User login
CERVICAL DISEASE
The author is a consultant to Merck & Co., Inc., GlaxoSmithKline, and Roche Molecular Diagnostics.
New data enhance our knowledge in two critical areas previously covered in this update: the human papillomavirus (HPV) vaccine and HPV DNA testing for cervical cancer screening.
Among findings published in 2007:
- results of phase-3 trials of the quadrivalent and bivalent HPV vaccines, which confirm the remarkable efficacy seen in phase-2 trials among women not previously exposed to the vaccine-targeted HPV types
- three large randomized cervical cancer screening trials from Canada, Sweden, and the Netherlands, which confirm the superiority of cervical cancer screening programs that add HPV DNA testing to cytology in women 30 years and older
- the much-anticipated update of consensus guidelines on the management of women with abnormal cervical cancer screening tests.
Efficacy of HPV vaccine approaches 100% in targeted population
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943.
These trials, referred to as FUTURE I and II, were multicenter, multicountry, double-blinded, placebo-controlled trials of the quadrivalent vaccine (Gardasil) that enrolled women 15 to 26 years of age (TABLE).1 Participants were followed for an average of 3 years after receiving the first of the series of three vaccinations. The efficacy of the vaccine at preventing high-grade neoplasia associated with vaccine-targeted HPV types (HPV 6, 11, 16, and 18) was 98% and 100% in the two trials among the “per-protocol,” susceptible population. That population was defined as women who had no evidence of exposure to the targeted HPV types, according to serologic or HPV DNA testing during the first 7 months of the trials, and who received all three vaccinations. This population is a good indicator of how the vaccine will work in adolescents who are not yet sexually active (TABLE).
Efficacy is high in key phase-3 trials of the HPV vaccine
| AUTHORS | HPV TYPES TARGETED | WOMEN (N) | FOLLOW-UP | ENDPOINT | VACCINE EFFICACY (%)* |
|---|---|---|---|---|---|
| Garland et al (FUTURE I) (2007) | 6, 11, 16, 18 | 4,499 | 3 years | CIN 2+ and adenocarcinoma in situ | 100 (95% CI, 94–100) |
| FUTURE II Study Group (2007) | 6, 11, 16, 18 | 12,167 | 3 years | CIN 2+ and adenocarcinoma in situ | 98 (95% CI, 86–100) |
| Joura et al1 (2007) | 6, 11, 16, 18 | 15,596 | 3 years | Vulvar intraepithelial neoplasia 2+ and vaginal intraepithelial neoplasia 2+ | 100 (95% CI, 72–100) |
| Paavonen et al (2007) | 16, 18 | 15,626 | 15 months | CIN 2+ | 90 (95% CI, 53–99) |
| * In women naïve to vaccine-targeted HPV types by serology and HPV DNA testing. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia. | |||||
The vaccine was less effective in the “intention-to-treat” population that included all women enrolled in the study regardless of HPV status. At 3 years, the vaccine reduced high-grade neoplasia associated with vaccine-targeted HPV types in this population by only 29% and 50% in the two trials.
When these results were published, some experts expressed concern and questioned the benefit of the vaccines.2 There is no reason for concern, however, because lower short-term efficacy in the intention-to-treat population was expected. Because the current generation of HPV vaccines does not have a measurable therapeutic effect, vaccination will not prevent cervical intraepithelial neoplasia (CIN) in women who are already infected with vaccine-targeted HPV types; nor will it cause regression of CIN lesions that are already present when the woman is vaccinated.
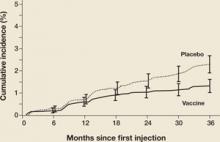
There were a number of women already infected with vaccine-targeted HPV types at enrollment in the “intention-to-treat” population. Some of these women developed CIN 2,3 associated with vaccine-targeted HPV strains during the first 18 months of the trial (FIGURE 1). However, with longer follow-up, the cumulative number of cases of CIN 2,3 plateaued in vaccinated women, whereas it continued to rise in the placebo arm. Thus, with longer follow-up, vaccine efficacy will improve. Therefore, both the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices recommend that all sexually active adolescents and young women be vaccinated through 26 years of age.
Figure 1 Cases of CIN 2,3 eventually plateau in vaccinated women
The graph charts the efficacy of the quadrivalent HPV vaccine in preventing CIN 2,3 in the intention-to-treat population of a phase-3 efficacy trial.
SOURCE: Garland et al. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Bivalent vaccine was 90.4% effective against CIN 2,3
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170.
This interim analysis of a phase-3 double-blind, placebo-controlled trial of the bivalent HPV vaccine (Cervarix), which targets HPV types 16 and 18, also was published last year. It enrolled more than 18,000 women 15 to 25 years old who had a mean length of follow-up of 15 months. The vaccine was 90.4% effective against CIN 2,3 associated with the targeted strains (types 16 and 18), the primary endpoint of the trial (TABLE). There was no significant difference in safety outcome between vaccine and placebo recipients.
This trial is ongoing, with final results expected in approximately 2 years. Based on interim findings, the vaccine has been approved for use in a number of countries, including all 27 European Union nations, and the manufacturer of the vaccine has filed an application for approval with the US Food and Drug Administration (FDA).
Screening is more effective when HPV testing is included—or used alone
Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772.
Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597.
Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588.
These major studies compared cytology alone with HPV DNA testing for high-risk strains (16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68) and found HPV testing—with or without cytology—to be superior to cytology alone.
In a trial from the Netherlands, Bulkmans and colleagues randomly assigned more than 17,000 women 29 years and older to cytologic screening only or a combination of cytology and HPV DNA testing. After 5 years of follow-up, all women were rescreened using both tests. The baseline screen including a combination of cytology and HPV DNA testing identified 70% more CIN 3 lesions and cancers than did cytology alone. More important, during the subsequent round of screening, CIN 3 lesions and cancers decreased by 55% in the group initially screened with both tests.
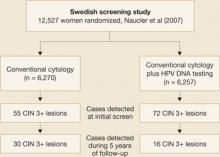
Naucler and associates had similar results in a prospective Swedish trial that randomized women to screening by cytology alone or a combination of cytology and HPV DNA testing. During the initial round of screening, 31% more CIN 3 lesions and cancers were detected in the group screened with both tests (FIGURE 2). In subsequent rounds of screening, 47% fewer CIN 3 lesions or cancers were identified in this group.
Figure 2 Screening protocol that includes HPV DNA testing is superior, large trial confirmsTaken together, these two prospective studies clearly demonstrate that the addition of HPV DNA testing to cytology increases detection of high-grade lesions and reduces the incidence of high-grade neoplasia and cancers detected subsequently.
HPV testing is more sensitive, only slightly less specific, than cytology
In a cross-sectional study from Canada, Mayrand and colleagues compared HPV DNA testing and cytology during a single round of screening in more than 10,000 women. The findings were consistent with those of previous studies showing HPV DNA testing to be significantly more sensitive but somewhat less specific than cytology.3
The sensitivity of HPV testing for CIN 2,3 was 95% (95% confidence interval [CI], 84–100), compared with 55% (95% CI, 34–77) for cytology. Specificity of HPV DNA testing and cytology was 94% and 97%, respectively. When the two tests were used together, sensitivity was 100% and specificity was 93%.
New consensus guidelines clarify screening in special populations
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355.
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345.
The 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests clarify management of special populations such as adolescents, postmenopausal women, and patients with cervical adenocarcinoma in situ. Although the 2001 guidelines were widely adopted in the United States as the standard for managing women with abnormal screening tests—more than 500,000 copies were downloaded from Web site of the American Society for Colposcopy and Cervical Pathology (ASCCP)4—it became apparent after their implementation in a variety of clinical settings that some clarification of the guidelines was needed.
In adolescents, treat abnormalities conservatively
A major theme of the 2006 guidelines is a more conservative approach to adolescent patients (ages 13 to 20 years). Although this population has a very low risk of developing invasive cervical cancer, women 15 to 19 years of age are very likely to be diagnosed with minor cytologic abnormalities such as atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesions (LSIL), owing to the very high prevalence of anogenital HPV infection in this age group.
Because most anogenital HPV infections will spontaneously clear, minor cytologic abnormalities are usually of little consequence in adolescents.
Therefore, the 2006 consensus guidelines discourage the use of colposcopy in adolescents who have ASC-US and LSIL. Instead, these patients should be followed with annual repeat cytology and referred to colposcopy only when a high-grade cytologic abnormality is identified or when a low-grade cytologic abnormality persists for 24 months.
HPV testing most informative in older women
The new guidelines expand the clinical indications for HPV DNA testing and provide recommendations for managing different combinations of cytology and HPV test results when screening women 30 years and older. For example, they emphasize the use of HPV DNA testing in postmenopausal women because recent studies clearly demonstrate that the prevalence of high-risk HPV DNA positivity is lower in postmenopausal women with ASC-US or LSIL than in younger women.
Use only FDA-approved HPV tests
With the expanded indications for HPV DNA testing, the new guidelines take pains to point out that HPV test methods that have not been approved by the FDA may not produce findings consistent with approved methods. This is a very important point because many laboratories have started using unapproved testing methods. Although these methods have been validated internally by the laboratories, they have not been through the rigorous evaluation required for FDA approval. The new guidelines therefore state: “Appropriate use of these guidelines requires that laboratories utilize only HPV tests that have been analytically and clinically validated with proven acceptable reproducibility, clinical sensitivity, specificity, and positive and negative predictive values for cervical cancer and verified precancer (CIN 2,3), as documented by FDA approval and/or publication in peer-reviewed scientific literature.”
The guidelines are accessible online at the ASCCP Web site at www.asccp.org/consensus/cytological.shtml.
1. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
2. Sawaya GF. Smith-McCune K. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
3. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
4. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA .2002;287:2120-2129.
The author is a consultant to Merck & Co., Inc., GlaxoSmithKline, and Roche Molecular Diagnostics.
New data enhance our knowledge in two critical areas previously covered in this update: the human papillomavirus (HPV) vaccine and HPV DNA testing for cervical cancer screening.
Among findings published in 2007:
- results of phase-3 trials of the quadrivalent and bivalent HPV vaccines, which confirm the remarkable efficacy seen in phase-2 trials among women not previously exposed to the vaccine-targeted HPV types
- three large randomized cervical cancer screening trials from Canada, Sweden, and the Netherlands, which confirm the superiority of cervical cancer screening programs that add HPV DNA testing to cytology in women 30 years and older
- the much-anticipated update of consensus guidelines on the management of women with abnormal cervical cancer screening tests.
Efficacy of HPV vaccine approaches 100% in targeted population
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943.
These trials, referred to as FUTURE I and II, were multicenter, multicountry, double-blinded, placebo-controlled trials of the quadrivalent vaccine (Gardasil) that enrolled women 15 to 26 years of age (TABLE).1 Participants were followed for an average of 3 years after receiving the first of the series of three vaccinations. The efficacy of the vaccine at preventing high-grade neoplasia associated with vaccine-targeted HPV types (HPV 6, 11, 16, and 18) was 98% and 100% in the two trials among the “per-protocol,” susceptible population. That population was defined as women who had no evidence of exposure to the targeted HPV types, according to serologic or HPV DNA testing during the first 7 months of the trials, and who received all three vaccinations. This population is a good indicator of how the vaccine will work in adolescents who are not yet sexually active (TABLE).
Efficacy is high in key phase-3 trials of the HPV vaccine
| AUTHORS | HPV TYPES TARGETED | WOMEN (N) | FOLLOW-UP | ENDPOINT | VACCINE EFFICACY (%)* |
|---|---|---|---|---|---|
| Garland et al (FUTURE I) (2007) | 6, 11, 16, 18 | 4,499 | 3 years | CIN 2+ and adenocarcinoma in situ | 100 (95% CI, 94–100) |
| FUTURE II Study Group (2007) | 6, 11, 16, 18 | 12,167 | 3 years | CIN 2+ and adenocarcinoma in situ | 98 (95% CI, 86–100) |
| Joura et al1 (2007) | 6, 11, 16, 18 | 15,596 | 3 years | Vulvar intraepithelial neoplasia 2+ and vaginal intraepithelial neoplasia 2+ | 100 (95% CI, 72–100) |
| Paavonen et al (2007) | 16, 18 | 15,626 | 15 months | CIN 2+ | 90 (95% CI, 53–99) |
| * In women naïve to vaccine-targeted HPV types by serology and HPV DNA testing. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia. | |||||
The vaccine was less effective in the “intention-to-treat” population that included all women enrolled in the study regardless of HPV status. At 3 years, the vaccine reduced high-grade neoplasia associated with vaccine-targeted HPV types in this population by only 29% and 50% in the two trials.
When these results were published, some experts expressed concern and questioned the benefit of the vaccines.2 There is no reason for concern, however, because lower short-term efficacy in the intention-to-treat population was expected. Because the current generation of HPV vaccines does not have a measurable therapeutic effect, vaccination will not prevent cervical intraepithelial neoplasia (CIN) in women who are already infected with vaccine-targeted HPV types; nor will it cause regression of CIN lesions that are already present when the woman is vaccinated.
There were a number of women already infected with vaccine-targeted HPV types at enrollment in the “intention-to-treat” population. Some of these women developed CIN 2,3 associated with vaccine-targeted HPV strains during the first 18 months of the trial (FIGURE 1). However, with longer follow-up, the cumulative number of cases of CIN 2,3 plateaued in vaccinated women, whereas it continued to rise in the placebo arm. Thus, with longer follow-up, vaccine efficacy will improve. Therefore, both the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices recommend that all sexually active adolescents and young women be vaccinated through 26 years of age.
Figure 1 Cases of CIN 2,3 eventually plateau in vaccinated women
The graph charts the efficacy of the quadrivalent HPV vaccine in preventing CIN 2,3 in the intention-to-treat population of a phase-3 efficacy trial.
SOURCE: Garland et al. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Bivalent vaccine was 90.4% effective against CIN 2,3
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170.
This interim analysis of a phase-3 double-blind, placebo-controlled trial of the bivalent HPV vaccine (Cervarix), which targets HPV types 16 and 18, also was published last year. It enrolled more than 18,000 women 15 to 25 years old who had a mean length of follow-up of 15 months. The vaccine was 90.4% effective against CIN 2,3 associated with the targeted strains (types 16 and 18), the primary endpoint of the trial (TABLE). There was no significant difference in safety outcome between vaccine and placebo recipients.
This trial is ongoing, with final results expected in approximately 2 years. Based on interim findings, the vaccine has been approved for use in a number of countries, including all 27 European Union nations, and the manufacturer of the vaccine has filed an application for approval with the US Food and Drug Administration (FDA).
Screening is more effective when HPV testing is included—or used alone
Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772.
Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597.
Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588.
These major studies compared cytology alone with HPV DNA testing for high-risk strains (16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68) and found HPV testing—with or without cytology—to be superior to cytology alone.
In a trial from the Netherlands, Bulkmans and colleagues randomly assigned more than 17,000 women 29 years and older to cytologic screening only or a combination of cytology and HPV DNA testing. After 5 years of follow-up, all women were rescreened using both tests. The baseline screen including a combination of cytology and HPV DNA testing identified 70% more CIN 3 lesions and cancers than did cytology alone. More important, during the subsequent round of screening, CIN 3 lesions and cancers decreased by 55% in the group initially screened with both tests.
Naucler and associates had similar results in a prospective Swedish trial that randomized women to screening by cytology alone or a combination of cytology and HPV DNA testing. During the initial round of screening, 31% more CIN 3 lesions and cancers were detected in the group screened with both tests (FIGURE 2). In subsequent rounds of screening, 47% fewer CIN 3 lesions or cancers were identified in this group.
Figure 2 Screening protocol that includes HPV DNA testing is superior, large trial confirmsTaken together, these two prospective studies clearly demonstrate that the addition of HPV DNA testing to cytology increases detection of high-grade lesions and reduces the incidence of high-grade neoplasia and cancers detected subsequently.
HPV testing is more sensitive, only slightly less specific, than cytology
In a cross-sectional study from Canada, Mayrand and colleagues compared HPV DNA testing and cytology during a single round of screening in more than 10,000 women. The findings were consistent with those of previous studies showing HPV DNA testing to be significantly more sensitive but somewhat less specific than cytology.3
The sensitivity of HPV testing for CIN 2,3 was 95% (95% confidence interval [CI], 84–100), compared with 55% (95% CI, 34–77) for cytology. Specificity of HPV DNA testing and cytology was 94% and 97%, respectively. When the two tests were used together, sensitivity was 100% and specificity was 93%.
New consensus guidelines clarify screening in special populations
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355.
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345.
The 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests clarify management of special populations such as adolescents, postmenopausal women, and patients with cervical adenocarcinoma in situ. Although the 2001 guidelines were widely adopted in the United States as the standard for managing women with abnormal screening tests—more than 500,000 copies were downloaded from Web site of the American Society for Colposcopy and Cervical Pathology (ASCCP)4—it became apparent after their implementation in a variety of clinical settings that some clarification of the guidelines was needed.
In adolescents, treat abnormalities conservatively
A major theme of the 2006 guidelines is a more conservative approach to adolescent patients (ages 13 to 20 years). Although this population has a very low risk of developing invasive cervical cancer, women 15 to 19 years of age are very likely to be diagnosed with minor cytologic abnormalities such as atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesions (LSIL), owing to the very high prevalence of anogenital HPV infection in this age group.
Because most anogenital HPV infections will spontaneously clear, minor cytologic abnormalities are usually of little consequence in adolescents.
Therefore, the 2006 consensus guidelines discourage the use of colposcopy in adolescents who have ASC-US and LSIL. Instead, these patients should be followed with annual repeat cytology and referred to colposcopy only when a high-grade cytologic abnormality is identified or when a low-grade cytologic abnormality persists for 24 months.
HPV testing most informative in older women
The new guidelines expand the clinical indications for HPV DNA testing and provide recommendations for managing different combinations of cytology and HPV test results when screening women 30 years and older. For example, they emphasize the use of HPV DNA testing in postmenopausal women because recent studies clearly demonstrate that the prevalence of high-risk HPV DNA positivity is lower in postmenopausal women with ASC-US or LSIL than in younger women.
Use only FDA-approved HPV tests
With the expanded indications for HPV DNA testing, the new guidelines take pains to point out that HPV test methods that have not been approved by the FDA may not produce findings consistent with approved methods. This is a very important point because many laboratories have started using unapproved testing methods. Although these methods have been validated internally by the laboratories, they have not been through the rigorous evaluation required for FDA approval. The new guidelines therefore state: “Appropriate use of these guidelines requires that laboratories utilize only HPV tests that have been analytically and clinically validated with proven acceptable reproducibility, clinical sensitivity, specificity, and positive and negative predictive values for cervical cancer and verified precancer (CIN 2,3), as documented by FDA approval and/or publication in peer-reviewed scientific literature.”
The guidelines are accessible online at the ASCCP Web site at www.asccp.org/consensus/cytological.shtml.
The author is a consultant to Merck & Co., Inc., GlaxoSmithKline, and Roche Molecular Diagnostics.
New data enhance our knowledge in two critical areas previously covered in this update: the human papillomavirus (HPV) vaccine and HPV DNA testing for cervical cancer screening.
Among findings published in 2007:
- results of phase-3 trials of the quadrivalent and bivalent HPV vaccines, which confirm the remarkable efficacy seen in phase-2 trials among women not previously exposed to the vaccine-targeted HPV types
- three large randomized cervical cancer screening trials from Canada, Sweden, and the Netherlands, which confirm the superiority of cervical cancer screening programs that add HPV DNA testing to cytology in women 30 years and older
- the much-anticipated update of consensus guidelines on the management of women with abnormal cervical cancer screening tests.
Efficacy of HPV vaccine approaches 100% in targeted population
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943.
These trials, referred to as FUTURE I and II, were multicenter, multicountry, double-blinded, placebo-controlled trials of the quadrivalent vaccine (Gardasil) that enrolled women 15 to 26 years of age (TABLE).1 Participants were followed for an average of 3 years after receiving the first of the series of three vaccinations. The efficacy of the vaccine at preventing high-grade neoplasia associated with vaccine-targeted HPV types (HPV 6, 11, 16, and 18) was 98% and 100% in the two trials among the “per-protocol,” susceptible population. That population was defined as women who had no evidence of exposure to the targeted HPV types, according to serologic or HPV DNA testing during the first 7 months of the trials, and who received all three vaccinations. This population is a good indicator of how the vaccine will work in adolescents who are not yet sexually active (TABLE).
Efficacy is high in key phase-3 trials of the HPV vaccine
| AUTHORS | HPV TYPES TARGETED | WOMEN (N) | FOLLOW-UP | ENDPOINT | VACCINE EFFICACY (%)* |
|---|---|---|---|---|---|
| Garland et al (FUTURE I) (2007) | 6, 11, 16, 18 | 4,499 | 3 years | CIN 2+ and adenocarcinoma in situ | 100 (95% CI, 94–100) |
| FUTURE II Study Group (2007) | 6, 11, 16, 18 | 12,167 | 3 years | CIN 2+ and adenocarcinoma in situ | 98 (95% CI, 86–100) |
| Joura et al1 (2007) | 6, 11, 16, 18 | 15,596 | 3 years | Vulvar intraepithelial neoplasia 2+ and vaginal intraepithelial neoplasia 2+ | 100 (95% CI, 72–100) |
| Paavonen et al (2007) | 16, 18 | 15,626 | 15 months | CIN 2+ | 90 (95% CI, 53–99) |
| * In women naïve to vaccine-targeted HPV types by serology and HPV DNA testing. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia. | |||||
The vaccine was less effective in the “intention-to-treat” population that included all women enrolled in the study regardless of HPV status. At 3 years, the vaccine reduced high-grade neoplasia associated with vaccine-targeted HPV types in this population by only 29% and 50% in the two trials.
When these results were published, some experts expressed concern and questioned the benefit of the vaccines.2 There is no reason for concern, however, because lower short-term efficacy in the intention-to-treat population was expected. Because the current generation of HPV vaccines does not have a measurable therapeutic effect, vaccination will not prevent cervical intraepithelial neoplasia (CIN) in women who are already infected with vaccine-targeted HPV types; nor will it cause regression of CIN lesions that are already present when the woman is vaccinated.
There were a number of women already infected with vaccine-targeted HPV types at enrollment in the “intention-to-treat” population. Some of these women developed CIN 2,3 associated with vaccine-targeted HPV strains during the first 18 months of the trial (FIGURE 1). However, with longer follow-up, the cumulative number of cases of CIN 2,3 plateaued in vaccinated women, whereas it continued to rise in the placebo arm. Thus, with longer follow-up, vaccine efficacy will improve. Therefore, both the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices recommend that all sexually active adolescents and young women be vaccinated through 26 years of age.
Figure 1 Cases of CIN 2,3 eventually plateau in vaccinated women
The graph charts the efficacy of the quadrivalent HPV vaccine in preventing CIN 2,3 in the intention-to-treat population of a phase-3 efficacy trial.
SOURCE: Garland et al. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Bivalent vaccine was 90.4% effective against CIN 2,3
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170.
This interim analysis of a phase-3 double-blind, placebo-controlled trial of the bivalent HPV vaccine (Cervarix), which targets HPV types 16 and 18, also was published last year. It enrolled more than 18,000 women 15 to 25 years old who had a mean length of follow-up of 15 months. The vaccine was 90.4% effective against CIN 2,3 associated with the targeted strains (types 16 and 18), the primary endpoint of the trial (TABLE). There was no significant difference in safety outcome between vaccine and placebo recipients.
This trial is ongoing, with final results expected in approximately 2 years. Based on interim findings, the vaccine has been approved for use in a number of countries, including all 27 European Union nations, and the manufacturer of the vaccine has filed an application for approval with the US Food and Drug Administration (FDA).
Screening is more effective when HPV testing is included—or used alone
Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772.
Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597.
Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588.
These major studies compared cytology alone with HPV DNA testing for high-risk strains (16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68) and found HPV testing—with or without cytology—to be superior to cytology alone.
In a trial from the Netherlands, Bulkmans and colleagues randomly assigned more than 17,000 women 29 years and older to cytologic screening only or a combination of cytology and HPV DNA testing. After 5 years of follow-up, all women were rescreened using both tests. The baseline screen including a combination of cytology and HPV DNA testing identified 70% more CIN 3 lesions and cancers than did cytology alone. More important, during the subsequent round of screening, CIN 3 lesions and cancers decreased by 55% in the group initially screened with both tests.
Naucler and associates had similar results in a prospective Swedish trial that randomized women to screening by cytology alone or a combination of cytology and HPV DNA testing. During the initial round of screening, 31% more CIN 3 lesions and cancers were detected in the group screened with both tests (FIGURE 2). In subsequent rounds of screening, 47% fewer CIN 3 lesions or cancers were identified in this group.
Figure 2 Screening protocol that includes HPV DNA testing is superior, large trial confirmsTaken together, these two prospective studies clearly demonstrate that the addition of HPV DNA testing to cytology increases detection of high-grade lesions and reduces the incidence of high-grade neoplasia and cancers detected subsequently.
HPV testing is more sensitive, only slightly less specific, than cytology
In a cross-sectional study from Canada, Mayrand and colleagues compared HPV DNA testing and cytology during a single round of screening in more than 10,000 women. The findings were consistent with those of previous studies showing HPV DNA testing to be significantly more sensitive but somewhat less specific than cytology.3
The sensitivity of HPV testing for CIN 2,3 was 95% (95% confidence interval [CI], 84–100), compared with 55% (95% CI, 34–77) for cytology. Specificity of HPV DNA testing and cytology was 94% and 97%, respectively. When the two tests were used together, sensitivity was 100% and specificity was 93%.
New consensus guidelines clarify screening in special populations
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355.
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345.
The 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests clarify management of special populations such as adolescents, postmenopausal women, and patients with cervical adenocarcinoma in situ. Although the 2001 guidelines were widely adopted in the United States as the standard for managing women with abnormal screening tests—more than 500,000 copies were downloaded from Web site of the American Society for Colposcopy and Cervical Pathology (ASCCP)4—it became apparent after their implementation in a variety of clinical settings that some clarification of the guidelines was needed.
In adolescents, treat abnormalities conservatively
A major theme of the 2006 guidelines is a more conservative approach to adolescent patients (ages 13 to 20 years). Although this population has a very low risk of developing invasive cervical cancer, women 15 to 19 years of age are very likely to be diagnosed with minor cytologic abnormalities such as atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesions (LSIL), owing to the very high prevalence of anogenital HPV infection in this age group.
Because most anogenital HPV infections will spontaneously clear, minor cytologic abnormalities are usually of little consequence in adolescents.
Therefore, the 2006 consensus guidelines discourage the use of colposcopy in adolescents who have ASC-US and LSIL. Instead, these patients should be followed with annual repeat cytology and referred to colposcopy only when a high-grade cytologic abnormality is identified or when a low-grade cytologic abnormality persists for 24 months.
HPV testing most informative in older women
The new guidelines expand the clinical indications for HPV DNA testing and provide recommendations for managing different combinations of cytology and HPV test results when screening women 30 years and older. For example, they emphasize the use of HPV DNA testing in postmenopausal women because recent studies clearly demonstrate that the prevalence of high-risk HPV DNA positivity is lower in postmenopausal women with ASC-US or LSIL than in younger women.
Use only FDA-approved HPV tests
With the expanded indications for HPV DNA testing, the new guidelines take pains to point out that HPV test methods that have not been approved by the FDA may not produce findings consistent with approved methods. This is a very important point because many laboratories have started using unapproved testing methods. Although these methods have been validated internally by the laboratories, they have not been through the rigorous evaluation required for FDA approval. The new guidelines therefore state: “Appropriate use of these guidelines requires that laboratories utilize only HPV tests that have been analytically and clinically validated with proven acceptable reproducibility, clinical sensitivity, specificity, and positive and negative predictive values for cervical cancer and verified precancer (CIN 2,3), as documented by FDA approval and/or publication in peer-reviewed scientific literature.”
The guidelines are accessible online at the ASCCP Web site at www.asccp.org/consensus/cytological.shtml.
1. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
2. Sawaya GF. Smith-McCune K. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
3. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
4. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA .2002;287:2120-2129.
1. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
2. Sawaya GF. Smith-McCune K. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
3. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
4. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA .2002;287:2120-2129.
What you need to know about cervical cancer, genital warts, and HPV
CERVICAL DISEASE
The year 2006 was a busy one for those of us engaged in cervical cancer prevention. The most notable development was approval by the US Food and Drug Administration of the human papillomavirus (HPV) quadrivalent vaccine in June, followed closely by guidelines for its use from the Advisory Committee on Immunization Practices (June) and the American College of Obstetricians and Gynecologists (September). Key issues related to the introduction of the HPV vaccine into clinical practice were reviewed in a roundtable discussion in the January 2007 issue of OBG Management.
Therefore, this update will depart, for the moment, from matters related to the vaccine and concentrate on several other critical areas:
- Testing for high-risk HPV types is useful. Large European cervical cancer screening trials confirm a benefit.
- Condoms and oral contraceptives—are they risk modifiers for HPV infection? Answers (“Yes” and “No,” respectively) come from new data.
- Loop electrosurgical excision carries obstetric risks. In fact, all types of excisional procedures produce similar pregnancy-related morbidity.
- Liquid-based cytology may not be superior to conventional cytology. So suggest new studies and a systematic review of the literature.
HPV testing outperforms cytology for screening
Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101.
Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765–774.
HPV DNA testing is more sensitive than cervical cytology, reduces specificity to only a moderate degree, and performs similarly in different parts of Europe and North America. Those are the findings of a review by Cuzick and colleagues of all recent large European and North American cervical cancer screening studies. To date, 4 large European trials and 1 from Mexico have directly compared HPV testing and cytology in women aged 30 years and older. Combined, these trials have enrolled over 60,000 women. In every study, testing for high-risk types of HPV using the commercially available Hybrid Capture 2 HPV DNA assay had a much higher sensitivity for identifying women with cervical intraepithelial neoplasia (CIN) 2,3 or cancer (86–97%) than did cervical cytology (34–74%). Moreover, the combination of cytology and HPV testing had a sensitivity ranging from 94% to 100% in the different studies. The average sensitivity of 98% for the combination of cytology and HPV testing means that there is a less than 1 in 1,000 chance of missing CIN 2,3 or cancer when women are screened with both tests (TABLE 1).
Specificity is reasonable, too
If you worry that using HPV DNA testing in women aged 30 and older will flag too many as high-risk HPV-positive and cause them unnecessary colposcopic examinations or anxiety, here is a comforting finding: The specificity of HPV DNA testing is not as low as many had feared—provided we limit screening to women aged 30 and older. In the 5 trials mentioned, the specificity of HPV DNA testing ranged from 92% to 97%. Even when HPV DNA testing and cytology were used together, the average specificity of the 2 tests combined was 93%. A specificity of 93% means that only 7 of 100 screened women who don’t have CIN 2,3 or cancer will be classified as “positive.”
To put this number into perspective, a 2003 survey of US cytology laboratories found a median rate of abnormal results of 6.9%.1 Therefore, in routine clinical practice, incorporation of HPV DNA testing into screening for women aged 30 and older is not expected to greatly increase the number of women requiring additional follow-up.
The single most important component of management is appropriate counseling. Even though there are fewer of these women than we anticipated, these patients need to be reassured that their risk of having a significant lesion (CIN 2,3 or cancer) is quite low—only about 1 in 20. They also need to know that about two thirds of women—even women aged 30 and older—are HPV-negative when they are retested in 12 months.
In addition, clinicians need to stress that positive HPV status is a risk factor for having or developing cervical disease, not an indication that disease is present. One analogy that patients readily understand is the relationship between other types of health risk factors, such as mild hypertension or mildly elevated serum cholesterol, and disease. These explanations help the patient understand why, in settings where genotyping for HPV 16 and 18 is not available, the best course of action is to wait 12 months and be retested.2
Which test should be used first?
Basic screening principles suggest that, whenever 2 tests are used in combination, the most sensitive test should be used first, with patients who test positive tested again using the second, more specific, test. These principles suggest we should be using HPV testing alone as the initial screening test and limiting the use of cytology to triage HPV-positive women. This sequence of testing could potentially be done in a “reflex” fashion.
The large Italian screening trial by Ronco and colleagues randomized 33,364 women aged 35 to 60 years to 2 different screening strategies: routine conventional cytology or liquid-based cytology with HPV testing.3 In the routine cytology arm, researchers identified only 51 cases of CIN 2,3 or cancer, but in the experimental arm, they identified 75 cases. A breakdown of the initial screening results in the women found to have CIN 2,3 or cancer in the experimental arm shows that cytology adds very little benefit. Only 2 of 75 women with CIN 2,3 or cancer were identified by cytology alone. In contrast, 21 (28%) of the cases of CIN 2,3 or cancer were in women who were high-risk HPV-positive and cytology-negative (TABLE 2).
TABLE 2
How cytology and HPV testing compare: Results from the Italian screening trial
| CYTOLOGY/HPV TEST | TOTAL NO. | CIN 2+ |
|---|---|---|
| ≥ASCUS/positive | 300 | 52 (69%) |
| ≥ASCUS/negative | 594 | 2 (3%) |
| Within normal limits/positive | 885 | 21 (28%) |
| Modified from Ronco G et al | ||
The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are under way.
OCs not linked to HPV infection, and condoms afford some protection
Vaccarella S, Lazcano-Ponce E, Castro-Garduno JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–1939.
Vaccarella S, Franceschi S, Herrero R, et al. Sexual behavior, condom use, and human papillomavirus: pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15:326–333.
Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–2654.
No question: Anogenital HPV infections are transmitted almost exclusively through intimate sexual contact.3 The standard markers of sexual exposure, such as the number of sexual partners and the number of partners that one’s partner has had, are key risk factors for infection with HPV. Women often ask whether other factors such as oral contraceptive (OC) use, diet, smoking, and condom use affect their risk for infection. But nonsexual risk factors are difficult to evaluate because the strength of sexual risk factors is so high.
To clarify the role played by non-sexual factors, the International Association for Research on Cancer (IARC) pooled data from multiple HPV prevalence studies involving more than 15,000 women from 14 different areas worldwide. This study clearly indicates that the use of OCs is not associated with HPV infection. Current, former, and never users of OCs all had the same risk of being HPV-positive. Therefore, although OCs are a risk factor for cervical cancer, the elevated risk cannot be explained by an increased susceptibility to HPV infection. This study also found that the menopausal transition had no clear effect on HPV infection.
New data show condoms to be more beneficial than not
Condoms are widely recognized as an effective barrier to the sexual transmission of HIV; their efficacy in blocking the transmission of other sexual diseases is less well documented. Most studies that have evaluated the impact of condom use on HPV infection have failed to find a beneficial effect. This may reflect the fact that condoms are often used inconsistently. In addition, there is a tendency to use condoms when having higher-risk sexual encounters, such as with a new partner.
In a recent study from Seattle, Winer and colleagues followed 82 female university students who first initiated sex while enrolled in the study or within 2 weeks of joining the study. The incidence of HPV infection was 38 per 100 patient-years of follow-up among women whose partners used condoms during all acts of intercourse, compared with 89.3 per 100 patient-years of follow-up among women whose partners used condoms less than 5% of the time. Risk reductions were observed for both high- and low-risk types of HPV.
Condoms also appeared to protect against the development of CIN. There were no cases of CIN during 32 patient-years among women whose partners consistently used condoms, compared with 14 cases of incident CIN during 97 patient-years of follow-up among women whose partners did not use condoms or who used them less consistently.
LEEP may have an adverse obstetric impact
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498.
Although most clinicians recognize that cold-knife conization has the potential to cause adverse obstetric outcomes, the same has not been recognized for loop electrosurgical excisional procedures (LEEP). In fact, most of the studies published in the early 1990s showed that LEEP had little impact on obstetric outcomes. Now we know better: Kyrgiou and colleagues conducted a systematic review and meta-analysis of the published literature on obstetric outcomes after treatment of CIN lesions, and found that all types of excisional procedures produce similar pregnancy-related morbidities.
LEEP had a significant association with preterm delivery (11% risk in treated women versus 7% in untreated women), low-birth-weight infants (8% in treated women versus 4% in untreated women), and premature rupture of membranes (5% in treated women versus 2% in untreated women). Although there were no significant increases in NICU admissions or perinatal mortality among the offspring of women who had undergone LEEP versus those who had not, nonsignificant increases were observed.
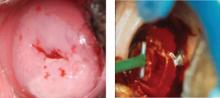
Similar increases in pregnancy-related morbidity were not observed among patients who underwent ablative procedures. This suggests that the amount of tissue that is removed during the LEEP (FIGURE 1) is important. Therefore, when treating CIN 2,3 lesions, especially in young women, consider using an ablative method such as cryotherapy or electrofulguration, unless colposcopy is unsatisfactory or there is a colposcopic or pathologic suspicion that an occult cancer is present.
FIGURE 1 CIN 2,3 and its treatment by LEEP
Is liquid-based cytology as sensitive as we thought?
Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122–132.
Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765–774.
Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC Jr. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006;118:957–962.
A major reappraisal of liquid-based cytology (LBC) is under way. When it was first introduced, LBC was believed to provide a significant advantage over conventional cervical cytology in terms of sensitivity for CIN 2,3 or cancer. However, most of the studies that compared the 2 modalities had severe methodological problems. Many utilized historical controls, and most others simply reported increases in the number of cases cytologically diagnosed as squamous intraepithelial lesions (SIL). Very few measured histologic endpoints, and the few studies that did failed to blind the pathologists evaluating the histology to the cytologic findings. Only 1 small study was randomized.
Focus on high-quality studies finds lower sensitivity for LBC
Recently, Davey and colleagues conducted a systematic review of the published literature comparing LBC with conventional cytology. A total of 56 studies were evaluated, 52 of which provided enough information to evaluate differences between the 2 methods in the detection of low-grade squamous intraepithelial lesions (LSIL) and high-grade SIL (HSIL). These 52 studies included more than 1.25 million slides.
None of the studies that were evaluated were judged to be of “ideal quality,” and only 5 were judged to be of “high quality.” When all of the studies are taken into account and combined, there appears to be an increase in the cytologic detection of LSIL and HSIL with the use of LBC. However, further evaluation showed marked differences in the results obtained by studies of different quality.
When only “high-quality” studies are analyzed, there is no indication that LBC increases the detection of HSIL. Davey and colleagues concluded that there is no evidence that LBC reduces the proportion of unsatisfactory slides or outperforms conventional cytology in identifying women with CIN 2,3. They also noted that large randomized trials are needed.
Little difference between modalities in randomized trials
After the systematic review was conducted, 2 large trials comparing LBC with conventional cytology were published. In the first trial, Taylor and colleagues collected samples from South African women and analyzed them in blinded fashion in US laboratories. In their carefully controlled study, 5,652 women received either LBC or conventional cytology (rotated on a 6-month basis), and all women underwent colposcopy and cervical biopsy. No significant difference was observed in the sensitivity of LBC and conventional cytology in the detection of CIN 2,3 or cancer. In fact, there was a nonsignificant increase in sensitivity with conventional cytology, compared with LBC. Positive predictive value was lower with LBC than with conventional cytology. This means that a smaller proportion of women with an abnormal result on LBC had CIN 2,3 or cancer identified at colposcopy than did women who had an abnormal result on conventional cytology.
Similarly, in a large trial from Italy, Ronco and colleagues also failed to find LBC to be more sensitive than conventional cytology. Their trial randomized 33,364 women to LBC or conventional cytology. The use of LBC did not increase the detection of CIN 2,3 or cancer, compared with conventional cytology, but did lead to a dramatic reduction (43%) in positive predictive value due to an increase in the number of abnormal samples.
A similar increase in minor cytologic abnormalities with the use of LBC is now well documented in the United States. According to surveys from the College of American Pathologists, the median percentile reporting rate of LSIL in US laboratories in 2003 was 1.4% for conventional cytology specimens and 2.4% for liquid-based specimens.1
Taken together (TABLE 3), these studies suggest that LBC has no greater sensitivity than conventional cytology and therefore does not solve the problems associated with the poor sensitivity of cervical cytology. However, LBC does have other advantages, the greatest being the availability of residual fluid for “reflex” HPV testing in women with ASC-US and for testing for other pathogens, such as Chlamydia.
TABLE 3
When conventional and liquid-based cytology are compared, the latter isn’t more sensitive
| STUDY | NO. WOMEN | CONVENTIONAL CYTOLOGY | LIQUID-BASED CYTOLOGY | ||
|---|---|---|---|---|---|
| SENSITIVITY* | PPV* | SENSITIVITY* | PPV* | ||
| Taylor et al | 5,652 | 84%† | 11.4 | 71% | 9.4 |
| Ronco et al | 33,364 | 70% | 11.4 | 74%† | 6.5 |
| PPV=positive predictive value | |||||
| *for detection of CIN 2,3 or cancer | |||||
| †Nonsignificant | |||||
Most cytologists find it easier to evaluate LBC specimens than conventional cytology specimens. Nor is it likely that cytology laboratories will want to switch back to conventional cytology now that the conversion to LBC has taken place.
Dr. Wright reports no financial relationships with any company whose products are mentioned in this article.
1. Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128:1224-1229.
2. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
3. Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 Suppl 3:S52-61.
The year 2006 was a busy one for those of us engaged in cervical cancer prevention. The most notable development was approval by the US Food and Drug Administration of the human papillomavirus (HPV) quadrivalent vaccine in June, followed closely by guidelines for its use from the Advisory Committee on Immunization Practices (June) and the American College of Obstetricians and Gynecologists (September). Key issues related to the introduction of the HPV vaccine into clinical practice were reviewed in a roundtable discussion in the January 2007 issue of OBG Management.
Therefore, this update will depart, for the moment, from matters related to the vaccine and concentrate on several other critical areas:
- Testing for high-risk HPV types is useful. Large European cervical cancer screening trials confirm a benefit.
- Condoms and oral contraceptives—are they risk modifiers for HPV infection? Answers (“Yes” and “No,” respectively) come from new data.
- Loop electrosurgical excision carries obstetric risks. In fact, all types of excisional procedures produce similar pregnancy-related morbidity.
- Liquid-based cytology may not be superior to conventional cytology. So suggest new studies and a systematic review of the literature.
HPV testing outperforms cytology for screening
Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101.
Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765–774.
HPV DNA testing is more sensitive than cervical cytology, reduces specificity to only a moderate degree, and performs similarly in different parts of Europe and North America. Those are the findings of a review by Cuzick and colleagues of all recent large European and North American cervical cancer screening studies. To date, 4 large European trials and 1 from Mexico have directly compared HPV testing and cytology in women aged 30 years and older. Combined, these trials have enrolled over 60,000 women. In every study, testing for high-risk types of HPV using the commercially available Hybrid Capture 2 HPV DNA assay had a much higher sensitivity for identifying women with cervical intraepithelial neoplasia (CIN) 2,3 or cancer (86–97%) than did cervical cytology (34–74%). Moreover, the combination of cytology and HPV testing had a sensitivity ranging from 94% to 100% in the different studies. The average sensitivity of 98% for the combination of cytology and HPV testing means that there is a less than 1 in 1,000 chance of missing CIN 2,3 or cancer when women are screened with both tests (TABLE 1).
Specificity is reasonable, too
If you worry that using HPV DNA testing in women aged 30 and older will flag too many as high-risk HPV-positive and cause them unnecessary colposcopic examinations or anxiety, here is a comforting finding: The specificity of HPV DNA testing is not as low as many had feared—provided we limit screening to women aged 30 and older. In the 5 trials mentioned, the specificity of HPV DNA testing ranged from 92% to 97%. Even when HPV DNA testing and cytology were used together, the average specificity of the 2 tests combined was 93%. A specificity of 93% means that only 7 of 100 screened women who don’t have CIN 2,3 or cancer will be classified as “positive.”
To put this number into perspective, a 2003 survey of US cytology laboratories found a median rate of abnormal results of 6.9%.1 Therefore, in routine clinical practice, incorporation of HPV DNA testing into screening for women aged 30 and older is not expected to greatly increase the number of women requiring additional follow-up.
The single most important component of management is appropriate counseling. Even though there are fewer of these women than we anticipated, these patients need to be reassured that their risk of having a significant lesion (CIN 2,3 or cancer) is quite low—only about 1 in 20. They also need to know that about two thirds of women—even women aged 30 and older—are HPV-negative when they are retested in 12 months.
In addition, clinicians need to stress that positive HPV status is a risk factor for having or developing cervical disease, not an indication that disease is present. One analogy that patients readily understand is the relationship between other types of health risk factors, such as mild hypertension or mildly elevated serum cholesterol, and disease. These explanations help the patient understand why, in settings where genotyping for HPV 16 and 18 is not available, the best course of action is to wait 12 months and be retested.2
Which test should be used first?
Basic screening principles suggest that, whenever 2 tests are used in combination, the most sensitive test should be used first, with patients who test positive tested again using the second, more specific, test. These principles suggest we should be using HPV testing alone as the initial screening test and limiting the use of cytology to triage HPV-positive women. This sequence of testing could potentially be done in a “reflex” fashion.
The large Italian screening trial by Ronco and colleagues randomized 33,364 women aged 35 to 60 years to 2 different screening strategies: routine conventional cytology or liquid-based cytology with HPV testing.3 In the routine cytology arm, researchers identified only 51 cases of CIN 2,3 or cancer, but in the experimental arm, they identified 75 cases. A breakdown of the initial screening results in the women found to have CIN 2,3 or cancer in the experimental arm shows that cytology adds very little benefit. Only 2 of 75 women with CIN 2,3 or cancer were identified by cytology alone. In contrast, 21 (28%) of the cases of CIN 2,3 or cancer were in women who were high-risk HPV-positive and cytology-negative (TABLE 2).
TABLE 2
How cytology and HPV testing compare: Results from the Italian screening trial
| CYTOLOGY/HPV TEST | TOTAL NO. | CIN 2+ |
|---|---|---|
| ≥ASCUS/positive | 300 | 52 (69%) |
| ≥ASCUS/negative | 594 | 2 (3%) |
| Within normal limits/positive | 885 | 21 (28%) |
| Modified from Ronco G et al | ||
The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are under way.
OCs not linked to HPV infection, and condoms afford some protection
Vaccarella S, Lazcano-Ponce E, Castro-Garduno JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–1939.
Vaccarella S, Franceschi S, Herrero R, et al. Sexual behavior, condom use, and human papillomavirus: pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15:326–333.
Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–2654.
No question: Anogenital HPV infections are transmitted almost exclusively through intimate sexual contact.3 The standard markers of sexual exposure, such as the number of sexual partners and the number of partners that one’s partner has had, are key risk factors for infection with HPV. Women often ask whether other factors such as oral contraceptive (OC) use, diet, smoking, and condom use affect their risk for infection. But nonsexual risk factors are difficult to evaluate because the strength of sexual risk factors is so high.
To clarify the role played by non-sexual factors, the International Association for Research on Cancer (IARC) pooled data from multiple HPV prevalence studies involving more than 15,000 women from 14 different areas worldwide. This study clearly indicates that the use of OCs is not associated with HPV infection. Current, former, and never users of OCs all had the same risk of being HPV-positive. Therefore, although OCs are a risk factor for cervical cancer, the elevated risk cannot be explained by an increased susceptibility to HPV infection. This study also found that the menopausal transition had no clear effect on HPV infection.
New data show condoms to be more beneficial than not
Condoms are widely recognized as an effective barrier to the sexual transmission of HIV; their efficacy in blocking the transmission of other sexual diseases is less well documented. Most studies that have evaluated the impact of condom use on HPV infection have failed to find a beneficial effect. This may reflect the fact that condoms are often used inconsistently. In addition, there is a tendency to use condoms when having higher-risk sexual encounters, such as with a new partner.
In a recent study from Seattle, Winer and colleagues followed 82 female university students who first initiated sex while enrolled in the study or within 2 weeks of joining the study. The incidence of HPV infection was 38 per 100 patient-years of follow-up among women whose partners used condoms during all acts of intercourse, compared with 89.3 per 100 patient-years of follow-up among women whose partners used condoms less than 5% of the time. Risk reductions were observed for both high- and low-risk types of HPV.
Condoms also appeared to protect against the development of CIN. There were no cases of CIN during 32 patient-years among women whose partners consistently used condoms, compared with 14 cases of incident CIN during 97 patient-years of follow-up among women whose partners did not use condoms or who used them less consistently.
LEEP may have an adverse obstetric impact
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498.
Although most clinicians recognize that cold-knife conization has the potential to cause adverse obstetric outcomes, the same has not been recognized for loop electrosurgical excisional procedures (LEEP). In fact, most of the studies published in the early 1990s showed that LEEP had little impact on obstetric outcomes. Now we know better: Kyrgiou and colleagues conducted a systematic review and meta-analysis of the published literature on obstetric outcomes after treatment of CIN lesions, and found that all types of excisional procedures produce similar pregnancy-related morbidities.
LEEP had a significant association with preterm delivery (11% risk in treated women versus 7% in untreated women), low-birth-weight infants (8% in treated women versus 4% in untreated women), and premature rupture of membranes (5% in treated women versus 2% in untreated women). Although there were no significant increases in NICU admissions or perinatal mortality among the offspring of women who had undergone LEEP versus those who had not, nonsignificant increases were observed.
Similar increases in pregnancy-related morbidity were not observed among patients who underwent ablative procedures. This suggests that the amount of tissue that is removed during the LEEP (FIGURE 1) is important. Therefore, when treating CIN 2,3 lesions, especially in young women, consider using an ablative method such as cryotherapy or electrofulguration, unless colposcopy is unsatisfactory or there is a colposcopic or pathologic suspicion that an occult cancer is present.
FIGURE 1 CIN 2,3 and its treatment by LEEP
Is liquid-based cytology as sensitive as we thought?
Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122–132.
Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765–774.
Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC Jr. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006;118:957–962.
A major reappraisal of liquid-based cytology (LBC) is under way. When it was first introduced, LBC was believed to provide a significant advantage over conventional cervical cytology in terms of sensitivity for CIN 2,3 or cancer. However, most of the studies that compared the 2 modalities had severe methodological problems. Many utilized historical controls, and most others simply reported increases in the number of cases cytologically diagnosed as squamous intraepithelial lesions (SIL). Very few measured histologic endpoints, and the few studies that did failed to blind the pathologists evaluating the histology to the cytologic findings. Only 1 small study was randomized.
Focus on high-quality studies finds lower sensitivity for LBC
Recently, Davey and colleagues conducted a systematic review of the published literature comparing LBC with conventional cytology. A total of 56 studies were evaluated, 52 of which provided enough information to evaluate differences between the 2 methods in the detection of low-grade squamous intraepithelial lesions (LSIL) and high-grade SIL (HSIL). These 52 studies included more than 1.25 million slides.
None of the studies that were evaluated were judged to be of “ideal quality,” and only 5 were judged to be of “high quality.” When all of the studies are taken into account and combined, there appears to be an increase in the cytologic detection of LSIL and HSIL with the use of LBC. However, further evaluation showed marked differences in the results obtained by studies of different quality.
When only “high-quality” studies are analyzed, there is no indication that LBC increases the detection of HSIL. Davey and colleagues concluded that there is no evidence that LBC reduces the proportion of unsatisfactory slides or outperforms conventional cytology in identifying women with CIN 2,3. They also noted that large randomized trials are needed.
Little difference between modalities in randomized trials
After the systematic review was conducted, 2 large trials comparing LBC with conventional cytology were published. In the first trial, Taylor and colleagues collected samples from South African women and analyzed them in blinded fashion in US laboratories. In their carefully controlled study, 5,652 women received either LBC or conventional cytology (rotated on a 6-month basis), and all women underwent colposcopy and cervical biopsy. No significant difference was observed in the sensitivity of LBC and conventional cytology in the detection of CIN 2,3 or cancer. In fact, there was a nonsignificant increase in sensitivity with conventional cytology, compared with LBC. Positive predictive value was lower with LBC than with conventional cytology. This means that a smaller proportion of women with an abnormal result on LBC had CIN 2,3 or cancer identified at colposcopy than did women who had an abnormal result on conventional cytology.
Similarly, in a large trial from Italy, Ronco and colleagues also failed to find LBC to be more sensitive than conventional cytology. Their trial randomized 33,364 women to LBC or conventional cytology. The use of LBC did not increase the detection of CIN 2,3 or cancer, compared with conventional cytology, but did lead to a dramatic reduction (43%) in positive predictive value due to an increase in the number of abnormal samples.
A similar increase in minor cytologic abnormalities with the use of LBC is now well documented in the United States. According to surveys from the College of American Pathologists, the median percentile reporting rate of LSIL in US laboratories in 2003 was 1.4% for conventional cytology specimens and 2.4% for liquid-based specimens.1
Taken together (TABLE 3), these studies suggest that LBC has no greater sensitivity than conventional cytology and therefore does not solve the problems associated with the poor sensitivity of cervical cytology. However, LBC does have other advantages, the greatest being the availability of residual fluid for “reflex” HPV testing in women with ASC-US and for testing for other pathogens, such as Chlamydia.
TABLE 3
When conventional and liquid-based cytology are compared, the latter isn’t more sensitive
| STUDY | NO. WOMEN | CONVENTIONAL CYTOLOGY | LIQUID-BASED CYTOLOGY | ||
|---|---|---|---|---|---|
| SENSITIVITY* | PPV* | SENSITIVITY* | PPV* | ||
| Taylor et al | 5,652 | 84%† | 11.4 | 71% | 9.4 |
| Ronco et al | 33,364 | 70% | 11.4 | 74%† | 6.5 |
| PPV=positive predictive value | |||||
| *for detection of CIN 2,3 or cancer | |||||
| †Nonsignificant | |||||
Most cytologists find it easier to evaluate LBC specimens than conventional cytology specimens. Nor is it likely that cytology laboratories will want to switch back to conventional cytology now that the conversion to LBC has taken place.
Dr. Wright reports no financial relationships with any company whose products are mentioned in this article.
The year 2006 was a busy one for those of us engaged in cervical cancer prevention. The most notable development was approval by the US Food and Drug Administration of the human papillomavirus (HPV) quadrivalent vaccine in June, followed closely by guidelines for its use from the Advisory Committee on Immunization Practices (June) and the American College of Obstetricians and Gynecologists (September). Key issues related to the introduction of the HPV vaccine into clinical practice were reviewed in a roundtable discussion in the January 2007 issue of OBG Management.
Therefore, this update will depart, for the moment, from matters related to the vaccine and concentrate on several other critical areas:
- Testing for high-risk HPV types is useful. Large European cervical cancer screening trials confirm a benefit.
- Condoms and oral contraceptives—are they risk modifiers for HPV infection? Answers (“Yes” and “No,” respectively) come from new data.
- Loop electrosurgical excision carries obstetric risks. In fact, all types of excisional procedures produce similar pregnancy-related morbidity.
- Liquid-based cytology may not be superior to conventional cytology. So suggest new studies and a systematic review of the literature.
HPV testing outperforms cytology for screening
Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101.
Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765–774.
HPV DNA testing is more sensitive than cervical cytology, reduces specificity to only a moderate degree, and performs similarly in different parts of Europe and North America. Those are the findings of a review by Cuzick and colleagues of all recent large European and North American cervical cancer screening studies. To date, 4 large European trials and 1 from Mexico have directly compared HPV testing and cytology in women aged 30 years and older. Combined, these trials have enrolled over 60,000 women. In every study, testing for high-risk types of HPV using the commercially available Hybrid Capture 2 HPV DNA assay had a much higher sensitivity for identifying women with cervical intraepithelial neoplasia (CIN) 2,3 or cancer (86–97%) than did cervical cytology (34–74%). Moreover, the combination of cytology and HPV testing had a sensitivity ranging from 94% to 100% in the different studies. The average sensitivity of 98% for the combination of cytology and HPV testing means that there is a less than 1 in 1,000 chance of missing CIN 2,3 or cancer when women are screened with both tests (TABLE 1).
Specificity is reasonable, too
If you worry that using HPV DNA testing in women aged 30 and older will flag too many as high-risk HPV-positive and cause them unnecessary colposcopic examinations or anxiety, here is a comforting finding: The specificity of HPV DNA testing is not as low as many had feared—provided we limit screening to women aged 30 and older. In the 5 trials mentioned, the specificity of HPV DNA testing ranged from 92% to 97%. Even when HPV DNA testing and cytology were used together, the average specificity of the 2 tests combined was 93%. A specificity of 93% means that only 7 of 100 screened women who don’t have CIN 2,3 or cancer will be classified as “positive.”
To put this number into perspective, a 2003 survey of US cytology laboratories found a median rate of abnormal results of 6.9%.1 Therefore, in routine clinical practice, incorporation of HPV DNA testing into screening for women aged 30 and older is not expected to greatly increase the number of women requiring additional follow-up.
The single most important component of management is appropriate counseling. Even though there are fewer of these women than we anticipated, these patients need to be reassured that their risk of having a significant lesion (CIN 2,3 or cancer) is quite low—only about 1 in 20. They also need to know that about two thirds of women—even women aged 30 and older—are HPV-negative when they are retested in 12 months.
In addition, clinicians need to stress that positive HPV status is a risk factor for having or developing cervical disease, not an indication that disease is present. One analogy that patients readily understand is the relationship between other types of health risk factors, such as mild hypertension or mildly elevated serum cholesterol, and disease. These explanations help the patient understand why, in settings where genotyping for HPV 16 and 18 is not available, the best course of action is to wait 12 months and be retested.2
Which test should be used first?
Basic screening principles suggest that, whenever 2 tests are used in combination, the most sensitive test should be used first, with patients who test positive tested again using the second, more specific, test. These principles suggest we should be using HPV testing alone as the initial screening test and limiting the use of cytology to triage HPV-positive women. This sequence of testing could potentially be done in a “reflex” fashion.
The large Italian screening trial by Ronco and colleagues randomized 33,364 women aged 35 to 60 years to 2 different screening strategies: routine conventional cytology or liquid-based cytology with HPV testing.3 In the routine cytology arm, researchers identified only 51 cases of CIN 2,3 or cancer, but in the experimental arm, they identified 75 cases. A breakdown of the initial screening results in the women found to have CIN 2,3 or cancer in the experimental arm shows that cytology adds very little benefit. Only 2 of 75 women with CIN 2,3 or cancer were identified by cytology alone. In contrast, 21 (28%) of the cases of CIN 2,3 or cancer were in women who were high-risk HPV-positive and cytology-negative (TABLE 2).
TABLE 2
How cytology and HPV testing compare: Results from the Italian screening trial
| CYTOLOGY/HPV TEST | TOTAL NO. | CIN 2+ |
|---|---|---|
| ≥ASCUS/positive | 300 | 52 (69%) |
| ≥ASCUS/negative | 594 | 2 (3%) |
| Within normal limits/positive | 885 | 21 (28%) |
| Modified from Ronco G et al | ||
The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are under way.
OCs not linked to HPV infection, and condoms afford some protection
Vaccarella S, Lazcano-Ponce E, Castro-Garduno JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–1939.
Vaccarella S, Franceschi S, Herrero R, et al. Sexual behavior, condom use, and human papillomavirus: pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15:326–333.
Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–2654.
No question: Anogenital HPV infections are transmitted almost exclusively through intimate sexual contact.3 The standard markers of sexual exposure, such as the number of sexual partners and the number of partners that one’s partner has had, are key risk factors for infection with HPV. Women often ask whether other factors such as oral contraceptive (OC) use, diet, smoking, and condom use affect their risk for infection. But nonsexual risk factors are difficult to evaluate because the strength of sexual risk factors is so high.
To clarify the role played by non-sexual factors, the International Association for Research on Cancer (IARC) pooled data from multiple HPV prevalence studies involving more than 15,000 women from 14 different areas worldwide. This study clearly indicates that the use of OCs is not associated with HPV infection. Current, former, and never users of OCs all had the same risk of being HPV-positive. Therefore, although OCs are a risk factor for cervical cancer, the elevated risk cannot be explained by an increased susceptibility to HPV infection. This study also found that the menopausal transition had no clear effect on HPV infection.
New data show condoms to be more beneficial than not
Condoms are widely recognized as an effective barrier to the sexual transmission of HIV; their efficacy in blocking the transmission of other sexual diseases is less well documented. Most studies that have evaluated the impact of condom use on HPV infection have failed to find a beneficial effect. This may reflect the fact that condoms are often used inconsistently. In addition, there is a tendency to use condoms when having higher-risk sexual encounters, such as with a new partner.
In a recent study from Seattle, Winer and colleagues followed 82 female university students who first initiated sex while enrolled in the study or within 2 weeks of joining the study. The incidence of HPV infection was 38 per 100 patient-years of follow-up among women whose partners used condoms during all acts of intercourse, compared with 89.3 per 100 patient-years of follow-up among women whose partners used condoms less than 5% of the time. Risk reductions were observed for both high- and low-risk types of HPV.
Condoms also appeared to protect against the development of CIN. There were no cases of CIN during 32 patient-years among women whose partners consistently used condoms, compared with 14 cases of incident CIN during 97 patient-years of follow-up among women whose partners did not use condoms or who used them less consistently.
LEEP may have an adverse obstetric impact
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498.
Although most clinicians recognize that cold-knife conization has the potential to cause adverse obstetric outcomes, the same has not been recognized for loop electrosurgical excisional procedures (LEEP). In fact, most of the studies published in the early 1990s showed that LEEP had little impact on obstetric outcomes. Now we know better: Kyrgiou and colleagues conducted a systematic review and meta-analysis of the published literature on obstetric outcomes after treatment of CIN lesions, and found that all types of excisional procedures produce similar pregnancy-related morbidities.
LEEP had a significant association with preterm delivery (11% risk in treated women versus 7% in untreated women), low-birth-weight infants (8% in treated women versus 4% in untreated women), and premature rupture of membranes (5% in treated women versus 2% in untreated women). Although there were no significant increases in NICU admissions or perinatal mortality among the offspring of women who had undergone LEEP versus those who had not, nonsignificant increases were observed.
Similar increases in pregnancy-related morbidity were not observed among patients who underwent ablative procedures. This suggests that the amount of tissue that is removed during the LEEP (FIGURE 1) is important. Therefore, when treating CIN 2,3 lesions, especially in young women, consider using an ablative method such as cryotherapy or electrofulguration, unless colposcopy is unsatisfactory or there is a colposcopic or pathologic suspicion that an occult cancer is present.
FIGURE 1 CIN 2,3 and its treatment by LEEP
Is liquid-based cytology as sensitive as we thought?
Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122–132.
Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765–774.
Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC Jr. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006;118:957–962.
A major reappraisal of liquid-based cytology (LBC) is under way. When it was first introduced, LBC was believed to provide a significant advantage over conventional cervical cytology in terms of sensitivity for CIN 2,3 or cancer. However, most of the studies that compared the 2 modalities had severe methodological problems. Many utilized historical controls, and most others simply reported increases in the number of cases cytologically diagnosed as squamous intraepithelial lesions (SIL). Very few measured histologic endpoints, and the few studies that did failed to blind the pathologists evaluating the histology to the cytologic findings. Only 1 small study was randomized.
Focus on high-quality studies finds lower sensitivity for LBC
Recently, Davey and colleagues conducted a systematic review of the published literature comparing LBC with conventional cytology. A total of 56 studies were evaluated, 52 of which provided enough information to evaluate differences between the 2 methods in the detection of low-grade squamous intraepithelial lesions (LSIL) and high-grade SIL (HSIL). These 52 studies included more than 1.25 million slides.
None of the studies that were evaluated were judged to be of “ideal quality,” and only 5 were judged to be of “high quality.” When all of the studies are taken into account and combined, there appears to be an increase in the cytologic detection of LSIL and HSIL with the use of LBC. However, further evaluation showed marked differences in the results obtained by studies of different quality.
When only “high-quality” studies are analyzed, there is no indication that LBC increases the detection of HSIL. Davey and colleagues concluded that there is no evidence that LBC reduces the proportion of unsatisfactory slides or outperforms conventional cytology in identifying women with CIN 2,3. They also noted that large randomized trials are needed.
Little difference between modalities in randomized trials
After the systematic review was conducted, 2 large trials comparing LBC with conventional cytology were published. In the first trial, Taylor and colleagues collected samples from South African women and analyzed them in blinded fashion in US laboratories. In their carefully controlled study, 5,652 women received either LBC or conventional cytology (rotated on a 6-month basis), and all women underwent colposcopy and cervical biopsy. No significant difference was observed in the sensitivity of LBC and conventional cytology in the detection of CIN 2,3 or cancer. In fact, there was a nonsignificant increase in sensitivity with conventional cytology, compared with LBC. Positive predictive value was lower with LBC than with conventional cytology. This means that a smaller proportion of women with an abnormal result on LBC had CIN 2,3 or cancer identified at colposcopy than did women who had an abnormal result on conventional cytology.
Similarly, in a large trial from Italy, Ronco and colleagues also failed to find LBC to be more sensitive than conventional cytology. Their trial randomized 33,364 women to LBC or conventional cytology. The use of LBC did not increase the detection of CIN 2,3 or cancer, compared with conventional cytology, but did lead to a dramatic reduction (43%) in positive predictive value due to an increase in the number of abnormal samples.
A similar increase in minor cytologic abnormalities with the use of LBC is now well documented in the United States. According to surveys from the College of American Pathologists, the median percentile reporting rate of LSIL in US laboratories in 2003 was 1.4% for conventional cytology specimens and 2.4% for liquid-based specimens.1
Taken together (TABLE 3), these studies suggest that LBC has no greater sensitivity than conventional cytology and therefore does not solve the problems associated with the poor sensitivity of cervical cytology. However, LBC does have other advantages, the greatest being the availability of residual fluid for “reflex” HPV testing in women with ASC-US and for testing for other pathogens, such as Chlamydia.
TABLE 3
When conventional and liquid-based cytology are compared, the latter isn’t more sensitive
| STUDY | NO. WOMEN | CONVENTIONAL CYTOLOGY | LIQUID-BASED CYTOLOGY | ||
|---|---|---|---|---|---|
| SENSITIVITY* | PPV* | SENSITIVITY* | PPV* | ||
| Taylor et al | 5,652 | 84%† | 11.4 | 71% | 9.4 |
| Ronco et al | 33,364 | 70% | 11.4 | 74%† | 6.5 |
| PPV=positive predictive value | |||||
| *for detection of CIN 2,3 or cancer | |||||
| †Nonsignificant | |||||
Most cytologists find it easier to evaluate LBC specimens than conventional cytology specimens. Nor is it likely that cytology laboratories will want to switch back to conventional cytology now that the conversion to LBC has taken place.
Dr. Wright reports no financial relationships with any company whose products are mentioned in this article.
1. Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128:1224-1229.
2. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
3. Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 Suppl 3:S52-61.
1. Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128:1224-1229.
2. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
3. Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 Suppl 3:S52-61.
EXPERT PANEL The new HPV vaccine: What the ObGyn needs to know
What once seemed far in the future is now a reality: the human papillomavirus (HPV) vaccine. The quadrivalent vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18 was approved last June by the US Food and Drug Administration (FDA) for clinical use in females 9 to 26 years old. Shortly after its approval, the Advisory Committee on Immunization Practices (ACIP) issued guidelines on who should be vaccinated.
In light of these developments, OBG Management invited Dr. Tom Wright to convene an expert panel to discuss the ACIP recommendations and ways of introducing the vaccine into practice.

“Vaccination can reduce the disease burden—even in a woman who has had multiple sexual partners”—Barbara S. Levy, MD

“Both the ACIP and ACOG suggest that we encourage ‘catch-up’ vaccination of sexually active women through 26 years”—Thomas C. Wright, MD

“Young people should not have to sneak around to get protection”—Mark DeFrancesco, MD, MBA

“The HPV vaccine is an ObGyn vaccine, and we should embrace it with vigor”—Stanley Gall, MD

“Even if we reach all at-risk young women with our vaccine program, they will still be at risk for infection with other high-risk HPV types”—Barbara S. Levy, MD
ACIP recommends vaccination at age 11 or 12
WRIGHT: Dr. DeFrancesco, would you review the ACIP recommendations for us?
DeFRANCESCO: Shortly after the FDA approved Gardasil, the quadrivalent vaccine (Merck, Whitehouse Station, NJ), the ACIP recommended routine vaccination with 3 doses for girls aged 11 or 12 years, but noted that vaccination is also acceptable for girls as young as 9 at the discretion of the physician or health-care provider. The new 2006–2007 Recommended Adult Immunization Schedule states that the HPV vaccine is “recommended for all women aged ≤26 years of age” (available at www.cdc.gov/nip/recs/adult-schedule).
Ideally, the vaccine should be given before the onset of sexual activity (ie, before a woman is exposed to the virus), but sexually active girls and women through 26 years should still be vaccinated, as they are not likely to have been exposed to all 4 HPV types covered by the vaccine.
ACOG recommendations mirror those of ACIP
WRIGHT: Dr. Gall, are ACOG’s recommendations similar to the ACIP’s?
GALL: Yes. They mirror those of the ACIP, as they recommend that:
Should sexually active women be vaccinated?
WRIGHT: Both the ACIP and ACOG suggest that we encourage “catch-up” vaccination of sexually active women through 26 years. However, many experts disagree, arguing that vaccination of this population may not be worth the effort.
Dr. Gall, why does ACOG recommend that sexually active women get vaccinated?
GALL: Data from the Merck Phase III trials indicate that only 25% of women at age 23 are either serologically or DNA positive for 1 of the 4 HPV types included in the quadrivalent vaccine and that only 0.1% of women are positive for all 4 vaccine HPV types. Data on HPV 16 from the National Health and Nutrition Examination Surveys (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), are in line with this estimate. It is pretty clear that most sexually active women aged 26 or younger will benefit from vaccination.
Vaccine may benefit even women with high-risk sexual practices
WRIGHT: OK, Dr. Levy, you heard Dr. Gall say we should vaccinate sexually active women. Are you convinced? What are you going to tell a 24-year-old single woman who has had 12 lifetime sexual partners?
LEVY: I would tell her that she is extremely likely to have been infected with 1 or more HPV strains—but unlikely to have been exposed to all 4 types present in the vaccine. I would also explain that the vaccine is almost 100% effective at preventing genital warts caused by HPV 6 and 11 and will prevent both infections and lesions with HPV types 16 and/or 18 if the patient has not been exposed to them.
The benefit for this 24-year-old may not be as great as it is for our primary target population of preteens not yet exposed to HPV, but vaccination can reduce the disease burden—even in a woman who has had multiple sexual partners.
If this patient has already had genital warts and an abnormal Pap smear, or is positive for high-risk HPV DNA, the benefit would be even lower. Ultimately, she will have to decide whether the cost is worth the lessened benefit in her situation.
Lessons learned from the hepatitis B experience
WRIGHT: One of the things we learned 20 years ago when we introduced the hepatitis B vaccine is that limiting vaccination to groups expected to gain the most benefit doesn’t work very well. With hepatitis B, we initially targeted only high-risk groups such as intravenous drug users, men who have sex with men, and health-care workers—but this strategy didn’t reduce the rate of hepatitis to the extent expected. Once we recommended universal vaccination of the general population, however, a rapid reduction in hepatitis B occurred.
In many respects HPV is like hepatitis B. I have heard some experts say that we may eventually vaccinate all at-risk women—essentially, all sexually active women.
WRIGHT: Women older than 26 are already asking whether they should be vaccinated. What do we know about the safety and efficacy of the vaccine in these women?
GALL: Even though the number of women infected with at least 1 HPV type, or who have evidence of such infection, exceeds 60% by age 50, only a small number of women will have been exposed to all 4 HPV types covered by the vaccine. Thus, it seems likely that sexually active women over age 26 will benefit from vaccination.
The safety data on the vaccine are excellent. In our experience, the quadrivalent vaccine has been less reactogenic than the influenza vaccine. There is no reason to suspect that the HPV vaccine will be less safe in women over age 26.
Recently, immunogenicity data for the bivalent vaccine—not yet approved by the FDA—were presented to the American Society of Clinical Oncology for women aged 26 to 55 years, and excellent immune responses were observed. All we need to recommend vaccination of sexually active women over age 26 is the efficacy data, and I see no reason to think that the HPV vaccine will not be effective.
Counsel older women about off-label use
WRIGHT: What would you tell a recently divorced 32-year-old who got married in college, has had only a couple of partners, and is beginning to date again?
DeFRANCESCO: The vaccine is approved and recommended only for females aged 9 to 26, so vaccinating an older woman would be off-label—or “off-recommendation,” as those who specialize in vaccination say.
We also know that the immune system is generally more responsive in younger people, although the immunogenicity data that Dr. Gall just mentioned indicate that the bivalent HPV vaccine is highly immunogenic in older women. I would be hard-pressed to deny the apparent protection of the vaccine to a 32-year-old woman simply because she is over age 26.
However, given the medicolegal climate, I would ensure that informed consent includes a caveat about use of the vaccine in someone outside the approved age range and makes it clear that the patient has acknowledged being informed about the “off-recommendation” use.
Readers will want to know that Phase III trials are now assessing the safety and efficacy of the quadrivalent and bivalent vaccines in women over 26; data should be available in the next couple of years.
WRIGHT: I agree completely. In today’s litigious world, it is vital to counsel women appropriately and obtain informed consent prior to any vaccination. One way to educate the patient about potential benefits and risks is by providing her with a Vaccine Information Sheet, available for download from the CDC’s Web site (www.cdc.gov/nip/publications/VIS/default.htm#hpv).
Can an adolescent give her own consent?
WRIGHT: It has not yet been fully clarified whether a sexually active adolescent can provide consent on her own, or whether a parent must sign the consent form. Most states have laws that allow at least some adolescents to seek reproductive services, as well as screening and treatment for sexually transmitted diseases, without parental notification. However, at several meetings I attended recently, lawyers specializing in the legal rights of adolescents said it remains unclear which of these state laws extend to the HPV vaccine.
DeFRANCESCO: I’ve been told the same thing by our legal advisors. Pending clarification, it is important to emphasize to our patients that the vaccine is a cancer vaccine, not a drug to prevent sexually transmitted infection. The vaccine does not give young people “permission” to have sex, but helps prevent them from ever developing cervical cancer. Young people should not have to sneak around to get this protection.
WRIGHT: One of the really big issues is how we are going to pay for the HPV vaccine, which has a list price of $120 per dose and requires 3 injections. If you add the cost of 3 office visits, that’s almost $500.
LEVY: Most insurance companies in our area have already determined that they will cover the HPV vaccine. Even carriers that are usually slow to make coverage decisions added the HPV vaccine to their list of covered services fairly promptly.
WRIGHT: That’s good news for people who have health insurance. What about women who don’t, or who have high deductibles or carve-outs for “preventive services”?
Patients may be willing to foot the bill
LEVY: I have been discussing the vaccine with eligible women and mothers of young girls for several months. Even before payers stepped up with coverage, no patient was seriously concerned about the cost. Clearly, this will not be true for everyone, but when offered an opportunity to avoid cancer, my patients have been happy to pay for it. In addition, many offices now accept credit cards, which may make it possible for patients to make payments over time.
I think success will depend in large part on how we educate our patients. I frequently discuss preventive care in the context of other things we do in our lives for “maintenance.” For example, none of us would expect our automobile insurance to cover the cost of changing the oil or buying new tires. The HPV vaccine is comparable: The costs incurred now may prevent significant health risks in the near future.
Further, the price of the vaccine series is quite low relative to the potential costs of office visits for follow-up of abnormal Pap smears or treatment of genital warts.
We must stress to our patients that Pap smears aid in the detection of cervical cancer precursors, but the vaccine is an opportunity to prevent cervical cancer.
The poor and uninsured have several alternatives
WRIGHT: In the predominantly Latin American neighborhood where I am located, there are many uninsured who simply cannot afford $500 for the full course of 3 vaccinations. For uninsured children and adolescents, or those on Medicaid up to age 19, the federally funded Vaccines for Children program will cover the cost. However, for women aged 19 and older, vaccinations are considered an “optional” benefit under Medicaid, which means that individual states must decide whether the HPV vaccine will be a covered service.
One bit of good news: Merck plans to provide free vaccines, including the HPV vaccine, to low-income and uninsured adults 19 years and older who visit private clinicians who already provide Merck vaccines. Although the details of this initiative have not been finalized, the program may help individuals in states that decide not to cover the HPV vaccine with Medicaid.
Vaccine appears safe near time of conception
WRIGHT: I have heard varying opinions about the level of risk vaccination poses if a woman becomes pregnant shortly afterward. What do the data show?
GALL: It is inevitable that this vaccine will be administered to some women who are not yet aware they are pregnant. In the Merck trials, more than 1,000 pregnancies occurred in both the vaccine and placebo groups. There were 15 abnormal infants in the vaccine group and 16 in the placebo group. The abnormalities were nonrepetitive and did not raise concern at the FDA.
Among the women who received an injection within 30 days of conception, there were 5 abnormalities, compared with none in the placebo group—but none of the abnormalities were repetitive and some involved such things as an extra digit.
The vaccine was accorded a pregnancy category B. This is a landmark for the FDA because no other vaccine has this designation, even those used extensively during pregnancy, such as the trivalent inactivated influenza vaccine (TIV) and hepatitis B.
At present, clinicians are asked to report any women who receive the quadrivalent vaccine and become pregnant, but I can foresee a time when we will administer this vaccine during pregnancy.
How ObGyns are reacting
WRIGHT: Dr. DeFrancesco, you are involved in managing almost 200 ObGyns. How do you expect the specialty to respond to the new vaccine?
DeFRANCESCO: Vaccination is not a traditional ObGyn responsibility, but I think most of us are comfortable administering other injections, such as Rho(D) immune globulin, leuprolide acetate, and depot medroxyprogesterone acetate, or even hepatitis B vaccines for our staff. The HPV vaccine is a different injection with a different purpose, but well within our expertise to administer. I am pleased to report that—in record time!—all our practices are offering the vaccine and implementing this new service. It clearly is the right thing to do.
ObGyns need to build a vaccination infrastructure
WRIGHT: There are related issues: maintaining stocks of vaccine in ObGyn offices, developing and using consent forms, and implementing a tracking system to make sure patients get all 3 injections of the vaccine. How are you addressing these issues?
DeFRANCESCO: We have implemented a clinical guideline consistent with the ACIP and ACOG policy statements, along with a model informed consent and insurance waiver within our large group practice. This helps us ensure that providers are up-to-date on the latest recommendations and are ready to provide this service.
If patients are concerned about the potential cost, we advise them to check with their carriers. We also ask them to sign a waiver that will permit us to bill the patient herself if she is not covered.
In addition, we have set up an account for each of our practices with the vaccine manufacturer so we can order vaccine on fairly short notice. We have recommended that our divisions each stock a reasonable number of doses to ensure enough supply to meet the demand expected in the very near future. We have also recommended that our practices go ahead and schedule the 2- and 6-month booster visits at the time of the patient’s first vaccination and counsel the patient about the importance of receiving all 3 doses.
ACOG hopes to create better (and bigger) vaccinators
GALL: In general, ObGyns don’t do a very good job at vaccinating. The last CDC survey I saw indicated that less than 60% of ObGyns collect immunization and infection histories from their patients, and only two thirds offer even a single vaccine.
WRIGHT: You have been working for several years to help the ObGyn community with vaccine implementation. What is ACOG doing to help physicians in private practice?
GALL: ACOG has promoted the concept that ObGyns should, and need to, become better vaccinators. A working committee on immunization has developed a program for practicing ObGyns. The concept is simple: The HPV vaccine is an ObGyn vaccine, and we should embrace it with vigor. If the ObGyn office gets set up to administer the HPV vaccine, why not administer other important vaccines such as TIV, hepatitis B, Tdap, MCV4, and herpes zoster?
This working committee has prepared a number of materials that should be available by the next annual clinical meeting:
We also plan presentations for the district and annual clinical meetings.
Is there a future for cervical cancer screening?
WRIGHT: Dr. Levy, can you explain why we are going to need to continue screening once the vaccine becomes widely used?
LEVY: For the next 30 to 40 years we will have a large population of women—already over age 26—who have not received the vaccine. These women will require ongoing screening for cervical cancer precursors throughout their lives.
Although the vaccine protects against HPV types 16 and 18, which cause 70% of cancer cases, and types 6 and 11, which cause 90% of genital warts, immunity to these HPV types will not protect a woman against the other 11 high-risk HPV types. These 11 types are not as commonly associated with cervical cancer or its precursors, but they do lead to cancer in some women and can still infect the cervix in women who have received the vaccine. Even if we reach all at-risk young women with our vaccine program, they will still be at risk—albeit lower risk—for cervical cancer due to infection with other high-risk HPV types.
One other point: We do not yet know how long the immunity from these vaccines will last. So it seems clear that screening will still be required to detect cervical cancer precursors and prevent cervical cancer from developing—even in women who have received the HPV vaccine.
Do we risk increasing the cancer rate?
WRIGHT: I worry about vaccination coverage. In the absence of state school requirements for the HPV vaccine, we are unlikely to get a high coverage rate among adolescents in the US. In several European countries, such as Germany, that have both recommendations and funding for universal hepatitis B vaccination of adolescents, only about 30% of adolescents have been vaccinated. The reason? These countries lack school requirements for the vaccination and have no school-based vaccination program. With the high prevalence of HPV infections in young, sexually active women in the US, we could actually increase our cervical cancer rate if we recommend reduced screening without ensuring high levels of vaccination coverage.
Dr. Gall, what do you predict for the next decade?
GALL: The future looks bright. A bipartisan bill was just introduced in the Michigan state legislature to add the HPV vaccine as a requirement for entry into junior high school. I expect more states to follow.
In general, adolescents are a poorly served group when it comes to health care because many fall out of the system. The HPV vaccine provides a great opportunity for us to encourage patients to bring their adolescent daughters to the office for a consultation. During this visit, we will conduct an “about the umbilicus” (ie, non-pelvic) physical exam, provide immunization, and discuss a number of topics such as contraception, menstruation, nutrition, etc. A critical step is to get state health departments and Medicaid officials off the dime and supporting the HPV vaccine.
Drs. Wright, DeFrancesco, Gall, and Levy report no financial relationships relevant to this article.
1. Gardasil [package insert]. Whitehouse Station, NJ: Merck.
Dr. Wright is an author of the 2001 Consensus Guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim Guidance for Use of HPV DNA testing for Primary Screening, and the 2001 Bethesda System. He is Professor of Pathology, Columbia University, New York.
What once seemed far in the future is now a reality: the human papillomavirus (HPV) vaccine. The quadrivalent vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18 was approved last June by the US Food and Drug Administration (FDA) for clinical use in females 9 to 26 years old. Shortly after its approval, the Advisory Committee on Immunization Practices (ACIP) issued guidelines on who should be vaccinated.
In light of these developments, OBG Management invited Dr. Tom Wright to convene an expert panel to discuss the ACIP recommendations and ways of introducing the vaccine into practice.

“Vaccination can reduce the disease burden—even in a woman who has had multiple sexual partners”—Barbara S. Levy, MD

“Both the ACIP and ACOG suggest that we encourage ‘catch-up’ vaccination of sexually active women through 26 years”—Thomas C. Wright, MD

“Young people should not have to sneak around to get protection”—Mark DeFrancesco, MD, MBA

“The HPV vaccine is an ObGyn vaccine, and we should embrace it with vigor”—Stanley Gall, MD

“Even if we reach all at-risk young women with our vaccine program, they will still be at risk for infection with other high-risk HPV types”—Barbara S. Levy, MD
ACIP recommends vaccination at age 11 or 12
WRIGHT: Dr. DeFrancesco, would you review the ACIP recommendations for us?
DeFRANCESCO: Shortly after the FDA approved Gardasil, the quadrivalent vaccine (Merck, Whitehouse Station, NJ), the ACIP recommended routine vaccination with 3 doses for girls aged 11 or 12 years, but noted that vaccination is also acceptable for girls as young as 9 at the discretion of the physician or health-care provider. The new 2006–2007 Recommended Adult Immunization Schedule states that the HPV vaccine is “recommended for all women aged ≤26 years of age” (available at www.cdc.gov/nip/recs/adult-schedule).
Ideally, the vaccine should be given before the onset of sexual activity (ie, before a woman is exposed to the virus), but sexually active girls and women through 26 years should still be vaccinated, as they are not likely to have been exposed to all 4 HPV types covered by the vaccine.
ACOG recommendations mirror those of ACIP
WRIGHT: Dr. Gall, are ACOG’s recommendations similar to the ACIP’s?
GALL: Yes. They mirror those of the ACIP, as they recommend that:
Should sexually active women be vaccinated?
WRIGHT: Both the ACIP and ACOG suggest that we encourage “catch-up” vaccination of sexually active women through 26 years. However, many experts disagree, arguing that vaccination of this population may not be worth the effort.
Dr. Gall, why does ACOG recommend that sexually active women get vaccinated?
GALL: Data from the Merck Phase III trials indicate that only 25% of women at age 23 are either serologically or DNA positive for 1 of the 4 HPV types included in the quadrivalent vaccine and that only 0.1% of women are positive for all 4 vaccine HPV types. Data on HPV 16 from the National Health and Nutrition Examination Surveys (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), are in line with this estimate. It is pretty clear that most sexually active women aged 26 or younger will benefit from vaccination.
Vaccine may benefit even women with high-risk sexual practices
WRIGHT: OK, Dr. Levy, you heard Dr. Gall say we should vaccinate sexually active women. Are you convinced? What are you going to tell a 24-year-old single woman who has had 12 lifetime sexual partners?
LEVY: I would tell her that she is extremely likely to have been infected with 1 or more HPV strains—but unlikely to have been exposed to all 4 types present in the vaccine. I would also explain that the vaccine is almost 100% effective at preventing genital warts caused by HPV 6 and 11 and will prevent both infections and lesions with HPV types 16 and/or 18 if the patient has not been exposed to them.
The benefit for this 24-year-old may not be as great as it is for our primary target population of preteens not yet exposed to HPV, but vaccination can reduce the disease burden—even in a woman who has had multiple sexual partners.
If this patient has already had genital warts and an abnormal Pap smear, or is positive for high-risk HPV DNA, the benefit would be even lower. Ultimately, she will have to decide whether the cost is worth the lessened benefit in her situation.
Lessons learned from the hepatitis B experience
WRIGHT: One of the things we learned 20 years ago when we introduced the hepatitis B vaccine is that limiting vaccination to groups expected to gain the most benefit doesn’t work very well. With hepatitis B, we initially targeted only high-risk groups such as intravenous drug users, men who have sex with men, and health-care workers—but this strategy didn’t reduce the rate of hepatitis to the extent expected. Once we recommended universal vaccination of the general population, however, a rapid reduction in hepatitis B occurred.
In many respects HPV is like hepatitis B. I have heard some experts say that we may eventually vaccinate all at-risk women—essentially, all sexually active women.
WRIGHT: Women older than 26 are already asking whether they should be vaccinated. What do we know about the safety and efficacy of the vaccine in these women?
GALL: Even though the number of women infected with at least 1 HPV type, or who have evidence of such infection, exceeds 60% by age 50, only a small number of women will have been exposed to all 4 HPV types covered by the vaccine. Thus, it seems likely that sexually active women over age 26 will benefit from vaccination.
The safety data on the vaccine are excellent. In our experience, the quadrivalent vaccine has been less reactogenic than the influenza vaccine. There is no reason to suspect that the HPV vaccine will be less safe in women over age 26.
Recently, immunogenicity data for the bivalent vaccine—not yet approved by the FDA—were presented to the American Society of Clinical Oncology for women aged 26 to 55 years, and excellent immune responses were observed. All we need to recommend vaccination of sexually active women over age 26 is the efficacy data, and I see no reason to think that the HPV vaccine will not be effective.
Counsel older women about off-label use
WRIGHT: What would you tell a recently divorced 32-year-old who got married in college, has had only a couple of partners, and is beginning to date again?
DeFRANCESCO: The vaccine is approved and recommended only for females aged 9 to 26, so vaccinating an older woman would be off-label—or “off-recommendation,” as those who specialize in vaccination say.
We also know that the immune system is generally more responsive in younger people, although the immunogenicity data that Dr. Gall just mentioned indicate that the bivalent HPV vaccine is highly immunogenic in older women. I would be hard-pressed to deny the apparent protection of the vaccine to a 32-year-old woman simply because she is over age 26.
However, given the medicolegal climate, I would ensure that informed consent includes a caveat about use of the vaccine in someone outside the approved age range and makes it clear that the patient has acknowledged being informed about the “off-recommendation” use.
Readers will want to know that Phase III trials are now assessing the safety and efficacy of the quadrivalent and bivalent vaccines in women over 26; data should be available in the next couple of years.
WRIGHT: I agree completely. In today’s litigious world, it is vital to counsel women appropriately and obtain informed consent prior to any vaccination. One way to educate the patient about potential benefits and risks is by providing her with a Vaccine Information Sheet, available for download from the CDC’s Web site (www.cdc.gov/nip/publications/VIS/default.htm#hpv).
Can an adolescent give her own consent?
WRIGHT: It has not yet been fully clarified whether a sexually active adolescent can provide consent on her own, or whether a parent must sign the consent form. Most states have laws that allow at least some adolescents to seek reproductive services, as well as screening and treatment for sexually transmitted diseases, without parental notification. However, at several meetings I attended recently, lawyers specializing in the legal rights of adolescents said it remains unclear which of these state laws extend to the HPV vaccine.
DeFRANCESCO: I’ve been told the same thing by our legal advisors. Pending clarification, it is important to emphasize to our patients that the vaccine is a cancer vaccine, not a drug to prevent sexually transmitted infection. The vaccine does not give young people “permission” to have sex, but helps prevent them from ever developing cervical cancer. Young people should not have to sneak around to get this protection.
WRIGHT: One of the really big issues is how we are going to pay for the HPV vaccine, which has a list price of $120 per dose and requires 3 injections. If you add the cost of 3 office visits, that’s almost $500.
LEVY: Most insurance companies in our area have already determined that they will cover the HPV vaccine. Even carriers that are usually slow to make coverage decisions added the HPV vaccine to their list of covered services fairly promptly.
WRIGHT: That’s good news for people who have health insurance. What about women who don’t, or who have high deductibles or carve-outs for “preventive services”?
Patients may be willing to foot the bill
LEVY: I have been discussing the vaccine with eligible women and mothers of young girls for several months. Even before payers stepped up with coverage, no patient was seriously concerned about the cost. Clearly, this will not be true for everyone, but when offered an opportunity to avoid cancer, my patients have been happy to pay for it. In addition, many offices now accept credit cards, which may make it possible for patients to make payments over time.
I think success will depend in large part on how we educate our patients. I frequently discuss preventive care in the context of other things we do in our lives for “maintenance.” For example, none of us would expect our automobile insurance to cover the cost of changing the oil or buying new tires. The HPV vaccine is comparable: The costs incurred now may prevent significant health risks in the near future.
Further, the price of the vaccine series is quite low relative to the potential costs of office visits for follow-up of abnormal Pap smears or treatment of genital warts.
We must stress to our patients that Pap smears aid in the detection of cervical cancer precursors, but the vaccine is an opportunity to prevent cervical cancer.
The poor and uninsured have several alternatives
WRIGHT: In the predominantly Latin American neighborhood where I am located, there are many uninsured who simply cannot afford $500 for the full course of 3 vaccinations. For uninsured children and adolescents, or those on Medicaid up to age 19, the federally funded Vaccines for Children program will cover the cost. However, for women aged 19 and older, vaccinations are considered an “optional” benefit under Medicaid, which means that individual states must decide whether the HPV vaccine will be a covered service.
One bit of good news: Merck plans to provide free vaccines, including the HPV vaccine, to low-income and uninsured adults 19 years and older who visit private clinicians who already provide Merck vaccines. Although the details of this initiative have not been finalized, the program may help individuals in states that decide not to cover the HPV vaccine with Medicaid.
Vaccine appears safe near time of conception
WRIGHT: I have heard varying opinions about the level of risk vaccination poses if a woman becomes pregnant shortly afterward. What do the data show?
GALL: It is inevitable that this vaccine will be administered to some women who are not yet aware they are pregnant. In the Merck trials, more than 1,000 pregnancies occurred in both the vaccine and placebo groups. There were 15 abnormal infants in the vaccine group and 16 in the placebo group. The abnormalities were nonrepetitive and did not raise concern at the FDA.
Among the women who received an injection within 30 days of conception, there were 5 abnormalities, compared with none in the placebo group—but none of the abnormalities were repetitive and some involved such things as an extra digit.
The vaccine was accorded a pregnancy category B. This is a landmark for the FDA because no other vaccine has this designation, even those used extensively during pregnancy, such as the trivalent inactivated influenza vaccine (TIV) and hepatitis B.
At present, clinicians are asked to report any women who receive the quadrivalent vaccine and become pregnant, but I can foresee a time when we will administer this vaccine during pregnancy.
How ObGyns are reacting
WRIGHT: Dr. DeFrancesco, you are involved in managing almost 200 ObGyns. How do you expect the specialty to respond to the new vaccine?
DeFRANCESCO: Vaccination is not a traditional ObGyn responsibility, but I think most of us are comfortable administering other injections, such as Rho(D) immune globulin, leuprolide acetate, and depot medroxyprogesterone acetate, or even hepatitis B vaccines for our staff. The HPV vaccine is a different injection with a different purpose, but well within our expertise to administer. I am pleased to report that—in record time!—all our practices are offering the vaccine and implementing this new service. It clearly is the right thing to do.
ObGyns need to build a vaccination infrastructure
WRIGHT: There are related issues: maintaining stocks of vaccine in ObGyn offices, developing and using consent forms, and implementing a tracking system to make sure patients get all 3 injections of the vaccine. How are you addressing these issues?
DeFRANCESCO: We have implemented a clinical guideline consistent with the ACIP and ACOG policy statements, along with a model informed consent and insurance waiver within our large group practice. This helps us ensure that providers are up-to-date on the latest recommendations and are ready to provide this service.
If patients are concerned about the potential cost, we advise them to check with their carriers. We also ask them to sign a waiver that will permit us to bill the patient herself if she is not covered.
In addition, we have set up an account for each of our practices with the vaccine manufacturer so we can order vaccine on fairly short notice. We have recommended that our divisions each stock a reasonable number of doses to ensure enough supply to meet the demand expected in the very near future. We have also recommended that our practices go ahead and schedule the 2- and 6-month booster visits at the time of the patient’s first vaccination and counsel the patient about the importance of receiving all 3 doses.
ACOG hopes to create better (and bigger) vaccinators
GALL: In general, ObGyns don’t do a very good job at vaccinating. The last CDC survey I saw indicated that less than 60% of ObGyns collect immunization and infection histories from their patients, and only two thirds offer even a single vaccine.
WRIGHT: You have been working for several years to help the ObGyn community with vaccine implementation. What is ACOG doing to help physicians in private practice?
GALL: ACOG has promoted the concept that ObGyns should, and need to, become better vaccinators. A working committee on immunization has developed a program for practicing ObGyns. The concept is simple: The HPV vaccine is an ObGyn vaccine, and we should embrace it with vigor. If the ObGyn office gets set up to administer the HPV vaccine, why not administer other important vaccines such as TIV, hepatitis B, Tdap, MCV4, and herpes zoster?
This working committee has prepared a number of materials that should be available by the next annual clinical meeting:
We also plan presentations for the district and annual clinical meetings.
Is there a future for cervical cancer screening?
WRIGHT: Dr. Levy, can you explain why we are going to need to continue screening once the vaccine becomes widely used?
LEVY: For the next 30 to 40 years we will have a large population of women—already over age 26—who have not received the vaccine. These women will require ongoing screening for cervical cancer precursors throughout their lives.
Although the vaccine protects against HPV types 16 and 18, which cause 70% of cancer cases, and types 6 and 11, which cause 90% of genital warts, immunity to these HPV types will not protect a woman against the other 11 high-risk HPV types. These 11 types are not as commonly associated with cervical cancer or its precursors, but they do lead to cancer in some women and can still infect the cervix in women who have received the vaccine. Even if we reach all at-risk young women with our vaccine program, they will still be at risk—albeit lower risk—for cervical cancer due to infection with other high-risk HPV types.
One other point: We do not yet know how long the immunity from these vaccines will last. So it seems clear that screening will still be required to detect cervical cancer precursors and prevent cervical cancer from developing—even in women who have received the HPV vaccine.
Do we risk increasing the cancer rate?
WRIGHT: I worry about vaccination coverage. In the absence of state school requirements for the HPV vaccine, we are unlikely to get a high coverage rate among adolescents in the US. In several European countries, such as Germany, that have both recommendations and funding for universal hepatitis B vaccination of adolescents, only about 30% of adolescents have been vaccinated. The reason? These countries lack school requirements for the vaccination and have no school-based vaccination program. With the high prevalence of HPV infections in young, sexually active women in the US, we could actually increase our cervical cancer rate if we recommend reduced screening without ensuring high levels of vaccination coverage.
Dr. Gall, what do you predict for the next decade?
GALL: The future looks bright. A bipartisan bill was just introduced in the Michigan state legislature to add the HPV vaccine as a requirement for entry into junior high school. I expect more states to follow.
In general, adolescents are a poorly served group when it comes to health care because many fall out of the system. The HPV vaccine provides a great opportunity for us to encourage patients to bring their adolescent daughters to the office for a consultation. During this visit, we will conduct an “about the umbilicus” (ie, non-pelvic) physical exam, provide immunization, and discuss a number of topics such as contraception, menstruation, nutrition, etc. A critical step is to get state health departments and Medicaid officials off the dime and supporting the HPV vaccine.
Drs. Wright, DeFrancesco, Gall, and Levy report no financial relationships relevant to this article.
What once seemed far in the future is now a reality: the human papillomavirus (HPV) vaccine. The quadrivalent vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18 was approved last June by the US Food and Drug Administration (FDA) for clinical use in females 9 to 26 years old. Shortly after its approval, the Advisory Committee on Immunization Practices (ACIP) issued guidelines on who should be vaccinated.
In light of these developments, OBG Management invited Dr. Tom Wright to convene an expert panel to discuss the ACIP recommendations and ways of introducing the vaccine into practice.

“Vaccination can reduce the disease burden—even in a woman who has had multiple sexual partners”—Barbara S. Levy, MD

“Both the ACIP and ACOG suggest that we encourage ‘catch-up’ vaccination of sexually active women through 26 years”—Thomas C. Wright, MD

“Young people should not have to sneak around to get protection”—Mark DeFrancesco, MD, MBA

“The HPV vaccine is an ObGyn vaccine, and we should embrace it with vigor”—Stanley Gall, MD

“Even if we reach all at-risk young women with our vaccine program, they will still be at risk for infection with other high-risk HPV types”—Barbara S. Levy, MD
ACIP recommends vaccination at age 11 or 12
WRIGHT: Dr. DeFrancesco, would you review the ACIP recommendations for us?
DeFRANCESCO: Shortly after the FDA approved Gardasil, the quadrivalent vaccine (Merck, Whitehouse Station, NJ), the ACIP recommended routine vaccination with 3 doses for girls aged 11 or 12 years, but noted that vaccination is also acceptable for girls as young as 9 at the discretion of the physician or health-care provider. The new 2006–2007 Recommended Adult Immunization Schedule states that the HPV vaccine is “recommended for all women aged ≤26 years of age” (available at www.cdc.gov/nip/recs/adult-schedule).
Ideally, the vaccine should be given before the onset of sexual activity (ie, before a woman is exposed to the virus), but sexually active girls and women through 26 years should still be vaccinated, as they are not likely to have been exposed to all 4 HPV types covered by the vaccine.
ACOG recommendations mirror those of ACIP
WRIGHT: Dr. Gall, are ACOG’s recommendations similar to the ACIP’s?
GALL: Yes. They mirror those of the ACIP, as they recommend that:
Should sexually active women be vaccinated?
WRIGHT: Both the ACIP and ACOG suggest that we encourage “catch-up” vaccination of sexually active women through 26 years. However, many experts disagree, arguing that vaccination of this population may not be worth the effort.
Dr. Gall, why does ACOG recommend that sexually active women get vaccinated?
GALL: Data from the Merck Phase III trials indicate that only 25% of women at age 23 are either serologically or DNA positive for 1 of the 4 HPV types included in the quadrivalent vaccine and that only 0.1% of women are positive for all 4 vaccine HPV types. Data on HPV 16 from the National Health and Nutrition Examination Surveys (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), are in line with this estimate. It is pretty clear that most sexually active women aged 26 or younger will benefit from vaccination.
Vaccine may benefit even women with high-risk sexual practices
WRIGHT: OK, Dr. Levy, you heard Dr. Gall say we should vaccinate sexually active women. Are you convinced? What are you going to tell a 24-year-old single woman who has had 12 lifetime sexual partners?
LEVY: I would tell her that she is extremely likely to have been infected with 1 or more HPV strains—but unlikely to have been exposed to all 4 types present in the vaccine. I would also explain that the vaccine is almost 100% effective at preventing genital warts caused by HPV 6 and 11 and will prevent both infections and lesions with HPV types 16 and/or 18 if the patient has not been exposed to them.
The benefit for this 24-year-old may not be as great as it is for our primary target population of preteens not yet exposed to HPV, but vaccination can reduce the disease burden—even in a woman who has had multiple sexual partners.
If this patient has already had genital warts and an abnormal Pap smear, or is positive for high-risk HPV DNA, the benefit would be even lower. Ultimately, she will have to decide whether the cost is worth the lessened benefit in her situation.
Lessons learned from the hepatitis B experience
WRIGHT: One of the things we learned 20 years ago when we introduced the hepatitis B vaccine is that limiting vaccination to groups expected to gain the most benefit doesn’t work very well. With hepatitis B, we initially targeted only high-risk groups such as intravenous drug users, men who have sex with men, and health-care workers—but this strategy didn’t reduce the rate of hepatitis to the extent expected. Once we recommended universal vaccination of the general population, however, a rapid reduction in hepatitis B occurred.
In many respects HPV is like hepatitis B. I have heard some experts say that we may eventually vaccinate all at-risk women—essentially, all sexually active women.
WRIGHT: Women older than 26 are already asking whether they should be vaccinated. What do we know about the safety and efficacy of the vaccine in these women?
GALL: Even though the number of women infected with at least 1 HPV type, or who have evidence of such infection, exceeds 60% by age 50, only a small number of women will have been exposed to all 4 HPV types covered by the vaccine. Thus, it seems likely that sexually active women over age 26 will benefit from vaccination.
The safety data on the vaccine are excellent. In our experience, the quadrivalent vaccine has been less reactogenic than the influenza vaccine. There is no reason to suspect that the HPV vaccine will be less safe in women over age 26.
Recently, immunogenicity data for the bivalent vaccine—not yet approved by the FDA—were presented to the American Society of Clinical Oncology for women aged 26 to 55 years, and excellent immune responses were observed. All we need to recommend vaccination of sexually active women over age 26 is the efficacy data, and I see no reason to think that the HPV vaccine will not be effective.
Counsel older women about off-label use
WRIGHT: What would you tell a recently divorced 32-year-old who got married in college, has had only a couple of partners, and is beginning to date again?
DeFRANCESCO: The vaccine is approved and recommended only for females aged 9 to 26, so vaccinating an older woman would be off-label—or “off-recommendation,” as those who specialize in vaccination say.
We also know that the immune system is generally more responsive in younger people, although the immunogenicity data that Dr. Gall just mentioned indicate that the bivalent HPV vaccine is highly immunogenic in older women. I would be hard-pressed to deny the apparent protection of the vaccine to a 32-year-old woman simply because she is over age 26.
However, given the medicolegal climate, I would ensure that informed consent includes a caveat about use of the vaccine in someone outside the approved age range and makes it clear that the patient has acknowledged being informed about the “off-recommendation” use.
Readers will want to know that Phase III trials are now assessing the safety and efficacy of the quadrivalent and bivalent vaccines in women over 26; data should be available in the next couple of years.
WRIGHT: I agree completely. In today’s litigious world, it is vital to counsel women appropriately and obtain informed consent prior to any vaccination. One way to educate the patient about potential benefits and risks is by providing her with a Vaccine Information Sheet, available for download from the CDC’s Web site (www.cdc.gov/nip/publications/VIS/default.htm#hpv).
Can an adolescent give her own consent?
WRIGHT: It has not yet been fully clarified whether a sexually active adolescent can provide consent on her own, or whether a parent must sign the consent form. Most states have laws that allow at least some adolescents to seek reproductive services, as well as screening and treatment for sexually transmitted diseases, without parental notification. However, at several meetings I attended recently, lawyers specializing in the legal rights of adolescents said it remains unclear which of these state laws extend to the HPV vaccine.
DeFRANCESCO: I’ve been told the same thing by our legal advisors. Pending clarification, it is important to emphasize to our patients that the vaccine is a cancer vaccine, not a drug to prevent sexually transmitted infection. The vaccine does not give young people “permission” to have sex, but helps prevent them from ever developing cervical cancer. Young people should not have to sneak around to get this protection.
WRIGHT: One of the really big issues is how we are going to pay for the HPV vaccine, which has a list price of $120 per dose and requires 3 injections. If you add the cost of 3 office visits, that’s almost $500.
LEVY: Most insurance companies in our area have already determined that they will cover the HPV vaccine. Even carriers that are usually slow to make coverage decisions added the HPV vaccine to their list of covered services fairly promptly.
WRIGHT: That’s good news for people who have health insurance. What about women who don’t, or who have high deductibles or carve-outs for “preventive services”?
Patients may be willing to foot the bill
LEVY: I have been discussing the vaccine with eligible women and mothers of young girls for several months. Even before payers stepped up with coverage, no patient was seriously concerned about the cost. Clearly, this will not be true for everyone, but when offered an opportunity to avoid cancer, my patients have been happy to pay for it. In addition, many offices now accept credit cards, which may make it possible for patients to make payments over time.
I think success will depend in large part on how we educate our patients. I frequently discuss preventive care in the context of other things we do in our lives for “maintenance.” For example, none of us would expect our automobile insurance to cover the cost of changing the oil or buying new tires. The HPV vaccine is comparable: The costs incurred now may prevent significant health risks in the near future.
Further, the price of the vaccine series is quite low relative to the potential costs of office visits for follow-up of abnormal Pap smears or treatment of genital warts.
We must stress to our patients that Pap smears aid in the detection of cervical cancer precursors, but the vaccine is an opportunity to prevent cervical cancer.
The poor and uninsured have several alternatives
WRIGHT: In the predominantly Latin American neighborhood where I am located, there are many uninsured who simply cannot afford $500 for the full course of 3 vaccinations. For uninsured children and adolescents, or those on Medicaid up to age 19, the federally funded Vaccines for Children program will cover the cost. However, for women aged 19 and older, vaccinations are considered an “optional” benefit under Medicaid, which means that individual states must decide whether the HPV vaccine will be a covered service.
One bit of good news: Merck plans to provide free vaccines, including the HPV vaccine, to low-income and uninsured adults 19 years and older who visit private clinicians who already provide Merck vaccines. Although the details of this initiative have not been finalized, the program may help individuals in states that decide not to cover the HPV vaccine with Medicaid.
Vaccine appears safe near time of conception
WRIGHT: I have heard varying opinions about the level of risk vaccination poses if a woman becomes pregnant shortly afterward. What do the data show?
GALL: It is inevitable that this vaccine will be administered to some women who are not yet aware they are pregnant. In the Merck trials, more than 1,000 pregnancies occurred in both the vaccine and placebo groups. There were 15 abnormal infants in the vaccine group and 16 in the placebo group. The abnormalities were nonrepetitive and did not raise concern at the FDA.
Among the women who received an injection within 30 days of conception, there were 5 abnormalities, compared with none in the placebo group—but none of the abnormalities were repetitive and some involved such things as an extra digit.
The vaccine was accorded a pregnancy category B. This is a landmark for the FDA because no other vaccine has this designation, even those used extensively during pregnancy, such as the trivalent inactivated influenza vaccine (TIV) and hepatitis B.
At present, clinicians are asked to report any women who receive the quadrivalent vaccine and become pregnant, but I can foresee a time when we will administer this vaccine during pregnancy.
How ObGyns are reacting
WRIGHT: Dr. DeFrancesco, you are involved in managing almost 200 ObGyns. How do you expect the specialty to respond to the new vaccine?
DeFRANCESCO: Vaccination is not a traditional ObGyn responsibility, but I think most of us are comfortable administering other injections, such as Rho(D) immune globulin, leuprolide acetate, and depot medroxyprogesterone acetate, or even hepatitis B vaccines for our staff. The HPV vaccine is a different injection with a different purpose, but well within our expertise to administer. I am pleased to report that—in record time!—all our practices are offering the vaccine and implementing this new service. It clearly is the right thing to do.
ObGyns need to build a vaccination infrastructure
WRIGHT: There are related issues: maintaining stocks of vaccine in ObGyn offices, developing and using consent forms, and implementing a tracking system to make sure patients get all 3 injections of the vaccine. How are you addressing these issues?
DeFRANCESCO: We have implemented a clinical guideline consistent with the ACIP and ACOG policy statements, along with a model informed consent and insurance waiver within our large group practice. This helps us ensure that providers are up-to-date on the latest recommendations and are ready to provide this service.
If patients are concerned about the potential cost, we advise them to check with their carriers. We also ask them to sign a waiver that will permit us to bill the patient herself if she is not covered.
In addition, we have set up an account for each of our practices with the vaccine manufacturer so we can order vaccine on fairly short notice. We have recommended that our divisions each stock a reasonable number of doses to ensure enough supply to meet the demand expected in the very near future. We have also recommended that our practices go ahead and schedule the 2- and 6-month booster visits at the time of the patient’s first vaccination and counsel the patient about the importance of receiving all 3 doses.
ACOG hopes to create better (and bigger) vaccinators
GALL: In general, ObGyns don’t do a very good job at vaccinating. The last CDC survey I saw indicated that less than 60% of ObGyns collect immunization and infection histories from their patients, and only two thirds offer even a single vaccine.
WRIGHT: You have been working for several years to help the ObGyn community with vaccine implementation. What is ACOG doing to help physicians in private practice?
GALL: ACOG has promoted the concept that ObGyns should, and need to, become better vaccinators. A working committee on immunization has developed a program for practicing ObGyns. The concept is simple: The HPV vaccine is an ObGyn vaccine, and we should embrace it with vigor. If the ObGyn office gets set up to administer the HPV vaccine, why not administer other important vaccines such as TIV, hepatitis B, Tdap, MCV4, and herpes zoster?
This working committee has prepared a number of materials that should be available by the next annual clinical meeting:
We also plan presentations for the district and annual clinical meetings.
Is there a future for cervical cancer screening?
WRIGHT: Dr. Levy, can you explain why we are going to need to continue screening once the vaccine becomes widely used?
LEVY: For the next 30 to 40 years we will have a large population of women—already over age 26—who have not received the vaccine. These women will require ongoing screening for cervical cancer precursors throughout their lives.
Although the vaccine protects against HPV types 16 and 18, which cause 70% of cancer cases, and types 6 and 11, which cause 90% of genital warts, immunity to these HPV types will not protect a woman against the other 11 high-risk HPV types. These 11 types are not as commonly associated with cervical cancer or its precursors, but they do lead to cancer in some women and can still infect the cervix in women who have received the vaccine. Even if we reach all at-risk young women with our vaccine program, they will still be at risk—albeit lower risk—for cervical cancer due to infection with other high-risk HPV types.
One other point: We do not yet know how long the immunity from these vaccines will last. So it seems clear that screening will still be required to detect cervical cancer precursors and prevent cervical cancer from developing—even in women who have received the HPV vaccine.
Do we risk increasing the cancer rate?
WRIGHT: I worry about vaccination coverage. In the absence of state school requirements for the HPV vaccine, we are unlikely to get a high coverage rate among adolescents in the US. In several European countries, such as Germany, that have both recommendations and funding for universal hepatitis B vaccination of adolescents, only about 30% of adolescents have been vaccinated. The reason? These countries lack school requirements for the vaccination and have no school-based vaccination program. With the high prevalence of HPV infections in young, sexually active women in the US, we could actually increase our cervical cancer rate if we recommend reduced screening without ensuring high levels of vaccination coverage.
Dr. Gall, what do you predict for the next decade?
GALL: The future looks bright. A bipartisan bill was just introduced in the Michigan state legislature to add the HPV vaccine as a requirement for entry into junior high school. I expect more states to follow.
In general, adolescents are a poorly served group when it comes to health care because many fall out of the system. The HPV vaccine provides a great opportunity for us to encourage patients to bring their adolescent daughters to the office for a consultation. During this visit, we will conduct an “about the umbilicus” (ie, non-pelvic) physical exam, provide immunization, and discuss a number of topics such as contraception, menstruation, nutrition, etc. A critical step is to get state health departments and Medicaid officials off the dime and supporting the HPV vaccine.
Drs. Wright, DeFrancesco, Gall, and Levy report no financial relationships relevant to this article.
1. Gardasil [package insert]. Whitehouse Station, NJ: Merck.
Dr. Wright is an author of the 2001 Consensus Guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim Guidance for Use of HPV DNA testing for Primary Screening, and the 2001 Bethesda System. He is Professor of Pathology, Columbia University, New York.
1. Gardasil [package insert]. Whitehouse Station, NJ: Merck.
Dr. Wright is an author of the 2001 Consensus Guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim Guidance for Use of HPV DNA testing for Primary Screening, and the 2001 Bethesda System. He is Professor of Pathology, Columbia University, New York.
Beyond the Pap
Introducing HPV vaccination
- Who to vaccinate
- Consent forms
- Tracking systems to assure all 3 visits
- Should you vaccinate “off recommendations”?
THE ROAD AHEAD
This new series in Obg Management will keep you up to date on the many changes expected to take place as a result of the 2006 Consensus Conference held in September, and the introduction of HPV vaccines.
Our whole approach to cervical cancer prevention is likely to change within the next few years. Today, many obstetricians and gynecologists still consider the annual Papanicolaou test “the gold standard.” This is going to change, however, with…
ObGyns nationwide follow guidelines
This article summarizes the September 2006 Consensus Conference, organized by the ASCCP. The goal was to keep pace with the explosion of new data from clinical trials published in the last 5 years. The expected release date for the 2006 Guidelines is in the Spring or Summer of 2007. The 2001 Consensus Guidelines for the Management of Women With Cytologic Abnormalities have been extremely successful—they are now utilized by most clinicians and managed care organizations nationwide. In the last 5 years alone, more than 500,000 copies of the management algorithms have been downloaded from the ASCCP’s Web site (http://www.asccp.org), and many more have been downloaded from the Journal of the American Medical Association and the national guideline clearinghouse.
In large part, this success derived from the fact that most professional societies and federal agencies that deal with cervical cancer screening partnered in their development. Although the ASCCP organized the effort, 29 professional organizations participated. This meant that when groups such as ACOG subsequently developed guidelines for their members, they closely mirrored the 2001 Consensus Guidelines.
The 2006 Consensus Conference included approximately 100 delegates, 28 national and international professional societies, and federal agencies, but the meeting itself was only the final step in a long process. For 18 months, 6 different working groups reviewed the literature and determined where changes were needed.
NEW GUIDELINES ARE EVIDENCE-BASED
“The working groups evaluated hundreds of manuscripts and studies to make certain that the new guidelines remain evidence-based”
Kathy Poole, ASCCP Executive Director
The working groups posted their findings on Internet-based bulletin boards that allowed anyone in the national or international screening community to have input.
NEW GUIDELINES ARE SIMPLER
“One of the key goals of the 2006 Guidelines is simplification”
Dr. Mark Spitzer, ASCCP President and Chairman, Department of Obstetrics and Gynecology, Brookdale University Hospital and Medical Center, Brooklyn
“Many clinicians who manage women with cytologic abnormalities are not obstetricians and gynecologists. Nurse practitioners and family practice clinicians who don’t spend their entire lives dealing with abnormal Pap smears are responsible for much of the cervical cancer screening that takes place nationally,” said Dr. Spitzer. “The 2001 Consensus Guidelines have sometimes been characterized as complex and difficult to follow by such clinicians.”
Atypical squamous cells
HPV DNA-positive ASC-US vs LSIL
No major changes were recommended for the general population with ASC-US. However, since the ALTS trial clearly demonstrated that women with ASC-US who are high-risk HPV DNA-positive are essentially identical to women with LSIL, an effort was made to ensure that recommendations for these 2 groups are identical.
IDENTICAL MANAGEMENT
“Current data clearly indicate that women with ASC-US who are HPV DNA-positive and women with LSIL have the same risk of having high-grade disease and should therefore be managed identically” Dr. Spitzer
Although new data resulted in a number of minor changes to ASC-US recommendations, there are more significant changes in management of ASC-US with “special circumstances.” The 2001 Guidelines identified postmenopausal women and immuno-suppressed women with ASC-US as “special circumstances” to be managed differently than the general population. New guidelines eliminate this distinction.
Limited colposcopy in adolescents
Previously there was no provision for managing adolescents (up to 20 years of age) with ASC-US differently than the general population. New guidelines add adolescents as a “special population,” with different management. This change is based on considerable data demonstrating that the risk of cervical cancer is extremely low in adolescents, and that more than two thirds of adolescents with ASC-US are referred to colposcopy if “reflex” HPV DNA testing or repeat cervical cytology is utilized. Based on the newer data, a more conservative approach, which greatly limits use of colposcopy, will be introduced.
ASC-H
A number of studies have confirmed a high prevalence of CIN 2,3 as well as a high prevalence of high-risk HPV DNA positivity in women with ASC-H compared with women with ASC-US. Therefore, no substantive changes were made in the management recommendations for ASC-H. However, the need for a complete review of cytology, colposcopy, and histology results is downplayed for ASC-H without high-grade CIN.
LSIL and CIN 1
Basic management did not change for the general population, but did change for adolescents and postmenopausal women:
Previously, management of CIN 1 depended on whether colposcopy was satisfactory. Either treatment or follow-up was acceptable for women with CIN 1 and satisfactory colposcopy, but a diagnostic excisional procedure was advised for unsatisfactory colposcopy.
This has changed. The importance of a satisfactory colposcopic examination is deemphasized, and conservative follow-up of CIN 1 (which really represents histological manifestations of a productive HPV infection rather than a cancer precursor) is now stressed. Treatment for CIN 1 is particularly discouraged among adolescents.
HSIL and CIN 2,3
Immediate “screen and treat” as a management strategy for HSIL in the general population is emphasized more. As with ASC-H, the need for a complete review of the cytology, colposcopy, and histology results for women with HSIL in whom CIN 2,3 is not identified is deemphasized.
New guidelines no longer require that women with HSIL who do not have biopsy-confirmed CIN 2,3 undergo a diagnostic excisional procedure.
Basic management of CIN 2,3 is modified in only minor ways; however, options for conservative management of adolescents with CIN 2,3 are expanded (as for other clinical scenarios such as ASC-US, LSIL, and HSIL in adolescents).
Atypical glandular cells
HPV DNA STATUS MATTERS
“Based on data obtained since the 2001 Consensus Conference, it now appears reasonable to incorporate knowledge of a woman’s HPV status in management of atypical glandular cell (AGC) cytological abnormalities”
Dr. Charles Dunton, Chair, AGC Working Group, and Director, Division of Gynecological Oncology, Albert Einstein Medical Center, Wynnewood, Pa
Although the 2001 recommendation that almost all women with AGC undergo colposcopy remains, recommended follow-up will be modified to take into account HPV DNA status. Women who are high-risk HPV DNA-negative can be followed less aggressively than those who are high-risk HPV DNA-positive.
Other new guidelines
These include specific guidelines for managing benign endometrial cells identified during routine cytology (in both postmenopausal and cycling women) and benign-appearing glandular cells identified on routine cytology in women who have had a hysterectomy, as well as comprehensive management recommendations for women with biopsy-confirmed cervical adenocarcinoma in situ.
HPV DNA testing
“The 2001 guidelines did not address HPV DNA testing as an adjunct to cervical cytology in women aged 30 and older because HPV DNA testing was not yet FDA approved for this use,” says Dr. Edward J. Wilkinson, Chair, HPV DNA Working Group, and Professor and Vice Chairman, Department of Pathology, University of Florida College of Medicine, Gainesville. Therefore, the Interim Guidance introduced by the ASCCP/NCI/ACS in 2004 was the basis for new recommendations. For the most part, the 2006 Consensus Conference formally adopted the Interim Guidance published in 2004.
Quite a bit of consideration was given to how management will change once the FDA approves genotyping assays that identify HPV 16 and 18 for clinical use. Once such assays become available, the new guidelines indicate that there is sufficient data to support the triage of women who are cytology-negative/HPV DNA-positive, using HPV genotyping assays that identify HPV 16 and 18.
Dr. Wright is a consultant to GlaxoSmithKline and a reference pathologist for Roche Molecular Diagnostics.
Introducing HPV vaccination
- Who to vaccinate
- Consent forms
- Tracking systems to assure all 3 visits
- Should you vaccinate “off recommendations”?
THE ROAD AHEAD
This new series in Obg Management will keep you up to date on the many changes expected to take place as a result of the 2006 Consensus Conference held in September, and the introduction of HPV vaccines.
Our whole approach to cervical cancer prevention is likely to change within the next few years. Today, many obstetricians and gynecologists still consider the annual Papanicolaou test “the gold standard.” This is going to change, however, with…
ObGyns nationwide follow guidelines
This article summarizes the September 2006 Consensus Conference, organized by the ASCCP. The goal was to keep pace with the explosion of new data from clinical trials published in the last 5 years. The expected release date for the 2006 Guidelines is in the Spring or Summer of 2007. The 2001 Consensus Guidelines for the Management of Women With Cytologic Abnormalities have been extremely successful—they are now utilized by most clinicians and managed care organizations nationwide. In the last 5 years alone, more than 500,000 copies of the management algorithms have been downloaded from the ASCCP’s Web site (http://www.asccp.org), and many more have been downloaded from the Journal of the American Medical Association and the national guideline clearinghouse.
In large part, this success derived from the fact that most professional societies and federal agencies that deal with cervical cancer screening partnered in their development. Although the ASCCP organized the effort, 29 professional organizations participated. This meant that when groups such as ACOG subsequently developed guidelines for their members, they closely mirrored the 2001 Consensus Guidelines.
The 2006 Consensus Conference included approximately 100 delegates, 28 national and international professional societies, and federal agencies, but the meeting itself was only the final step in a long process. For 18 months, 6 different working groups reviewed the literature and determined where changes were needed.
NEW GUIDELINES ARE EVIDENCE-BASED
“The working groups evaluated hundreds of manuscripts and studies to make certain that the new guidelines remain evidence-based”
Kathy Poole, ASCCP Executive Director
The working groups posted their findings on Internet-based bulletin boards that allowed anyone in the national or international screening community to have input.
NEW GUIDELINES ARE SIMPLER
“One of the key goals of the 2006 Guidelines is simplification”
Dr. Mark Spitzer, ASCCP President and Chairman, Department of Obstetrics and Gynecology, Brookdale University Hospital and Medical Center, Brooklyn
“Many clinicians who manage women with cytologic abnormalities are not obstetricians and gynecologists. Nurse practitioners and family practice clinicians who don’t spend their entire lives dealing with abnormal Pap smears are responsible for much of the cervical cancer screening that takes place nationally,” said Dr. Spitzer. “The 2001 Consensus Guidelines have sometimes been characterized as complex and difficult to follow by such clinicians.”
Atypical squamous cells
HPV DNA-positive ASC-US vs LSIL
No major changes were recommended for the general population with ASC-US. However, since the ALTS trial clearly demonstrated that women with ASC-US who are high-risk HPV DNA-positive are essentially identical to women with LSIL, an effort was made to ensure that recommendations for these 2 groups are identical.
IDENTICAL MANAGEMENT
“Current data clearly indicate that women with ASC-US who are HPV DNA-positive and women with LSIL have the same risk of having high-grade disease and should therefore be managed identically” Dr. Spitzer
Although new data resulted in a number of minor changes to ASC-US recommendations, there are more significant changes in management of ASC-US with “special circumstances.” The 2001 Guidelines identified postmenopausal women and immuno-suppressed women with ASC-US as “special circumstances” to be managed differently than the general population. New guidelines eliminate this distinction.
Limited colposcopy in adolescents
Previously there was no provision for managing adolescents (up to 20 years of age) with ASC-US differently than the general population. New guidelines add adolescents as a “special population,” with different management. This change is based on considerable data demonstrating that the risk of cervical cancer is extremely low in adolescents, and that more than two thirds of adolescents with ASC-US are referred to colposcopy if “reflex” HPV DNA testing or repeat cervical cytology is utilized. Based on the newer data, a more conservative approach, which greatly limits use of colposcopy, will be introduced.
ASC-H
A number of studies have confirmed a high prevalence of CIN 2,3 as well as a high prevalence of high-risk HPV DNA positivity in women with ASC-H compared with women with ASC-US. Therefore, no substantive changes were made in the management recommendations for ASC-H. However, the need for a complete review of cytology, colposcopy, and histology results is downplayed for ASC-H without high-grade CIN.
LSIL and CIN 1
Basic management did not change for the general population, but did change for adolescents and postmenopausal women:
Previously, management of CIN 1 depended on whether colposcopy was satisfactory. Either treatment or follow-up was acceptable for women with CIN 1 and satisfactory colposcopy, but a diagnostic excisional procedure was advised for unsatisfactory colposcopy.
This has changed. The importance of a satisfactory colposcopic examination is deemphasized, and conservative follow-up of CIN 1 (which really represents histological manifestations of a productive HPV infection rather than a cancer precursor) is now stressed. Treatment for CIN 1 is particularly discouraged among adolescents.
HSIL and CIN 2,3
Immediate “screen and treat” as a management strategy for HSIL in the general population is emphasized more. As with ASC-H, the need for a complete review of the cytology, colposcopy, and histology results for women with HSIL in whom CIN 2,3 is not identified is deemphasized.
New guidelines no longer require that women with HSIL who do not have biopsy-confirmed CIN 2,3 undergo a diagnostic excisional procedure.
Basic management of CIN 2,3 is modified in only minor ways; however, options for conservative management of adolescents with CIN 2,3 are expanded (as for other clinical scenarios such as ASC-US, LSIL, and HSIL in adolescents).
Atypical glandular cells
HPV DNA STATUS MATTERS
“Based on data obtained since the 2001 Consensus Conference, it now appears reasonable to incorporate knowledge of a woman’s HPV status in management of atypical glandular cell (AGC) cytological abnormalities”
Dr. Charles Dunton, Chair, AGC Working Group, and Director, Division of Gynecological Oncology, Albert Einstein Medical Center, Wynnewood, Pa
Although the 2001 recommendation that almost all women with AGC undergo colposcopy remains, recommended follow-up will be modified to take into account HPV DNA status. Women who are high-risk HPV DNA-negative can be followed less aggressively than those who are high-risk HPV DNA-positive.
Other new guidelines
These include specific guidelines for managing benign endometrial cells identified during routine cytology (in both postmenopausal and cycling women) and benign-appearing glandular cells identified on routine cytology in women who have had a hysterectomy, as well as comprehensive management recommendations for women with biopsy-confirmed cervical adenocarcinoma in situ.
HPV DNA testing
“The 2001 guidelines did not address HPV DNA testing as an adjunct to cervical cytology in women aged 30 and older because HPV DNA testing was not yet FDA approved for this use,” says Dr. Edward J. Wilkinson, Chair, HPV DNA Working Group, and Professor and Vice Chairman, Department of Pathology, University of Florida College of Medicine, Gainesville. Therefore, the Interim Guidance introduced by the ASCCP/NCI/ACS in 2004 was the basis for new recommendations. For the most part, the 2006 Consensus Conference formally adopted the Interim Guidance published in 2004.
Quite a bit of consideration was given to how management will change once the FDA approves genotyping assays that identify HPV 16 and 18 for clinical use. Once such assays become available, the new guidelines indicate that there is sufficient data to support the triage of women who are cytology-negative/HPV DNA-positive, using HPV genotyping assays that identify HPV 16 and 18.
Introducing HPV vaccination
- Who to vaccinate
- Consent forms
- Tracking systems to assure all 3 visits
- Should you vaccinate “off recommendations”?
THE ROAD AHEAD
This new series in Obg Management will keep you up to date on the many changes expected to take place as a result of the 2006 Consensus Conference held in September, and the introduction of HPV vaccines.
Our whole approach to cervical cancer prevention is likely to change within the next few years. Today, many obstetricians and gynecologists still consider the annual Papanicolaou test “the gold standard.” This is going to change, however, with…
ObGyns nationwide follow guidelines
This article summarizes the September 2006 Consensus Conference, organized by the ASCCP. The goal was to keep pace with the explosion of new data from clinical trials published in the last 5 years. The expected release date for the 2006 Guidelines is in the Spring or Summer of 2007. The 2001 Consensus Guidelines for the Management of Women With Cytologic Abnormalities have been extremely successful—they are now utilized by most clinicians and managed care organizations nationwide. In the last 5 years alone, more than 500,000 copies of the management algorithms have been downloaded from the ASCCP’s Web site (http://www.asccp.org), and many more have been downloaded from the Journal of the American Medical Association and the national guideline clearinghouse.
In large part, this success derived from the fact that most professional societies and federal agencies that deal with cervical cancer screening partnered in their development. Although the ASCCP organized the effort, 29 professional organizations participated. This meant that when groups such as ACOG subsequently developed guidelines for their members, they closely mirrored the 2001 Consensus Guidelines.
The 2006 Consensus Conference included approximately 100 delegates, 28 national and international professional societies, and federal agencies, but the meeting itself was only the final step in a long process. For 18 months, 6 different working groups reviewed the literature and determined where changes were needed.
NEW GUIDELINES ARE EVIDENCE-BASED
“The working groups evaluated hundreds of manuscripts and studies to make certain that the new guidelines remain evidence-based”
Kathy Poole, ASCCP Executive Director
The working groups posted their findings on Internet-based bulletin boards that allowed anyone in the national or international screening community to have input.
NEW GUIDELINES ARE SIMPLER
“One of the key goals of the 2006 Guidelines is simplification”
Dr. Mark Spitzer, ASCCP President and Chairman, Department of Obstetrics and Gynecology, Brookdale University Hospital and Medical Center, Brooklyn
“Many clinicians who manage women with cytologic abnormalities are not obstetricians and gynecologists. Nurse practitioners and family practice clinicians who don’t spend their entire lives dealing with abnormal Pap smears are responsible for much of the cervical cancer screening that takes place nationally,” said Dr. Spitzer. “The 2001 Consensus Guidelines have sometimes been characterized as complex and difficult to follow by such clinicians.”
Atypical squamous cells
HPV DNA-positive ASC-US vs LSIL
No major changes were recommended for the general population with ASC-US. However, since the ALTS trial clearly demonstrated that women with ASC-US who are high-risk HPV DNA-positive are essentially identical to women with LSIL, an effort was made to ensure that recommendations for these 2 groups are identical.
IDENTICAL MANAGEMENT
“Current data clearly indicate that women with ASC-US who are HPV DNA-positive and women with LSIL have the same risk of having high-grade disease and should therefore be managed identically” Dr. Spitzer
Although new data resulted in a number of minor changes to ASC-US recommendations, there are more significant changes in management of ASC-US with “special circumstances.” The 2001 Guidelines identified postmenopausal women and immuno-suppressed women with ASC-US as “special circumstances” to be managed differently than the general population. New guidelines eliminate this distinction.
Limited colposcopy in adolescents
Previously there was no provision for managing adolescents (up to 20 years of age) with ASC-US differently than the general population. New guidelines add adolescents as a “special population,” with different management. This change is based on considerable data demonstrating that the risk of cervical cancer is extremely low in adolescents, and that more than two thirds of adolescents with ASC-US are referred to colposcopy if “reflex” HPV DNA testing or repeat cervical cytology is utilized. Based on the newer data, a more conservative approach, which greatly limits use of colposcopy, will be introduced.
ASC-H
A number of studies have confirmed a high prevalence of CIN 2,3 as well as a high prevalence of high-risk HPV DNA positivity in women with ASC-H compared with women with ASC-US. Therefore, no substantive changes were made in the management recommendations for ASC-H. However, the need for a complete review of cytology, colposcopy, and histology results is downplayed for ASC-H without high-grade CIN.
LSIL and CIN 1
Basic management did not change for the general population, but did change for adolescents and postmenopausal women:
Previously, management of CIN 1 depended on whether colposcopy was satisfactory. Either treatment or follow-up was acceptable for women with CIN 1 and satisfactory colposcopy, but a diagnostic excisional procedure was advised for unsatisfactory colposcopy.
This has changed. The importance of a satisfactory colposcopic examination is deemphasized, and conservative follow-up of CIN 1 (which really represents histological manifestations of a productive HPV infection rather than a cancer precursor) is now stressed. Treatment for CIN 1 is particularly discouraged among adolescents.
HSIL and CIN 2,3
Immediate “screen and treat” as a management strategy for HSIL in the general population is emphasized more. As with ASC-H, the need for a complete review of the cytology, colposcopy, and histology results for women with HSIL in whom CIN 2,3 is not identified is deemphasized.
New guidelines no longer require that women with HSIL who do not have biopsy-confirmed CIN 2,3 undergo a diagnostic excisional procedure.
Basic management of CIN 2,3 is modified in only minor ways; however, options for conservative management of adolescents with CIN 2,3 are expanded (as for other clinical scenarios such as ASC-US, LSIL, and HSIL in adolescents).
Atypical glandular cells
HPV DNA STATUS MATTERS
“Based on data obtained since the 2001 Consensus Conference, it now appears reasonable to incorporate knowledge of a woman’s HPV status in management of atypical glandular cell (AGC) cytological abnormalities”
Dr. Charles Dunton, Chair, AGC Working Group, and Director, Division of Gynecological Oncology, Albert Einstein Medical Center, Wynnewood, Pa
Although the 2001 recommendation that almost all women with AGC undergo colposcopy remains, recommended follow-up will be modified to take into account HPV DNA status. Women who are high-risk HPV DNA-negative can be followed less aggressively than those who are high-risk HPV DNA-positive.
Other new guidelines
These include specific guidelines for managing benign endometrial cells identified during routine cytology (in both postmenopausal and cycling women) and benign-appearing glandular cells identified on routine cytology in women who have had a hysterectomy, as well as comprehensive management recommendations for women with biopsy-confirmed cervical adenocarcinoma in situ.
HPV DNA testing
“The 2001 guidelines did not address HPV DNA testing as an adjunct to cervical cytology in women aged 30 and older because HPV DNA testing was not yet FDA approved for this use,” says Dr. Edward J. Wilkinson, Chair, HPV DNA Working Group, and Professor and Vice Chairman, Department of Pathology, University of Florida College of Medicine, Gainesville. Therefore, the Interim Guidance introduced by the ASCCP/NCI/ACS in 2004 was the basis for new recommendations. For the most part, the 2006 Consensus Conference formally adopted the Interim Guidance published in 2004.
Quite a bit of consideration was given to how management will change once the FDA approves genotyping assays that identify HPV 16 and 18 for clinical use. Once such assays become available, the new guidelines indicate that there is sufficient data to support the triage of women who are cytology-negative/HPV DNA-positive, using HPV genotyping assays that identify HPV 16 and 18.
Dr. Wright is a consultant to GlaxoSmithKline and a reference pathologist for Roche Molecular Diagnostics.
Dr. Wright is a consultant to GlaxoSmithKline and a reference pathologist for Roche Molecular Diagnostics.
The HPV vaccine: A good start
Last month the Food and Drug Administration approved the first vaccine (Gardisil) to prevent infection with specific types of human papillomavirus (HPV). The approval is the culmination of 2 decades of work in a variety of disciplines that have clearly demonstrated that infection with specific types of high-risk HPV is required for the development of cervical cancer—and that the development of HPV-associated disease can be prevented through vaccination.
How and when will HPV vaccine be put to use?
It is important to recognize that FDA approval does not mean the HPV vaccine will be widely used. Five components are necessary for successful introduction:
1. FDA approval
FDA approval is based solely on the safety and effectiveness of the vaccine.
Indications. The FDA determined that the quadrivalent HPV vaccine is indicated for prevention of HPV 6, 11, 16, and 18 associated cervical cancer, genital warts, CIN, AIS, and VIN 2,3 and VAIN 2,3.
The indication applies to girls and women 9 to 26 years of age. The indication in girls 9 to 15 years of age, who were not included in the Phase II and III trials, was based on immunological “bridging” studies that demonstrated better immune responses to vaccination in this group than in the older group enrolled in the trials.
2. Federal-level recommendations
The second component of a successful vaccine introduction is the support of the Advisory Committee on Immunization Practices (ACIP),2 a congressionally mandated federal advisory committee coordinated by the Centers for Disease Control and Prevention (CDC). The ACIP advises the Secretary of Health and Human Services, as well as the Director of the CDC, on appropriate immunization practices for the United States.
Within several months of the June 2006 FDA approval, the ACIP will make a recommendation on the vaccine’s use. When making its recommendation, the ACIP takes into account the burden of disease in the population, safety and efficacy, and health economics of vaccination—will it be cost-effective?
Funding and insurance coverage at stake. The ACIP also determines whether the vaccine will be acceptable to physicians and consumers and what programmatic issues are associated with its introduction.
- Public funding. The ACIP recommendation strongly influences standard of practice, and is relied upon by the federal government and states for determining whether to include a vaccine in public funding programs.
- Insurers likewise look to the ACIP for guidance when setting reimbursement policy.
3. Achieving adequate funding
The third component of a successful vaccine introduction is achieving adequate funding. Federal and state programs such as the Vaccines for Children (VFC) program currently pay for more than half of all childhood vaccinations. Therefore it is important that these programs include the HPV vaccine in order to ensure it is widely available, especially among lower socio-economic groups.
4. Recommendations by professional societies
Societies such as the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians, whose members provide most of the vaccinations in the US, have representatives who work directly with the ACIP. Many societies make their own vaccination recommendations, as well.
5. Advocacy and education
Other groups increase awareness of the need for vaccination among consumers, public health and government officials, and clinicians.
The quadrivalent vaccine consists of recombinant viral-like particles (VLPs) derived from the 4 different types of HPV (6, 11, 16, and 18) which, combined, account for approximately 70% of all invasive cervical cancers, 60% of high-grade CIN lesions, and more than 90% of genital warts, both in the United States and globally.
The biotechnology. The VLPs used in the HPV vaccines are produced by cloning the major viral capsid genes (L1) from the 4 different HPV types, inserting them into plasmids, and then producing large amounts of the L1 proteins of each of the HPV types separately in yeast. The recombinant L1 proteins are subsequently self-assembled into VLPs that structurally appear identical to HPV virions, but which lack both DNA and RNA.
Therefore, the VLPs are completely non-infectious and non-oncogenic. The purified VLPs are then mixed with an aluminum-containing adjuvant to produce the final vaccine.
Administration. It is administered intramuscularly as 3 injections over a 6-month period: at enrollment and at 2 and 6 months.
A bivalent vaccine for HPV 16 and 18 produced by GlaxoSmithKline is based on similar technology, and will most likely become available in 2007.
Clinical trials: 100% effective, well-accepted, safe
FDA approval of the quadrivalent HPV vaccine came approximately 18 months earlier than expected because, in the Phase III trials, the vaccine proved much more effective than originally predicted, thus shortening the length of follow-up needed to reach the endpoints.
Efficacy and safety studies. Generally, the vaccine appears to be well-accepted and safe. To date, the efficacy and safety of the quadrivalent vaccine have been evaluated in 4 Phase II and III studies that included 20,541 females aged 16 to 26 who were followed for a median of 2 to 4 years in the different studies.1 The endpoint for these trials was biopsy-confirmed high-grade cervical neoplasia, including CIN 2,3 and adenocarcinoma in-situ (AIS). The primary analyses of efficacy were conducted in the “per protocol population,” which consisted of women who received all 3 vaccinations and who were both DNA and serologically negative for the relevant HPV types during the 6-month vaccination period.
In each of the 4 trials, the quadrivalent vaccine was found to be 100% efficacious against HPV 16 and 18 associated CIN 2,3 and AIS. Similarly, the vaccine was also highly efficacious against HPV 6, 11, 16, or 18 associated genital warts.
Adverse events. Although injection site pain, swelling, erythema, and pruritus were increased in vaccine recipients compared to placebo, few subjects (0.1%) discontinued the study as a result of adverse experiences.
Varicella vaccine success story. Varicella vaccine was approved in the 1990s, but both physicians and the public were slow to accept it, in part because it was relatively expensive for physicians to buy, and insurance companies were slow to pay for vaccination. What brought about widespread adoption of varicella vaccination was education on the dangers of chicken pox, directed to clinicians, patients, and government officials. This led to appreciation of the benefits of vaccination. As a result, states changed their school entry requirements, and today, almost all children receive the varicella vaccine.
The unknowns
It is important to recognize that with all new vaccines there are unknowns when they are first introduced.
What will the ACIP recommend?
One of the biggest concerns about the HPV vaccines is that we do not yet know who the ACIP will recommend for vaccination. In the clinical trials, the HPV vaccine did not appear effective in women already exposed to HPV. Since the cumulative incidence of HPV infection in young women is approximately 40% within 2 years of initiating intercourse, we will need to target adolescents prior to onset of sexual activity.3 In the US, 7.4% of adolescents report having begun having sexual intercourse by 13 years of age, and about one third by the ninth grade.4 Therefore the most likely primary target population will be young girls 11 to 12 years of age. It is also likely that there will be a recommendation for “catch-up” vaccination of older girls and young women.
What is the duration of protection afforded by HPV vaccination?
We know that the vaccine appears to provide protection for at least 4 years, but if we vaccinate 11- to 12-year-old girls, will they require a booster later in life?5
How will HPV vaccination affect cervical screening?
Once vaccination is widespread, a significant reduction in CIN 2,3 will occur, necessitating changes in the age to start screening and screening frequency. However, it is unclear what percentage of the population will need to be vaccinated before we change screening policy.
Will HPV vaccine be efficacious in women over the age of 26 years?
Vaccine trials are currently underway in older women, but until these trials are completed, all we can tell our older, sexually active patients is that we simply don’t know if the vaccine will benefit them.
Will parents, adolescents, and the public at large accept the vaccine?
As with all issues relating to sexual behavior, it is likely that opinions will differ as to the acceptability of vaccinating young adolescents against a sexually transmitted disease.
1. Gardasil package insert. Released June 2006.
2. Orenstein S, Rodewald L, Hinman A. Immunization in the United States. In: Plotkin SA, Orenstein S, editors. Vaccines. 4th ed. Philadelphia: WB Saunders Company; 2004:1357-1386.
3. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Whalen L, Grunbaum J, Kinchen S, McManus T, Shanklin S, Kann L. Middle School: Youth Risk Behavior Survey 2003. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005.
5. Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
Dr. Wright is the chairman of the upcoming ASCCP (American Society for Colposcopy and Cervical Pathology) 2006 Consensus Conference. He is an author of the 2001 Consensus Guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim Guidance for Use of HPV DNA testing for Primary Screening, and the 2001 Bethesda System. He is a Professor of Pathology, Columbia University, New York City.
Dr. Wright serves as a consultant to GlaxoSmithKline.
Last month the Food and Drug Administration approved the first vaccine (Gardisil) to prevent infection with specific types of human papillomavirus (HPV). The approval is the culmination of 2 decades of work in a variety of disciplines that have clearly demonstrated that infection with specific types of high-risk HPV is required for the development of cervical cancer—and that the development of HPV-associated disease can be prevented through vaccination.
How and when will HPV vaccine be put to use?
It is important to recognize that FDA approval does not mean the HPV vaccine will be widely used. Five components are necessary for successful introduction:
1. FDA approval
FDA approval is based solely on the safety and effectiveness of the vaccine.
Indications. The FDA determined that the quadrivalent HPV vaccine is indicated for prevention of HPV 6, 11, 16, and 18 associated cervical cancer, genital warts, CIN, AIS, and VIN 2,3 and VAIN 2,3.
The indication applies to girls and women 9 to 26 years of age. The indication in girls 9 to 15 years of age, who were not included in the Phase II and III trials, was based on immunological “bridging” studies that demonstrated better immune responses to vaccination in this group than in the older group enrolled in the trials.
2. Federal-level recommendations
The second component of a successful vaccine introduction is the support of the Advisory Committee on Immunization Practices (ACIP),2 a congressionally mandated federal advisory committee coordinated by the Centers for Disease Control and Prevention (CDC). The ACIP advises the Secretary of Health and Human Services, as well as the Director of the CDC, on appropriate immunization practices for the United States.
Within several months of the June 2006 FDA approval, the ACIP will make a recommendation on the vaccine’s use. When making its recommendation, the ACIP takes into account the burden of disease in the population, safety and efficacy, and health economics of vaccination—will it be cost-effective?
Funding and insurance coverage at stake. The ACIP also determines whether the vaccine will be acceptable to physicians and consumers and what programmatic issues are associated with its introduction.
- Public funding. The ACIP recommendation strongly influences standard of practice, and is relied upon by the federal government and states for determining whether to include a vaccine in public funding programs.
- Insurers likewise look to the ACIP for guidance when setting reimbursement policy.
3. Achieving adequate funding
The third component of a successful vaccine introduction is achieving adequate funding. Federal and state programs such as the Vaccines for Children (VFC) program currently pay for more than half of all childhood vaccinations. Therefore it is important that these programs include the HPV vaccine in order to ensure it is widely available, especially among lower socio-economic groups.
4. Recommendations by professional societies
Societies such as the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians, whose members provide most of the vaccinations in the US, have representatives who work directly with the ACIP. Many societies make their own vaccination recommendations, as well.
5. Advocacy and education
Other groups increase awareness of the need for vaccination among consumers, public health and government officials, and clinicians.
The quadrivalent vaccine consists of recombinant viral-like particles (VLPs) derived from the 4 different types of HPV (6, 11, 16, and 18) which, combined, account for approximately 70% of all invasive cervical cancers, 60% of high-grade CIN lesions, and more than 90% of genital warts, both in the United States and globally.
The biotechnology. The VLPs used in the HPV vaccines are produced by cloning the major viral capsid genes (L1) from the 4 different HPV types, inserting them into plasmids, and then producing large amounts of the L1 proteins of each of the HPV types separately in yeast. The recombinant L1 proteins are subsequently self-assembled into VLPs that structurally appear identical to HPV virions, but which lack both DNA and RNA.
Therefore, the VLPs are completely non-infectious and non-oncogenic. The purified VLPs are then mixed with an aluminum-containing adjuvant to produce the final vaccine.
Administration. It is administered intramuscularly as 3 injections over a 6-month period: at enrollment and at 2 and 6 months.
A bivalent vaccine for HPV 16 and 18 produced by GlaxoSmithKline is based on similar technology, and will most likely become available in 2007.
Clinical trials: 100% effective, well-accepted, safe
FDA approval of the quadrivalent HPV vaccine came approximately 18 months earlier than expected because, in the Phase III trials, the vaccine proved much more effective than originally predicted, thus shortening the length of follow-up needed to reach the endpoints.
Efficacy and safety studies. Generally, the vaccine appears to be well-accepted and safe. To date, the efficacy and safety of the quadrivalent vaccine have been evaluated in 4 Phase II and III studies that included 20,541 females aged 16 to 26 who were followed for a median of 2 to 4 years in the different studies.1 The endpoint for these trials was biopsy-confirmed high-grade cervical neoplasia, including CIN 2,3 and adenocarcinoma in-situ (AIS). The primary analyses of efficacy were conducted in the “per protocol population,” which consisted of women who received all 3 vaccinations and who were both DNA and serologically negative for the relevant HPV types during the 6-month vaccination period.
In each of the 4 trials, the quadrivalent vaccine was found to be 100% efficacious against HPV 16 and 18 associated CIN 2,3 and AIS. Similarly, the vaccine was also highly efficacious against HPV 6, 11, 16, or 18 associated genital warts.
Adverse events. Although injection site pain, swelling, erythema, and pruritus were increased in vaccine recipients compared to placebo, few subjects (0.1%) discontinued the study as a result of adverse experiences.
Varicella vaccine success story. Varicella vaccine was approved in the 1990s, but both physicians and the public were slow to accept it, in part because it was relatively expensive for physicians to buy, and insurance companies were slow to pay for vaccination. What brought about widespread adoption of varicella vaccination was education on the dangers of chicken pox, directed to clinicians, patients, and government officials. This led to appreciation of the benefits of vaccination. As a result, states changed their school entry requirements, and today, almost all children receive the varicella vaccine.
The unknowns
It is important to recognize that with all new vaccines there are unknowns when they are first introduced.
What will the ACIP recommend?
One of the biggest concerns about the HPV vaccines is that we do not yet know who the ACIP will recommend for vaccination. In the clinical trials, the HPV vaccine did not appear effective in women already exposed to HPV. Since the cumulative incidence of HPV infection in young women is approximately 40% within 2 years of initiating intercourse, we will need to target adolescents prior to onset of sexual activity.3 In the US, 7.4% of adolescents report having begun having sexual intercourse by 13 years of age, and about one third by the ninth grade.4 Therefore the most likely primary target population will be young girls 11 to 12 years of age. It is also likely that there will be a recommendation for “catch-up” vaccination of older girls and young women.
What is the duration of protection afforded by HPV vaccination?
We know that the vaccine appears to provide protection for at least 4 years, but if we vaccinate 11- to 12-year-old girls, will they require a booster later in life?5
How will HPV vaccination affect cervical screening?
Once vaccination is widespread, a significant reduction in CIN 2,3 will occur, necessitating changes in the age to start screening and screening frequency. However, it is unclear what percentage of the population will need to be vaccinated before we change screening policy.
Will HPV vaccine be efficacious in women over the age of 26 years?
Vaccine trials are currently underway in older women, but until these trials are completed, all we can tell our older, sexually active patients is that we simply don’t know if the vaccine will benefit them.
Will parents, adolescents, and the public at large accept the vaccine?
As with all issues relating to sexual behavior, it is likely that opinions will differ as to the acceptability of vaccinating young adolescents against a sexually transmitted disease.
Last month the Food and Drug Administration approved the first vaccine (Gardisil) to prevent infection with specific types of human papillomavirus (HPV). The approval is the culmination of 2 decades of work in a variety of disciplines that have clearly demonstrated that infection with specific types of high-risk HPV is required for the development of cervical cancer—and that the development of HPV-associated disease can be prevented through vaccination.
How and when will HPV vaccine be put to use?
It is important to recognize that FDA approval does not mean the HPV vaccine will be widely used. Five components are necessary for successful introduction:
1. FDA approval
FDA approval is based solely on the safety and effectiveness of the vaccine.
Indications. The FDA determined that the quadrivalent HPV vaccine is indicated for prevention of HPV 6, 11, 16, and 18 associated cervical cancer, genital warts, CIN, AIS, and VIN 2,3 and VAIN 2,3.
The indication applies to girls and women 9 to 26 years of age. The indication in girls 9 to 15 years of age, who were not included in the Phase II and III trials, was based on immunological “bridging” studies that demonstrated better immune responses to vaccination in this group than in the older group enrolled in the trials.
2. Federal-level recommendations
The second component of a successful vaccine introduction is the support of the Advisory Committee on Immunization Practices (ACIP),2 a congressionally mandated federal advisory committee coordinated by the Centers for Disease Control and Prevention (CDC). The ACIP advises the Secretary of Health and Human Services, as well as the Director of the CDC, on appropriate immunization practices for the United States.
Within several months of the June 2006 FDA approval, the ACIP will make a recommendation on the vaccine’s use. When making its recommendation, the ACIP takes into account the burden of disease in the population, safety and efficacy, and health economics of vaccination—will it be cost-effective?
Funding and insurance coverage at stake. The ACIP also determines whether the vaccine will be acceptable to physicians and consumers and what programmatic issues are associated with its introduction.
- Public funding. The ACIP recommendation strongly influences standard of practice, and is relied upon by the federal government and states for determining whether to include a vaccine in public funding programs.
- Insurers likewise look to the ACIP for guidance when setting reimbursement policy.
3. Achieving adequate funding
The third component of a successful vaccine introduction is achieving adequate funding. Federal and state programs such as the Vaccines for Children (VFC) program currently pay for more than half of all childhood vaccinations. Therefore it is important that these programs include the HPV vaccine in order to ensure it is widely available, especially among lower socio-economic groups.
4. Recommendations by professional societies
Societies such as the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians, whose members provide most of the vaccinations in the US, have representatives who work directly with the ACIP. Many societies make their own vaccination recommendations, as well.
5. Advocacy and education
Other groups increase awareness of the need for vaccination among consumers, public health and government officials, and clinicians.
The quadrivalent vaccine consists of recombinant viral-like particles (VLPs) derived from the 4 different types of HPV (6, 11, 16, and 18) which, combined, account for approximately 70% of all invasive cervical cancers, 60% of high-grade CIN lesions, and more than 90% of genital warts, both in the United States and globally.
The biotechnology. The VLPs used in the HPV vaccines are produced by cloning the major viral capsid genes (L1) from the 4 different HPV types, inserting them into plasmids, and then producing large amounts of the L1 proteins of each of the HPV types separately in yeast. The recombinant L1 proteins are subsequently self-assembled into VLPs that structurally appear identical to HPV virions, but which lack both DNA and RNA.
Therefore, the VLPs are completely non-infectious and non-oncogenic. The purified VLPs are then mixed with an aluminum-containing adjuvant to produce the final vaccine.
Administration. It is administered intramuscularly as 3 injections over a 6-month period: at enrollment and at 2 and 6 months.
A bivalent vaccine for HPV 16 and 18 produced by GlaxoSmithKline is based on similar technology, and will most likely become available in 2007.
Clinical trials: 100% effective, well-accepted, safe
FDA approval of the quadrivalent HPV vaccine came approximately 18 months earlier than expected because, in the Phase III trials, the vaccine proved much more effective than originally predicted, thus shortening the length of follow-up needed to reach the endpoints.
Efficacy and safety studies. Generally, the vaccine appears to be well-accepted and safe. To date, the efficacy and safety of the quadrivalent vaccine have been evaluated in 4 Phase II and III studies that included 20,541 females aged 16 to 26 who were followed for a median of 2 to 4 years in the different studies.1 The endpoint for these trials was biopsy-confirmed high-grade cervical neoplasia, including CIN 2,3 and adenocarcinoma in-situ (AIS). The primary analyses of efficacy were conducted in the “per protocol population,” which consisted of women who received all 3 vaccinations and who were both DNA and serologically negative for the relevant HPV types during the 6-month vaccination period.
In each of the 4 trials, the quadrivalent vaccine was found to be 100% efficacious against HPV 16 and 18 associated CIN 2,3 and AIS. Similarly, the vaccine was also highly efficacious against HPV 6, 11, 16, or 18 associated genital warts.
Adverse events. Although injection site pain, swelling, erythema, and pruritus were increased in vaccine recipients compared to placebo, few subjects (0.1%) discontinued the study as a result of adverse experiences.
Varicella vaccine success story. Varicella vaccine was approved in the 1990s, but both physicians and the public were slow to accept it, in part because it was relatively expensive for physicians to buy, and insurance companies were slow to pay for vaccination. What brought about widespread adoption of varicella vaccination was education on the dangers of chicken pox, directed to clinicians, patients, and government officials. This led to appreciation of the benefits of vaccination. As a result, states changed their school entry requirements, and today, almost all children receive the varicella vaccine.
The unknowns
It is important to recognize that with all new vaccines there are unknowns when they are first introduced.
What will the ACIP recommend?
One of the biggest concerns about the HPV vaccines is that we do not yet know who the ACIP will recommend for vaccination. In the clinical trials, the HPV vaccine did not appear effective in women already exposed to HPV. Since the cumulative incidence of HPV infection in young women is approximately 40% within 2 years of initiating intercourse, we will need to target adolescents prior to onset of sexual activity.3 In the US, 7.4% of adolescents report having begun having sexual intercourse by 13 years of age, and about one third by the ninth grade.4 Therefore the most likely primary target population will be young girls 11 to 12 years of age. It is also likely that there will be a recommendation for “catch-up” vaccination of older girls and young women.
What is the duration of protection afforded by HPV vaccination?
We know that the vaccine appears to provide protection for at least 4 years, but if we vaccinate 11- to 12-year-old girls, will they require a booster later in life?5
How will HPV vaccination affect cervical screening?
Once vaccination is widespread, a significant reduction in CIN 2,3 will occur, necessitating changes in the age to start screening and screening frequency. However, it is unclear what percentage of the population will need to be vaccinated before we change screening policy.
Will HPV vaccine be efficacious in women over the age of 26 years?
Vaccine trials are currently underway in older women, but until these trials are completed, all we can tell our older, sexually active patients is that we simply don’t know if the vaccine will benefit them.
Will parents, adolescents, and the public at large accept the vaccine?
As with all issues relating to sexual behavior, it is likely that opinions will differ as to the acceptability of vaccinating young adolescents against a sexually transmitted disease.
1. Gardasil package insert. Released June 2006.
2. Orenstein S, Rodewald L, Hinman A. Immunization in the United States. In: Plotkin SA, Orenstein S, editors. Vaccines. 4th ed. Philadelphia: WB Saunders Company; 2004:1357-1386.
3. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Whalen L, Grunbaum J, Kinchen S, McManus T, Shanklin S, Kann L. Middle School: Youth Risk Behavior Survey 2003. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005.
5. Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
Dr. Wright is the chairman of the upcoming ASCCP (American Society for Colposcopy and Cervical Pathology) 2006 Consensus Conference. He is an author of the 2001 Consensus Guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim Guidance for Use of HPV DNA testing for Primary Screening, and the 2001 Bethesda System. He is a Professor of Pathology, Columbia University, New York City.
Dr. Wright serves as a consultant to GlaxoSmithKline.
1. Gardasil package insert. Released June 2006.
2. Orenstein S, Rodewald L, Hinman A. Immunization in the United States. In: Plotkin SA, Orenstein S, editors. Vaccines. 4th ed. Philadelphia: WB Saunders Company; 2004:1357-1386.
3. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Whalen L, Grunbaum J, Kinchen S, McManus T, Shanklin S, Kann L. Middle School: Youth Risk Behavior Survey 2003. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005.
5. Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
Dr. Wright is the chairman of the upcoming ASCCP (American Society for Colposcopy and Cervical Pathology) 2006 Consensus Conference. He is an author of the 2001 Consensus Guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim Guidance for Use of HPV DNA testing for Primary Screening, and the 2001 Bethesda System. He is a Professor of Pathology, Columbia University, New York City.
Dr. Wright serves as a consultant to GlaxoSmithKline.
IN THIS ARTICLE