women: 15 lb to 25 lb
- obese women: 15 lb or more
Preconception: Control hypertension and diabetes
The negative impact that excess weight has on pregnancy begins even before conception (TABLE 1). For example, obese women are more likely to have chronic hypertension and diabetes. In 1 study, researchers reported the incidence of chronic hypertension among obese patients (defined as those weighing 300 lb or more) to be 33%, compared with 5% among controls, while diabetes occurred in 15% of obese patients and 3% of controls.9
Through preconception counseling and management, practitioners can improve pregnancy outcomes among patients with these medical complications. Strict glucose control of pregestational diabetes, for example, decreases the risk of congenital malformations. The 4-fold increase in malformations related to poor glucose control during embryogenesis is diminished if preconceptional glycosylated hemoglobin levels are in the normal range.10
Note that hypertension may be falsely diagnosed in an obese woman if an inappropriately small cuff is used. When taking the blood pressure (BP) of these patients, therefore, clinicians should make sure the length of the cuff is 1.5 times the upper arm circumference or that the inflatable bladder of the cuff encircles at least 80% of the arm.11 For women with an arm circumference of more than 41 cm, use a thigh cuff to ensure an accurate measurement.
In general, any hypertensive woman of childbearing age should take only agents with documented fetal safety. Drugs such as angiotensin-converting enzyme inhibitors should not be used due to their association with oligohydramnios, fetal hypocalvaria, and neonatal renal failure.
TABLE 1
Obstetric concerns among obese patients
| Preconception | Pregestational diabetes mellitus |
| Chronic hypertension | |
| Antepartum period | Gestational diabetes |
| Preeclampsia | |
| Deep venous thrombosis | |
| Stillbirth | |
| Intrapartum period | Induction |
| Cesarean delivery | |
| Poor VBAC success | |
| Macrosomia | |
| Postpartum period | Prolonged hospitalization |
| Cesarean complications | |
| Wound infection | |
| VBAC = vaginal birth after cesarean | |
Antepartum
Gestational diabetes and preeclampsia. During pregnancy, all obese patients—even those without a history of hypertension or diabetes—have an increased risk of gestational diabetes and preeclampsia. Baeten et al12 recently reported the odds ratios for gestational diabetes, preeclampsia, and eclampsia in the obese nulliparous patient as 5.2, 3.3, and 3.0, respectively.
What are the reasons for this? For one, obesity and pregnancy are both associated with increased insulin resistance. The combination of these 2 conditions can overwhelm the pancreas and unmask any small abnormality in its ability to secrete insulin.
The pathophysiology of preeclampsia is less clearly understood and, therefore, so is its link with obesity. However, Stone et al13 theorized that the relationship between obesity and hyperlipidemia is what leads to preeclampsia. Hyperlipidemia damages endothelial cells through lipid peroxidases. This damage leads to increased vasoconstriction and platelet aggregation.
The obese gravida should undergo early glucose screening along with regular blood pressure measurements.
For the obese patient, clinicians should place increased emphasis on preeclampsia and gestational diabetes screening and prevention. The obese gravida should undergo early glucose screening along with regular BP measurements. Several studies have investigated possible interventions for women at high risk for pregnancy-induced hypertension. In 1 systematic review of 41 randomized controlled trials, aspirin was associated with a 15% reduction in the relative risk of preeclampsia (95% confidence interval, 0.78 to 0.92), with no increase in adverse outcomes.14 Another systematic review found that calcium supplementation (at least 1 g per day) can reduce the risk of preeclampsia by 30%.15 Still, no trials have examined aspirin or calcium supplementation among obese patients; the clinician must therefore weigh the benefits of these prophylactic measures.
Deep venous thrombosis. Along with preeclampsia and gestational diabetes, deep venous thrombosis and its complications—which include maternal mortality—are seen more frequently in the obese patient. One 10-year review in Minnesota looked at weight distributions for mothers who died. Researchers found that 12% of this population, compared with 2% of the control group, had prepregnancy weights greater than 200 lb.16 The leading cause of death among the obese group was pulmonary embolus.
Fetal death. A large, population-based cohort study reported a relationship between maternal obesity and fetal death.17 Among nulliparous women in this study, the risk of late fetal death (stillbirth occurring at 28 weeks’ gestation or later) increased as the BMI rose. The obese woman was 4 times as likely to have a late fetal death as the lean woman. In parous women, the risk was only increased in the obese BMI category—rather than in all classifications of BMI. After excluding women with hypertensive diseases and diabetes, the association persisted. Huang et al18 supported these findings by identifying maternal prepregnancy weight greater than 68 kg as a risk factor for unexplained fetal deaths, even after controlling for maternal diabetes and hypertensive disease.
Intrapartum
Labor induction. Obesity is associated with an increased likelihood of labor induction. Gross et al19 reported that 15% of obese women (over 90 kg) had labor induced, compared with 8% of controls (P.0001 ekblad and grenman>20 also showed a significantly higher induction rate in obese patients and those with excessive weight gain during pregnancy.
Cesarean delivery. The effect of obesity on cesarean delivery rates has been debated, but most studies indicate a direct correlation (TABLE 2). Kaiser and Kirby21 showed that even among low-risk patients in a nurse-midwifery service, a BMI above 29 was associated with a 3-fold to 4-fold increase in cesarean delivery. A study by Cnattingius et al17 demonstrated that the effect of BMI on cesarean rates also was influenced by maternal height: Short obese women had the highest cesarean rate (36%), followed by (in decreasing order) short, lean women; tall, obese women; and, finally, tall, lean women.
VBAC. These findings raise a natural follow-up question: What is the success rate of vaginal birth after cesarean (VBAC) among obese parturients? Among 30 women weighing more than 300 lb at conception, Chauhan et al22 noted a VBAC success rate of less than 15%.This is much lower than the general success rate of 60% to 80% quoted in the ACOG practice bulletin on VBAC.23 Grobman et al24 reported that VBAC is cost-effective among women with 1 prior cesarean delivery only if the success rate is above 40%; it is therefore worth pondering whether VBAC should be attempted in overweight patients.
TABLE 2
Obesity and cesarean delivery rates
| AUTHORS | NUMBER OF SUBJECTS | OBESITY DEFINED AS | RATE OF CESAREAN DELIVERY | COMMENTS |
|---|---|---|---|---|
| Baeten et al, 200112 | 9,817 | BMI≥30 | Increased | — |
| Kaiser and Kirby, 200121 | 452 | BMI≥29 | Increased* | Population was low risk without prior cesarean. |
| Kumari, 200137 | 188 | BMI >40 | Increased* | Elective* and emergency cesareans examined. |
| Steinfeld et al, 200038 | 168 | BMI≥29 | Increased* | Excluded elective cesareans and those performed due to fetal malpresentation and previa. |
| Jensen et al, 199939 | 163 | BMI≥30 | Increased | Excluded patients with prior cesarean. |
| Ranta et al, 199540 | 53 | BMI≥30 | Increased | — |
| Issacs et al, 19949 | 117 | >300 lb | Increased* | Primary and repeat cesareans examined. |
| Hood and Dewan, 199325 | 117 | >300 lb | Increased* | Elective and emergency* cesareans examined. |
| Ekblad and Grenman, 199220 | 77 | ≥20%† | Increased | Emergency cesareans examined. |
| Perlow et al, 199235 | 111 | >300 lb | Increased* | Primary* cesareans and those performed due to fetal distress examined. |
| Pongthai, 199041 | 741 | ≥80 kg | Increased* | Primary and repeat* cesareans examined. |
| Johnson et al, 198730 | 588 | >113.6 kg | Increased* | Primary cesareans examined only. |
| Garbaciak et al, 198536 | 1,889 | >120%† | Increased* | Primary cesareans examined only. |
| Gross et al, 198019 | 279 | ≥90 kg | Increased | Repeat cesareans omitted. |
| Edwards et al, 197827 | 208 | >50%‡ | Increased | — |
| BMI = body mass index | ||||
| *Significant increase | ||||
| †Over ideal body weight for height | ||||
| ‡Above standard weight for height on the Metropolitan Life Insurance tables | ||||
Postpartum: Longer hospitalization
Although they did not provide the reasons, Hood and Dewan25 linked obesity with prolonged postpartum hospitalization. They found obese patients to have significantly longer hospital stays, regardless of the type of delivery:
- Following vaginal delivery, postpartum hospitalization was 3.8±2.4 days among overweight patients and 2.9±2.1 days among controls.
- After cesarean delivery, obese patients were in the hospital for 7.3±5.0 days; nonobese, for 5.4±3.1 days.
Cesarean complications
Obesity is a specific risk factor for several operative complications, including hemorrhage during surgery, postoperative wound infections, aspiration, and pulmonary embolism. A case-control study by Naef et al26 revealed that a weight of more than 250 lb has an odds ratio of 13.1 (95% confidence interval, 1.7 to 102.7) for hemorrhage (decrease in hematocrit of 10% or greater, estimated blood loss greater than 1,500 mL, or packed red blood cell administration) during abdominal delivery.
Multiple studies have shown obesity to be a risk factor for postoperative wound infections.27-30 For example, Johnson et al30 reported a wound infection rate of 37.6% for the obese parturient and 10.2% for those of normal weight (P.001>
The link between excess weight and infectious morbidity may be secondary to the increased subcutaneous tissue layer and accumulation of loculated fluid. In 2000, Vermillion et al31 published a study that looked at 140 women who had cesarean deliveries. Initially, a univariate analysis identified the risk factors for wound infection as maternal weight (a mean of 82.8 kg±18.6 kg in the uninfected population versus 99.4 kg±33.3 kg in the infected population), BMI (44.5±2.1 for uninfected versus 49.7±6.3 for infected), and thickness of subcutaneous tissue (2.3 cm±1.2 cm for uninfected versus 4.1 cm±1.8 cm for infected). After a multiple logistic regression analysis, however, subcutaneous tissue thickness was the only significant risk factor confirmed. A potential explanation for this finding is that the blood supply to subcutaneous fat is relatively poor.
Reducing infection. By modifying surgical techniques, physicians may be able to decrease the rate of wound infection among overweight parturients. Naumann et al32 randomized closure versus nonclosure of the subcutaneous tissue in 245 patients with at least 2 cm of adipose tissue. There was a significant difference in the incidence of overall wound disruptions (14.5% versus 26.6%)—specifically, seroma formation (5.1% versus 17.2%)—between the closure and nonclosure groups, respectively, but no significant difference in wound infections (6% versus 7.8%).
There is no consistent evidence that obesity alone is associated with poor perinatal outcome.
Allaire et al33 showed that the use of a subcutaneous drain or subcutaneous suture decreased the rates of wound infection or separation among obese women undergoing cesarean delivery. The incidence dropped from 30.8% when neither was used to 15.4% with suture and 4.2% with a drain.
While several investigators have noted the increased rate of postoperative complications among obese parturients, few have systematically analyzed their etiology. Wolfe et al34 reviewed the antepartum and intrapartum variables among 107 consecutive obese parturients (all at least 200 lb) who had cesarean deliveries. Using multivariate analysis, the investigators noted that various degrees of obesity, preexisting medical conditions, the type of skin incision, and the type of anesthesia were not risk factors for postpartum infectious sequelae. Only 2 factors—both of which were under the control of physicians—contributed to morbidity: duration of cesarean delivery and operative blood loss. According to their regression equation, if surgical time was decreased from 1.5 hours to 1 hour, the postoperative stay would decrease by 1 day. These authors did not comment on the estimated blood loss or drop in hematocrit threshold that would minimize postoperative complications.
What about the neonate?
Interestingly, there is no consistent evidence that obesity alone is associated with poor perinatal outcome. A case-control study by Perlow et al35 reported the outcomes of 111 neonates born to obese mothers. These infants were more likely to weigh less than 2,500 g or more than 4,000 g, to have intrauterine growth restriction, and to require admission to a neonatal intensive care unit. However, when patients with prepregnancy diagnoses of chronic hypertension or insulin-requiring diabetes mellitus were excluded, perinatal outcome was similar for obese and nonobese mothers. Garbaciak et al36 reported similar results: They showed that only obese patients with antepartum complications had an increase in perinatal mortality. Two other studies showed no increase in perinatal morbidity or mortality among obese subjects.19,27 It seems, therefore, that the risk for adverse perinatal outcomes may be related to underlying medical diseases rather than excessive weight.
Research has also linked infant birth weight to maternal weight. Studies have shown the incidence of macrosomic infants (birth weight of at least 4,000 g) to be higher in obese women, even in the absence of antenatal complications.19,25,36 Specifically, Gross et al19 concluded that the increase in macrosomic and large-for-gestational-age infants (defined as over 90% of weight for gestational age) born to obese mothers cannot be explained by the presence of maternal diabetes. They noted that the frequency of macrosomia was 15.1% and large-for-gestational-age was 31% among obese patients, while the incidence of diabetes mellitus was only 9%. However, Perlow et al35 demonstrated that the increased rate of macrosomia disappeared if patients with preexisting medical conditions were excluded.
Studies also have shown that newborns of obese patients have weight problems as they age. Edwards et al27 noted that at 1 year, infants of obese mothers were significantly more overweight than those of controls. Specifically, 47% of the infants of obese mothers were above the 75th percentile for weight-length, compared to 22% in the control group.
Counsel weight reduction
Obesity is a major health risk for both the general and obstetric populations. Fortunately, this risk can be addressed through lifestyle modification. Though we lack studies demonstrating improved peripartum outcomes with weight reduction, there is no reason to doubt that weight loss will decrease the rate of adverse events. Ob/Gyns caring for obese patients should inform these women of the unfavorable pregnancy outcomes secondary to excessive weight and encourage preconception weight control.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Lu GC, Rouse DJ, DeBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Obstet Gynecol. 2001;185:845-849.
2. Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States 1991-1998. JAMA. 1999;282:1519-1522.
3. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, Md: National Heart, Lung, and Blood Institute; June 1998;1-226.
4. American College of Obstetricians and Gynecologists. ACOG Education Bulletin #229: nutrition and women. Washington, DC: ACOG; 1996.
5. Institute of Medicine subcommittee on nutritional status and weight gain during pregnancy. Nutrition During Pregnancy. Washington, DC: National Academy Press; 1990.
6. Cogswell ME, Serdula MK, Hungerford DW, Yip R. Gestational weight gain among average-weight and overweight women—what is excessive? Am J Obstet Gynecol. 1995;172:705-712.
7. Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal weight women: effects of gestational weight change. Obstet Gynecol. 1996;87:389-394.
8. Parker JD, Abrams B. Prenatal weight gain advice: an examination of the recent prenatal weight gain recommendations of the Institute of Medicine. Obstet Gynecol. 1992;79:664-669.
9. Isaacs JD, Magann EF, Martin RW, Chauhan SP, Morrison JC. Obstetric challenges of massive obesity complicating pregnancy. J Perinatol. 1994;14:10-14.
10. American College of Obstetricians and Gynecologists. ACOG Technical Bulletin #200: diabetes and pregnancy. Washington, DC: ACOG; 2000.
11. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
12. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91:436-440.
13. Stone JL, Lockwood CJ, Berkowitz GS, Alvarez M, Lapinski R, Berkowitz RL. Risk factors for preeclampsia. Obstet Gynecol. 1994;83:357-361.
14. Knight M, Duley L, Henderson-Smart D, et al. The effectiveness and safety of antiplatelet agents for the prevention and treatment of preeclampsia. In: The Cochrane Library, Issue 4, 2000. Oxford: Update Software. Search date 1999. Primary sources: Cochrane Pregnancy and Childbirth Group Trials Register, conference proceedings.
15. Atallah AN, Hofmeyr GJ, Duley L. Calcium supplementation during pregnancy to prevent hypertensive disorders and related adverse outcomes. In: The Cochrane Library, Issue 4, 2000. Oxford: Updated Software. Search date 2000. Primary sources: Cochrane Pregnancy and Childbirth Group Trials Register.
16. Maeder EC, Barno A, Mecklenburg F. Obesity: a maternal high-risk factor. Obstet Gynecol. 1975;45:669-671.
17. Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147-152.
18. Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts RC. Determinants of unexplained antepartum fetal death. Obstet Gynecol. 2000;95:215-221.
19. Gross T, Sokol RJ, King KC. Obesity in pregnancy: risks and outcome. Obstet Gynecol. 1980;56:446-450.
20. Ekblad U, Grenman S. Maternal weight, weight gain during pregnancy and pregnancy outcome. Int J Gynecol Obstet. 1992;39:277-283.
21. Kaiser PS, Kirby RS. Obesity as a risk factor for cesarean in a low risk population. Obstet Gynecol. 2001;97:39-43.
22. Chauhan SP, Magann EF, Carroll CS, Barrilleaux PS, Scardo JA, Martin JN. Mode of delivery for the morbidly obese with prior cesarean delivery: vaginal versus repeat cesarean section. Am J Obstet Gynecol. 2001;185:349-354.
23. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #5: vaginal birth after previous cesarean delivery. Washington, DC: ACOG; 2000.
24. Grobman WA, Peaceman AM, Socol ML. Cost-effectiveness of elective cesarean delivery after one prior low transverse cesarean. Obstet Gynecol. 2000;95:745-751.
25. Hood DD, Dewan DM. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210-1218.
26. Naef RW, Chauhan SP, Chevalier SP, Roberts WE, Meydrech EF, Morrison JC. Prediction of hemorrhage at cesarean delivery. Obstet Gynecol. 1994;83:923-926.
27. Edwards LE, Dickes WF, Alton IR, Hakanson EY. Pregnancy in the massively obese: course, outcome, and obesity prognosis of the infant. Am J Obstet Gynecol. 1978;131:479-483.
28. Pelle H, Jepsen OB, Larsen SO, et al. Wound infection after cesarean section. Infect Control. 1986;7:456-461.
29. Nielsen TF, Hokegard K. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
30. Johnson SR, Kolberg BH, Varner MW, Railsback LD. Maternal obesity and pregnancy. Surg Gynecol Obstet. 1987;164:431-437.
31. Vermillion ST, Lamoutte C, Soper DE, Verdeja A. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
32. Naumann RW, Hauth JC, Owen J, Hodgkins PM, Lincoln T. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
33. Allaire AD, Fisch J, McMahon MJ. Subcutaneous drain vs suture in obese women undergoing cesarean delivery. J Reprod Med. 2000;45:327-331.
34. Wolfe HM, Gross TL, Sokol RJ, Bottoms SF, Thompson KL. Determinants of morbidity in obese women delivered by cesarean. Obstet Gynecol. 1998;71:691-696.
35. Perlow JH, Morgan MA, Montgomery D, Towers CV, Porto M. Perinatal outcome in pregnancy complicated by massive obesity. Am J Obstet Gynecol. 1992;167:958-962.
36. Garbaciak JA, Jr, Richter M, Miller S, Barton JJ. Maternal weight and pregnancy complications. Obstet Gynecol. 1985;152:238-245.
37. Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynecol Obstet. 2001;73:101-107.
38. Steinfeld JD, Valentine S, Lerer T, Ingardia CJ, Wax JR, Curry SL. Obesity-related complications of pregnancy vary by race. J Matern Fetal Med. 2000;9:238-241.
39. Jensen H, Agger AO, Rasmussen KL. The influence of prepregnancy body mass index on labor complications. Acta Obstet Gynecol Scand. 1999;78:799-802.
40. Ranta P, Jouppila P, Spalding M, Jouppila R. The effect of maternal obesity on labour and labour pain. Anaesthesia. 1995;50:322-326.
41. Pongthai S. Labour and delivery of obese parturients. J Med Assoc Thai. 1990;73:52-56.
1. Lu GC, Rouse DJ, DeBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Obstet Gynecol. 2001;185:845-849.
2. Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States 1991-1998. JAMA. 1999;282:1519-1522.
3. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, Md: National Heart, Lung, and Blood Institute; June 1998;1-226.
4. American College of Obstetricians and Gynecologists. ACOG Education Bulletin #229: nutrition and women. Washington, DC: ACOG; 1996.
5. Institute of Medicine subcommittee on nutritional status and weight gain during pregnancy. Nutrition During Pregnancy. Washington, DC: National Academy Press; 1990.
6. Cogswell ME, Serdula MK, Hungerford DW, Yip R. Gestational weight gain among average-weight and overweight women—what is excessive? Am J Obstet Gynecol. 1995;172:705-712.
7. Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal weight women: effects of gestational weight change. Obstet Gynecol. 1996;87:389-394.
8. Parker JD, Abrams B. Prenatal weight gain advice: an examination of the recent prenatal weight gain recommendations of the Institute of Medicine. Obstet Gynecol. 1992;79:664-669.
9. Isaacs JD, Magann EF, Martin RW, Chauhan SP, Morrison JC. Obstetric challenges of massive obesity complicating pregnancy. J Perinatol. 1994;14:10-14.
10. American College of Obstetricians and Gynecologists. ACOG Technical Bulletin #200: diabetes and pregnancy. Washington, DC: ACOG; 2000.
11. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
12. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91:436-440.
13. Stone JL, Lockwood CJ, Berkowitz GS, Alvarez M, Lapinski R, Berkowitz RL. Risk factors for preeclampsia. Obstet Gynecol. 1994;83:357-361.
14. Knight M, Duley L, Henderson-Smart D, et al. The effectiveness and safety of antiplatelet agents for the prevention and treatment of preeclampsia. In: The Cochrane Library, Issue 4, 2000. Oxford: Update Software. Search date 1999. Primary sources: Cochrane Pregnancy and Childbirth Group Trials Register, conference proceedings.
15. Atallah AN, Hofmeyr GJ, Duley L. Calcium supplementation during pregnancy to prevent hypertensive disorders and related adverse outcomes. In: The Cochrane Library, Issue 4, 2000. Oxford: Updated Software. Search date 2000. Primary sources: Cochrane Pregnancy and Childbirth Group Trials Register.
16. Maeder EC, Barno A, Mecklenburg F. Obesity: a maternal high-risk factor. Obstet Gynecol. 1975;45:669-671.
17. Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147-152.
18. Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts RC. Determinants of unexplained antepartum fetal death. Obstet Gynecol. 2000;95:215-221.
19. Gross T, Sokol RJ, King KC. Obesity in pregnancy: risks and outcome. Obstet Gynecol. 1980;56:446-450.
20. Ekblad U, Grenman S. Maternal weight, weight gain during pregnancy and pregnancy outcome. Int J Gynecol Obstet. 1992;39:277-283.
21. Kaiser PS, Kirby RS. Obesity as a risk factor for cesarean in a low risk population. Obstet Gynecol. 2001;97:39-43.
22. Chauhan SP, Magann EF, Carroll CS, Barrilleaux PS, Scardo JA, Martin JN. Mode of delivery for the morbidly obese with prior cesarean delivery: vaginal versus repeat cesarean section. Am J Obstet Gynecol. 2001;185:349-354.
23. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #5: vaginal birth after previous cesarean delivery. Washington, DC: ACOG; 2000.
24. Grobman WA, Peaceman AM, Socol ML. Cost-effectiveness of elective cesarean delivery after one prior low transverse cesarean. Obstet Gynecol. 2000;95:745-751.
25. Hood DD, Dewan DM. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210-1218.
26. Naef RW, Chauhan SP, Chevalier SP, Roberts WE, Meydrech EF, Morrison JC. Prediction of hemorrhage at cesarean delivery. Obstet Gynecol. 1994;83:923-926.
27. Edwards LE, Dickes WF, Alton IR, Hakanson EY. Pregnancy in the massively obese: course, outcome, and obesity prognosis of the infant. Am J Obstet Gynecol. 1978;131:479-483.
28. Pelle H, Jepsen OB, Larsen SO, et al. Wound infection after cesarean section. Infect Control. 1986;7:456-461.
29. Nielsen TF, Hokegard K. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
30. Johnson SR, Kolberg BH, Varner MW, Railsback LD. Maternal obesity and pregnancy. Surg Gynecol Obstet. 1987;164:431-437.
31. Vermillion ST, Lamoutte C, Soper DE, Verdeja A. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
32. Naumann RW, Hauth JC, Owen J, Hodgkins PM, Lincoln T. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
33. Allaire AD, Fisch J, McMahon MJ. Subcutaneous drain vs suture in obese women undergoing cesarean delivery. J Reprod Med. 2000;45:327-331.
34. Wolfe HM, Gross TL, Sokol RJ, Bottoms SF, Thompson KL. Determinants of morbidity in obese women delivered by cesarean. Obstet Gynecol. 1998;71:691-696.
35. Perlow JH, Morgan MA, Montgomery D, Towers CV, Porto M. Perinatal outcome in pregnancy complicated by massive obesity. Am J Obstet Gynecol. 1992;167:958-962.
36. Garbaciak JA, Jr, Richter M, Miller S, Barton JJ. Maternal weight and pregnancy complications. Obstet Gynecol. 1985;152:238-245.
37. Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynecol Obstet. 2001;73:101-107.
38. Steinfeld JD, Valentine S, Lerer T, Ingardia CJ, Wax JR, Curry SL. Obesity-related complications of pregnancy vary by race. J Matern Fetal Med. 2000;9:238-241.
39. Jensen H, Agger AO, Rasmussen KL. The influence of prepregnancy body mass index on labor complications. Acta Obstet Gynecol Scand. 1999;78:799-802.
40. Ranta P, Jouppila P, Spalding M, Jouppila R. The effect of maternal obesity on labour and labour pain. Anaesthesia. 1995;50:322-326.
41. Pongthai S. Labour and delivery of obese parturients. J Med Assoc Thai. 1990;73:52-56.