User login
Helping patients kick the "other" habit
- Nicotine replacement therapy may be useful for short-term treatment of cravings, but may not improve cessation rates among patients who use smokeless tobacco (B).
- Patients who use >3 cans of smokeless tobacco a day may need higher than normal doses (42 mg/day) of nicotine replacement therapy (B).
- Evidence is insufficient to support the routine use of bupropion (Zyban) for smokeless tobacco cessation. It should be initiated at the physician’s discretion (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Through with Chew Week” and the “Great American Spitout.” Do they ring a bell?
If you answered no, you’re not alone.
“Through with Chew Week” was established by the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) in 1989, but it hasn’t quite garnered the same kind of recognition as the American Cancer Society’s Great American Smokeout.
When it comes to tobacco use—and more importantly, cessation—smokeless tobacco just doesn’t generate the kind of attention that cigarette smoking does. In fact, smokeless tobacco’s low profile extends beyond talk of “smokeouts” and “spitouts” to research on effective ways to quit.
We found this out first-hand when we conducted a literature search of Medline, PubMed, and a number of other databases to learn which cessation methods have proven efficacy. What we learned is that not only is research on the subject of smokeless tobacco cessation limited, but there is no recommended medication therapy to help these patients quit. Specifically:
- Nicotine-replacement therapies have failed to demonstrate a clear benefit in smokeless tobacco cessation, but under-dosing may be a factor for some patients.
- Bupropion’s (Zyban) usefulness in smokeless tobacco cessation is unclear. Data, thus far, have been inconclusive.
- Varenicline’s (Chantix) usefulness in smokeless tobacco cessation is unknown. There are no published case reports or clinical trials on the subject.
Millions of “chew” users are at risk
Tobacco use is the leading preventable cause of premature death in the United States, with more than 440,000 Americans dying of tobacco-related disease each year.1 Cigarette smoking is by far the most common form of tobacco used; however, smokeless tobacco, also known as chew, spit tobacco, or snuff, is used by 8.2 million Americans.2 More men than women use tobacco products overall, and smokeless tobacco, in particular.2
Health risks include MI. Specific health risks associated with smokeless tobacco include cancer of the oral cavity and pharynx, oral and periodontal disease, tooth decay, and pregnancy-related problems.1 (See “Smokeless tobacco was to blame”)
In addition, an international, case-control study evaluating the risk of myocardial infarction associated with various forms of tobacco use found an increased risk of myocardial infarction associated with smokeless tobacco use compared to non-tobacco users (odds ratio [OR]=2.23; 95% confidence interval [CI], 1.41-3.52).3
Notably, smokeless tobacco users in the study who also smoked cigarettes had the highest risk of all tobacco users when compared to non-users (OR=4.09; 95% CI, 2.98-5.61). These findings demonstrate that nicotine dependence is detrimental to health—regardless of the form of tobacco used. Even more worrisome is the notion that risk may actually be increased when multiple forms of tobacco are used by the same patient.
Patients want medication to help them quit
Current guidelines for tobacco cessation recommend that patients using smokeless tobacco should be identified, urged to quit, and treated with counseling interventions.4 Despite this recommendation, many patients are interested in using a medication to aid them in their quit effort, and many physicians would like to prescribe medication to help patients succeed.
To that end, it seemed logical to us that the same treatments used for smoking cessation would also be effective for smokeless tobacco cessation, given that the underlying problem—despite the form of tobacco used—is nicotine dependence. So we did a literature review to determine the optimal treatment for smokeless tobacco cessation. Our search included: Medline (1950-2007), PubMed (1966-2006), International Pharmaceutical Abstracts (1970-2006), Science Direct, CINAHL, PsycArticles, and Dissertation Abstracts. We used the following search terms: smokeless tobacco, spit tobacco, chew tobacco, cessation, bupropion, nicotine, and nicotine replacement. (Searches in Medline for nicotine replacement therapy in smokeless tobacco cessation were limited to clinical trials.) What we found were a limited number of studies, which we’ve summarized here.
Nicotine patch is useful, but to what degree?
We reviewed four studies involving the use of a nicotine patch for smokeless tobacco cessation (TABLE 1). In chronological order:
Study #1: 15-mg patch. The first study to evaluate the nicotine patch was a randomized, double-blind, placebo controlled trial published in 1999. A 15-mg nicotine patch was used by approximately 420 patients.5 Patients included in the trial were at least 18 years of age, nonsmokers, and used at least 1 can of smokeless tobacco per week. The main outcome of this trial was cessation at 6 months.
A significant difference was found in abstinence rates between patients treated with active therapy compared to those treated with placebo early in the study; however, by study end, there were no significant differences between groups. The medication was well tolerated, though patients receiving active therapy did report an increase in adverse effects related to patch use, such as rash and itching.
TABLE 1
Conflicting findings on the nicotine patch for smokeless tobacco cessation
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Howard-Pitney5 (1999) | 420 patients • ≥18 years, nonsmokers • ≥1 can/week | • Nicotine patch (15 mg) • Placebo patch | • Written materials • Telephone contact | 6 months | No significant difference in cessation rates | N/A |
| Hatsukami6 (2000) | 400 patients • 1 can/week | • Nicotine patch (began on 22 mg and tapered to 7 mg by study end) + mint snuff • Nicotine patch (same tapering as above) + no mint snuff • Placebo patch + mint snuff • Placebo patch + no mint snuff | • Written materials • 10 minute behavioral counseling | 10 weeks | Patients receiving active NRT had significantly higher quit rates at 10 and 15 weeks (P=0.002 and 0.016, respectively) | Patients with mint snuff: • 10 weeks: 4.3 • 15 weeks: 5.5 Patients w/o mint snuff: • 10 weeks: 14.3 • 15 weeks: 20 |
| Stotts7 (2003) | 300 patients • 14- to 19- year-old males • ST use ≥5 days/week | • Nicotine patch • Placebo patch • Usual care | • Patch: Six, 50- minute behavioral counseling sessions • Usual care: one, 5- to 10-minute counseling session with one follow-up telephone contact | 1 year | No significant difference in cessation rates at 1 year | N/A |
| Ebbert8 (2007) | 42 patients • ≥18 years, in good health • ≥3 cans or pouches/day | • Nicotine patch (21, 42, or 63 mg/day) • Placebo patch | • None | 3 days | NRT with 42 mg/day provided similar levels of nicotine to active ST users | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; ST, smokeless tobacco. | ||||||
Study #2: Nicotine plus mint snuff. The second trial to evaluate nicotine patches randomized approximately 400 patients to 1 of 4 groups: active nicotine patch plus mint snuff (non-nicotine mint-leaf product), active nicotine patch and no mint snuff, placebo plus mint snuff, or placebo patch and no mint snuff.6 To be enrolled in the study, patients had to use 1 can of smokeless tobacco per week and express a desire to quit. Patients given the active nicotine patch were begun on 22 mg and tapered to 7 mg by the end of 10 weeks. At 10 weeks, continuous abstinence and abstinence since the last clinic visit were evaluated. (Researchers tracked the patients for a total of 62 weeks.)
Patients who received active nicotine patches had significantly higher cessation rates compared to placebo at 10 (P=0.002) and 15 (P=0.016) weeks, but not at any other time during the study. No information regarding adverse effects was reported.
Study #3: Patch in adolescents. In a third, randomized, placebo-controlled trial, the effect of nicotine replacement in adolescents was evaluated. Patients were randomized to an active patch, placebo patch, or usual care (1 counseling session with a follow-up phone call).7
Patients who received an active patch also received 6 weeks of behavior counseling sessions. Smokeless tobacco users were further stratified according to light/moderate or heavy use according to saliva cotinine levels: light/moderate users were started on 14 mg and heavy users on 21 mg of nicotine. All patients were tapered to 7 mg over a 6-week period. Approximately 300 males between the ages of 14 and 19 who used smokeless tobacco at least 5 days a week were enrolled in the study.
Cessation rates at 1 year were not statistically significant between the placebo and active patch (P=0.22). The nicotine patch was well tolerated, with the exception of 5 patients who experienced skin irritation or headache; however, 3 patients were removed from patch therapy due to skin hyper-reaction or headache.
Study #4: Dosing. A fourth study, by Ebbert et al,8 addressed potential under-dosing of nicotine patches. This study was a randomized, double-blind, placebo controlled trial that evaluated 21, 42, or 63 mg/day of nicotine delivered by patch or placebo in 42 patients. (The 21 mg/day dose is the highest recommended starting dose for smokers.) The patients had to be 18 years of age or older, in good health, and use 3 or more cans/pouches of smokeless tobacco per day.
The study took place in 3 phases: outpatient preadmission, inpatient research phase, and an outpatient follow-up phase. The inpatient phase measured nicotine and cotinine concentrations during active smokeless tobacco use and while receiving treatment or placebo. Patients treated with the 42 mg/day patch had levels closer to those measured during active smokeless tobacco use, while patients treated with 63 mg/day tended to be “over-replaced” and experienced more severe adverse effects, such as nausea, vomiting, and headache. Overall, nicotine replacement therapy was well tolerated throughout the trial.
Nicotine gum: No lasting benefit
The earliest trial to evaluate nicotine gum for smokeless tobacco cessation was conducted by Boyle9 and was only available in abstract form (TABLE 2). This randomized, double-blind, placebo-controlled trial evaluated 2 mg of nicotine gum vs placebo in 100 patients. The main outcome was quit rates at the end of 6 weeks. The quit rate was similar for each group, and did not reach statistical significance (no P value provided). No information regarding adverse effects was reported.
A second trial10 to evaluate nicotine gum was also a randomized placebo-controlled trial that enrolled just over 200 adult patients that used ≥1 tin of smokeless tobacco per week (TABLE 2). Patients were randomized to 2 mg of nicotine gum or placebo and group therapy sessions or minimal behavioral intervention contact for a 2-month treatment period.
This trial did show a significant difference at week 4 (P<0.01) in point prevalence abstinence—that is, the number of people reporting to be abstinent at the 4-week mark, regardless of whether their abstinence was continuous or not. Significance, however, was lost by week 8. Cessation rates were also significant at 1- and 6-month follow-up exams (P<0.05), but were not maintained at the 12-month follow-up.
TABLE 2
Nicotine gum for smokeless tobacco cessation: Benefit doesn’t last
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Boyle9 (1992) | 100 patients | • Nicotine gum (2 mg) • Placebo gum | • Weekly group meetings | 6 weeks | No significant difference at 6 weeks | N/A |
| Hatsukami10 (1996) | 200 adult patients • ≥1 tin/week | • Nicotine gum (2 mg) • Placebo gum | • 8 group therapy sessions or • Minimal behavioral intervention | 12 months (8 weeks of treatment) | Point prevalence abstinence* significantly different at 4 weeks (P<0.01); no significant difference at 8 weeks | • Patients in group therapy at 4 weeks: 42 • Patients with minimal intervention at 4 weeks: 7.8 |
| NNT, number needed to treat. | ||||||
| * Point prevalence abstinence is the number of patients reporting to be abstinent on a given day. | ||||||
Continuous abstinence rates were similar for all groups except the 2 mg nicotine and minimal intervention group. Patients in this group had significantly lower abstinence rates from all other groups (P<0.003). No information regarding adverse effects was reported.
Case report suggests bupropion has potential
Bupropion is a non-nicotine smoking cessation therapy that inhibits the uptake of norepinephrine and dopamine. It is unclear how bupropion aids in smoking cessation, though its ability to do so appears to be related to its dopaminergic properties.11
Given its utility in treatment for smoking cessation, it seems plausible that the drug may serve as an effective treatment for smokeless tobacco, as well. However, the literature regarding the use of bupropion for smokeless tobacco cessation is even more limited than that for nicotine replacement therapy.
Berigan and Deagle first described the use of bupropion for smokeless tobacco cessation in 1999 (TABLE 3).12 Their case report focused on a 31-year-old man who reported using 1 can of smokeless tobacco per day for 11 years. The patient reported having tried nicotine patches and attempting to quit “cold turkey,” with limited success.
The patient was started on a trial of bupropion along with approximately 1 month of behavioral counseling. After 1 week of medication, the patient reported a reduction in cravings. By 5 weeks, the patient reported no use of smokeless tobacco. A total of 10 weeks of medication was given to the patient and he experienced no withdrawal effects after cessation. At 8 months, the patient was still tobacco free. The patient also reported few adverse effects from bupropion.
2 bupropion clinical trials are less encouraging
We found 2 clinical trials evaluating sustained-release bupropion 300 mg/day (TABLE 3). The first was a randomized, placebo-controlled trial in which patients received 7 weeks of active or placebo medication, and were then followed (with minimal counseling) for an additional 5 weeks.13 A total of 70 men, 18 years of age and older, were enrolled in the study; all had expressed a desire to quit and used at least one-half can of smokeless tobacco a day in the past year.
The primary endpoint was smokeless tobacco abstinence during weeks 4 through 7 of the trial. At the end of week 7 (end of treatment), significantly more patients in the treatment group were free of smokeless tobacco, compared to the placebo group (OR=2.73; 95% CI, 1.07-7.72; P=0.04). However, significance was lost by week 12 (OR=1.93; 95% CI, 0.71-5.47; P=0.2). Bupropion was well tolerated throughout this trial and no serious adverse effects were reported.
TABLE 3
Usefulness of bupropion for smokeless tobacco cessation is unclear
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Berigan12 (1999) | 1 can/day | • Bupropion | • Behavioral counseling | 10 weeks | Patient in case report remained ST free at 8 months | N/A |
| Glover13 (2002) | 70 patients • ≥18 year-old- men • ≥½ can/day | • Bupropion SR • Placebo | • Telephone sessions (weeks 9-11) | 3 months | Patients receiving active NRT had significantly higher quit rates by week 7 (P=0.04) | 4.3 |
| Dale14 (2002) | 68 patients • ≥18 years • Regularly used ST | • Bupropion SR • Placebo | • 10 minute behavioral counseling | 24 weeks | No significant difference | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; SR, sustained release; ST, smokeless tobacco. | ||||||
The second trial14 evaluating the efficacy of bupropion for smokeless tobacco cessation was also a randomized, placebo-controlled trial. Enrollees were randomly assigned to bupropion SR 300 mg/day or matching placebo for 12 weeks. In addition, minimal behavioral intervention was provided until week 24. Researchers enrolled 68 patients; all but one were men. Participants in this study were 18 years of age or older who regularly used smokeless tobacco. The efficacy of bupropion SR for nicotine dependence was the primary endpoint evaluated.
At the completion of the treatment phase, more patients in the bupropion group reported cessation (44%) compared to placebo (26%). The difference between the 2 groups was nonsignificant and remained so until the end of the study. By study end, each group had a reported cessation rate of 29%.
One patient experienced a diffuse skin rash that resulted in discontinuation of the study medication; otherwise buproprion was well tolerated.
A product that shows promise for smoking cessation is varenicline, which was approved by the FDA in May 2006.15 Varenicline acts as an agonist at the α4β2 neuronal nicotinic acetylcholine receptors. Its action at this receptor subtype blocks the ability of nicotine to bind to the receptor and is therefore thought to blunt the feeling of reward experienced by smokers.16 In addition, the medication may possess some nicotine-like action at the nicotinic receptor that may decrease the amount of withdrawal symptoms experienced by patients.15
In theory, this mechanism of action may help smokeless tobacco users to succeed in their quit attempts. Unfortunately, though, there have been no published case reports or clinical trials to date regarding the use of varenicline in the treatment of smokeless tobacco cessation.
Helping patients despite the information gap
Our knowledge of the effectiveness of various tools in smokeless tobacco cessation is hampered on a number of fronts.
Nicotine replacement therapy. The conflicting evidence regarding nicotine replacement therapy may be the result of a number of factors. First, although many of the trials used a randomized, placebo-controlled design, there were a number of variations in the patient populations studied, inconsistencies in the amount and type of counseling used, and differences in the amount of smokeless tobacco used by the patients.
These 2 cases illustrate the damage that chew can do
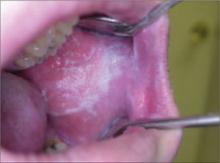
Oral leukoplakia
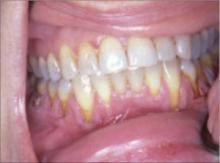
Gum recession
This 24-year-old patient sought treatment for an asymptomatic oral lesion that he noticed while brushing his teeth. The patient, who did not smoke tobacco and drank alcohol socially on weekends, told us that he had started chewing tobacco while playing baseball in high school 8 years earlier. He said that over the past 3 years, he had increased the habit.
On examination, we noted a well-defined, macular, white lesion on his left buccal mucosa. The lesion was not painful with palpation and remained unchanged when scraped with a tongue depressor.
Our patient had an oral leukoplakia, which can result from the chronic use of chewing tobacco. Cessation of the habit typically results in the resolution of the lesion in approximately 4 weeks. That was the case with our patient: He quit the habit and in 4 weeks, 80% of the lesion resolved. He was closely monitored, and at 3 months the lesion was undetectable.
If, however, a patient discontinues the habit and there is no change in the lesion after 4 weeks, biopsy is indicated. The most serious consequence of a malignant transformation of leukoplakia is oral squamous cell carcinoma.
A 30-year-old man was referred to us for evaluation of gum recession that had worsened over the past year. The patient complained that his teeth were sensitive to hot and cold drinks, but had no other symptoms. He had a 5-year history of smokeless tobacco use and said he usually placed the tobacco along his inner vestibule. He said that he did not smoke tobacco, nor did he drink alcohol.
On examination, we noted that the patient had localized recession along the cervical area of his lower teeth. With manipulation, there was bleeding from the gingival surface. His teeth were otherwise in good shape and there were no other lesions within the oral mucosa.
Gingival recession is a common consequence of smokeless tobacco use. Treatment consists of a gingival graft, in which palatal connective tissue is removed and used to reestablish gingival attachment to the tooth. (Tissue harvesting surgery from the palate can be quite painful.) Left untreated, teeth can become loose and fall out.
We advised our patient to follow up for gingival grafting, but he did not return for his follow-up visit.
Denise Rizzolo, PA-C, PhD is a physician assistant at Care Station in Springfield, NJ and Thomas A. Chiodo, DDS, is an oral and maxillofacial surgeon in private practice in Somerville, NJ. Dr. Chiodo also teaches at UMDNJ Dental School in Newark, NJ; [email protected]
Additionally, recent evidence suggests it may be important to consider dosing nicotine replacement according to number of cans of smokeless tobacco the patient uses per week. Heavy smokeless tobacco users may require higher initial doses than routinely used for smoking cessation therapy (eg, 42 mg/day if >3 cans of smokeless tobacco/day used).8
Further clinical research is needed to validate this recommendation, as well as to give specific dosing recommendations.
Nicotine gum. Two issues come to mind when considering the research on nicotine gum. First, the studies had relatively small sample sizes and it’s possible that the studies were not properly designed to detect a difference between groups.6,7 Second, the concept of dosing nicotine gum based on the number of cans of smokeless tobacco used per day has not been explored.
Bupropion. Studies involving bupropion and smokeless tobacco cessation share several of the limitations we’ve just discussed. Differing results reported in the literature regarding bupropion’s effectiveness makes its potential benefit unclear. Use should be determined on a case-by-case basis with the understanding that it may—or may not—be useful in decreasing cravings or increasing abstinence rates.
Varenicline. There is currently no literature available to help us evaluate the usefulness of varenicline in smokeless tobacco cessation. The mechanism of action of varenicline is such that use in smokeless tobacco cessation is plausible. Again, consideration of patients on a case-by-case basis is warranted.
One size does not fit all
Although nicotine dependence is the underlying problem for patients who utilize smokeless tobacco, current literature does not support a “one-size-fits-all” approach to treatment of various forms of tobacco abuse. Further clinical investigations are needed to determine the true utility of bupropion and varenicline, as well as the appropriate dosing of nicotine replacement therapy when prescribed for smokeless tobacco cessation.
1. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Tobacco Use: Prevention, Cessation and Control. Ann Intern Med 2006;145:839-844.
2. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. Available at: www.oas.samhsa.gov/nsduh/2k6nsduh/2k6results.pdf. Accessed February 26, 2008.
3. Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658.
4. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
5. Howard-Pitney B, Killen JD, Fortmann SP. Quitting Chew: Results from a Randomized Trial Using Nicotine Patches. Exp Clin Psychopharmacol. 1999;7:362-371.
6. Hatsukami DK, Grillo M, Boyle R, et al. Treatment of Spit Tobacco Users with Transdermal Nicotine System and Mint Snuff. J Consult Clin Psychol. l2000;68:241-249.
7. Stotts RC, Roberson PK, Hanna EY, Jones SK, Smith CK. A Randomised Clinical Trial of Nicotine Patches for Treatment of Spit Tobacco Addiction Among Adolescents. Tob Control. 2003;12(S4):iv11-15.
8. Ebbert JO, Post JA, Moyer, TP, et al. Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug Alcohol Depend. 2007;89:223-226.
9. Boyle RG. Smokeless Tobacco Cessation with Nicotine Replacement: A Randomized Clinical Trial. Dissertation Abstracts International. 1992;54:825.-
10. Hatsukami D, Jensen J, Allen S, Grillo M, Bliss R. Effects of Behavioral and Pharmacological Treatment on Smokeless Tobacco Users. J Consult Clin Psychol. 1996;64:153-161.
11. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; August 2007.
12. Berigan TR, Deagle EA. Treatment of Smokeless Tobacco Addiction with Bupropion and Behavior Modification. J Am Med Assoc. 1999;281:233.-
13. Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A Comparison of Sustained-Release Bupropion and Placebo for Smokeless Tobacco Cessation. Am J Health Behav. 2002;26:389-393.
14. Dale LC, Ebbert JO, Schroeder DR, et al. Bupropion for the Treatment of Nicotine Dependence in Spit Tobacco Users: A Pilot Study. Nicotine Tob Res. 2002;4:267-274.
15. US Food and Drug Administration. FDA Approves Novel Medication for Smoking Cessation. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01370.html. Accessed March 6, 2007.
16. Chantix [package insert]. New York, NY: Pfizer Labs; January 2008.
Correspondence Terri M. Wensel, PharmD, 316 North Main Street, Campus Box 3087, Wingate, NC 28174; [email protected].
- Nicotine replacement therapy may be useful for short-term treatment of cravings, but may not improve cessation rates among patients who use smokeless tobacco (B).
- Patients who use >3 cans of smokeless tobacco a day may need higher than normal doses (42 mg/day) of nicotine replacement therapy (B).
- Evidence is insufficient to support the routine use of bupropion (Zyban) for smokeless tobacco cessation. It should be initiated at the physician’s discretion (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Through with Chew Week” and the “Great American Spitout.” Do they ring a bell?
If you answered no, you’re not alone.
“Through with Chew Week” was established by the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) in 1989, but it hasn’t quite garnered the same kind of recognition as the American Cancer Society’s Great American Smokeout.
When it comes to tobacco use—and more importantly, cessation—smokeless tobacco just doesn’t generate the kind of attention that cigarette smoking does. In fact, smokeless tobacco’s low profile extends beyond talk of “smokeouts” and “spitouts” to research on effective ways to quit.
We found this out first-hand when we conducted a literature search of Medline, PubMed, and a number of other databases to learn which cessation methods have proven efficacy. What we learned is that not only is research on the subject of smokeless tobacco cessation limited, but there is no recommended medication therapy to help these patients quit. Specifically:
- Nicotine-replacement therapies have failed to demonstrate a clear benefit in smokeless tobacco cessation, but under-dosing may be a factor for some patients.
- Bupropion’s (Zyban) usefulness in smokeless tobacco cessation is unclear. Data, thus far, have been inconclusive.
- Varenicline’s (Chantix) usefulness in smokeless tobacco cessation is unknown. There are no published case reports or clinical trials on the subject.
Millions of “chew” users are at risk
Tobacco use is the leading preventable cause of premature death in the United States, with more than 440,000 Americans dying of tobacco-related disease each year.1 Cigarette smoking is by far the most common form of tobacco used; however, smokeless tobacco, also known as chew, spit tobacco, or snuff, is used by 8.2 million Americans.2 More men than women use tobacco products overall, and smokeless tobacco, in particular.2
Health risks include MI. Specific health risks associated with smokeless tobacco include cancer of the oral cavity and pharynx, oral and periodontal disease, tooth decay, and pregnancy-related problems.1 (See “Smokeless tobacco was to blame”)
In addition, an international, case-control study evaluating the risk of myocardial infarction associated with various forms of tobacco use found an increased risk of myocardial infarction associated with smokeless tobacco use compared to non-tobacco users (odds ratio [OR]=2.23; 95% confidence interval [CI], 1.41-3.52).3
Notably, smokeless tobacco users in the study who also smoked cigarettes had the highest risk of all tobacco users when compared to non-users (OR=4.09; 95% CI, 2.98-5.61). These findings demonstrate that nicotine dependence is detrimental to health—regardless of the form of tobacco used. Even more worrisome is the notion that risk may actually be increased when multiple forms of tobacco are used by the same patient.
Patients want medication to help them quit
Current guidelines for tobacco cessation recommend that patients using smokeless tobacco should be identified, urged to quit, and treated with counseling interventions.4 Despite this recommendation, many patients are interested in using a medication to aid them in their quit effort, and many physicians would like to prescribe medication to help patients succeed.
To that end, it seemed logical to us that the same treatments used for smoking cessation would also be effective for smokeless tobacco cessation, given that the underlying problem—despite the form of tobacco used—is nicotine dependence. So we did a literature review to determine the optimal treatment for smokeless tobacco cessation. Our search included: Medline (1950-2007), PubMed (1966-2006), International Pharmaceutical Abstracts (1970-2006), Science Direct, CINAHL, PsycArticles, and Dissertation Abstracts. We used the following search terms: smokeless tobacco, spit tobacco, chew tobacco, cessation, bupropion, nicotine, and nicotine replacement. (Searches in Medline for nicotine replacement therapy in smokeless tobacco cessation were limited to clinical trials.) What we found were a limited number of studies, which we’ve summarized here.
Nicotine patch is useful, but to what degree?
We reviewed four studies involving the use of a nicotine patch for smokeless tobacco cessation (TABLE 1). In chronological order:
Study #1: 15-mg patch. The first study to evaluate the nicotine patch was a randomized, double-blind, placebo controlled trial published in 1999. A 15-mg nicotine patch was used by approximately 420 patients.5 Patients included in the trial were at least 18 years of age, nonsmokers, and used at least 1 can of smokeless tobacco per week. The main outcome of this trial was cessation at 6 months.
A significant difference was found in abstinence rates between patients treated with active therapy compared to those treated with placebo early in the study; however, by study end, there were no significant differences between groups. The medication was well tolerated, though patients receiving active therapy did report an increase in adverse effects related to patch use, such as rash and itching.
TABLE 1
Conflicting findings on the nicotine patch for smokeless tobacco cessation
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Howard-Pitney5 (1999) | 420 patients • ≥18 years, nonsmokers • ≥1 can/week | • Nicotine patch (15 mg) • Placebo patch | • Written materials • Telephone contact | 6 months | No significant difference in cessation rates | N/A |
| Hatsukami6 (2000) | 400 patients • 1 can/week | • Nicotine patch (began on 22 mg and tapered to 7 mg by study end) + mint snuff • Nicotine patch (same tapering as above) + no mint snuff • Placebo patch + mint snuff • Placebo patch + no mint snuff | • Written materials • 10 minute behavioral counseling | 10 weeks | Patients receiving active NRT had significantly higher quit rates at 10 and 15 weeks (P=0.002 and 0.016, respectively) | Patients with mint snuff: • 10 weeks: 4.3 • 15 weeks: 5.5 Patients w/o mint snuff: • 10 weeks: 14.3 • 15 weeks: 20 |
| Stotts7 (2003) | 300 patients • 14- to 19- year-old males • ST use ≥5 days/week | • Nicotine patch • Placebo patch • Usual care | • Patch: Six, 50- minute behavioral counseling sessions • Usual care: one, 5- to 10-minute counseling session with one follow-up telephone contact | 1 year | No significant difference in cessation rates at 1 year | N/A |
| Ebbert8 (2007) | 42 patients • ≥18 years, in good health • ≥3 cans or pouches/day | • Nicotine patch (21, 42, or 63 mg/day) • Placebo patch | • None | 3 days | NRT with 42 mg/day provided similar levels of nicotine to active ST users | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; ST, smokeless tobacco. | ||||||
Study #2: Nicotine plus mint snuff. The second trial to evaluate nicotine patches randomized approximately 400 patients to 1 of 4 groups: active nicotine patch plus mint snuff (non-nicotine mint-leaf product), active nicotine patch and no mint snuff, placebo plus mint snuff, or placebo patch and no mint snuff.6 To be enrolled in the study, patients had to use 1 can of smokeless tobacco per week and express a desire to quit. Patients given the active nicotine patch were begun on 22 mg and tapered to 7 mg by the end of 10 weeks. At 10 weeks, continuous abstinence and abstinence since the last clinic visit were evaluated. (Researchers tracked the patients for a total of 62 weeks.)
Patients who received active nicotine patches had significantly higher cessation rates compared to placebo at 10 (P=0.002) and 15 (P=0.016) weeks, but not at any other time during the study. No information regarding adverse effects was reported.
Study #3: Patch in adolescents. In a third, randomized, placebo-controlled trial, the effect of nicotine replacement in adolescents was evaluated. Patients were randomized to an active patch, placebo patch, or usual care (1 counseling session with a follow-up phone call).7
Patients who received an active patch also received 6 weeks of behavior counseling sessions. Smokeless tobacco users were further stratified according to light/moderate or heavy use according to saliva cotinine levels: light/moderate users were started on 14 mg and heavy users on 21 mg of nicotine. All patients were tapered to 7 mg over a 6-week period. Approximately 300 males between the ages of 14 and 19 who used smokeless tobacco at least 5 days a week were enrolled in the study.
Cessation rates at 1 year were not statistically significant between the placebo and active patch (P=0.22). The nicotine patch was well tolerated, with the exception of 5 patients who experienced skin irritation or headache; however, 3 patients were removed from patch therapy due to skin hyper-reaction or headache.
Study #4: Dosing. A fourth study, by Ebbert et al,8 addressed potential under-dosing of nicotine patches. This study was a randomized, double-blind, placebo controlled trial that evaluated 21, 42, or 63 mg/day of nicotine delivered by patch or placebo in 42 patients. (The 21 mg/day dose is the highest recommended starting dose for smokers.) The patients had to be 18 years of age or older, in good health, and use 3 or more cans/pouches of smokeless tobacco per day.
The study took place in 3 phases: outpatient preadmission, inpatient research phase, and an outpatient follow-up phase. The inpatient phase measured nicotine and cotinine concentrations during active smokeless tobacco use and while receiving treatment or placebo. Patients treated with the 42 mg/day patch had levels closer to those measured during active smokeless tobacco use, while patients treated with 63 mg/day tended to be “over-replaced” and experienced more severe adverse effects, such as nausea, vomiting, and headache. Overall, nicotine replacement therapy was well tolerated throughout the trial.
Nicotine gum: No lasting benefit
The earliest trial to evaluate nicotine gum for smokeless tobacco cessation was conducted by Boyle9 and was only available in abstract form (TABLE 2). This randomized, double-blind, placebo-controlled trial evaluated 2 mg of nicotine gum vs placebo in 100 patients. The main outcome was quit rates at the end of 6 weeks. The quit rate was similar for each group, and did not reach statistical significance (no P value provided). No information regarding adverse effects was reported.
A second trial10 to evaluate nicotine gum was also a randomized placebo-controlled trial that enrolled just over 200 adult patients that used ≥1 tin of smokeless tobacco per week (TABLE 2). Patients were randomized to 2 mg of nicotine gum or placebo and group therapy sessions or minimal behavioral intervention contact for a 2-month treatment period.
This trial did show a significant difference at week 4 (P<0.01) in point prevalence abstinence—that is, the number of people reporting to be abstinent at the 4-week mark, regardless of whether their abstinence was continuous or not. Significance, however, was lost by week 8. Cessation rates were also significant at 1- and 6-month follow-up exams (P<0.05), but were not maintained at the 12-month follow-up.
TABLE 2
Nicotine gum for smokeless tobacco cessation: Benefit doesn’t last
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Boyle9 (1992) | 100 patients | • Nicotine gum (2 mg) • Placebo gum | • Weekly group meetings | 6 weeks | No significant difference at 6 weeks | N/A |
| Hatsukami10 (1996) | 200 adult patients • ≥1 tin/week | • Nicotine gum (2 mg) • Placebo gum | • 8 group therapy sessions or • Minimal behavioral intervention | 12 months (8 weeks of treatment) | Point prevalence abstinence* significantly different at 4 weeks (P<0.01); no significant difference at 8 weeks | • Patients in group therapy at 4 weeks: 42 • Patients with minimal intervention at 4 weeks: 7.8 |
| NNT, number needed to treat. | ||||||
| * Point prevalence abstinence is the number of patients reporting to be abstinent on a given day. | ||||||
Continuous abstinence rates were similar for all groups except the 2 mg nicotine and minimal intervention group. Patients in this group had significantly lower abstinence rates from all other groups (P<0.003). No information regarding adverse effects was reported.
Case report suggests bupropion has potential
Bupropion is a non-nicotine smoking cessation therapy that inhibits the uptake of norepinephrine and dopamine. It is unclear how bupropion aids in smoking cessation, though its ability to do so appears to be related to its dopaminergic properties.11
Given its utility in treatment for smoking cessation, it seems plausible that the drug may serve as an effective treatment for smokeless tobacco, as well. However, the literature regarding the use of bupropion for smokeless tobacco cessation is even more limited than that for nicotine replacement therapy.
Berigan and Deagle first described the use of bupropion for smokeless tobacco cessation in 1999 (TABLE 3).12 Their case report focused on a 31-year-old man who reported using 1 can of smokeless tobacco per day for 11 years. The patient reported having tried nicotine patches and attempting to quit “cold turkey,” with limited success.
The patient was started on a trial of bupropion along with approximately 1 month of behavioral counseling. After 1 week of medication, the patient reported a reduction in cravings. By 5 weeks, the patient reported no use of smokeless tobacco. A total of 10 weeks of medication was given to the patient and he experienced no withdrawal effects after cessation. At 8 months, the patient was still tobacco free. The patient also reported few adverse effects from bupropion.
2 bupropion clinical trials are less encouraging
We found 2 clinical trials evaluating sustained-release bupropion 300 mg/day (TABLE 3). The first was a randomized, placebo-controlled trial in which patients received 7 weeks of active or placebo medication, and were then followed (with minimal counseling) for an additional 5 weeks.13 A total of 70 men, 18 years of age and older, were enrolled in the study; all had expressed a desire to quit and used at least one-half can of smokeless tobacco a day in the past year.
The primary endpoint was smokeless tobacco abstinence during weeks 4 through 7 of the trial. At the end of week 7 (end of treatment), significantly more patients in the treatment group were free of smokeless tobacco, compared to the placebo group (OR=2.73; 95% CI, 1.07-7.72; P=0.04). However, significance was lost by week 12 (OR=1.93; 95% CI, 0.71-5.47; P=0.2). Bupropion was well tolerated throughout this trial and no serious adverse effects were reported.
TABLE 3
Usefulness of bupropion for smokeless tobacco cessation is unclear
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Berigan12 (1999) | 1 can/day | • Bupropion | • Behavioral counseling | 10 weeks | Patient in case report remained ST free at 8 months | N/A |
| Glover13 (2002) | 70 patients • ≥18 year-old- men • ≥½ can/day | • Bupropion SR • Placebo | • Telephone sessions (weeks 9-11) | 3 months | Patients receiving active NRT had significantly higher quit rates by week 7 (P=0.04) | 4.3 |
| Dale14 (2002) | 68 patients • ≥18 years • Regularly used ST | • Bupropion SR • Placebo | • 10 minute behavioral counseling | 24 weeks | No significant difference | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; SR, sustained release; ST, smokeless tobacco. | ||||||
The second trial14 evaluating the efficacy of bupropion for smokeless tobacco cessation was also a randomized, placebo-controlled trial. Enrollees were randomly assigned to bupropion SR 300 mg/day or matching placebo for 12 weeks. In addition, minimal behavioral intervention was provided until week 24. Researchers enrolled 68 patients; all but one were men. Participants in this study were 18 years of age or older who regularly used smokeless tobacco. The efficacy of bupropion SR for nicotine dependence was the primary endpoint evaluated.
At the completion of the treatment phase, more patients in the bupropion group reported cessation (44%) compared to placebo (26%). The difference between the 2 groups was nonsignificant and remained so until the end of the study. By study end, each group had a reported cessation rate of 29%.
One patient experienced a diffuse skin rash that resulted in discontinuation of the study medication; otherwise buproprion was well tolerated.
A product that shows promise for smoking cessation is varenicline, which was approved by the FDA in May 2006.15 Varenicline acts as an agonist at the α4β2 neuronal nicotinic acetylcholine receptors. Its action at this receptor subtype blocks the ability of nicotine to bind to the receptor and is therefore thought to blunt the feeling of reward experienced by smokers.16 In addition, the medication may possess some nicotine-like action at the nicotinic receptor that may decrease the amount of withdrawal symptoms experienced by patients.15
In theory, this mechanism of action may help smokeless tobacco users to succeed in their quit attempts. Unfortunately, though, there have been no published case reports or clinical trials to date regarding the use of varenicline in the treatment of smokeless tobacco cessation.
Helping patients despite the information gap
Our knowledge of the effectiveness of various tools in smokeless tobacco cessation is hampered on a number of fronts.
Nicotine replacement therapy. The conflicting evidence regarding nicotine replacement therapy may be the result of a number of factors. First, although many of the trials used a randomized, placebo-controlled design, there were a number of variations in the patient populations studied, inconsistencies in the amount and type of counseling used, and differences in the amount of smokeless tobacco used by the patients.
These 2 cases illustrate the damage that chew can do
Oral leukoplakia
Gum recession
This 24-year-old patient sought treatment for an asymptomatic oral lesion that he noticed while brushing his teeth. The patient, who did not smoke tobacco and drank alcohol socially on weekends, told us that he had started chewing tobacco while playing baseball in high school 8 years earlier. He said that over the past 3 years, he had increased the habit.
On examination, we noted a well-defined, macular, white lesion on his left buccal mucosa. The lesion was not painful with palpation and remained unchanged when scraped with a tongue depressor.
Our patient had an oral leukoplakia, which can result from the chronic use of chewing tobacco. Cessation of the habit typically results in the resolution of the lesion in approximately 4 weeks. That was the case with our patient: He quit the habit and in 4 weeks, 80% of the lesion resolved. He was closely monitored, and at 3 months the lesion was undetectable.
If, however, a patient discontinues the habit and there is no change in the lesion after 4 weeks, biopsy is indicated. The most serious consequence of a malignant transformation of leukoplakia is oral squamous cell carcinoma.
A 30-year-old man was referred to us for evaluation of gum recession that had worsened over the past year. The patient complained that his teeth were sensitive to hot and cold drinks, but had no other symptoms. He had a 5-year history of smokeless tobacco use and said he usually placed the tobacco along his inner vestibule. He said that he did not smoke tobacco, nor did he drink alcohol.
On examination, we noted that the patient had localized recession along the cervical area of his lower teeth. With manipulation, there was bleeding from the gingival surface. His teeth were otherwise in good shape and there were no other lesions within the oral mucosa.
Gingival recession is a common consequence of smokeless tobacco use. Treatment consists of a gingival graft, in which palatal connective tissue is removed and used to reestablish gingival attachment to the tooth. (Tissue harvesting surgery from the palate can be quite painful.) Left untreated, teeth can become loose and fall out.
We advised our patient to follow up for gingival grafting, but he did not return for his follow-up visit.
Denise Rizzolo, PA-C, PhD is a physician assistant at Care Station in Springfield, NJ and Thomas A. Chiodo, DDS, is an oral and maxillofacial surgeon in private practice in Somerville, NJ. Dr. Chiodo also teaches at UMDNJ Dental School in Newark, NJ; [email protected]
Additionally, recent evidence suggests it may be important to consider dosing nicotine replacement according to number of cans of smokeless tobacco the patient uses per week. Heavy smokeless tobacco users may require higher initial doses than routinely used for smoking cessation therapy (eg, 42 mg/day if >3 cans of smokeless tobacco/day used).8
Further clinical research is needed to validate this recommendation, as well as to give specific dosing recommendations.
Nicotine gum. Two issues come to mind when considering the research on nicotine gum. First, the studies had relatively small sample sizes and it’s possible that the studies were not properly designed to detect a difference between groups.6,7 Second, the concept of dosing nicotine gum based on the number of cans of smokeless tobacco used per day has not been explored.
Bupropion. Studies involving bupropion and smokeless tobacco cessation share several of the limitations we’ve just discussed. Differing results reported in the literature regarding bupropion’s effectiveness makes its potential benefit unclear. Use should be determined on a case-by-case basis with the understanding that it may—or may not—be useful in decreasing cravings or increasing abstinence rates.
Varenicline. There is currently no literature available to help us evaluate the usefulness of varenicline in smokeless tobacco cessation. The mechanism of action of varenicline is such that use in smokeless tobacco cessation is plausible. Again, consideration of patients on a case-by-case basis is warranted.
One size does not fit all
Although nicotine dependence is the underlying problem for patients who utilize smokeless tobacco, current literature does not support a “one-size-fits-all” approach to treatment of various forms of tobacco abuse. Further clinical investigations are needed to determine the true utility of bupropion and varenicline, as well as the appropriate dosing of nicotine replacement therapy when prescribed for smokeless tobacco cessation.
- Nicotine replacement therapy may be useful for short-term treatment of cravings, but may not improve cessation rates among patients who use smokeless tobacco (B).
- Patients who use >3 cans of smokeless tobacco a day may need higher than normal doses (42 mg/day) of nicotine replacement therapy (B).
- Evidence is insufficient to support the routine use of bupropion (Zyban) for smokeless tobacco cessation. It should be initiated at the physician’s discretion (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Through with Chew Week” and the “Great American Spitout.” Do they ring a bell?
If you answered no, you’re not alone.
“Through with Chew Week” was established by the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) in 1989, but it hasn’t quite garnered the same kind of recognition as the American Cancer Society’s Great American Smokeout.
When it comes to tobacco use—and more importantly, cessation—smokeless tobacco just doesn’t generate the kind of attention that cigarette smoking does. In fact, smokeless tobacco’s low profile extends beyond talk of “smokeouts” and “spitouts” to research on effective ways to quit.
We found this out first-hand when we conducted a literature search of Medline, PubMed, and a number of other databases to learn which cessation methods have proven efficacy. What we learned is that not only is research on the subject of smokeless tobacco cessation limited, but there is no recommended medication therapy to help these patients quit. Specifically:
- Nicotine-replacement therapies have failed to demonstrate a clear benefit in smokeless tobacco cessation, but under-dosing may be a factor for some patients.
- Bupropion’s (Zyban) usefulness in smokeless tobacco cessation is unclear. Data, thus far, have been inconclusive.
- Varenicline’s (Chantix) usefulness in smokeless tobacco cessation is unknown. There are no published case reports or clinical trials on the subject.
Millions of “chew” users are at risk
Tobacco use is the leading preventable cause of premature death in the United States, with more than 440,000 Americans dying of tobacco-related disease each year.1 Cigarette smoking is by far the most common form of tobacco used; however, smokeless tobacco, also known as chew, spit tobacco, or snuff, is used by 8.2 million Americans.2 More men than women use tobacco products overall, and smokeless tobacco, in particular.2
Health risks include MI. Specific health risks associated with smokeless tobacco include cancer of the oral cavity and pharynx, oral and periodontal disease, tooth decay, and pregnancy-related problems.1 (See “Smokeless tobacco was to blame”)
In addition, an international, case-control study evaluating the risk of myocardial infarction associated with various forms of tobacco use found an increased risk of myocardial infarction associated with smokeless tobacco use compared to non-tobacco users (odds ratio [OR]=2.23; 95% confidence interval [CI], 1.41-3.52).3
Notably, smokeless tobacco users in the study who also smoked cigarettes had the highest risk of all tobacco users when compared to non-users (OR=4.09; 95% CI, 2.98-5.61). These findings demonstrate that nicotine dependence is detrimental to health—regardless of the form of tobacco used. Even more worrisome is the notion that risk may actually be increased when multiple forms of tobacco are used by the same patient.
Patients want medication to help them quit
Current guidelines for tobacco cessation recommend that patients using smokeless tobacco should be identified, urged to quit, and treated with counseling interventions.4 Despite this recommendation, many patients are interested in using a medication to aid them in their quit effort, and many physicians would like to prescribe medication to help patients succeed.
To that end, it seemed logical to us that the same treatments used for smoking cessation would also be effective for smokeless tobacco cessation, given that the underlying problem—despite the form of tobacco used—is nicotine dependence. So we did a literature review to determine the optimal treatment for smokeless tobacco cessation. Our search included: Medline (1950-2007), PubMed (1966-2006), International Pharmaceutical Abstracts (1970-2006), Science Direct, CINAHL, PsycArticles, and Dissertation Abstracts. We used the following search terms: smokeless tobacco, spit tobacco, chew tobacco, cessation, bupropion, nicotine, and nicotine replacement. (Searches in Medline for nicotine replacement therapy in smokeless tobacco cessation were limited to clinical trials.) What we found were a limited number of studies, which we’ve summarized here.
Nicotine patch is useful, but to what degree?
We reviewed four studies involving the use of a nicotine patch for smokeless tobacco cessation (TABLE 1). In chronological order:
Study #1: 15-mg patch. The first study to evaluate the nicotine patch was a randomized, double-blind, placebo controlled trial published in 1999. A 15-mg nicotine patch was used by approximately 420 patients.5 Patients included in the trial were at least 18 years of age, nonsmokers, and used at least 1 can of smokeless tobacco per week. The main outcome of this trial was cessation at 6 months.
A significant difference was found in abstinence rates between patients treated with active therapy compared to those treated with placebo early in the study; however, by study end, there were no significant differences between groups. The medication was well tolerated, though patients receiving active therapy did report an increase in adverse effects related to patch use, such as rash and itching.
TABLE 1
Conflicting findings on the nicotine patch for smokeless tobacco cessation
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Howard-Pitney5 (1999) | 420 patients • ≥18 years, nonsmokers • ≥1 can/week | • Nicotine patch (15 mg) • Placebo patch | • Written materials • Telephone contact | 6 months | No significant difference in cessation rates | N/A |
| Hatsukami6 (2000) | 400 patients • 1 can/week | • Nicotine patch (began on 22 mg and tapered to 7 mg by study end) + mint snuff • Nicotine patch (same tapering as above) + no mint snuff • Placebo patch + mint snuff • Placebo patch + no mint snuff | • Written materials • 10 minute behavioral counseling | 10 weeks | Patients receiving active NRT had significantly higher quit rates at 10 and 15 weeks (P=0.002 and 0.016, respectively) | Patients with mint snuff: • 10 weeks: 4.3 • 15 weeks: 5.5 Patients w/o mint snuff: • 10 weeks: 14.3 • 15 weeks: 20 |
| Stotts7 (2003) | 300 patients • 14- to 19- year-old males • ST use ≥5 days/week | • Nicotine patch • Placebo patch • Usual care | • Patch: Six, 50- minute behavioral counseling sessions • Usual care: one, 5- to 10-minute counseling session with one follow-up telephone contact | 1 year | No significant difference in cessation rates at 1 year | N/A |
| Ebbert8 (2007) | 42 patients • ≥18 years, in good health • ≥3 cans or pouches/day | • Nicotine patch (21, 42, or 63 mg/day) • Placebo patch | • None | 3 days | NRT with 42 mg/day provided similar levels of nicotine to active ST users | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; ST, smokeless tobacco. | ||||||
Study #2: Nicotine plus mint snuff. The second trial to evaluate nicotine patches randomized approximately 400 patients to 1 of 4 groups: active nicotine patch plus mint snuff (non-nicotine mint-leaf product), active nicotine patch and no mint snuff, placebo plus mint snuff, or placebo patch and no mint snuff.6 To be enrolled in the study, patients had to use 1 can of smokeless tobacco per week and express a desire to quit. Patients given the active nicotine patch were begun on 22 mg and tapered to 7 mg by the end of 10 weeks. At 10 weeks, continuous abstinence and abstinence since the last clinic visit were evaluated. (Researchers tracked the patients for a total of 62 weeks.)
Patients who received active nicotine patches had significantly higher cessation rates compared to placebo at 10 (P=0.002) and 15 (P=0.016) weeks, but not at any other time during the study. No information regarding adverse effects was reported.
Study #3: Patch in adolescents. In a third, randomized, placebo-controlled trial, the effect of nicotine replacement in adolescents was evaluated. Patients were randomized to an active patch, placebo patch, or usual care (1 counseling session with a follow-up phone call).7
Patients who received an active patch also received 6 weeks of behavior counseling sessions. Smokeless tobacco users were further stratified according to light/moderate or heavy use according to saliva cotinine levels: light/moderate users were started on 14 mg and heavy users on 21 mg of nicotine. All patients were tapered to 7 mg over a 6-week period. Approximately 300 males between the ages of 14 and 19 who used smokeless tobacco at least 5 days a week were enrolled in the study.
Cessation rates at 1 year were not statistically significant between the placebo and active patch (P=0.22). The nicotine patch was well tolerated, with the exception of 5 patients who experienced skin irritation or headache; however, 3 patients were removed from patch therapy due to skin hyper-reaction or headache.
Study #4: Dosing. A fourth study, by Ebbert et al,8 addressed potential under-dosing of nicotine patches. This study was a randomized, double-blind, placebo controlled trial that evaluated 21, 42, or 63 mg/day of nicotine delivered by patch or placebo in 42 patients. (The 21 mg/day dose is the highest recommended starting dose for smokers.) The patients had to be 18 years of age or older, in good health, and use 3 or more cans/pouches of smokeless tobacco per day.
The study took place in 3 phases: outpatient preadmission, inpatient research phase, and an outpatient follow-up phase. The inpatient phase measured nicotine and cotinine concentrations during active smokeless tobacco use and while receiving treatment or placebo. Patients treated with the 42 mg/day patch had levels closer to those measured during active smokeless tobacco use, while patients treated with 63 mg/day tended to be “over-replaced” and experienced more severe adverse effects, such as nausea, vomiting, and headache. Overall, nicotine replacement therapy was well tolerated throughout the trial.
Nicotine gum: No lasting benefit
The earliest trial to evaluate nicotine gum for smokeless tobacco cessation was conducted by Boyle9 and was only available in abstract form (TABLE 2). This randomized, double-blind, placebo-controlled trial evaluated 2 mg of nicotine gum vs placebo in 100 patients. The main outcome was quit rates at the end of 6 weeks. The quit rate was similar for each group, and did not reach statistical significance (no P value provided). No information regarding adverse effects was reported.
A second trial10 to evaluate nicotine gum was also a randomized placebo-controlled trial that enrolled just over 200 adult patients that used ≥1 tin of smokeless tobacco per week (TABLE 2). Patients were randomized to 2 mg of nicotine gum or placebo and group therapy sessions or minimal behavioral intervention contact for a 2-month treatment period.
This trial did show a significant difference at week 4 (P<0.01) in point prevalence abstinence—that is, the number of people reporting to be abstinent at the 4-week mark, regardless of whether their abstinence was continuous or not. Significance, however, was lost by week 8. Cessation rates were also significant at 1- and 6-month follow-up exams (P<0.05), but were not maintained at the 12-month follow-up.
TABLE 2
Nicotine gum for smokeless tobacco cessation: Benefit doesn’t last
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Boyle9 (1992) | 100 patients | • Nicotine gum (2 mg) • Placebo gum | • Weekly group meetings | 6 weeks | No significant difference at 6 weeks | N/A |
| Hatsukami10 (1996) | 200 adult patients • ≥1 tin/week | • Nicotine gum (2 mg) • Placebo gum | • 8 group therapy sessions or • Minimal behavioral intervention | 12 months (8 weeks of treatment) | Point prevalence abstinence* significantly different at 4 weeks (P<0.01); no significant difference at 8 weeks | • Patients in group therapy at 4 weeks: 42 • Patients with minimal intervention at 4 weeks: 7.8 |
| NNT, number needed to treat. | ||||||
| * Point prevalence abstinence is the number of patients reporting to be abstinent on a given day. | ||||||
Continuous abstinence rates were similar for all groups except the 2 mg nicotine and minimal intervention group. Patients in this group had significantly lower abstinence rates from all other groups (P<0.003). No information regarding adverse effects was reported.
Case report suggests bupropion has potential
Bupropion is a non-nicotine smoking cessation therapy that inhibits the uptake of norepinephrine and dopamine. It is unclear how bupropion aids in smoking cessation, though its ability to do so appears to be related to its dopaminergic properties.11
Given its utility in treatment for smoking cessation, it seems plausible that the drug may serve as an effective treatment for smokeless tobacco, as well. However, the literature regarding the use of bupropion for smokeless tobacco cessation is even more limited than that for nicotine replacement therapy.
Berigan and Deagle first described the use of bupropion for smokeless tobacco cessation in 1999 (TABLE 3).12 Their case report focused on a 31-year-old man who reported using 1 can of smokeless tobacco per day for 11 years. The patient reported having tried nicotine patches and attempting to quit “cold turkey,” with limited success.
The patient was started on a trial of bupropion along with approximately 1 month of behavioral counseling. After 1 week of medication, the patient reported a reduction in cravings. By 5 weeks, the patient reported no use of smokeless tobacco. A total of 10 weeks of medication was given to the patient and he experienced no withdrawal effects after cessation. At 8 months, the patient was still tobacco free. The patient also reported few adverse effects from bupropion.
2 bupropion clinical trials are less encouraging
We found 2 clinical trials evaluating sustained-release bupropion 300 mg/day (TABLE 3). The first was a randomized, placebo-controlled trial in which patients received 7 weeks of active or placebo medication, and were then followed (with minimal counseling) for an additional 5 weeks.13 A total of 70 men, 18 years of age and older, were enrolled in the study; all had expressed a desire to quit and used at least one-half can of smokeless tobacco a day in the past year.
The primary endpoint was smokeless tobacco abstinence during weeks 4 through 7 of the trial. At the end of week 7 (end of treatment), significantly more patients in the treatment group were free of smokeless tobacco, compared to the placebo group (OR=2.73; 95% CI, 1.07-7.72; P=0.04). However, significance was lost by week 12 (OR=1.93; 95% CI, 0.71-5.47; P=0.2). Bupropion was well tolerated throughout this trial and no serious adverse effects were reported.
TABLE 3
Usefulness of bupropion for smokeless tobacco cessation is unclear
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Berigan12 (1999) | 1 can/day | • Bupropion | • Behavioral counseling | 10 weeks | Patient in case report remained ST free at 8 months | N/A |
| Glover13 (2002) | 70 patients • ≥18 year-old- men • ≥½ can/day | • Bupropion SR • Placebo | • Telephone sessions (weeks 9-11) | 3 months | Patients receiving active NRT had significantly higher quit rates by week 7 (P=0.04) | 4.3 |
| Dale14 (2002) | 68 patients • ≥18 years • Regularly used ST | • Bupropion SR • Placebo | • 10 minute behavioral counseling | 24 weeks | No significant difference | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; SR, sustained release; ST, smokeless tobacco. | ||||||
The second trial14 evaluating the efficacy of bupropion for smokeless tobacco cessation was also a randomized, placebo-controlled trial. Enrollees were randomly assigned to bupropion SR 300 mg/day or matching placebo for 12 weeks. In addition, minimal behavioral intervention was provided until week 24. Researchers enrolled 68 patients; all but one were men. Participants in this study were 18 years of age or older who regularly used smokeless tobacco. The efficacy of bupropion SR for nicotine dependence was the primary endpoint evaluated.
At the completion of the treatment phase, more patients in the bupropion group reported cessation (44%) compared to placebo (26%). The difference between the 2 groups was nonsignificant and remained so until the end of the study. By study end, each group had a reported cessation rate of 29%.
One patient experienced a diffuse skin rash that resulted in discontinuation of the study medication; otherwise buproprion was well tolerated.
A product that shows promise for smoking cessation is varenicline, which was approved by the FDA in May 2006.15 Varenicline acts as an agonist at the α4β2 neuronal nicotinic acetylcholine receptors. Its action at this receptor subtype blocks the ability of nicotine to bind to the receptor and is therefore thought to blunt the feeling of reward experienced by smokers.16 In addition, the medication may possess some nicotine-like action at the nicotinic receptor that may decrease the amount of withdrawal symptoms experienced by patients.15
In theory, this mechanism of action may help smokeless tobacco users to succeed in their quit attempts. Unfortunately, though, there have been no published case reports or clinical trials to date regarding the use of varenicline in the treatment of smokeless tobacco cessation.
Helping patients despite the information gap
Our knowledge of the effectiveness of various tools in smokeless tobacco cessation is hampered on a number of fronts.
Nicotine replacement therapy. The conflicting evidence regarding nicotine replacement therapy may be the result of a number of factors. First, although many of the trials used a randomized, placebo-controlled design, there were a number of variations in the patient populations studied, inconsistencies in the amount and type of counseling used, and differences in the amount of smokeless tobacco used by the patients.
These 2 cases illustrate the damage that chew can do
Oral leukoplakia
Gum recession
This 24-year-old patient sought treatment for an asymptomatic oral lesion that he noticed while brushing his teeth. The patient, who did not smoke tobacco and drank alcohol socially on weekends, told us that he had started chewing tobacco while playing baseball in high school 8 years earlier. He said that over the past 3 years, he had increased the habit.
On examination, we noted a well-defined, macular, white lesion on his left buccal mucosa. The lesion was not painful with palpation and remained unchanged when scraped with a tongue depressor.
Our patient had an oral leukoplakia, which can result from the chronic use of chewing tobacco. Cessation of the habit typically results in the resolution of the lesion in approximately 4 weeks. That was the case with our patient: He quit the habit and in 4 weeks, 80% of the lesion resolved. He was closely monitored, and at 3 months the lesion was undetectable.
If, however, a patient discontinues the habit and there is no change in the lesion after 4 weeks, biopsy is indicated. The most serious consequence of a malignant transformation of leukoplakia is oral squamous cell carcinoma.
A 30-year-old man was referred to us for evaluation of gum recession that had worsened over the past year. The patient complained that his teeth were sensitive to hot and cold drinks, but had no other symptoms. He had a 5-year history of smokeless tobacco use and said he usually placed the tobacco along his inner vestibule. He said that he did not smoke tobacco, nor did he drink alcohol.
On examination, we noted that the patient had localized recession along the cervical area of his lower teeth. With manipulation, there was bleeding from the gingival surface. His teeth were otherwise in good shape and there were no other lesions within the oral mucosa.
Gingival recession is a common consequence of smokeless tobacco use. Treatment consists of a gingival graft, in which palatal connective tissue is removed and used to reestablish gingival attachment to the tooth. (Tissue harvesting surgery from the palate can be quite painful.) Left untreated, teeth can become loose and fall out.
We advised our patient to follow up for gingival grafting, but he did not return for his follow-up visit.
Denise Rizzolo, PA-C, PhD is a physician assistant at Care Station in Springfield, NJ and Thomas A. Chiodo, DDS, is an oral and maxillofacial surgeon in private practice in Somerville, NJ. Dr. Chiodo also teaches at UMDNJ Dental School in Newark, NJ; [email protected]
Additionally, recent evidence suggests it may be important to consider dosing nicotine replacement according to number of cans of smokeless tobacco the patient uses per week. Heavy smokeless tobacco users may require higher initial doses than routinely used for smoking cessation therapy (eg, 42 mg/day if >3 cans of smokeless tobacco/day used).8
Further clinical research is needed to validate this recommendation, as well as to give specific dosing recommendations.
Nicotine gum. Two issues come to mind when considering the research on nicotine gum. First, the studies had relatively small sample sizes and it’s possible that the studies were not properly designed to detect a difference between groups.6,7 Second, the concept of dosing nicotine gum based on the number of cans of smokeless tobacco used per day has not been explored.
Bupropion. Studies involving bupropion and smokeless tobacco cessation share several of the limitations we’ve just discussed. Differing results reported in the literature regarding bupropion’s effectiveness makes its potential benefit unclear. Use should be determined on a case-by-case basis with the understanding that it may—or may not—be useful in decreasing cravings or increasing abstinence rates.
Varenicline. There is currently no literature available to help us evaluate the usefulness of varenicline in smokeless tobacco cessation. The mechanism of action of varenicline is such that use in smokeless tobacco cessation is plausible. Again, consideration of patients on a case-by-case basis is warranted.
One size does not fit all
Although nicotine dependence is the underlying problem for patients who utilize smokeless tobacco, current literature does not support a “one-size-fits-all” approach to treatment of various forms of tobacco abuse. Further clinical investigations are needed to determine the true utility of bupropion and varenicline, as well as the appropriate dosing of nicotine replacement therapy when prescribed for smokeless tobacco cessation.
1. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Tobacco Use: Prevention, Cessation and Control. Ann Intern Med 2006;145:839-844.
2. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. Available at: www.oas.samhsa.gov/nsduh/2k6nsduh/2k6results.pdf. Accessed February 26, 2008.
3. Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658.
4. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
5. Howard-Pitney B, Killen JD, Fortmann SP. Quitting Chew: Results from a Randomized Trial Using Nicotine Patches. Exp Clin Psychopharmacol. 1999;7:362-371.
6. Hatsukami DK, Grillo M, Boyle R, et al. Treatment of Spit Tobacco Users with Transdermal Nicotine System and Mint Snuff. J Consult Clin Psychol. l2000;68:241-249.
7. Stotts RC, Roberson PK, Hanna EY, Jones SK, Smith CK. A Randomised Clinical Trial of Nicotine Patches for Treatment of Spit Tobacco Addiction Among Adolescents. Tob Control. 2003;12(S4):iv11-15.
8. Ebbert JO, Post JA, Moyer, TP, et al. Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug Alcohol Depend. 2007;89:223-226.
9. Boyle RG. Smokeless Tobacco Cessation with Nicotine Replacement: A Randomized Clinical Trial. Dissertation Abstracts International. 1992;54:825.-
10. Hatsukami D, Jensen J, Allen S, Grillo M, Bliss R. Effects of Behavioral and Pharmacological Treatment on Smokeless Tobacco Users. J Consult Clin Psychol. 1996;64:153-161.
11. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; August 2007.
12. Berigan TR, Deagle EA. Treatment of Smokeless Tobacco Addiction with Bupropion and Behavior Modification. J Am Med Assoc. 1999;281:233.-
13. Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A Comparison of Sustained-Release Bupropion and Placebo for Smokeless Tobacco Cessation. Am J Health Behav. 2002;26:389-393.
14. Dale LC, Ebbert JO, Schroeder DR, et al. Bupropion for the Treatment of Nicotine Dependence in Spit Tobacco Users: A Pilot Study. Nicotine Tob Res. 2002;4:267-274.
15. US Food and Drug Administration. FDA Approves Novel Medication for Smoking Cessation. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01370.html. Accessed March 6, 2007.
16. Chantix [package insert]. New York, NY: Pfizer Labs; January 2008.
Correspondence Terri M. Wensel, PharmD, 316 North Main Street, Campus Box 3087, Wingate, NC 28174; [email protected].
1. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Tobacco Use: Prevention, Cessation and Control. Ann Intern Med 2006;145:839-844.
2. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. Available at: www.oas.samhsa.gov/nsduh/2k6nsduh/2k6results.pdf. Accessed February 26, 2008.
3. Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658.
4. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
5. Howard-Pitney B, Killen JD, Fortmann SP. Quitting Chew: Results from a Randomized Trial Using Nicotine Patches. Exp Clin Psychopharmacol. 1999;7:362-371.
6. Hatsukami DK, Grillo M, Boyle R, et al. Treatment of Spit Tobacco Users with Transdermal Nicotine System and Mint Snuff. J Consult Clin Psychol. l2000;68:241-249.
7. Stotts RC, Roberson PK, Hanna EY, Jones SK, Smith CK. A Randomised Clinical Trial of Nicotine Patches for Treatment of Spit Tobacco Addiction Among Adolescents. Tob Control. 2003;12(S4):iv11-15.
8. Ebbert JO, Post JA, Moyer, TP, et al. Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug Alcohol Depend. 2007;89:223-226.
9. Boyle RG. Smokeless Tobacco Cessation with Nicotine Replacement: A Randomized Clinical Trial. Dissertation Abstracts International. 1992;54:825.-
10. Hatsukami D, Jensen J, Allen S, Grillo M, Bliss R. Effects of Behavioral and Pharmacological Treatment on Smokeless Tobacco Users. J Consult Clin Psychol. 1996;64:153-161.
11. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; August 2007.
12. Berigan TR, Deagle EA. Treatment of Smokeless Tobacco Addiction with Bupropion and Behavior Modification. J Am Med Assoc. 1999;281:233.-
13. Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A Comparison of Sustained-Release Bupropion and Placebo for Smokeless Tobacco Cessation. Am J Health Behav. 2002;26:389-393.
14. Dale LC, Ebbert JO, Schroeder DR, et al. Bupropion for the Treatment of Nicotine Dependence in Spit Tobacco Users: A Pilot Study. Nicotine Tob Res. 2002;4:267-274.
15. US Food and Drug Administration. FDA Approves Novel Medication for Smoking Cessation. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01370.html. Accessed March 6, 2007.
16. Chantix [package insert]. New York, NY: Pfizer Labs; January 2008.
Correspondence Terri M. Wensel, PharmD, 316 North Main Street, Campus Box 3087, Wingate, NC 28174; [email protected].