User login
Understanding cultural barriers in hepatitis B virus infection
Asian Americans represent 4% of the population in the United States, and their share of the US population is projected to grow to 9% by 2050.1 These numbers are significant because of the high prevalence of hepatitis B virus (HBV) infection in this community and the cultural barriers to its effective management.
Appreciating the impact of cultural barriers on health care among Asian Americans requires an understanding of the diversity of the Asian continent, which is composed of 52 countries where 100 languages and dialects are spoken. Within each region are religious, cultural, and societal differences. Asians have immigrated to the United States over the course of several generations, and the era in which they immigrated may affect their ability to understand English, integrate into American culture, and navigate the US health care system. Successful integration into American life favors those whose families immigrated several generations earlier.
The overall prevalence of HBV infection in the United States is 0.4%2; however, estimates of prevalence range from 5% to 15% in Asian American populations, and are as high as 20% in some Pacific Rim populations.3,4 The prevalence of HBV infection in Asian Americans differs by subpopulation, with the highest prevalence among immigrants from Vietnam, Laos, and China, and the lowest among those from Japan.
Of the approximately 1 million Americans estimated to be infected with HBV as of 2005, more than 750,000 had access to health care; of these, 205,000 were diagnosed with HBV infection,5 suggesting substantial underdiagnosis. Referrals to specialists were even fewer (175,000), and only about 31,000 patients chronically infected with HBV received antiviral treatment, a figure that has likely increased with greater awareness of HBV and the availability of new antiviral medications.
BARRIERS TO DIAGNOSIS AND TREATMENT
The barriers to effective management of HBV infection in Asian Americans include cultural, socioeconomic, and accessibility issues (see “Case: Stigma and cultural barriers lead to inadequate care”).

Language and linguistic isolation
Limited proficiency in English is a large, if not the largest, barrier to effective management of chronic HBV infection. According to the US Census Bureau, a person with limited English proficiency is one who does not speak English “very well.”6 This terminology has implications for allocation of federal government resources; ie, the percentage of a community’s residents with limited English proficiency is a criterion for receipt of governmental grants and other forms of assistance, including translation services.6
Linguistic isolation, another barrier to medical care, is lack of an English-speaking household member who is older than 14 years.7 By this definition, more than one-third of Korean, Taiwanese, Chinese, Hmong, and Bangladeshi households, and almost half of Vietnamese households, are linguistically isolated, with limited ability to communicate with health care providers.8
Lack of health insurance and its correlates
The high percentage of Asian immigrants without health insurance is a challenge to providing adequate health care. Health insurance coverage is lacking for about one-third of Korean immigrants, about one in five immigrants from Southeast Asia and South Asia, and about 15% of Filipino and Chinese immigrants.9
One reason for the large proportion of uninsured among these groups is the high rate of small business ownership among Asian Americans and the difficulty that small business owners have in obtaining affordable health insurance coverage. In addition, although Asian Americans are as likely as other US residents to be employed full time, their employment options may be less likely to include health insurance benefits.
Poverty affects the ability to acquire health insurance. Although the popular image of the Asian immigrant is an educated person with high earning potential, the reality is that poverty strikes immigrants from Southeast Asia at a high rate. Almost 40% of the Hmong population, for example, lives below the poverty level, and poverty rates among the Cambodian, Bangladeshi, Malaysian, and several other Asian subpopulations are nearly as high.8
Citizenship correlates with the ability to obtain health insurance; it is estimated that 42% to 57% of noncitizens lack health insurance, compared with 15% of citizens.8 Only half of Asian immigrants become naturalized citizens, with wide variability among subgroups. Two-thirds of Filipinos who immigrate to the United States eventually become naturalized compared with less than one-third of Malaysian, Japanese, Indonesian, and Hmong immigrants.8
Educational achievement is associated with attainment of financial security and health insurance. The vast majority of Taiwanese, Japanese, Filipino, and Korean Americans obtain a high school education or higher, with correspondingly higher rates of health insurance coverage. Among those from Southeast Asia (Hmong, Cambodians, Laotians, and Vietnamese), whose immigration to this country is relatively recent, fewer than half complete a high school education.8
Health care workforce representation
Certain Asian subgroups are underrepresented in the racial composition of the US health care workforce; this imbalance may affect accessibility to the health care system and adherence to medical prescriptions and instructions among underrepresented groups. Racial concordance between patient and health care provider is associated with greater patient participation in care, according to the Institute of Medicine.10 In addition to racial similarity, linguistic similarity enhances communication and adherence to instructions.
Belief systems and attitudes toward health care
An immigrant patient’s religious beliefs and cultural attitudes toward Western medicine may pose difficulties in successfully managing disease. Many Asian Americans are Buddhists, who may believe that suffering is an integral part of life; proactively seeking medical care may not be imperative for them. Confucianism, the worship of ancestors and the subjugation of the self to the well-being of the family, is a common belief system among Asians that may inhibit the desire to seek needed medical care. For example, a family elder may instruct a young man not to seek medical care for his HBV infection because this would jeopardize his siblings’ marriage prospects. Taoism involves the belief that perfection is achieved when events are allowed to take the more natural course. Intervention is therefore frowned upon.
Some belief systems may impede care because they incorporate indifference toward suffering. Many Hmong believe that the length of life is predetermined, so lifesaving care is pointless. Cultural value may be placed on stoicism, discouraging visits to health care providers. A belief that disease is caused by supernatural events rather than organic etiologies is another perception that serves as a barrier to seeking medical care.
Distrust of, or unfamiliarity with, Western medicine may delay care, and the resulting poor outcomes may be falsely attributed to Western medicine itself. In some cultures, there is a pervasive belief that a physician can touch the pulse and identify the problem. Some Laotians believe that immunizations are dangerous for a baby’s spirit, and therefore forgo immunization against HBV when it is indicated.
The patient’s relationship with his or her health care provider is an important determinant of quality of care and willingness to continue to receive care. The best possible scenario is concordance in language and culture. Asian cultures emphasize politeness, respect for authority, filial piety, and avoidance of shame. Because Asian patients often view physicians as authority figures, they may not ask questions or voice reservations or fears about their treatment regimens; instead, they may express their agreement with physicians’ advice, but with no intent to return or follow instructions.
Infection with HBV carries a stigma about the mode of transmission that can interfere with patients’ daily lives. A study of attitudes about HBV found that HBV-infected patients feel less welcome to stay overnight or share the same bathroom at friends’ or relatives’ houses, that noninfected persons fear that the disease may be passed to them by HBV-positive friends, and that HBV-infected patients are concerned about whether their choices may have led to the infection.11
OVERCOMING BARRIERS
Sensitivity to cultural attitudes may enhance communication and the likelihood that patients will accept physicians’ recommendations. Several office visits may be necessary to confirm that a patient is receptive to the health care provider’s instructions and is adhering to them. Referral to access programs can aid communication. For example, most cities have community centers where patients can seek medical advice from physicians who speak the patients’ language; these centers also may provide native-language materials and interpreters.
Offering reassurance to patients in their own language and in a culturally sensitive setting will help break down barriers and improve care. Patients who are educated about HBV transmission and the availability of an effective vaccine may be instrumental in preventing transmission of the disease to household members.
Cultural sensitivity training will benefit health care providers and staffs whose patients include Asian Americans. Educational programs should be specific to the needs of the community, as different subpopulations have different needs. Resource materials are available for such training; for example, the federal government’s Office of Minority Health Web site (http://www.omhrc.gov/) offers links to resources for cultural training. In addition to educating themselves and their staffs, health care providers have a responsibility to advocate for funding and equal access to care, and for the creation of more cultural and community health centers that can serve as resources to overcome cultural barriers.
DISCUSSION
Robert G. Gish, MD: How often are herbal remedies tried for chronic HBV infection in the patients you see, especially in the Vietnamese population?
Tram T. Tran, MD: Once patients are diagnosed with chronic HBV infection, the use of herbal remedies is very high; it approaches 80% in my practice. Patients may not admit to it unless you ask them specifically, because they know herbal remedies may be somewhat frowned upon by Western physicians. If you are careful and ask very gently about their use of herbals, they will tell you that they do believe in herbal medicines pretty strongly.
Morris Sherman, MD, PhD: I’d like to emphasize the need to be able to communicate with patients in their own language. In Toronto, 50% of the population was born outside of Canada. We have a huge immigrant population; given the nature of hepatology, we have many patients from Southeast and South Asia, and from all over the world, who don’t speak English. My hospital has a multilingual interpreter service, which we use freely. Scarcely a day goes by without two or three interpreters coming to the clinic to talk to patients, and as a result it’s rare that I can’t make myself understood. Maybe what I’ve said hasn’t been accepted, but patients can at least understand what I’m saying.
William D. Carey, MD: I interview many applicants for our medical school, and many of them are Asians, including Hmong and Vietnamese. With the high value that most of these groups put on education and their success with educational attainment, is their access to care improving? Are we doing a better job of training nurses, allied health personnel, and physicians to deal with this problem?
Dr. Tran: I think so, yes. For instance, the Southeast Asian immigrant population arrived in two different eras. The Vietnamese who immigrated in 1975 have been in the United States longer and in general have been able to attain a higher level of education than those who came later. The group that arrived earlier is therefore more likely to have health insurance, and it has been easier to get them into the health care system. More recent immigrants have had more difficulty navigating the system. In general, their socioeconomic status and therefore access to care is directly related to how long they’ve been in the country.
- President’s Advisory Commission on Asian Americans and Pacific Islanders. Asian Americans and Pacific Islanders: a people looking forward. Action for access and partnerships in the 21st century. Interim report to the president and the nation. http://permanent.access.gpo.gov/lps17931/www.aapi.gov/intreport.htm. Published January 2001. Accessed December 21, 2008.
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Hepatitis B index. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm. Updated July 8, 2008. Accessed January 21, 2009.
- Do S. The natural history of hepatitis B in Asian Americans. Asian Am Pac Isl J Health 2001; 9:141–153.
- Stanford University School of Medicine. FAQ about hepatitis B. Asian Liver Center Web site. http://liver.stanford.edu/Education/faq.html. Updated July 10, 2008. Accessed January 21, 2009.
- Di Bisceglie AM, Keeffe E, Atillasoy E, Varshneya R, Bergstein G. Management of chronic hepatitis B—an analysis of physician practices [DDW abstract M918]. Gastroenterology 2005; 128(suppl 2):A739.
- US Census Bureau. American community survey. US Census Bureau Web site. http://www.census.gov/acs/www/SBasics/SQuest/fact_pdf/P%2013%20factsheetlanguageathome2.pdf. Published January 29, 2004. Accessed January 21, 2009.
- Lestina FA. Analysis of the linguistically isolated population in Census 2000. http://www.census.gov/pred/www/rpts/A.5a.pdf. Published September 30, 2003. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Diverse communities, diverse experiences: the status of Asian Americans and Pacific Islanders in the U.S. http://www.apiahf.org/resources/pdf/Diverse%20Communities%20Diverse%20Experiences.pdf. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Race, ethnicity and health care fact sheet. Henry J. Kaiser Family Foundation Web site. http://www.kff.org/minorityhealth/upload/7745.pdf. Published April 2008. Accessed January 21, 2009.
- Smedley BD, Stith AY, Nelson AR, eds; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. http://www.nap.edu/openbook.php?isbn=030908265X. Published 2003. Accessed January 21, 2009.
- Speigel BMR, Bollus R, Han S, et al. Development and validation of a disease-targeted quality of life instrument in chronic hepatitis B: the hepatitis B quality of life instrument, version 1.0. Hepatology 2007; 46:113–121.
Asian Americans represent 4% of the population in the United States, and their share of the US population is projected to grow to 9% by 2050.1 These numbers are significant because of the high prevalence of hepatitis B virus (HBV) infection in this community and the cultural barriers to its effective management.
Appreciating the impact of cultural barriers on health care among Asian Americans requires an understanding of the diversity of the Asian continent, which is composed of 52 countries where 100 languages and dialects are spoken. Within each region are religious, cultural, and societal differences. Asians have immigrated to the United States over the course of several generations, and the era in which they immigrated may affect their ability to understand English, integrate into American culture, and navigate the US health care system. Successful integration into American life favors those whose families immigrated several generations earlier.
The overall prevalence of HBV infection in the United States is 0.4%2; however, estimates of prevalence range from 5% to 15% in Asian American populations, and are as high as 20% in some Pacific Rim populations.3,4 The prevalence of HBV infection in Asian Americans differs by subpopulation, with the highest prevalence among immigrants from Vietnam, Laos, and China, and the lowest among those from Japan.
Of the approximately 1 million Americans estimated to be infected with HBV as of 2005, more than 750,000 had access to health care; of these, 205,000 were diagnosed with HBV infection,5 suggesting substantial underdiagnosis. Referrals to specialists were even fewer (175,000), and only about 31,000 patients chronically infected with HBV received antiviral treatment, a figure that has likely increased with greater awareness of HBV and the availability of new antiviral medications.
BARRIERS TO DIAGNOSIS AND TREATMENT
The barriers to effective management of HBV infection in Asian Americans include cultural, socioeconomic, and accessibility issues (see “Case: Stigma and cultural barriers lead to inadequate care”).

Language and linguistic isolation
Limited proficiency in English is a large, if not the largest, barrier to effective management of chronic HBV infection. According to the US Census Bureau, a person with limited English proficiency is one who does not speak English “very well.”6 This terminology has implications for allocation of federal government resources; ie, the percentage of a community’s residents with limited English proficiency is a criterion for receipt of governmental grants and other forms of assistance, including translation services.6
Linguistic isolation, another barrier to medical care, is lack of an English-speaking household member who is older than 14 years.7 By this definition, more than one-third of Korean, Taiwanese, Chinese, Hmong, and Bangladeshi households, and almost half of Vietnamese households, are linguistically isolated, with limited ability to communicate with health care providers.8
Lack of health insurance and its correlates
The high percentage of Asian immigrants without health insurance is a challenge to providing adequate health care. Health insurance coverage is lacking for about one-third of Korean immigrants, about one in five immigrants from Southeast Asia and South Asia, and about 15% of Filipino and Chinese immigrants.9
One reason for the large proportion of uninsured among these groups is the high rate of small business ownership among Asian Americans and the difficulty that small business owners have in obtaining affordable health insurance coverage. In addition, although Asian Americans are as likely as other US residents to be employed full time, their employment options may be less likely to include health insurance benefits.
Poverty affects the ability to acquire health insurance. Although the popular image of the Asian immigrant is an educated person with high earning potential, the reality is that poverty strikes immigrants from Southeast Asia at a high rate. Almost 40% of the Hmong population, for example, lives below the poverty level, and poverty rates among the Cambodian, Bangladeshi, Malaysian, and several other Asian subpopulations are nearly as high.8
Citizenship correlates with the ability to obtain health insurance; it is estimated that 42% to 57% of noncitizens lack health insurance, compared with 15% of citizens.8 Only half of Asian immigrants become naturalized citizens, with wide variability among subgroups. Two-thirds of Filipinos who immigrate to the United States eventually become naturalized compared with less than one-third of Malaysian, Japanese, Indonesian, and Hmong immigrants.8
Educational achievement is associated with attainment of financial security and health insurance. The vast majority of Taiwanese, Japanese, Filipino, and Korean Americans obtain a high school education or higher, with correspondingly higher rates of health insurance coverage. Among those from Southeast Asia (Hmong, Cambodians, Laotians, and Vietnamese), whose immigration to this country is relatively recent, fewer than half complete a high school education.8
Health care workforce representation
Certain Asian subgroups are underrepresented in the racial composition of the US health care workforce; this imbalance may affect accessibility to the health care system and adherence to medical prescriptions and instructions among underrepresented groups. Racial concordance between patient and health care provider is associated with greater patient participation in care, according to the Institute of Medicine.10 In addition to racial similarity, linguistic similarity enhances communication and adherence to instructions.
Belief systems and attitudes toward health care
An immigrant patient’s religious beliefs and cultural attitudes toward Western medicine may pose difficulties in successfully managing disease. Many Asian Americans are Buddhists, who may believe that suffering is an integral part of life; proactively seeking medical care may not be imperative for them. Confucianism, the worship of ancestors and the subjugation of the self to the well-being of the family, is a common belief system among Asians that may inhibit the desire to seek needed medical care. For example, a family elder may instruct a young man not to seek medical care for his HBV infection because this would jeopardize his siblings’ marriage prospects. Taoism involves the belief that perfection is achieved when events are allowed to take the more natural course. Intervention is therefore frowned upon.
Some belief systems may impede care because they incorporate indifference toward suffering. Many Hmong believe that the length of life is predetermined, so lifesaving care is pointless. Cultural value may be placed on stoicism, discouraging visits to health care providers. A belief that disease is caused by supernatural events rather than organic etiologies is another perception that serves as a barrier to seeking medical care.
Distrust of, or unfamiliarity with, Western medicine may delay care, and the resulting poor outcomes may be falsely attributed to Western medicine itself. In some cultures, there is a pervasive belief that a physician can touch the pulse and identify the problem. Some Laotians believe that immunizations are dangerous for a baby’s spirit, and therefore forgo immunization against HBV when it is indicated.
The patient’s relationship with his or her health care provider is an important determinant of quality of care and willingness to continue to receive care. The best possible scenario is concordance in language and culture. Asian cultures emphasize politeness, respect for authority, filial piety, and avoidance of shame. Because Asian patients often view physicians as authority figures, they may not ask questions or voice reservations or fears about their treatment regimens; instead, they may express their agreement with physicians’ advice, but with no intent to return or follow instructions.
Infection with HBV carries a stigma about the mode of transmission that can interfere with patients’ daily lives. A study of attitudes about HBV found that HBV-infected patients feel less welcome to stay overnight or share the same bathroom at friends’ or relatives’ houses, that noninfected persons fear that the disease may be passed to them by HBV-positive friends, and that HBV-infected patients are concerned about whether their choices may have led to the infection.11
OVERCOMING BARRIERS
Sensitivity to cultural attitudes may enhance communication and the likelihood that patients will accept physicians’ recommendations. Several office visits may be necessary to confirm that a patient is receptive to the health care provider’s instructions and is adhering to them. Referral to access programs can aid communication. For example, most cities have community centers where patients can seek medical advice from physicians who speak the patients’ language; these centers also may provide native-language materials and interpreters.
Offering reassurance to patients in their own language and in a culturally sensitive setting will help break down barriers and improve care. Patients who are educated about HBV transmission and the availability of an effective vaccine may be instrumental in preventing transmission of the disease to household members.
Cultural sensitivity training will benefit health care providers and staffs whose patients include Asian Americans. Educational programs should be specific to the needs of the community, as different subpopulations have different needs. Resource materials are available for such training; for example, the federal government’s Office of Minority Health Web site (http://www.omhrc.gov/) offers links to resources for cultural training. In addition to educating themselves and their staffs, health care providers have a responsibility to advocate for funding and equal access to care, and for the creation of more cultural and community health centers that can serve as resources to overcome cultural barriers.
DISCUSSION
Robert G. Gish, MD: How often are herbal remedies tried for chronic HBV infection in the patients you see, especially in the Vietnamese population?
Tram T. Tran, MD: Once patients are diagnosed with chronic HBV infection, the use of herbal remedies is very high; it approaches 80% in my practice. Patients may not admit to it unless you ask them specifically, because they know herbal remedies may be somewhat frowned upon by Western physicians. If you are careful and ask very gently about their use of herbals, they will tell you that they do believe in herbal medicines pretty strongly.
Morris Sherman, MD, PhD: I’d like to emphasize the need to be able to communicate with patients in their own language. In Toronto, 50% of the population was born outside of Canada. We have a huge immigrant population; given the nature of hepatology, we have many patients from Southeast and South Asia, and from all over the world, who don’t speak English. My hospital has a multilingual interpreter service, which we use freely. Scarcely a day goes by without two or three interpreters coming to the clinic to talk to patients, and as a result it’s rare that I can’t make myself understood. Maybe what I’ve said hasn’t been accepted, but patients can at least understand what I’m saying.
William D. Carey, MD: I interview many applicants for our medical school, and many of them are Asians, including Hmong and Vietnamese. With the high value that most of these groups put on education and their success with educational attainment, is their access to care improving? Are we doing a better job of training nurses, allied health personnel, and physicians to deal with this problem?
Dr. Tran: I think so, yes. For instance, the Southeast Asian immigrant population arrived in two different eras. The Vietnamese who immigrated in 1975 have been in the United States longer and in general have been able to attain a higher level of education than those who came later. The group that arrived earlier is therefore more likely to have health insurance, and it has been easier to get them into the health care system. More recent immigrants have had more difficulty navigating the system. In general, their socioeconomic status and therefore access to care is directly related to how long they’ve been in the country.
Asian Americans represent 4% of the population in the United States, and their share of the US population is projected to grow to 9% by 2050.1 These numbers are significant because of the high prevalence of hepatitis B virus (HBV) infection in this community and the cultural barriers to its effective management.
Appreciating the impact of cultural barriers on health care among Asian Americans requires an understanding of the diversity of the Asian continent, which is composed of 52 countries where 100 languages and dialects are spoken. Within each region are religious, cultural, and societal differences. Asians have immigrated to the United States over the course of several generations, and the era in which they immigrated may affect their ability to understand English, integrate into American culture, and navigate the US health care system. Successful integration into American life favors those whose families immigrated several generations earlier.
The overall prevalence of HBV infection in the United States is 0.4%2; however, estimates of prevalence range from 5% to 15% in Asian American populations, and are as high as 20% in some Pacific Rim populations.3,4 The prevalence of HBV infection in Asian Americans differs by subpopulation, with the highest prevalence among immigrants from Vietnam, Laos, and China, and the lowest among those from Japan.
Of the approximately 1 million Americans estimated to be infected with HBV as of 2005, more than 750,000 had access to health care; of these, 205,000 were diagnosed with HBV infection,5 suggesting substantial underdiagnosis. Referrals to specialists were even fewer (175,000), and only about 31,000 patients chronically infected with HBV received antiviral treatment, a figure that has likely increased with greater awareness of HBV and the availability of new antiviral medications.
BARRIERS TO DIAGNOSIS AND TREATMENT
The barriers to effective management of HBV infection in Asian Americans include cultural, socioeconomic, and accessibility issues (see “Case: Stigma and cultural barriers lead to inadequate care”).

Language and linguistic isolation
Limited proficiency in English is a large, if not the largest, barrier to effective management of chronic HBV infection. According to the US Census Bureau, a person with limited English proficiency is one who does not speak English “very well.”6 This terminology has implications for allocation of federal government resources; ie, the percentage of a community’s residents with limited English proficiency is a criterion for receipt of governmental grants and other forms of assistance, including translation services.6
Linguistic isolation, another barrier to medical care, is lack of an English-speaking household member who is older than 14 years.7 By this definition, more than one-third of Korean, Taiwanese, Chinese, Hmong, and Bangladeshi households, and almost half of Vietnamese households, are linguistically isolated, with limited ability to communicate with health care providers.8
Lack of health insurance and its correlates
The high percentage of Asian immigrants without health insurance is a challenge to providing adequate health care. Health insurance coverage is lacking for about one-third of Korean immigrants, about one in five immigrants from Southeast Asia and South Asia, and about 15% of Filipino and Chinese immigrants.9
One reason for the large proportion of uninsured among these groups is the high rate of small business ownership among Asian Americans and the difficulty that small business owners have in obtaining affordable health insurance coverage. In addition, although Asian Americans are as likely as other US residents to be employed full time, their employment options may be less likely to include health insurance benefits.
Poverty affects the ability to acquire health insurance. Although the popular image of the Asian immigrant is an educated person with high earning potential, the reality is that poverty strikes immigrants from Southeast Asia at a high rate. Almost 40% of the Hmong population, for example, lives below the poverty level, and poverty rates among the Cambodian, Bangladeshi, Malaysian, and several other Asian subpopulations are nearly as high.8
Citizenship correlates with the ability to obtain health insurance; it is estimated that 42% to 57% of noncitizens lack health insurance, compared with 15% of citizens.8 Only half of Asian immigrants become naturalized citizens, with wide variability among subgroups. Two-thirds of Filipinos who immigrate to the United States eventually become naturalized compared with less than one-third of Malaysian, Japanese, Indonesian, and Hmong immigrants.8
Educational achievement is associated with attainment of financial security and health insurance. The vast majority of Taiwanese, Japanese, Filipino, and Korean Americans obtain a high school education or higher, with correspondingly higher rates of health insurance coverage. Among those from Southeast Asia (Hmong, Cambodians, Laotians, and Vietnamese), whose immigration to this country is relatively recent, fewer than half complete a high school education.8
Health care workforce representation
Certain Asian subgroups are underrepresented in the racial composition of the US health care workforce; this imbalance may affect accessibility to the health care system and adherence to medical prescriptions and instructions among underrepresented groups. Racial concordance between patient and health care provider is associated with greater patient participation in care, according to the Institute of Medicine.10 In addition to racial similarity, linguistic similarity enhances communication and adherence to instructions.
Belief systems and attitudes toward health care
An immigrant patient’s religious beliefs and cultural attitudes toward Western medicine may pose difficulties in successfully managing disease. Many Asian Americans are Buddhists, who may believe that suffering is an integral part of life; proactively seeking medical care may not be imperative for them. Confucianism, the worship of ancestors and the subjugation of the self to the well-being of the family, is a common belief system among Asians that may inhibit the desire to seek needed medical care. For example, a family elder may instruct a young man not to seek medical care for his HBV infection because this would jeopardize his siblings’ marriage prospects. Taoism involves the belief that perfection is achieved when events are allowed to take the more natural course. Intervention is therefore frowned upon.
Some belief systems may impede care because they incorporate indifference toward suffering. Many Hmong believe that the length of life is predetermined, so lifesaving care is pointless. Cultural value may be placed on stoicism, discouraging visits to health care providers. A belief that disease is caused by supernatural events rather than organic etiologies is another perception that serves as a barrier to seeking medical care.
Distrust of, or unfamiliarity with, Western medicine may delay care, and the resulting poor outcomes may be falsely attributed to Western medicine itself. In some cultures, there is a pervasive belief that a physician can touch the pulse and identify the problem. Some Laotians believe that immunizations are dangerous for a baby’s spirit, and therefore forgo immunization against HBV when it is indicated.
The patient’s relationship with his or her health care provider is an important determinant of quality of care and willingness to continue to receive care. The best possible scenario is concordance in language and culture. Asian cultures emphasize politeness, respect for authority, filial piety, and avoidance of shame. Because Asian patients often view physicians as authority figures, they may not ask questions or voice reservations or fears about their treatment regimens; instead, they may express their agreement with physicians’ advice, but with no intent to return or follow instructions.
Infection with HBV carries a stigma about the mode of transmission that can interfere with patients’ daily lives. A study of attitudes about HBV found that HBV-infected patients feel less welcome to stay overnight or share the same bathroom at friends’ or relatives’ houses, that noninfected persons fear that the disease may be passed to them by HBV-positive friends, and that HBV-infected patients are concerned about whether their choices may have led to the infection.11
OVERCOMING BARRIERS
Sensitivity to cultural attitudes may enhance communication and the likelihood that patients will accept physicians’ recommendations. Several office visits may be necessary to confirm that a patient is receptive to the health care provider’s instructions and is adhering to them. Referral to access programs can aid communication. For example, most cities have community centers where patients can seek medical advice from physicians who speak the patients’ language; these centers also may provide native-language materials and interpreters.
Offering reassurance to patients in their own language and in a culturally sensitive setting will help break down barriers and improve care. Patients who are educated about HBV transmission and the availability of an effective vaccine may be instrumental in preventing transmission of the disease to household members.
Cultural sensitivity training will benefit health care providers and staffs whose patients include Asian Americans. Educational programs should be specific to the needs of the community, as different subpopulations have different needs. Resource materials are available for such training; for example, the federal government’s Office of Minority Health Web site (http://www.omhrc.gov/) offers links to resources for cultural training. In addition to educating themselves and their staffs, health care providers have a responsibility to advocate for funding and equal access to care, and for the creation of more cultural and community health centers that can serve as resources to overcome cultural barriers.
DISCUSSION
Robert G. Gish, MD: How often are herbal remedies tried for chronic HBV infection in the patients you see, especially in the Vietnamese population?
Tram T. Tran, MD: Once patients are diagnosed with chronic HBV infection, the use of herbal remedies is very high; it approaches 80% in my practice. Patients may not admit to it unless you ask them specifically, because they know herbal remedies may be somewhat frowned upon by Western physicians. If you are careful and ask very gently about their use of herbals, they will tell you that they do believe in herbal medicines pretty strongly.
Morris Sherman, MD, PhD: I’d like to emphasize the need to be able to communicate with patients in their own language. In Toronto, 50% of the population was born outside of Canada. We have a huge immigrant population; given the nature of hepatology, we have many patients from Southeast and South Asia, and from all over the world, who don’t speak English. My hospital has a multilingual interpreter service, which we use freely. Scarcely a day goes by without two or three interpreters coming to the clinic to talk to patients, and as a result it’s rare that I can’t make myself understood. Maybe what I’ve said hasn’t been accepted, but patients can at least understand what I’m saying.
William D. Carey, MD: I interview many applicants for our medical school, and many of them are Asians, including Hmong and Vietnamese. With the high value that most of these groups put on education and their success with educational attainment, is their access to care improving? Are we doing a better job of training nurses, allied health personnel, and physicians to deal with this problem?
Dr. Tran: I think so, yes. For instance, the Southeast Asian immigrant population arrived in two different eras. The Vietnamese who immigrated in 1975 have been in the United States longer and in general have been able to attain a higher level of education than those who came later. The group that arrived earlier is therefore more likely to have health insurance, and it has been easier to get them into the health care system. More recent immigrants have had more difficulty navigating the system. In general, their socioeconomic status and therefore access to care is directly related to how long they’ve been in the country.
- President’s Advisory Commission on Asian Americans and Pacific Islanders. Asian Americans and Pacific Islanders: a people looking forward. Action for access and partnerships in the 21st century. Interim report to the president and the nation. http://permanent.access.gpo.gov/lps17931/www.aapi.gov/intreport.htm. Published January 2001. Accessed December 21, 2008.
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Hepatitis B index. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm. Updated July 8, 2008. Accessed January 21, 2009.
- Do S. The natural history of hepatitis B in Asian Americans. Asian Am Pac Isl J Health 2001; 9:141–153.
- Stanford University School of Medicine. FAQ about hepatitis B. Asian Liver Center Web site. http://liver.stanford.edu/Education/faq.html. Updated July 10, 2008. Accessed January 21, 2009.
- Di Bisceglie AM, Keeffe E, Atillasoy E, Varshneya R, Bergstein G. Management of chronic hepatitis B—an analysis of physician practices [DDW abstract M918]. Gastroenterology 2005; 128(suppl 2):A739.
- US Census Bureau. American community survey. US Census Bureau Web site. http://www.census.gov/acs/www/SBasics/SQuest/fact_pdf/P%2013%20factsheetlanguageathome2.pdf. Published January 29, 2004. Accessed January 21, 2009.
- Lestina FA. Analysis of the linguistically isolated population in Census 2000. http://www.census.gov/pred/www/rpts/A.5a.pdf. Published September 30, 2003. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Diverse communities, diverse experiences: the status of Asian Americans and Pacific Islanders in the U.S. http://www.apiahf.org/resources/pdf/Diverse%20Communities%20Diverse%20Experiences.pdf. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Race, ethnicity and health care fact sheet. Henry J. Kaiser Family Foundation Web site. http://www.kff.org/minorityhealth/upload/7745.pdf. Published April 2008. Accessed January 21, 2009.
- Smedley BD, Stith AY, Nelson AR, eds; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. http://www.nap.edu/openbook.php?isbn=030908265X. Published 2003. Accessed January 21, 2009.
- Speigel BMR, Bollus R, Han S, et al. Development and validation of a disease-targeted quality of life instrument in chronic hepatitis B: the hepatitis B quality of life instrument, version 1.0. Hepatology 2007; 46:113–121.
- President’s Advisory Commission on Asian Americans and Pacific Islanders. Asian Americans and Pacific Islanders: a people looking forward. Action for access and partnerships in the 21st century. Interim report to the president and the nation. http://permanent.access.gpo.gov/lps17931/www.aapi.gov/intreport.htm. Published January 2001. Accessed December 21, 2008.
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Hepatitis B index. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm. Updated July 8, 2008. Accessed January 21, 2009.
- Do S. The natural history of hepatitis B in Asian Americans. Asian Am Pac Isl J Health 2001; 9:141–153.
- Stanford University School of Medicine. FAQ about hepatitis B. Asian Liver Center Web site. http://liver.stanford.edu/Education/faq.html. Updated July 10, 2008. Accessed January 21, 2009.
- Di Bisceglie AM, Keeffe E, Atillasoy E, Varshneya R, Bergstein G. Management of chronic hepatitis B—an analysis of physician practices [DDW abstract M918]. Gastroenterology 2005; 128(suppl 2):A739.
- US Census Bureau. American community survey. US Census Bureau Web site. http://www.census.gov/acs/www/SBasics/SQuest/fact_pdf/P%2013%20factsheetlanguageathome2.pdf. Published January 29, 2004. Accessed January 21, 2009.
- Lestina FA. Analysis of the linguistically isolated population in Census 2000. http://www.census.gov/pred/www/rpts/A.5a.pdf. Published September 30, 2003. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Diverse communities, diverse experiences: the status of Asian Americans and Pacific Islanders in the U.S. http://www.apiahf.org/resources/pdf/Diverse%20Communities%20Diverse%20Experiences.pdf. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Race, ethnicity and health care fact sheet. Henry J. Kaiser Family Foundation Web site. http://www.kff.org/minorityhealth/upload/7745.pdf. Published April 2008. Accessed January 21, 2009.
- Smedley BD, Stith AY, Nelson AR, eds; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. http://www.nap.edu/openbook.php?isbn=030908265X. Published 2003. Accessed January 21, 2009.
- Speigel BMR, Bollus R, Han S, et al. Development and validation of a disease-targeted quality of life instrument in chronic hepatitis B: the hepatitis B quality of life instrument, version 1.0. Hepatology 2007; 46:113–121.
KEY POINTS
- Some Asian Americans have limited proficiency in English and are isolated linguistically, limiting their ability to communicate with health care providers.
- Asian Americans may view Western medicine with suspicion, causing delays in seeking care and making it difficult to successfully manage chronic HBV infection.
- Sensitivity to cultural attitudes may enhance communication and the likelihood that immigrant patients will accept health care providers’ recommendations; cultural sensitivity training may be helpful.
Management of hepatitis B in pregnancy: Weighing the options
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
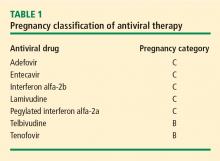
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
KEY POINTS
- Hepatitis B immune globulin at the time of birth plus three doses of the recombinant hepatitis B vaccine over the first 6 months of life is up to 95% effective in
preventing perinatal transmission. - Despite successful screening and vaccination, perinatal transmission of HBV is still possible if maternal viral load is high.
- Antiviral treatment during the third trimester of pregnancy may reduce perinatal transmission of HBV; the benefit appears most pronounced with high maternal viremia.