User login
Review of Strategies to Reduce Central Line-Associated Bloodstream Infection (CLABSI) and Catheter-Associated Urinary Tract Infection (CAUTI) in Adult ICUs
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
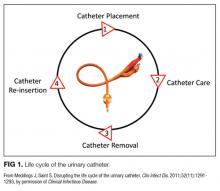
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
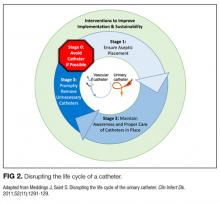
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
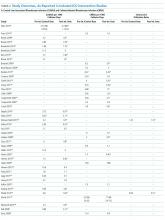
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
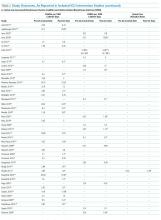
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
© 2018 Society of Hospital Medicine
Fuller Road, Ann Arbor, MI 48105; Telephone: 734-845-3460; Fax: 734-845-3290, E-mail: [email protected]
Caught red‐handed
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.
The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets 100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.

The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets 100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.

The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets 100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.