User login
Esophageal Cancer: Current Diagnosis and Management
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
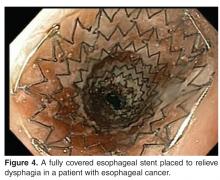
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.