User login
Medication for alcohol use disorder: Which agents work best?
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
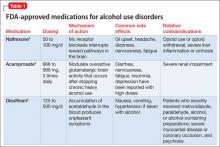
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
Antipsychotics in dementia: Beyond ‘black-box’ warnings
Diagnosed 7 years ago with Alzheimer’s disease (AD), Mrs. B, age 82, resides in an assisted living facility whose staff is trained to care for older persons with dementia. Over the past 2 months she has shown an escalating pattern of psychosis and aggression, despite one-to-one attention and verbal reassurance.
At first Mrs. B’s psychosis was restricted to occasional rape accusations during assisted bathing and aggression manifested by banging her hand repetitively on furniture, causing skin tears. In the last week, she has been accusing staff and patients of stealing her belongings and has assaulted a staff member and another resident. When supervisors at the facility advise Mrs. B’s husband that she can no longer stay there, he takes her to a local emergency room, from which she is admitted involuntarily to a geriatric psychiatry inpatient unit.
For many patients and families, the most problematic aspects of dementia are neuropsychiatric symptoms—depression, sleep disturbance, psychosis, and aggression. Psychosis affects approximately 40% of persons with AD, whereas ≥80% of persons with dementia experience agitation at some point in the illness.1 These symptoms can lead to:
- caregiver morbidity
- poor patient quality of life
- early patient institutionalization.2
Although no drug has been FDA-approved for treating dementia’s neuropsychiatric symptoms, psychiatrists often use off-label psychotropics—especially antipsychotics—to ameliorate them. This practice is controversial because of public perception that antipsychotics are used in dementia patients to create “zombies” to lighten healthcare workers’ burden. Nonetheless, because dementia patients with psychosis and severe agitation/aggression can pose risks to themselves and those around them, efforts to treat these symptoms are warranted.
The FDA warned prescribers in 2003 of increased risk of “cerebrovascular adverse events including stroke” in dementia patients treated with risperidone vs placebo. Similar cerebrovascular warnings have been issued for olanzapine and aripiprazole. Although the absolute risk difference was generally 1% to 2% between antipsychotic- and placebo-treated patients, the relative risk was approximately 2 times higher with antipsychotics because the prevalence of these events is low in both groups.3
Perhaps more daunting, after a meta-analysis of 17 trials using atypical antipsychotics in elderly patients with dementia-related psychosis, the FDA in 2005 issued a black-box warning of increased mortality risk with atypical antipsychotics (relative risk 1.6 to 1.7) vs placebo. The mortality rate in antipsychotic-treated patients was about 4.5%, compared with about 2.6% in the placebo group. Although causes of death varied, most were cardiovascular (heart failure, sudden death) or infectious (pneumonia). This warning was applied to atypical antipsychotics as a class. As with cerebrovascular risks, the absolute mortality risk difference was 1% to 2%.4
The FDA’s “black-box” warnings about using atypical agents in patients with dementia add another layer of complexity to your treatment decisions (Box).3,4 The public is well served by evidence identifying risks associated with prescription medications, but the FDA data do little to help millions of families answer the question, “And so, what now?”
Recognizing that solid empiric evidence is lacking, we attempt to address this lingering question for clinicians, patients, and caregivers who must deal with these symptoms while science tries to provide a more definitive answer.
5-step evaluation
A 5-step initial evaluation of persons with dementia who present with psychosis and/or agitation/aggression includes establishing the frequency, severity, and cause of these symptoms as well as the effectiveness of past treatments and strategies (Algorithm).5
Because adverse drug effects are a potentially reversible cause of psychosis and agitation, review the patient’s drug list—including “as needed” medications—from records at a facility or from family report. Mrs. B’s record from the assisted living facility reveals she was receiving:
- atenolol, 25 mg/d
- aspirin, 81 mg/d
- extended-release oxybutynin, 10 mg at bedtime
- psyllium, one packet daily
- hydrocodone/acetaminophen, 5/500 mg every 4 hours as needed for pain
- lorazepam, 1 mg every 6 hours as needed for agitation
- diphenhydramine, 25 mg at bedtime
- paroxetine, 20 mg/d
- haloperidol, 5 mg at bedtime
- memantine, 10 mg twice a day.
Mrs. B’s medication list is revealing for reasons that, unfortunately, are not rare. She is receiving 3 anticholinergic medications—oxybutynin, diphenhydramine, and paroxetine—that may be worsening her mental status and behavior directly through CNS effects, possibly in combination with frequent benzodiazepine use.
Anticholinergics also can lead to behavior changes via peripheral side effects. Constipation and urinary retention may cause discomfort that an aphasic patient “acts out.” A patient may be experiencing pain related to these side effects and receiving opioid analgesics, which can worsen constipation and urinary retention. Uncontrolled pain related to musculoskeletal disease or neuropathy may merit treatment that will reduce behavioral disturbances.
Mrs. B also was being catheterized every 8 hours as needed for urinary retention. The invasive and unpleasant nature of urinary catheterization is likely to worsen behavior and increases the risk of one of the most common “asymptomatic” etiologies of behavioral symptoms in dementia—urinary tract infection (UTI).
Algorithm
5-step evaluation of dementia patients
with psychosis and/or agitation/aggression*
1. How dangerous is the situation?
|
| ↓ |
2. Establish a clear diagnosis/etiology for the symptoms
|
| ↓ |
3. Establish symptom severity and frequency, including:
|
| ↓ |
| 4. Explore past treatments/caregiver strategies used to address the symptoms and their success and/or problematic outcomes |
| ↓ |
| 5. Discuss with the patient/decision-maker what is and is not known about possible risks and benefits of pharmacologic and nonpharmacologic treatments for psychosis and agitation/aggression in dementia |
| Source: Reference 5 |
| * Agitation is defined as “inappropriate verbal, vocal, or motor activity that is not judged by an outside observer to be an obvious outcome of the needs or confusion of the individual”24 |
CASE CONTINUED: Persistent agitation
After evaluating Mrs. B, the psychiatrist limits her medications to atenolol, aspirin, psyllium, and memantine, and begins to taper lorazepam and paroxetine. Laboratory, radiologic, and physical examinations reveal UTI, fecal impaction, bladder distension, and mild hyponatremia. She is given a phosphosoda enema and ciprofloxacin, 250 mg/d for 5 days.
Despite one-to-one nursing care, frequent reorientation, and attempts to interest her in art therapy, Mrs. B remains agitated and postures to strike staff members and other patients. She denies pain or discomfort. Fearing that someone might be injured, the nurse pages the on-duty psychiatrist.
The nurse then calls Mr. B, who has durable power of attorney for his wife’s healthcare. When the nurse advises Mr. B that the psychiatrist has ordered risperidone, 0.5 mg, he immediately interjects that the psychiatrist at the assisted living facility told him haloperidol should be used for his wife’s symptoms because other antipsychotics can cause strokes and death.
Typical vs atypical antipsychotics
Mrs. B’s nurse may have to delay administering risperidone while she puts Mr. B in contact with the psychiatrist. In an emergent situation when well-trained staff have assessed for common reversible causes of agitation and tried reasonable nonpharmacologic means to calm the patient, few people would argue against using medication to preserve the safety of the patient and others. To avoid questions such as this during a crisis, obtain informed consent at admission from the patient or (more likely) the proxy decision-maker for medications you anticipate the patient might receive during hospitalization.
The larger question is whether typical antipsychotics are preferred for dementia-related psychosis and agitation/aggression because the FDA has not issued the same global black-box warning for this class. Astute clinicians realize that a lack of evidence of harm is not evidence of a lack of harm. In fact, since the black-box warnings for atypical antipsychotics in dementia emerged, several studies have examined whether the same risks exist for typical agents.
Evidence regarding risk of stroke and death with the use of typical and atypical antipsychotics in patients with dementia is summarized in Table 1.6-13 Most evidence, including numerous studies in the past year, comes from retrospective database analyses. Prospective head-to-head comparisons of atypical and typical antipsychotics in dementia are scarce, and future prospective comparisons would be unethical.
No evidence suggests that typical antipsychotics mitigate the risks of stroke or death in dementia compared with atypical agents. Moreover, typical agents are more likely than atypicals to cause movement-related side effects—especially tardive dyskinesia and parkinsonism—in older adults with dementia.14
Table 1
Typical antipsychotics: Safer than atypicals for older patients?
| Study | Population | Summarized results |
|---|---|---|
| Mortality | ||
| Nasrallah et al6 | VA patients age ≥65 taking haloperidol or an atypical antipsychotic (n=1,553) | Approximately 4 times higher rate of death in those receiving haloperidol compared with those receiving atypicals |
| Wang et al8 | Pennsylvania adults age ≥65 with prescription coverage taking antipsychotics (n=22,890) | Typicals had higher relative risk (RR) of death at all time points over 180 days (RR 1.27 to 1.56), both in persons with and without dementia; higher risk associated with increased typical doses |
| Gill et al10 | Canadians age >65 with dementia (n=27,259 matched pairs) | Mortality rate was higher for users of typical vs atypical antipsychotics (RR 1.26 to 1.55) |
| Kales et al11 | VA patients age >65 prescribed psychotropics after a dementia diagnosis (n=10,615) | Risk of death similar for atypical and typical antipsychotics |
| Schneeweiss et al7 | Cancer-free Canadians age ≥65 taking antipsychotics (n=37,241) | Higher mortality rates for those taking typical antipsychotics than those taking atypicals (RR 1.47); higher mortality associated with higher typical doses |
| Trifirò et al9 | Adults age >65 with dementia receiving antipsychotics in Italy (n=2,385) | Equivalent rates of mortality in those taking typical and atypical antipsychotics |
| Stroke | ||
| Gill et al12 | Canadians age ≥65 with dementia receiving antipsychotics (n=32,710) | Equivalent rates of ischemic stroke in those taking atypical and typical agents compared with those receiving atypicals |
| Liperoti et al13 | Nursing home residents with dementia hospitalized for stroke or TIA and matched controls (n=4,788) | Rates of cerebrovascular adverse events equivalent between users of atypical and typical antipsychotics |
| VA: Veterans Affairs; TIA: transient ischemic attack | ||
CASE CONTINUED: Moderate relief from risperidone
After the psychiatrist explains the data on atypical vs typical antipsychotics in dementia—and the lack of FDA-approved treatments—Mr. B consents to the use of risperidone. He believes his wife would have wanted to try a medication with a moderate chance of relieving her internal distress and preventing her from harming anyone.
Risperidone provides moderate relief of Mrs. B’s aggression and paranoia. The next day Mr. B visits the unit and asks to speak with the psychiatrist. Although he appreciates the staff’s caring attitude, he says, “There must be safer or better ways to deal with these symptoms than medications like risperidone. I just don’t want the guilt of causing my wife to have a stroke or pass away.” He also asks, “How long will she have to take this medication?”
Evidence for efficacy
In addition to discussing antipsychotics’ risk in dementia, we also need to highlight their efficacy and effectiveness. A recent meta-analysis of 15 randomized controlled trials of atypical antipsychotics for agitation and/or psychosis in dementia included studies with risperidone, olanzapine, aripiprazole, and quetiapine.3 Most study participants were institutionalized, female, and had AD.
Psychosis scores improved in pooled studies of risperidone, whereas global neuropsychiatric disturbance improved with risperidone and aripiprazole. Effects were more notable in:
- persons without psychosis
- those living in nursing homes
- patients with severe cognitive impairment.
Subsequent placebo-controlled trials of risperidone, quetiapine, and aripiprazole—most focusing on patients with AD—reveal that atypical and typical antipsychotics have modest efficacy in reducing aggression and psychosis.15-19 However, to some extent the National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness Study for Alzheimer’s Disease (CATIE-AD)—the largest nonindustry-funded study conducted to address this question—called this conclusion into question.20 Risperidone and olanzapine (but not quetiapine) were efficacious in that fewer patients taking them vs placebo dropped out because of lack of efficacy. Antipsychotics were not effective overall, however, because the primary outcome—all-cause discontinuation rate—was similar for all 3 drugs and placebo. This indicates that on average these medications’ side effect burden may offset their efficacy, though individual patients’ responses may vary.
Alternatives to antipsychotics
Mr. B also raised the issue of treatment alternatives, such as no treatment, other psychotropics (Table 2),5,21 and nonpharmacologic methods (Table 3).22
“No treatment” does not imply a lack of assessment or intervention. Always examine patients for iatrogenic, medical, psychosocial, or other precipitants of behavioral symptoms. No treatment may be viable in mild to moderate cases but is impractical for patients with severe psychosis or agitation. Untreated, these symptoms could compromise safety or leave the patient without housing options.
Although possibly underused because of time constraints, reimbursement issues, or lack of training, nonpharmacologic strategies to treat aggression and psychosis in dementia are appealing alternatives to antipsychotics. Little empiric evidence supports nonpharmacologic strategies, however.22
Treatment decisions need to consider patients’ and caregivers’ value systems. Proxy decision-makers should examine treatment decisions in terms of how they believe the patient would view the alternatives. Without a specific advance directive, however, even well-intentioned decision-makers are likely to “contaminate” decisions with their own values and interests.
After discussing with the decision-maker various treatments’ risks and benefits, it might be useful to ask, for example, “If Mrs. B could have foreseen her behaviors 10 years ago, what do you think she would have wanted us to do? Some people might have been mortified by the thought of attacking other people, whereas other people would not mind this as much as the fear of being ‘overmedicated.’ Which end of the spectrum do you think she would have leaned toward?”
When medical research does not offer clear answers for the “right” next clinical step, clinicians can:
- acknowledge our own limits and those of human knowledge
- engage the caregiver (or, when appropriate, the patient) in shared decision-making, recognizing that some people will appreciate the opportunity for “equal partnership” whereas others will want us to decide based on our best clinical judgment.
Table 2
Pharmacologic alternatives to antipsychotics: What the evidence says
| Treatment | Evidence/results |
|---|---|
| Selective serotonin reuptake inhibitors | 2 positive studies with citalopram (more effective than placebo for agitation in 1 trial and equivalent to risperidone for psychosis and agitation with greater tolerability in the other); 2 negative trials with sertraline |
| Other antidepressants | 1 study showed trazodone was equivalent to haloperidol for agitation, with greater tolerability; another found trazodone was no different from placebo; other agents have only case reports or open-label trials |
| Anticonvulsants | 3 trials showed divalproex was equivalent to placebo; 2 positive trials for carbamazepine, but tolerability problems in both; other agents tried only in case reports or open-label trials |
| Benzodiazepines/anxiolytics | 3 trials showed oxazepam, alprazolam, diphenhydramine, and buspirone were equivalent to haloperidol in effects on agitation, but none used a placebo control; trials had problematic methodologies and indicated cognitive worsening with some agents (especially diphenhydramine) |
| Cognitive enhancers | Some evidence of modest benefit in mostly post-hoc data analyses in trials designed to assess cognitive variables and often among participants with overall mild psychiatric symptoms; prospective studies of rivastigmine and donepezil specifically designed to assess neuropsychiatric symptoms have found no difference compared with placebo |
| Miscellaneous drugs | Failed trial of transdermal estrogen in men; small study showed propranolol (average dose 106 mg/d) more effective than placebo |
| Source: References 5,21 | |
Table 3
How well do psychosocial/behavioral therapies manage
psychosis/agitation in dementia?*
| Treatment | Evidence/results |
|---|---|
| Caregiver psychoeducation/support | Several positive RCTs (evidence grade A) |
| Music therapy | 6 RCTs, generally positive in the short term (evidence grade B) |
| Cognitive stimulation therapy | Three-quarters of RCTs showed some benefit (evidence grade B) |
| Snoezelen therapy (controlled multisensory stimulation) | 3 RCTs with positive short-term benefits (evidence grade B) |
| Behavioral management therapies (by professionals) | Largest RCTs with some benefits (grade B) |
| Staff training/education | Several positive studies of fair-to-good methodologic quality (evidence grade B) |
| Reality orientation therapy | Best RCT showed no benefit (evidence grade D) |
| Teaching caregivers behavioral management techniques | Overall inconsistent results (evidence grade D) |
| Simulated presence therapy | Only 1 RCT which was negative (evidence grade D) |
| Validation therapy | 1-year RCT with mixed results (evidence grade D) |
| Reminiscence therapy | A few small studies with mixed methodologies (evidence grade D) |
| Therapeutic activity programs (such as exercise, puzzle play) | Varied methods and inconsistent results (evidence grade D) |
| Physical environmental stimulation (such as altered visual stimuli, mirrors, signs) | Generally poor methodology and inconsistent results; best results with obscuring exits to decrease exit-seeking (evidence grade D) |
| * Evidence grades from A (strongest) to D (weakest) were assigned in a review by Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021 | |
| RCT: randomized controlled trial | |
| Source: Reference 22 | |
Duration of treatment
Limited evidence leaves psychiatrists largely on our own in regards to how long to continue pharmacotherapy with antipsychotics. Neuropsychiatric symptoms such as psychosis and agitation exhibit variable patterns. Symptoms may wax and wane for unclear reasons.
Given the tenuous nature of the risk-benefit profile for atypical antipsychotics in dementia, consider a gradual taper for persons with dementia who remain asymptomatic after 3 to 6 months of atypical antipsychotic treatment. Monitor them closely for symptom recurrence.5
Carefully consider the necessary duration of antipsychotic therapy in patients (such as Mrs. B) in whom you can identify possibly reversible precipitants of psychosis and aggression. Patients may have a delayed beneficial response to the correction of precipitating factors such as medical illness, physical discomfort, or medication side effects.
Mrs. B received risperidone, but evidence for efficacy and safety in dementia-related psychosis or agitation does not yet significantly distinguish among the atypical agents (except that data are limited for ziprasidone and clozapine). Usual starting and target doses are provided in Table 4.23
Table 4
Atypicals in dementia: Starting and target doses
| Drug | Starting dose | Target dose |
|---|---|---|
| Aripiprazole | 2.5 to 5 mg/d | 7.5 to 12.5 mg/d |
| Olanzapine | 2.5 to 5 mg/d | 5 to 10 mg/d |
| Quetiapine | 12.5 to 25 mg/d | 50 to 200 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 0.5 to 1.5 mg/d |
| Source: Reference 23 | ||
Related resources
- Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
- American Association for Geriatric Psychiatry position statement: principles of care for persons with dementia resulting from Alzheimer disease. www.aagponline.org/prof/position_caredmnalz.asp.
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Atenolol • Tenormin
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Ciprofloxacin • Ciloxan
- Citalopram • Celexa
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Donepezil • Aricept
- Haloperidol • Haldol
- Hydrocodone/acetaminophen • Lortab, Vicodin
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Sertraline • Zoloft
- Trazodone • Desyrel
- Ziprasidone • Geodon
Disclosure
Dr. Meeks receives research/grant support from the John A. Hartford Foundation, the Mental Health Research Foundation, NARSAD, and the U.S. Department of Health and Human Services’ Health Resources and Services Administration.
Dr. Jeste receives research/grant support from the John A. Hartford Foundation, the National Institute of Aging, and the National Institute of Mental Health. AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, and Janssen, L.P. provide free medications for an NIMH-funded study for which Dr. Jeste is the principal investigator.
1. Jeste DV, Meeks TW, Kim DS, Zubenko GS. Research agenda for DSM-V: diagnostic categories and criteria for neuropsychiatry syndromes in dementia. J Geriatr Psychiatry Neurol 2006;19:160-71.
2. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090-7.
3. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210.
4. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43.
5. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on the use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
6. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry 2004;12:437-9.
7. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176:627-32.
8. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335-41.
9. Trifirò G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf 2007;16:538-44.
10. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775-86.
11. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007;164:1568-76.
12. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330:445.-
13. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry 2005;66:1090-6.
14. Jeste DV, Lacro JP, Nguyen HA, et al. Lower incidence of tardive dyskinesia with risperidone versus haloperidol. J Am Geriatr Soc 1999;47:716-9.
15. Rainer M, Haushofer M, Pfolz H, et al. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry 2007;22:395-403.
16. Holmes C, Wilkinson D, Dean C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry 2007;22:380-1.
17. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
18. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res 2007;4:81-93.
19. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007;15:918-31.
20. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
21. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
22. Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
23. Jeste D, Meeks T. To prescribe or not to prescribe? Atypical antipsychotic drugs in patients with dementia. South Med J 2007;100:961-3.
24. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review and critique. Am J Geriatr Psychiatry 2001;9:361-81.
Diagnosed 7 years ago with Alzheimer’s disease (AD), Mrs. B, age 82, resides in an assisted living facility whose staff is trained to care for older persons with dementia. Over the past 2 months she has shown an escalating pattern of psychosis and aggression, despite one-to-one attention and verbal reassurance.
At first Mrs. B’s psychosis was restricted to occasional rape accusations during assisted bathing and aggression manifested by banging her hand repetitively on furniture, causing skin tears. In the last week, she has been accusing staff and patients of stealing her belongings and has assaulted a staff member and another resident. When supervisors at the facility advise Mrs. B’s husband that she can no longer stay there, he takes her to a local emergency room, from which she is admitted involuntarily to a geriatric psychiatry inpatient unit.
For many patients and families, the most problematic aspects of dementia are neuropsychiatric symptoms—depression, sleep disturbance, psychosis, and aggression. Psychosis affects approximately 40% of persons with AD, whereas ≥80% of persons with dementia experience agitation at some point in the illness.1 These symptoms can lead to:
- caregiver morbidity
- poor patient quality of life
- early patient institutionalization.2
Although no drug has been FDA-approved for treating dementia’s neuropsychiatric symptoms, psychiatrists often use off-label psychotropics—especially antipsychotics—to ameliorate them. This practice is controversial because of public perception that antipsychotics are used in dementia patients to create “zombies” to lighten healthcare workers’ burden. Nonetheless, because dementia patients with psychosis and severe agitation/aggression can pose risks to themselves and those around them, efforts to treat these symptoms are warranted.
The FDA warned prescribers in 2003 of increased risk of “cerebrovascular adverse events including stroke” in dementia patients treated with risperidone vs placebo. Similar cerebrovascular warnings have been issued for olanzapine and aripiprazole. Although the absolute risk difference was generally 1% to 2% between antipsychotic- and placebo-treated patients, the relative risk was approximately 2 times higher with antipsychotics because the prevalence of these events is low in both groups.3
Perhaps more daunting, after a meta-analysis of 17 trials using atypical antipsychotics in elderly patients with dementia-related psychosis, the FDA in 2005 issued a black-box warning of increased mortality risk with atypical antipsychotics (relative risk 1.6 to 1.7) vs placebo. The mortality rate in antipsychotic-treated patients was about 4.5%, compared with about 2.6% in the placebo group. Although causes of death varied, most were cardiovascular (heart failure, sudden death) or infectious (pneumonia). This warning was applied to atypical antipsychotics as a class. As with cerebrovascular risks, the absolute mortality risk difference was 1% to 2%.4
The FDA’s “black-box” warnings about using atypical agents in patients with dementia add another layer of complexity to your treatment decisions (Box).3,4 The public is well served by evidence identifying risks associated with prescription medications, but the FDA data do little to help millions of families answer the question, “And so, what now?”
Recognizing that solid empiric evidence is lacking, we attempt to address this lingering question for clinicians, patients, and caregivers who must deal with these symptoms while science tries to provide a more definitive answer.
5-step evaluation
A 5-step initial evaluation of persons with dementia who present with psychosis and/or agitation/aggression includes establishing the frequency, severity, and cause of these symptoms as well as the effectiveness of past treatments and strategies (Algorithm).5
Because adverse drug effects are a potentially reversible cause of psychosis and agitation, review the patient’s drug list—including “as needed” medications—from records at a facility or from family report. Mrs. B’s record from the assisted living facility reveals she was receiving:
- atenolol, 25 mg/d
- aspirin, 81 mg/d
- extended-release oxybutynin, 10 mg at bedtime
- psyllium, one packet daily
- hydrocodone/acetaminophen, 5/500 mg every 4 hours as needed for pain
- lorazepam, 1 mg every 6 hours as needed for agitation
- diphenhydramine, 25 mg at bedtime
- paroxetine, 20 mg/d
- haloperidol, 5 mg at bedtime
- memantine, 10 mg twice a day.
Mrs. B’s medication list is revealing for reasons that, unfortunately, are not rare. She is receiving 3 anticholinergic medications—oxybutynin, diphenhydramine, and paroxetine—that may be worsening her mental status and behavior directly through CNS effects, possibly in combination with frequent benzodiazepine use.
Anticholinergics also can lead to behavior changes via peripheral side effects. Constipation and urinary retention may cause discomfort that an aphasic patient “acts out.” A patient may be experiencing pain related to these side effects and receiving opioid analgesics, which can worsen constipation and urinary retention. Uncontrolled pain related to musculoskeletal disease or neuropathy may merit treatment that will reduce behavioral disturbances.
Mrs. B also was being catheterized every 8 hours as needed for urinary retention. The invasive and unpleasant nature of urinary catheterization is likely to worsen behavior and increases the risk of one of the most common “asymptomatic” etiologies of behavioral symptoms in dementia—urinary tract infection (UTI).
Algorithm
5-step evaluation of dementia patients
with psychosis and/or agitation/aggression*
1. How dangerous is the situation?
|
| ↓ |
2. Establish a clear diagnosis/etiology for the symptoms
|
| ↓ |
3. Establish symptom severity and frequency, including:
|
| ↓ |
| 4. Explore past treatments/caregiver strategies used to address the symptoms and their success and/or problematic outcomes |
| ↓ |
| 5. Discuss with the patient/decision-maker what is and is not known about possible risks and benefits of pharmacologic and nonpharmacologic treatments for psychosis and agitation/aggression in dementia |
| Source: Reference 5 |
| * Agitation is defined as “inappropriate verbal, vocal, or motor activity that is not judged by an outside observer to be an obvious outcome of the needs or confusion of the individual”24 |
CASE CONTINUED: Persistent agitation
After evaluating Mrs. B, the psychiatrist limits her medications to atenolol, aspirin, psyllium, and memantine, and begins to taper lorazepam and paroxetine. Laboratory, radiologic, and physical examinations reveal UTI, fecal impaction, bladder distension, and mild hyponatremia. She is given a phosphosoda enema and ciprofloxacin, 250 mg/d for 5 days.
Despite one-to-one nursing care, frequent reorientation, and attempts to interest her in art therapy, Mrs. B remains agitated and postures to strike staff members and other patients. She denies pain or discomfort. Fearing that someone might be injured, the nurse pages the on-duty psychiatrist.
The nurse then calls Mr. B, who has durable power of attorney for his wife’s healthcare. When the nurse advises Mr. B that the psychiatrist has ordered risperidone, 0.5 mg, he immediately interjects that the psychiatrist at the assisted living facility told him haloperidol should be used for his wife’s symptoms because other antipsychotics can cause strokes and death.
Typical vs atypical antipsychotics
Mrs. B’s nurse may have to delay administering risperidone while she puts Mr. B in contact with the psychiatrist. In an emergent situation when well-trained staff have assessed for common reversible causes of agitation and tried reasonable nonpharmacologic means to calm the patient, few people would argue against using medication to preserve the safety of the patient and others. To avoid questions such as this during a crisis, obtain informed consent at admission from the patient or (more likely) the proxy decision-maker for medications you anticipate the patient might receive during hospitalization.
The larger question is whether typical antipsychotics are preferred for dementia-related psychosis and agitation/aggression because the FDA has not issued the same global black-box warning for this class. Astute clinicians realize that a lack of evidence of harm is not evidence of a lack of harm. In fact, since the black-box warnings for atypical antipsychotics in dementia emerged, several studies have examined whether the same risks exist for typical agents.
Evidence regarding risk of stroke and death with the use of typical and atypical antipsychotics in patients with dementia is summarized in Table 1.6-13 Most evidence, including numerous studies in the past year, comes from retrospective database analyses. Prospective head-to-head comparisons of atypical and typical antipsychotics in dementia are scarce, and future prospective comparisons would be unethical.
No evidence suggests that typical antipsychotics mitigate the risks of stroke or death in dementia compared with atypical agents. Moreover, typical agents are more likely than atypicals to cause movement-related side effects—especially tardive dyskinesia and parkinsonism—in older adults with dementia.14
Table 1
Typical antipsychotics: Safer than atypicals for older patients?
| Study | Population | Summarized results |
|---|---|---|
| Mortality | ||
| Nasrallah et al6 | VA patients age ≥65 taking haloperidol or an atypical antipsychotic (n=1,553) | Approximately 4 times higher rate of death in those receiving haloperidol compared with those receiving atypicals |
| Wang et al8 | Pennsylvania adults age ≥65 with prescription coverage taking antipsychotics (n=22,890) | Typicals had higher relative risk (RR) of death at all time points over 180 days (RR 1.27 to 1.56), both in persons with and without dementia; higher risk associated with increased typical doses |
| Gill et al10 | Canadians age >65 with dementia (n=27,259 matched pairs) | Mortality rate was higher for users of typical vs atypical antipsychotics (RR 1.26 to 1.55) |
| Kales et al11 | VA patients age >65 prescribed psychotropics after a dementia diagnosis (n=10,615) | Risk of death similar for atypical and typical antipsychotics |
| Schneeweiss et al7 | Cancer-free Canadians age ≥65 taking antipsychotics (n=37,241) | Higher mortality rates for those taking typical antipsychotics than those taking atypicals (RR 1.47); higher mortality associated with higher typical doses |
| Trifirò et al9 | Adults age >65 with dementia receiving antipsychotics in Italy (n=2,385) | Equivalent rates of mortality in those taking typical and atypical antipsychotics |
| Stroke | ||
| Gill et al12 | Canadians age ≥65 with dementia receiving antipsychotics (n=32,710) | Equivalent rates of ischemic stroke in those taking atypical and typical agents compared with those receiving atypicals |
| Liperoti et al13 | Nursing home residents with dementia hospitalized for stroke or TIA and matched controls (n=4,788) | Rates of cerebrovascular adverse events equivalent between users of atypical and typical antipsychotics |
| VA: Veterans Affairs; TIA: transient ischemic attack | ||
CASE CONTINUED: Moderate relief from risperidone
After the psychiatrist explains the data on atypical vs typical antipsychotics in dementia—and the lack of FDA-approved treatments—Mr. B consents to the use of risperidone. He believes his wife would have wanted to try a medication with a moderate chance of relieving her internal distress and preventing her from harming anyone.
Risperidone provides moderate relief of Mrs. B’s aggression and paranoia. The next day Mr. B visits the unit and asks to speak with the psychiatrist. Although he appreciates the staff’s caring attitude, he says, “There must be safer or better ways to deal with these symptoms than medications like risperidone. I just don’t want the guilt of causing my wife to have a stroke or pass away.” He also asks, “How long will she have to take this medication?”
Evidence for efficacy
In addition to discussing antipsychotics’ risk in dementia, we also need to highlight their efficacy and effectiveness. A recent meta-analysis of 15 randomized controlled trials of atypical antipsychotics for agitation and/or psychosis in dementia included studies with risperidone, olanzapine, aripiprazole, and quetiapine.3 Most study participants were institutionalized, female, and had AD.
Psychosis scores improved in pooled studies of risperidone, whereas global neuropsychiatric disturbance improved with risperidone and aripiprazole. Effects were more notable in:
- persons without psychosis
- those living in nursing homes
- patients with severe cognitive impairment.
Subsequent placebo-controlled trials of risperidone, quetiapine, and aripiprazole—most focusing on patients with AD—reveal that atypical and typical antipsychotics have modest efficacy in reducing aggression and psychosis.15-19 However, to some extent the National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness Study for Alzheimer’s Disease (CATIE-AD)—the largest nonindustry-funded study conducted to address this question—called this conclusion into question.20 Risperidone and olanzapine (but not quetiapine) were efficacious in that fewer patients taking them vs placebo dropped out because of lack of efficacy. Antipsychotics were not effective overall, however, because the primary outcome—all-cause discontinuation rate—was similar for all 3 drugs and placebo. This indicates that on average these medications’ side effect burden may offset their efficacy, though individual patients’ responses may vary.
Alternatives to antipsychotics
Mr. B also raised the issue of treatment alternatives, such as no treatment, other psychotropics (Table 2),5,21 and nonpharmacologic methods (Table 3).22
“No treatment” does not imply a lack of assessment or intervention. Always examine patients for iatrogenic, medical, psychosocial, or other precipitants of behavioral symptoms. No treatment may be viable in mild to moderate cases but is impractical for patients with severe psychosis or agitation. Untreated, these symptoms could compromise safety or leave the patient without housing options.
Although possibly underused because of time constraints, reimbursement issues, or lack of training, nonpharmacologic strategies to treat aggression and psychosis in dementia are appealing alternatives to antipsychotics. Little empiric evidence supports nonpharmacologic strategies, however.22
Treatment decisions need to consider patients’ and caregivers’ value systems. Proxy decision-makers should examine treatment decisions in terms of how they believe the patient would view the alternatives. Without a specific advance directive, however, even well-intentioned decision-makers are likely to “contaminate” decisions with their own values and interests.
After discussing with the decision-maker various treatments’ risks and benefits, it might be useful to ask, for example, “If Mrs. B could have foreseen her behaviors 10 years ago, what do you think she would have wanted us to do? Some people might have been mortified by the thought of attacking other people, whereas other people would not mind this as much as the fear of being ‘overmedicated.’ Which end of the spectrum do you think she would have leaned toward?”
When medical research does not offer clear answers for the “right” next clinical step, clinicians can:
- acknowledge our own limits and those of human knowledge
- engage the caregiver (or, when appropriate, the patient) in shared decision-making, recognizing that some people will appreciate the opportunity for “equal partnership” whereas others will want us to decide based on our best clinical judgment.
Table 2
Pharmacologic alternatives to antipsychotics: What the evidence says
| Treatment | Evidence/results |
|---|---|
| Selective serotonin reuptake inhibitors | 2 positive studies with citalopram (more effective than placebo for agitation in 1 trial and equivalent to risperidone for psychosis and agitation with greater tolerability in the other); 2 negative trials with sertraline |
| Other antidepressants | 1 study showed trazodone was equivalent to haloperidol for agitation, with greater tolerability; another found trazodone was no different from placebo; other agents have only case reports or open-label trials |
| Anticonvulsants | 3 trials showed divalproex was equivalent to placebo; 2 positive trials for carbamazepine, but tolerability problems in both; other agents tried only in case reports or open-label trials |
| Benzodiazepines/anxiolytics | 3 trials showed oxazepam, alprazolam, diphenhydramine, and buspirone were equivalent to haloperidol in effects on agitation, but none used a placebo control; trials had problematic methodologies and indicated cognitive worsening with some agents (especially diphenhydramine) |
| Cognitive enhancers | Some evidence of modest benefit in mostly post-hoc data analyses in trials designed to assess cognitive variables and often among participants with overall mild psychiatric symptoms; prospective studies of rivastigmine and donepezil specifically designed to assess neuropsychiatric symptoms have found no difference compared with placebo |
| Miscellaneous drugs | Failed trial of transdermal estrogen in men; small study showed propranolol (average dose 106 mg/d) more effective than placebo |
| Source: References 5,21 | |
Table 3
How well do psychosocial/behavioral therapies manage
psychosis/agitation in dementia?*
| Treatment | Evidence/results |
|---|---|
| Caregiver psychoeducation/support | Several positive RCTs (evidence grade A) |
| Music therapy | 6 RCTs, generally positive in the short term (evidence grade B) |
| Cognitive stimulation therapy | Three-quarters of RCTs showed some benefit (evidence grade B) |
| Snoezelen therapy (controlled multisensory stimulation) | 3 RCTs with positive short-term benefits (evidence grade B) |
| Behavioral management therapies (by professionals) | Largest RCTs with some benefits (grade B) |
| Staff training/education | Several positive studies of fair-to-good methodologic quality (evidence grade B) |
| Reality orientation therapy | Best RCT showed no benefit (evidence grade D) |
| Teaching caregivers behavioral management techniques | Overall inconsistent results (evidence grade D) |
| Simulated presence therapy | Only 1 RCT which was negative (evidence grade D) |
| Validation therapy | 1-year RCT with mixed results (evidence grade D) |
| Reminiscence therapy | A few small studies with mixed methodologies (evidence grade D) |
| Therapeutic activity programs (such as exercise, puzzle play) | Varied methods and inconsistent results (evidence grade D) |
| Physical environmental stimulation (such as altered visual stimuli, mirrors, signs) | Generally poor methodology and inconsistent results; best results with obscuring exits to decrease exit-seeking (evidence grade D) |
| * Evidence grades from A (strongest) to D (weakest) were assigned in a review by Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021 | |
| RCT: randomized controlled trial | |
| Source: Reference 22 | |
Duration of treatment
Limited evidence leaves psychiatrists largely on our own in regards to how long to continue pharmacotherapy with antipsychotics. Neuropsychiatric symptoms such as psychosis and agitation exhibit variable patterns. Symptoms may wax and wane for unclear reasons.
Given the tenuous nature of the risk-benefit profile for atypical antipsychotics in dementia, consider a gradual taper for persons with dementia who remain asymptomatic after 3 to 6 months of atypical antipsychotic treatment. Monitor them closely for symptom recurrence.5
Carefully consider the necessary duration of antipsychotic therapy in patients (such as Mrs. B) in whom you can identify possibly reversible precipitants of psychosis and aggression. Patients may have a delayed beneficial response to the correction of precipitating factors such as medical illness, physical discomfort, or medication side effects.
Mrs. B received risperidone, but evidence for efficacy and safety in dementia-related psychosis or agitation does not yet significantly distinguish among the atypical agents (except that data are limited for ziprasidone and clozapine). Usual starting and target doses are provided in Table 4.23
Table 4
Atypicals in dementia: Starting and target doses
| Drug | Starting dose | Target dose |
|---|---|---|
| Aripiprazole | 2.5 to 5 mg/d | 7.5 to 12.5 mg/d |
| Olanzapine | 2.5 to 5 mg/d | 5 to 10 mg/d |
| Quetiapine | 12.5 to 25 mg/d | 50 to 200 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 0.5 to 1.5 mg/d |
| Source: Reference 23 | ||
Related resources
- Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
- American Association for Geriatric Psychiatry position statement: principles of care for persons with dementia resulting from Alzheimer disease. www.aagponline.org/prof/position_caredmnalz.asp.
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Atenolol • Tenormin
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Ciprofloxacin • Ciloxan
- Citalopram • Celexa
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Donepezil • Aricept
- Haloperidol • Haldol
- Hydrocodone/acetaminophen • Lortab, Vicodin
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Sertraline • Zoloft
- Trazodone • Desyrel
- Ziprasidone • Geodon
Disclosure
Dr. Meeks receives research/grant support from the John A. Hartford Foundation, the Mental Health Research Foundation, NARSAD, and the U.S. Department of Health and Human Services’ Health Resources and Services Administration.
Dr. Jeste receives research/grant support from the John A. Hartford Foundation, the National Institute of Aging, and the National Institute of Mental Health. AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, and Janssen, L.P. provide free medications for an NIMH-funded study for which Dr. Jeste is the principal investigator.
Diagnosed 7 years ago with Alzheimer’s disease (AD), Mrs. B, age 82, resides in an assisted living facility whose staff is trained to care for older persons with dementia. Over the past 2 months she has shown an escalating pattern of psychosis and aggression, despite one-to-one attention and verbal reassurance.
At first Mrs. B’s psychosis was restricted to occasional rape accusations during assisted bathing and aggression manifested by banging her hand repetitively on furniture, causing skin tears. In the last week, she has been accusing staff and patients of stealing her belongings and has assaulted a staff member and another resident. When supervisors at the facility advise Mrs. B’s husband that she can no longer stay there, he takes her to a local emergency room, from which she is admitted involuntarily to a geriatric psychiatry inpatient unit.
For many patients and families, the most problematic aspects of dementia are neuropsychiatric symptoms—depression, sleep disturbance, psychosis, and aggression. Psychosis affects approximately 40% of persons with AD, whereas ≥80% of persons with dementia experience agitation at some point in the illness.1 These symptoms can lead to:
- caregiver morbidity
- poor patient quality of life
- early patient institutionalization.2
Although no drug has been FDA-approved for treating dementia’s neuropsychiatric symptoms, psychiatrists often use off-label psychotropics—especially antipsychotics—to ameliorate them. This practice is controversial because of public perception that antipsychotics are used in dementia patients to create “zombies” to lighten healthcare workers’ burden. Nonetheless, because dementia patients with psychosis and severe agitation/aggression can pose risks to themselves and those around them, efforts to treat these symptoms are warranted.
The FDA warned prescribers in 2003 of increased risk of “cerebrovascular adverse events including stroke” in dementia patients treated with risperidone vs placebo. Similar cerebrovascular warnings have been issued for olanzapine and aripiprazole. Although the absolute risk difference was generally 1% to 2% between antipsychotic- and placebo-treated patients, the relative risk was approximately 2 times higher with antipsychotics because the prevalence of these events is low in both groups.3
Perhaps more daunting, after a meta-analysis of 17 trials using atypical antipsychotics in elderly patients with dementia-related psychosis, the FDA in 2005 issued a black-box warning of increased mortality risk with atypical antipsychotics (relative risk 1.6 to 1.7) vs placebo. The mortality rate in antipsychotic-treated patients was about 4.5%, compared with about 2.6% in the placebo group. Although causes of death varied, most were cardiovascular (heart failure, sudden death) or infectious (pneumonia). This warning was applied to atypical antipsychotics as a class. As with cerebrovascular risks, the absolute mortality risk difference was 1% to 2%.4
The FDA’s “black-box” warnings about using atypical agents in patients with dementia add another layer of complexity to your treatment decisions (Box).3,4 The public is well served by evidence identifying risks associated with prescription medications, but the FDA data do little to help millions of families answer the question, “And so, what now?”
Recognizing that solid empiric evidence is lacking, we attempt to address this lingering question for clinicians, patients, and caregivers who must deal with these symptoms while science tries to provide a more definitive answer.
5-step evaluation
A 5-step initial evaluation of persons with dementia who present with psychosis and/or agitation/aggression includes establishing the frequency, severity, and cause of these symptoms as well as the effectiveness of past treatments and strategies (Algorithm).5
Because adverse drug effects are a potentially reversible cause of psychosis and agitation, review the patient’s drug list—including “as needed” medications—from records at a facility or from family report. Mrs. B’s record from the assisted living facility reveals she was receiving:
- atenolol, 25 mg/d
- aspirin, 81 mg/d
- extended-release oxybutynin, 10 mg at bedtime
- psyllium, one packet daily
- hydrocodone/acetaminophen, 5/500 mg every 4 hours as needed for pain
- lorazepam, 1 mg every 6 hours as needed for agitation
- diphenhydramine, 25 mg at bedtime
- paroxetine, 20 mg/d
- haloperidol, 5 mg at bedtime
- memantine, 10 mg twice a day.
Mrs. B’s medication list is revealing for reasons that, unfortunately, are not rare. She is receiving 3 anticholinergic medications—oxybutynin, diphenhydramine, and paroxetine—that may be worsening her mental status and behavior directly through CNS effects, possibly in combination with frequent benzodiazepine use.
Anticholinergics also can lead to behavior changes via peripheral side effects. Constipation and urinary retention may cause discomfort that an aphasic patient “acts out.” A patient may be experiencing pain related to these side effects and receiving opioid analgesics, which can worsen constipation and urinary retention. Uncontrolled pain related to musculoskeletal disease or neuropathy may merit treatment that will reduce behavioral disturbances.
Mrs. B also was being catheterized every 8 hours as needed for urinary retention. The invasive and unpleasant nature of urinary catheterization is likely to worsen behavior and increases the risk of one of the most common “asymptomatic” etiologies of behavioral symptoms in dementia—urinary tract infection (UTI).
Algorithm
5-step evaluation of dementia patients
with psychosis and/or agitation/aggression*
1. How dangerous is the situation?
|
| ↓ |
2. Establish a clear diagnosis/etiology for the symptoms
|
| ↓ |
3. Establish symptom severity and frequency, including:
|
| ↓ |
| 4. Explore past treatments/caregiver strategies used to address the symptoms and their success and/or problematic outcomes |
| ↓ |
| 5. Discuss with the patient/decision-maker what is and is not known about possible risks and benefits of pharmacologic and nonpharmacologic treatments for psychosis and agitation/aggression in dementia |
| Source: Reference 5 |
| * Agitation is defined as “inappropriate verbal, vocal, or motor activity that is not judged by an outside observer to be an obvious outcome of the needs or confusion of the individual”24 |
CASE CONTINUED: Persistent agitation
After evaluating Mrs. B, the psychiatrist limits her medications to atenolol, aspirin, psyllium, and memantine, and begins to taper lorazepam and paroxetine. Laboratory, radiologic, and physical examinations reveal UTI, fecal impaction, bladder distension, and mild hyponatremia. She is given a phosphosoda enema and ciprofloxacin, 250 mg/d for 5 days.
Despite one-to-one nursing care, frequent reorientation, and attempts to interest her in art therapy, Mrs. B remains agitated and postures to strike staff members and other patients. She denies pain or discomfort. Fearing that someone might be injured, the nurse pages the on-duty psychiatrist.
The nurse then calls Mr. B, who has durable power of attorney for his wife’s healthcare. When the nurse advises Mr. B that the psychiatrist has ordered risperidone, 0.5 mg, he immediately interjects that the psychiatrist at the assisted living facility told him haloperidol should be used for his wife’s symptoms because other antipsychotics can cause strokes and death.
Typical vs atypical antipsychotics
Mrs. B’s nurse may have to delay administering risperidone while she puts Mr. B in contact with the psychiatrist. In an emergent situation when well-trained staff have assessed for common reversible causes of agitation and tried reasonable nonpharmacologic means to calm the patient, few people would argue against using medication to preserve the safety of the patient and others. To avoid questions such as this during a crisis, obtain informed consent at admission from the patient or (more likely) the proxy decision-maker for medications you anticipate the patient might receive during hospitalization.
The larger question is whether typical antipsychotics are preferred for dementia-related psychosis and agitation/aggression because the FDA has not issued the same global black-box warning for this class. Astute clinicians realize that a lack of evidence of harm is not evidence of a lack of harm. In fact, since the black-box warnings for atypical antipsychotics in dementia emerged, several studies have examined whether the same risks exist for typical agents.
Evidence regarding risk of stroke and death with the use of typical and atypical antipsychotics in patients with dementia is summarized in Table 1.6-13 Most evidence, including numerous studies in the past year, comes from retrospective database analyses. Prospective head-to-head comparisons of atypical and typical antipsychotics in dementia are scarce, and future prospective comparisons would be unethical.
No evidence suggests that typical antipsychotics mitigate the risks of stroke or death in dementia compared with atypical agents. Moreover, typical agents are more likely than atypicals to cause movement-related side effects—especially tardive dyskinesia and parkinsonism—in older adults with dementia.14
Table 1
Typical antipsychotics: Safer than atypicals for older patients?
| Study | Population | Summarized results |
|---|---|---|
| Mortality | ||
| Nasrallah et al6 | VA patients age ≥65 taking haloperidol or an atypical antipsychotic (n=1,553) | Approximately 4 times higher rate of death in those receiving haloperidol compared with those receiving atypicals |
| Wang et al8 | Pennsylvania adults age ≥65 with prescription coverage taking antipsychotics (n=22,890) | Typicals had higher relative risk (RR) of death at all time points over 180 days (RR 1.27 to 1.56), both in persons with and without dementia; higher risk associated with increased typical doses |
| Gill et al10 | Canadians age >65 with dementia (n=27,259 matched pairs) | Mortality rate was higher for users of typical vs atypical antipsychotics (RR 1.26 to 1.55) |
| Kales et al11 | VA patients age >65 prescribed psychotropics after a dementia diagnosis (n=10,615) | Risk of death similar for atypical and typical antipsychotics |
| Schneeweiss et al7 | Cancer-free Canadians age ≥65 taking antipsychotics (n=37,241) | Higher mortality rates for those taking typical antipsychotics than those taking atypicals (RR 1.47); higher mortality associated with higher typical doses |
| Trifirò et al9 | Adults age >65 with dementia receiving antipsychotics in Italy (n=2,385) | Equivalent rates of mortality in those taking typical and atypical antipsychotics |
| Stroke | ||
| Gill et al12 | Canadians age ≥65 with dementia receiving antipsychotics (n=32,710) | Equivalent rates of ischemic stroke in those taking atypical and typical agents compared with those receiving atypicals |
| Liperoti et al13 | Nursing home residents with dementia hospitalized for stroke or TIA and matched controls (n=4,788) | Rates of cerebrovascular adverse events equivalent between users of atypical and typical antipsychotics |
| VA: Veterans Affairs; TIA: transient ischemic attack | ||
CASE CONTINUED: Moderate relief from risperidone
After the psychiatrist explains the data on atypical vs typical antipsychotics in dementia—and the lack of FDA-approved treatments—Mr. B consents to the use of risperidone. He believes his wife would have wanted to try a medication with a moderate chance of relieving her internal distress and preventing her from harming anyone.
Risperidone provides moderate relief of Mrs. B’s aggression and paranoia. The next day Mr. B visits the unit and asks to speak with the psychiatrist. Although he appreciates the staff’s caring attitude, he says, “There must be safer or better ways to deal with these symptoms than medications like risperidone. I just don’t want the guilt of causing my wife to have a stroke or pass away.” He also asks, “How long will she have to take this medication?”
Evidence for efficacy
In addition to discussing antipsychotics’ risk in dementia, we also need to highlight their efficacy and effectiveness. A recent meta-analysis of 15 randomized controlled trials of atypical antipsychotics for agitation and/or psychosis in dementia included studies with risperidone, olanzapine, aripiprazole, and quetiapine.3 Most study participants were institutionalized, female, and had AD.
Psychosis scores improved in pooled studies of risperidone, whereas global neuropsychiatric disturbance improved with risperidone and aripiprazole. Effects were more notable in:
- persons without psychosis
- those living in nursing homes
- patients with severe cognitive impairment.
Subsequent placebo-controlled trials of risperidone, quetiapine, and aripiprazole—most focusing on patients with AD—reveal that atypical and typical antipsychotics have modest efficacy in reducing aggression and psychosis.15-19 However, to some extent the National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness Study for Alzheimer’s Disease (CATIE-AD)—the largest nonindustry-funded study conducted to address this question—called this conclusion into question.20 Risperidone and olanzapine (but not quetiapine) were efficacious in that fewer patients taking them vs placebo dropped out because of lack of efficacy. Antipsychotics were not effective overall, however, because the primary outcome—all-cause discontinuation rate—was similar for all 3 drugs and placebo. This indicates that on average these medications’ side effect burden may offset their efficacy, though individual patients’ responses may vary.
Alternatives to antipsychotics
Mr. B also raised the issue of treatment alternatives, such as no treatment, other psychotropics (Table 2),5,21 and nonpharmacologic methods (Table 3).22
“No treatment” does not imply a lack of assessment or intervention. Always examine patients for iatrogenic, medical, psychosocial, or other precipitants of behavioral symptoms. No treatment may be viable in mild to moderate cases but is impractical for patients with severe psychosis or agitation. Untreated, these symptoms could compromise safety or leave the patient without housing options.
Although possibly underused because of time constraints, reimbursement issues, or lack of training, nonpharmacologic strategies to treat aggression and psychosis in dementia are appealing alternatives to antipsychotics. Little empiric evidence supports nonpharmacologic strategies, however.22
Treatment decisions need to consider patients’ and caregivers’ value systems. Proxy decision-makers should examine treatment decisions in terms of how they believe the patient would view the alternatives. Without a specific advance directive, however, even well-intentioned decision-makers are likely to “contaminate” decisions with their own values and interests.
After discussing with the decision-maker various treatments’ risks and benefits, it might be useful to ask, for example, “If Mrs. B could have foreseen her behaviors 10 years ago, what do you think she would have wanted us to do? Some people might have been mortified by the thought of attacking other people, whereas other people would not mind this as much as the fear of being ‘overmedicated.’ Which end of the spectrum do you think she would have leaned toward?”
When medical research does not offer clear answers for the “right” next clinical step, clinicians can:
- acknowledge our own limits and those of human knowledge
- engage the caregiver (or, when appropriate, the patient) in shared decision-making, recognizing that some people will appreciate the opportunity for “equal partnership” whereas others will want us to decide based on our best clinical judgment.
Table 2
Pharmacologic alternatives to antipsychotics: What the evidence says
| Treatment | Evidence/results |
|---|---|
| Selective serotonin reuptake inhibitors | 2 positive studies with citalopram (more effective than placebo for agitation in 1 trial and equivalent to risperidone for psychosis and agitation with greater tolerability in the other); 2 negative trials with sertraline |
| Other antidepressants | 1 study showed trazodone was equivalent to haloperidol for agitation, with greater tolerability; another found trazodone was no different from placebo; other agents have only case reports or open-label trials |
| Anticonvulsants | 3 trials showed divalproex was equivalent to placebo; 2 positive trials for carbamazepine, but tolerability problems in both; other agents tried only in case reports or open-label trials |
| Benzodiazepines/anxiolytics | 3 trials showed oxazepam, alprazolam, diphenhydramine, and buspirone were equivalent to haloperidol in effects on agitation, but none used a placebo control; trials had problematic methodologies and indicated cognitive worsening with some agents (especially diphenhydramine) |
| Cognitive enhancers | Some evidence of modest benefit in mostly post-hoc data analyses in trials designed to assess cognitive variables and often among participants with overall mild psychiatric symptoms; prospective studies of rivastigmine and donepezil specifically designed to assess neuropsychiatric symptoms have found no difference compared with placebo |
| Miscellaneous drugs | Failed trial of transdermal estrogen in men; small study showed propranolol (average dose 106 mg/d) more effective than placebo |
| Source: References 5,21 | |
Table 3
How well do psychosocial/behavioral therapies manage
psychosis/agitation in dementia?*
| Treatment | Evidence/results |
|---|---|
| Caregiver psychoeducation/support | Several positive RCTs (evidence grade A) |
| Music therapy | 6 RCTs, generally positive in the short term (evidence grade B) |
| Cognitive stimulation therapy | Three-quarters of RCTs showed some benefit (evidence grade B) |
| Snoezelen therapy (controlled multisensory stimulation) | 3 RCTs with positive short-term benefits (evidence grade B) |
| Behavioral management therapies (by professionals) | Largest RCTs with some benefits (grade B) |
| Staff training/education | Several positive studies of fair-to-good methodologic quality (evidence grade B) |
| Reality orientation therapy | Best RCT showed no benefit (evidence grade D) |
| Teaching caregivers behavioral management techniques | Overall inconsistent results (evidence grade D) |
| Simulated presence therapy | Only 1 RCT which was negative (evidence grade D) |
| Validation therapy | 1-year RCT with mixed results (evidence grade D) |
| Reminiscence therapy | A few small studies with mixed methodologies (evidence grade D) |
| Therapeutic activity programs (such as exercise, puzzle play) | Varied methods and inconsistent results (evidence grade D) |
| Physical environmental stimulation (such as altered visual stimuli, mirrors, signs) | Generally poor methodology and inconsistent results; best results with obscuring exits to decrease exit-seeking (evidence grade D) |
| * Evidence grades from A (strongest) to D (weakest) were assigned in a review by Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021 | |
| RCT: randomized controlled trial | |
| Source: Reference 22 | |
Duration of treatment
Limited evidence leaves psychiatrists largely on our own in regards to how long to continue pharmacotherapy with antipsychotics. Neuropsychiatric symptoms such as psychosis and agitation exhibit variable patterns. Symptoms may wax and wane for unclear reasons.
Given the tenuous nature of the risk-benefit profile for atypical antipsychotics in dementia, consider a gradual taper for persons with dementia who remain asymptomatic after 3 to 6 months of atypical antipsychotic treatment. Monitor them closely for symptom recurrence.5
Carefully consider the necessary duration of antipsychotic therapy in patients (such as Mrs. B) in whom you can identify possibly reversible precipitants of psychosis and aggression. Patients may have a delayed beneficial response to the correction of precipitating factors such as medical illness, physical discomfort, or medication side effects.
Mrs. B received risperidone, but evidence for efficacy and safety in dementia-related psychosis or agitation does not yet significantly distinguish among the atypical agents (except that data are limited for ziprasidone and clozapine). Usual starting and target doses are provided in Table 4.23
Table 4
Atypicals in dementia: Starting and target doses
| Drug | Starting dose | Target dose |
|---|---|---|
| Aripiprazole | 2.5 to 5 mg/d | 7.5 to 12.5 mg/d |
| Olanzapine | 2.5 to 5 mg/d | 5 to 10 mg/d |
| Quetiapine | 12.5 to 25 mg/d | 50 to 200 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 0.5 to 1.5 mg/d |
| Source: Reference 23 | ||
Related resources
- Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
- American Association for Geriatric Psychiatry position statement: principles of care for persons with dementia resulting from Alzheimer disease. www.aagponline.org/prof/position_caredmnalz.asp.
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Atenolol • Tenormin
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Ciprofloxacin • Ciloxan
- Citalopram • Celexa
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Donepezil • Aricept
- Haloperidol • Haldol
- Hydrocodone/acetaminophen • Lortab, Vicodin
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Sertraline • Zoloft
- Trazodone • Desyrel
- Ziprasidone • Geodon
Disclosure
Dr. Meeks receives research/grant support from the John A. Hartford Foundation, the Mental Health Research Foundation, NARSAD, and the U.S. Department of Health and Human Services’ Health Resources and Services Administration.
Dr. Jeste receives research/grant support from the John A. Hartford Foundation, the National Institute of Aging, and the National Institute of Mental Health. AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, and Janssen, L.P. provide free medications for an NIMH-funded study for which Dr. Jeste is the principal investigator.
1. Jeste DV, Meeks TW, Kim DS, Zubenko GS. Research agenda for DSM-V: diagnostic categories and criteria for neuropsychiatry syndromes in dementia. J Geriatr Psychiatry Neurol 2006;19:160-71.
2. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090-7.
3. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210.
4. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43.
5. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on the use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
6. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry 2004;12:437-9.
7. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176:627-32.
8. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335-41.
9. Trifirò G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf 2007;16:538-44.
10. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775-86.
11. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007;164:1568-76.
12. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330:445.-
13. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry 2005;66:1090-6.
14. Jeste DV, Lacro JP, Nguyen HA, et al. Lower incidence of tardive dyskinesia with risperidone versus haloperidol. J Am Geriatr Soc 1999;47:716-9.
15. Rainer M, Haushofer M, Pfolz H, et al. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry 2007;22:395-403.
16. Holmes C, Wilkinson D, Dean C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry 2007;22:380-1.
17. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
18. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res 2007;4:81-93.
19. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007;15:918-31.
20. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
21. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
22. Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
23. Jeste D, Meeks T. To prescribe or not to prescribe? Atypical antipsychotic drugs in patients with dementia. South Med J 2007;100:961-3.
24. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review and critique. Am J Geriatr Psychiatry 2001;9:361-81.
1. Jeste DV, Meeks TW, Kim DS, Zubenko GS. Research agenda for DSM-V: diagnostic categories and criteria for neuropsychiatry syndromes in dementia. J Geriatr Psychiatry Neurol 2006;19:160-71.
2. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090-7.
3. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210.
4. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43.
5. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on the use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
6. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry 2004;12:437-9.
7. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176:627-32.
8. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335-41.
9. Trifirò G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf 2007;16:538-44.
10. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775-86.
11. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007;164:1568-76.
12. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330:445.-
13. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry 2005;66:1090-6.
14. Jeste DV, Lacro JP, Nguyen HA, et al. Lower incidence of tardive dyskinesia with risperidone versus haloperidol. J Am Geriatr Soc 1999;47:716-9.
15. Rainer M, Haushofer M, Pfolz H, et al. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry 2007;22:395-403.
16. Holmes C, Wilkinson D, Dean C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry 2007;22:380-1.
17. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
18. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res 2007;4:81-93.
19. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007;15:918-31.
20. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
21. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
22. Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
23. Jeste D, Meeks T. To prescribe or not to prescribe? Atypical antipsychotic drugs in patients with dementia. South Med J 2007;100:961-3.
24. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review and critique. Am J Geriatr Psychiatry 2001;9:361-81.