User login
Neuropsychiatric impairment in a septic shock survivor
The effect of sepsis survivorship on cognition is a substantially under-recognized public health problem.1 Sepsis survivorship has implications for patients’ families and the health care system.2 Research has demonstrated that older patients may develop impaired cognition and functional capacity after severe sepsis3; limited evidence shows neurocognitive decline in non-geriatric patients.3 There are no reports of exacerbation of psychiatric illness after severe sepsis or septic shock, and existing literature indicates that the causative factors, epidemiology, and predisposition that may worsen psychiatric illness after septic shock are poorly defined.
Case: Sepsis-induced cognitive decline?
Following an intensive care admission for septic shock, Mr. J, age 49, presents to the outpatient behavioral medicine department with worsening mood, lethargy, agitation, suicidal ideations, hallucinations, and poor work performance for 10 months. He was diagnosed with major depressive disorder 13 years prior, but has no history of hospitalization for psychiatric illness. His depressive symptoms respond well to paroxetine, 60 mg/d. Subsequently, Mr. J becomes delusional, has intense command hallucinations, and attempts suicide, resulting in hospitalization. Neuropsychological testing reveals dementia and significant psychiatric distress, including elevated levels of depression and suicidal ideation. He is stabilized with duloxetine, 90 mg/d, and quetiapine, 50 mg/d. Two years later, Mr. J still exhibits cognitive and psychiatric disturbances.
Long-term results
The underlying mechanism of septic shock on the brain may be similar to the mechanisms that exacerbate psychiatric illnesses. This case validates the use of neuropsychological testing in septic shock survivors and encourages recognition of the effect septic shock has on neuropsychiatric illness.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers
of competing products.
References
1. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794.
2. Safdieh J. Cognition after sepsis. Neurology Alert. 2010; 29(4):30-31.
3. Williams GS. Older severe sepsis survivors are at risk for cognitive and developmental disability. Pulmonary Reviews. 2010;15(12):17-18.
The effect of sepsis survivorship on cognition is a substantially under-recognized public health problem.1 Sepsis survivorship has implications for patients’ families and the health care system.2 Research has demonstrated that older patients may develop impaired cognition and functional capacity after severe sepsis3; limited evidence shows neurocognitive decline in non-geriatric patients.3 There are no reports of exacerbation of psychiatric illness after severe sepsis or septic shock, and existing literature indicates that the causative factors, epidemiology, and predisposition that may worsen psychiatric illness after septic shock are poorly defined.
Case: Sepsis-induced cognitive decline?
Following an intensive care admission for septic shock, Mr. J, age 49, presents to the outpatient behavioral medicine department with worsening mood, lethargy, agitation, suicidal ideations, hallucinations, and poor work performance for 10 months. He was diagnosed with major depressive disorder 13 years prior, but has no history of hospitalization for psychiatric illness. His depressive symptoms respond well to paroxetine, 60 mg/d. Subsequently, Mr. J becomes delusional, has intense command hallucinations, and attempts suicide, resulting in hospitalization. Neuropsychological testing reveals dementia and significant psychiatric distress, including elevated levels of depression and suicidal ideation. He is stabilized with duloxetine, 90 mg/d, and quetiapine, 50 mg/d. Two years later, Mr. J still exhibits cognitive and psychiatric disturbances.
Long-term results
The underlying mechanism of septic shock on the brain may be similar to the mechanisms that exacerbate psychiatric illnesses. This case validates the use of neuropsychological testing in septic shock survivors and encourages recognition of the effect septic shock has on neuropsychiatric illness.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers
of competing products.
References
1. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794.
2. Safdieh J. Cognition after sepsis. Neurology Alert. 2010; 29(4):30-31.
3. Williams GS. Older severe sepsis survivors are at risk for cognitive and developmental disability. Pulmonary Reviews. 2010;15(12):17-18.
The effect of sepsis survivorship on cognition is a substantially under-recognized public health problem.1 Sepsis survivorship has implications for patients’ families and the health care system.2 Research has demonstrated that older patients may develop impaired cognition and functional capacity after severe sepsis3; limited evidence shows neurocognitive decline in non-geriatric patients.3 There are no reports of exacerbation of psychiatric illness after severe sepsis or septic shock, and existing literature indicates that the causative factors, epidemiology, and predisposition that may worsen psychiatric illness after septic shock are poorly defined.
Case: Sepsis-induced cognitive decline?
Following an intensive care admission for septic shock, Mr. J, age 49, presents to the outpatient behavioral medicine department with worsening mood, lethargy, agitation, suicidal ideations, hallucinations, and poor work performance for 10 months. He was diagnosed with major depressive disorder 13 years prior, but has no history of hospitalization for psychiatric illness. His depressive symptoms respond well to paroxetine, 60 mg/d. Subsequently, Mr. J becomes delusional, has intense command hallucinations, and attempts suicide, resulting in hospitalization. Neuropsychological testing reveals dementia and significant psychiatric distress, including elevated levels of depression and suicidal ideation. He is stabilized with duloxetine, 90 mg/d, and quetiapine, 50 mg/d. Two years later, Mr. J still exhibits cognitive and psychiatric disturbances.
Long-term results
The underlying mechanism of septic shock on the brain may be similar to the mechanisms that exacerbate psychiatric illnesses. This case validates the use of neuropsychological testing in septic shock survivors and encourages recognition of the effect septic shock has on neuropsychiatric illness.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers
of competing products.
References
1. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794.
2. Safdieh J. Cognition after sepsis. Neurology Alert. 2010; 29(4):30-31.
3. Williams GS. Older severe sepsis survivors are at risk for cognitive and developmental disability. Pulmonary Reviews. 2010;15(12):17-18.
Neuroleptic malignant syndrome: Don’t let your guard down yet
When second-generation antipsychotics (SGAs) were introduced, clinicians hoped the drugs would not have the potential to cause neuroleptic malignant syndrome (NMS).1 Since then, however, case reports have made it clear that SGAs—like first-generation antipsychotics (FGAs)—can precipitate this life-threatening neurologic emergency.
To help you protect your patients receiving SGAs, this article explains how to:
- identify those at risk
- recognize the different NMS presentations associated with each SGA
- continue antipsychotic treatment for a patient with a history of NMS.
CASE STUDY: A drug-induced disorder
Mrs. Z, age 39, has a history of multiple hospitalizations for schizoaffective disorder complicated by poor compliance and a history of benzodiazepine abuse. This time she was admitted with increased auditory hallucinations and paranoid delusions of her family trying to poison her. Despite multiple haloperidol injections (5 mg IM q4h prn), Mrs. Z continued to have hallucinations and remained agitated.
Haloperidol was discontinued and ziprasidone (20 mg IM q4h prn) was started. After 3 days, Mrs. Z became less agitated and had fewer hallucinations. The IM route was discontinued and oral ziprasidone was started at 40 mg bid, then titrated to 80 mg bid after 2 days. On the third day after titration, Mrs. Z fell twice. She hit her head in one fall, but a brain CT to rule out bleeding was normal.
The next day, Mrs. Z became more confused and developed fever, tremor, urinary incontinence, and a severe headache. She became obtunded, was intubated, and was transferred to the intensive care unit of a tertiary care center.
On admission, her temperature was 103° F (39.4° C); she had severe muscle rigidity and blood pressure of 85/60 mm Hg. Creatine phosphokinase (CPK) was 2,559 U/L (normal 24 to 170 U/L). Liver enzymes were elevated: alanine transaminase was 202 U/L (normal 13 to 50 U/L), and aspartate transaminase (AST) was 190 U/L (normal 15 to 46 U/L). At 140 μg/dL, Mrs. Z’s serum iron was within normal limits (40 to 150 μg/dL).
Neuroleptic malignant syndrome
Clinical manifestations of NMS range from typical—as defined by the DSM-IV-TR (Table 1)2,3—to atypical, without:
Table 1
DSM-IV-TR definition of NMS*
| Hyperthermia (>38° C) and |
| Muscle rigidity and |
At least 2 of the following:
|
| * Symptoms must be associated with the use of neuroleptic medication, and other central and systemic causes of hyperthermia must be excluded. |
| CPK: creatine phosphokinase; NMS: neuroleptic malignant syndrome |
| Source: DSM-IV-TR |
Many conditions resemble NMS (Table 2). Because NMS can be fatal without emergent diagnosis and treatment, maintain a high index of suspicion for this condition whenever you prescribe antipsychotics.
Table 2
NMS differential diagnosis
| Primary CNS disorders |
| CNS vasculitis |
| Infarctions |
| Infections |
| Parkinson’s disease |
| Status epilepticus |
| Trauma |
| Tumors |
| Systemic disorders |
| Acute porphyria |
| Autoimmune disorders |
| Dehydration |
| Heat stroke |
| Hyperthyroidism |
| Infections |
| Pheochromocytoma |
| Tetanus |
| Psychiatric disorders |
| Idiopathic lethal catatonia |
| Medication-related disorders |
| Anticholinergic syndrome |
| Drug intoxication |
| Levodopa syndrome |
| Malignant hyperthermia |
| Serotonin syndrome |
NMS is believed to be caused by reduced dopamine activity in the brain associated with dopamine antagonists, interruptions in nigrostriatal dopamine pathways, or withdrawal of dopaminergic medications.3 However, dopamine D2 receptor blocking potential is not directly linked to the occurrence of NMS.6 Other mechanisms include genetic susceptibility and different CNS neurotransmitter disturbances.7
NMS develops in an estimated 0.02% to 2.5% of patients treated with antipsychotics.8-10 The syndrome appears to occur slightly less frequently with SGAs than with FGAs.6,10
Risk factors. NMS can develop at any age, in men and women, and in patients with psychiatric or medical illness.11,12 In addition to antipsychotics, other medications—including antiemetics and sedatives—can cause NMS. The syndrome has been triggered when Parkinson’s disease patients stop taking or reduce the dose of a dopamine agonist or switch from 1 dopamine agonist to another.13,14
Symptoms usually develop during the first 2 weeks of pharmacotherapy but may start after the initial dose or during long-term stable therapy.15 Although some studies found NMS development to be dose-independent, multiple cases have demonstrated an association with dose changes. Death occurs from dysautonomic manifestations and systemic complications.
An elevated risk for NMS may exist in patients with:
- mood disorders
- preexisting catatonia16
- complicated medical and neurologic disorders, such as encephalitis or mental retardation17
- poor functional and physiologic status3
- concurrent lithium treatment
- IM injection of an antipsychotic
- use of a high-potency antipsychotic, such as haloperidol
- psychomotor agitation.
Other potential risk factors include dehydration, adolescent age, male gender, low serum iron concentrations, relatively high antipsychotic dosages, and mental retardation or prior structural brain injury.18-20
NMS and SGAs
We reviewed 88 reports of NMS cases associated with 6 SGAs: olanzapine, clozapine, risperidone, ziprasidone, quetiapine, and aripiprazole. In this article, we cite representative cases only; readers interested in the full literature search can find this evidence and its references in the Case Reports Table.
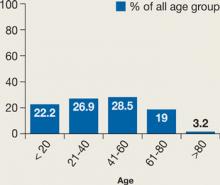
NMS cases were fairly evenly distributed across all age groups (Figure 1). SGAs were implicated in NMS when used as monotherapy in 9 cases (10%) and in combination with other psychotropics in 41 cases (47%). We could not find medication regimen data for 38 cases (43%).
Figure 1
NMS incidence across age groups
Incidence is dispersed fairly evenly; elderly patients may be less likely to be prescribed an antipsychotic than other age groups.
Source: Reference 5Our review suggests that a history of NMS is a risk factor for developing another episode. Twenty cases showed a clear history of NMS, and 2 cases reported 3 different NMS episodes in each patient.19,21
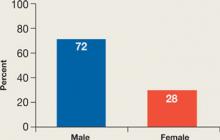
In the cases we reviewed, NMS developed more often among men than women (Figure 2). The reason is not clear. One hypothesis suggests that men are more likely to present with severe agitation that requires aggressive antipsychotic treatment.14,22
Figure 2
NMS: More common in men
Men may be at higher risk because they are more likely to present with severe agitation and receive larger doses of potent antipsychotics.
Source: References 14,22Previous reports suggested that parenteral antipsychotic administration might increase NMS risk. Most NMS cases in our review involved oral administration, perhaps because parenteral SGAs have become available only recently. In the future, increased use of parenteral SGAs might increase the incidence of NMS.
The NMS mortality rate associated with SGAs was lower than that linked to FGAs.6 This finding, however, may be influenced by increasing awareness of NMS among physicians, resulting in earlier diagnosis and treatment.6
Findings for specific SGAs
Aripiprazole. Because aripiprazole is the newest SGA, data on its association with NMS are limited. Our review looked at 2 cases. Both patients had atypical NMS features, including absence of fever and mild CPK elevation. In 1 case, aripiprazole was used to treat agitation in a 13-year-old girl with history of NMS. This resulted in a mild increase in tachycardia and brief worsening of serum CPK but did not significantly affect temperature, respiratory rate, or blood pressure.
Clozapine. Several NMS cases have been connected to clozapine monotherapy (6 cases) or combination therapy (22 cases). Compared with NMS caused by other antipsychotics, clozapine-induced NMS occurred sooner after patients started the drug or restarted it after discontinuation. NMS has developed in patients receiving chronic steady doses of clozapine, after dosage increases, and after other medications have been added.
Clozapine-treated patients need to be closely monitored for agranulocytosis symptoms, so any other adverse effects—such as initial symptoms of NMS—likely will be detected early. Some reports suggested that clozapine-induced NMS may feature fewer extrapyramidal side effects and a lower-than-typical increase in CPK. In the cases we reviewed, however, NMS presentations ranged from typical—with a highly elevated CPK—to mild with no rigidity and mild or no CPK elevation. Two of 28 cases reported neurologic sequelae, including severe truncal ataxia and dysmetria.
Clozapine has been used to treat patients with a history of NMS who experience psychotic relapse. In several cases, however, NMS recurred after clozapine was started. In 1 case, a third rechallenge with slow titration of clozapine was successful.
Olanzapine. Some studies have found olanzapine-induced NMS to be rare (rate ≤0.01%), but our review found 36 such cases. Ten patients (30%) had a history of NMS. Olanzapine dosing did not correlate with NMS—in 11 cases NMS occurred with daily doses ≤10 mg.
As with clozapine, the presentation of olanzapine-induced NMS varies widely. Onset from within 8 hours of starting olanzapine to after 2½ years of stable olanzapine dosing has been reported. Some cases have featured a typical NMS presentation. Atypical presentations have included:
- extremely elevated serum sodium
- absence of rigidity
- normal CPK
- generalized tonic-clonic seizures preceding NMS onset
- anterograde amnesia
- deficits in learning verbal information.
Olanzapine challenge for patients with a history of NMS often has triggered recurring NMS.
Quetiapine. NMS has been reported in patients receiving quetiapine monotherapy and combination therapy. Patients who previously experienced NMS after taking an FGA have developed quetiapine-induced NMS, as have some with a history of Lewy body disease. Two patients treated with quetiapine developed CPK elevations to almost 9,000 U/L (normal <171 U/L)—without other NMS features—that improved after discontinuing the medication.
Risperidone. NMS among patients taking risperidone occurs more frequently in those with history of NMS or who restart risperidone after discontinuation. Time to NMS occurrence after starting risperidone varies from hours to months. Atypical presentations include delayed fever, delayed muscle rigidity, massive intestinal bleeding, massive CPK elevation (such as 46,420 U/L), and hyponatremia instead of hypernatremia.
Ziprasidone. Administering IM ziprasidone or combining any form of the drug with other psychotropics increases NMS risk. Although most cases featured typical presentations, 1 case reported absence of muscle rigidity, which is present in >90% of patients with NMS associated with FGAs.
NMS sequelae related to SGAs
Brain injury following NMS can cause truncal ataxia, limb ataxia, athetosis, hemiballismus, dysmetria, dysarthria, sensory function problems, balance problems, persistent amnesia, difficulties comprehending commands, attention problems, and electroencephalograph or MRI abnormalities.23,24 Postmortem studies of patients with NMS have revealed cerebellar degeneration, reduction of Purkinje and granule cells, and gliosis in the dentate nucleus.25,26
Why some patients develop sequelae after NMS while others recover is unknown. Sustained hyperpyrexia, preexisting medical or neurologic disorders, polypharmacy, prolonged courses, and delayed diagnosis may play a role.25-27
CASE CONTINUED: A complicated illness
Mrs. Z was diagnosed with NMS. Ziprasidone was discontinued, and supportive treatment, bromocriptine (2.5 mg po qid), and lorazepam (2 mg IV qid) were started. Temperatures of 101° to 103° F (38.3° to 39.4° C) persisted for the next 2 days. This hyperthermia was difficult to control because of suspected meningitis.
The team started ceftriaxone (2 gm IV q12h) while awaiting lumbar puncture results. CSF showed mild white blood cell elevation of 20/cu mm (normal 0 to 5/cu mm) with 62% neutrophils (normal 0 to 6%), normal protein, normal glucose, and negative cultures. After 2 days of antibiotic therapy, the patient developed diarrhea and was diagnosed with Clostridium difficile-associated colitis, a side effect of the antibiotic.
Treatment is mainly supportive
Recognizing NMS signs is the first and most important step to quick diagnosis and early medical intervention. Recommendations for medical treatment of NMS vary widely, but most stress stopping the triggering drug and initiating supportive care (Table 3).27-29
Several medications have been used off-label to treat NMS based on anecdotal clinical reports. Benzodiazepines such as parenteral lorazepam, 1 to 2 mg every 6 to 8 hours, have been used to treat catatonic symptoms.30 Dopamine agonists—including bromocriptine, 2.5 mg every 8 hours—have reduced the duration and mortality of NMS but have the potential to worsen psychotic symptoms and cause hypotension and emesis.30
Table 3
Treating NMS: Where to start
| Stop offending agent(s) |
Provide intensive hemodynamic and supportive care:
|
CASE CONTINUED: Resuming antipsychotic Tx
Five days after intubation, Mrs. Z started to improve and was extubated successfully. However, she developed severe truncal ataxia, upper extremity tremors (resting and intentional), athetosis, hemiballismus, dysmetria, and dystonia. She continued to experience hallucinations after transfer back to the psychiatric floor.
Oral olanzapine challenge was started at 2.5 mg/d and titrated up to 10 mg/d over the next 7 days. Her psychotic symptoms showed mild improvement but her ataxic movements worsened and she fell frequently. Benztropine, 1 mg po bid, was added to her regimen and helped with the tremor. She was transferred for rehabilitation and eventually discharged home.
If a patient needs antipsychotics
If a patient who has experienced NMS continues to need pharmacotherapy for psychosis, wait 1 or 2 weeks after NMS symptoms resolve before restarting any antipsychotic.31 Although most patients can be treated safely with an antipsychotic after having NMS, clearly document the indications and your discussions with the patients and their families.
No conclusive evidence indicates which antipsychotic might lower a patient’s risk of recurrent NMS. Using an FGA in patients who recover from NMS carries a 30% risk of recurrent episodes.3 Data on the recurrence of NMS with SGAs are inconclusive. No relationship was found between relapse rate and patients’ age or sex.32
Regardless of which drug you choose, start with a low dosage and titrate slowly. You also can protect patients by reducing risk factors for NMS, such as dehydration, and considering alternate therapies such as electroconvulsive therapy, when appropriate.
This paper was among those entered in the 2007 Promising New Investigators competition sponsored by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition was “New insights on psychotropic drug safety and side effects.”
Current Psychiatry is honored to publish this peer-reviewed, evidenced-based article on a clinically important topic for practicing psychiatrists.
NMSIS is dedicated to reducing morbidity and mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing support information for medical professionals, patients, and families; and improving scientific understanding of these conditions through research.
Related resources
- Neuroleptic Malignant Syndrome Information Service. http://nmsis.org.
- National Institute of Neurological Disorders and Stroke. Neuroleptic malignant syndrome information page www.ninds.nih.gov/disorders/neuroleptic_syndrome/neuroleptic_syndrome.htm.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Ceftriaxone • Rocephin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118:145-52.
2. Thornberg SA, Ereshefsky L. Neuroleptic malignant syndrome associated with clozapine monotherapy. Pharmacotherapy 1993;13:510-4.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
4. Rodriguez OP, Dowell MS. A case report of neuroleptic malignant syndrome without fever in a patient given aripiprazole. J Okla State Med Assoc 2006;9(7):435-8.
5. Kogoj A, Velikonja I. Olanzapine induced neuroleptic malignant syndrome—a case review. Hum Psychopharmacol 2003;18(4):301-9.
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65(4):464-70.
7. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30:314-21.
8. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985;142:1137.-
9. Addonizio G, Susman VL, Roth SD. Symptoms of neuroleptic malignant syndrome in 82 consecutive inpatients. Am J Psychiatry 1986;143:1587-90.
10. Pope HG, Keck PE, McElroy SL. Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry 1986;143:1227-33.
11. Chungh DS, Kim BN, Cho SC. Neuroleptic malignant syndrome due to three atypical antipsychotics in a child. J Psychopharmacol 2005;19(4):422-5.
12. Suh H, Bronson B, Martin R. Neuroleptic malignant syndrome and low-dose olanzapine. Am J Psychiatry 2003;160(4):796.-
13. Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry 1989;50:18.-
14. Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf 1998;19:73.-
15. Pope HG, Jr, Aizley HG, Keck PE, Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry 1991;52:208.-
16. White DAC, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry 1991;158:419-21.
17. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18:349-51.
18. Apple JE, Van Hauer G. Neuroleptic malignant syndrome associated with olanzapine therapy. Psychosomatics 1999;40(3):267-8.
19. Margolese HC, Chouinard G. Olanzapine-induced neuroleptic malignant syndrome with mental retardation. Am J Psychiatry 1999;156(7):1115-6.
20. Boyd RD. Neuroleptic malignant syndrome and mental retardation: review and analysis of 29 cases. Am J Ment Retard 1993;98:143-55.
21. Malyuk R, Gibson B, Procyshyn RM, Kang N. Olanzapine associated weight gain, hyperglycemia and neuroleptic malignant syndrome: case report. Int J Geriatr Psychiatry 2002;17(4):326-8.
22. Zun LS. A prospective study of the complication rate of use of patient restraint in the emergency department. J Emerg Med 2003;24(2):119-24.
23. Labuda A, Cullen N. Brain injury following neuroleptic malignant syndrome: case report and review of the literature. Brain Inj 2006;20(7):775-8.
24. Manto M, Goldman S, Hildebrand J. Cerebellar gait ataxia following neuroleptic malignant syndrome. J Neurol 1996;243(1):101-2.
25. Lee S, Merriam A, Kim TS, et al. Cerebellar degeneration in neuroleptic malignant syndrome: neuropathologic findings and review of the literature concerning heat-related nervous system injury. J Neurol Neurosurg Psychiatry 1989;52(3):387-91.
26. Naramoto A, Koizumi N, Itoh N, Shigematsu H. An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol Jpn 1993;43(1-2):55-8.
27. Gratz SS, Levinson DF, Simpson GM. The treatment and management of neuroleptic malignant syndrome. Prog Neuropsychopharmacol Biol Psychiatry 1992;16(4):425-43.
28. Scheftner WA, Shulman RB. Treatment choice in neuroleptic malignant syndrome. Convuls Ther 1992;8:267-79.
29. Harsch HH. Neuroleptic malignant syndrome: physiological and laboratory findings in a series of nine cases. J Clin Psychiatry 1987;48:328-33.
30. Caroff SN. Neuroleptic malignant syndrome: still a risk, but which patients may be in danger? Current Psychiatry 2003;2:36-42.
31. Wells AJ, Sommi RW, Crismon ML. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm 1988;22:475-80.
32. Susman VL, Addonizio G. Recurrence of neuroleptic malignant syndrome. J Nerv Ment Dis 1988;176:234-41.
When second-generation antipsychotics (SGAs) were introduced, clinicians hoped the drugs would not have the potential to cause neuroleptic malignant syndrome (NMS).1 Since then, however, case reports have made it clear that SGAs—like first-generation antipsychotics (FGAs)—can precipitate this life-threatening neurologic emergency.
To help you protect your patients receiving SGAs, this article explains how to:
- identify those at risk
- recognize the different NMS presentations associated with each SGA
- continue antipsychotic treatment for a patient with a history of NMS.
CASE STUDY: A drug-induced disorder
Mrs. Z, age 39, has a history of multiple hospitalizations for schizoaffective disorder complicated by poor compliance and a history of benzodiazepine abuse. This time she was admitted with increased auditory hallucinations and paranoid delusions of her family trying to poison her. Despite multiple haloperidol injections (5 mg IM q4h prn), Mrs. Z continued to have hallucinations and remained agitated.
Haloperidol was discontinued and ziprasidone (20 mg IM q4h prn) was started. After 3 days, Mrs. Z became less agitated and had fewer hallucinations. The IM route was discontinued and oral ziprasidone was started at 40 mg bid, then titrated to 80 mg bid after 2 days. On the third day after titration, Mrs. Z fell twice. She hit her head in one fall, but a brain CT to rule out bleeding was normal.
The next day, Mrs. Z became more confused and developed fever, tremor, urinary incontinence, and a severe headache. She became obtunded, was intubated, and was transferred to the intensive care unit of a tertiary care center.
On admission, her temperature was 103° F (39.4° C); she had severe muscle rigidity and blood pressure of 85/60 mm Hg. Creatine phosphokinase (CPK) was 2,559 U/L (normal 24 to 170 U/L). Liver enzymes were elevated: alanine transaminase was 202 U/L (normal 13 to 50 U/L), and aspartate transaminase (AST) was 190 U/L (normal 15 to 46 U/L). At 140 μg/dL, Mrs. Z’s serum iron was within normal limits (40 to 150 μg/dL).
Neuroleptic malignant syndrome
Clinical manifestations of NMS range from typical—as defined by the DSM-IV-TR (Table 1)2,3—to atypical, without:
Table 1
DSM-IV-TR definition of NMS*
| Hyperthermia (>38° C) and |
| Muscle rigidity and |
At least 2 of the following:
|
| * Symptoms must be associated with the use of neuroleptic medication, and other central and systemic causes of hyperthermia must be excluded. |
| CPK: creatine phosphokinase; NMS: neuroleptic malignant syndrome |
| Source: DSM-IV-TR |
Many conditions resemble NMS (Table 2). Because NMS can be fatal without emergent diagnosis and treatment, maintain a high index of suspicion for this condition whenever you prescribe antipsychotics.
Table 2
NMS differential diagnosis
| Primary CNS disorders |
| CNS vasculitis |
| Infarctions |
| Infections |
| Parkinson’s disease |
| Status epilepticus |
| Trauma |
| Tumors |
| Systemic disorders |
| Acute porphyria |
| Autoimmune disorders |
| Dehydration |
| Heat stroke |
| Hyperthyroidism |
| Infections |
| Pheochromocytoma |
| Tetanus |
| Psychiatric disorders |
| Idiopathic lethal catatonia |
| Medication-related disorders |
| Anticholinergic syndrome |
| Drug intoxication |
| Levodopa syndrome |
| Malignant hyperthermia |
| Serotonin syndrome |
NMS is believed to be caused by reduced dopamine activity in the brain associated with dopamine antagonists, interruptions in nigrostriatal dopamine pathways, or withdrawal of dopaminergic medications.3 However, dopamine D2 receptor blocking potential is not directly linked to the occurrence of NMS.6 Other mechanisms include genetic susceptibility and different CNS neurotransmitter disturbances.7
NMS develops in an estimated 0.02% to 2.5% of patients treated with antipsychotics.8-10 The syndrome appears to occur slightly less frequently with SGAs than with FGAs.6,10
Risk factors. NMS can develop at any age, in men and women, and in patients with psychiatric or medical illness.11,12 In addition to antipsychotics, other medications—including antiemetics and sedatives—can cause NMS. The syndrome has been triggered when Parkinson’s disease patients stop taking or reduce the dose of a dopamine agonist or switch from 1 dopamine agonist to another.13,14
Symptoms usually develop during the first 2 weeks of pharmacotherapy but may start after the initial dose or during long-term stable therapy.15 Although some studies found NMS development to be dose-independent, multiple cases have demonstrated an association with dose changes. Death occurs from dysautonomic manifestations and systemic complications.
An elevated risk for NMS may exist in patients with:
- mood disorders
- preexisting catatonia16
- complicated medical and neurologic disorders, such as encephalitis or mental retardation17
- poor functional and physiologic status3
- concurrent lithium treatment
- IM injection of an antipsychotic
- use of a high-potency antipsychotic, such as haloperidol
- psychomotor agitation.
Other potential risk factors include dehydration, adolescent age, male gender, low serum iron concentrations, relatively high antipsychotic dosages, and mental retardation or prior structural brain injury.18-20
NMS and SGAs
We reviewed 88 reports of NMS cases associated with 6 SGAs: olanzapine, clozapine, risperidone, ziprasidone, quetiapine, and aripiprazole. In this article, we cite representative cases only; readers interested in the full literature search can find this evidence and its references in the Case Reports Table.
NMS cases were fairly evenly distributed across all age groups (Figure 1). SGAs were implicated in NMS when used as monotherapy in 9 cases (10%) and in combination with other psychotropics in 41 cases (47%). We could not find medication regimen data for 38 cases (43%).
Figure 1
NMS incidence across age groups
Incidence is dispersed fairly evenly; elderly patients may be less likely to be prescribed an antipsychotic than other age groups.
Source: Reference 5Our review suggests that a history of NMS is a risk factor for developing another episode. Twenty cases showed a clear history of NMS, and 2 cases reported 3 different NMS episodes in each patient.19,21
In the cases we reviewed, NMS developed more often among men than women (Figure 2). The reason is not clear. One hypothesis suggests that men are more likely to present with severe agitation that requires aggressive antipsychotic treatment.14,22
Figure 2
NMS: More common in men
Men may be at higher risk because they are more likely to present with severe agitation and receive larger doses of potent antipsychotics.
Source: References 14,22Previous reports suggested that parenteral antipsychotic administration might increase NMS risk. Most NMS cases in our review involved oral administration, perhaps because parenteral SGAs have become available only recently. In the future, increased use of parenteral SGAs might increase the incidence of NMS.
The NMS mortality rate associated with SGAs was lower than that linked to FGAs.6 This finding, however, may be influenced by increasing awareness of NMS among physicians, resulting in earlier diagnosis and treatment.6
Findings for specific SGAs
Aripiprazole. Because aripiprazole is the newest SGA, data on its association with NMS are limited. Our review looked at 2 cases. Both patients had atypical NMS features, including absence of fever and mild CPK elevation. In 1 case, aripiprazole was used to treat agitation in a 13-year-old girl with history of NMS. This resulted in a mild increase in tachycardia and brief worsening of serum CPK but did not significantly affect temperature, respiratory rate, or blood pressure.
Clozapine. Several NMS cases have been connected to clozapine monotherapy (6 cases) or combination therapy (22 cases). Compared with NMS caused by other antipsychotics, clozapine-induced NMS occurred sooner after patients started the drug or restarted it after discontinuation. NMS has developed in patients receiving chronic steady doses of clozapine, after dosage increases, and after other medications have been added.
Clozapine-treated patients need to be closely monitored for agranulocytosis symptoms, so any other adverse effects—such as initial symptoms of NMS—likely will be detected early. Some reports suggested that clozapine-induced NMS may feature fewer extrapyramidal side effects and a lower-than-typical increase in CPK. In the cases we reviewed, however, NMS presentations ranged from typical—with a highly elevated CPK—to mild with no rigidity and mild or no CPK elevation. Two of 28 cases reported neurologic sequelae, including severe truncal ataxia and dysmetria.
Clozapine has been used to treat patients with a history of NMS who experience psychotic relapse. In several cases, however, NMS recurred after clozapine was started. In 1 case, a third rechallenge with slow titration of clozapine was successful.
Olanzapine. Some studies have found olanzapine-induced NMS to be rare (rate ≤0.01%), but our review found 36 such cases. Ten patients (30%) had a history of NMS. Olanzapine dosing did not correlate with NMS—in 11 cases NMS occurred with daily doses ≤10 mg.
As with clozapine, the presentation of olanzapine-induced NMS varies widely. Onset from within 8 hours of starting olanzapine to after 2½ years of stable olanzapine dosing has been reported. Some cases have featured a typical NMS presentation. Atypical presentations have included:
- extremely elevated serum sodium
- absence of rigidity
- normal CPK
- generalized tonic-clonic seizures preceding NMS onset
- anterograde amnesia
- deficits in learning verbal information.
Olanzapine challenge for patients with a history of NMS often has triggered recurring NMS.
Quetiapine. NMS has been reported in patients receiving quetiapine monotherapy and combination therapy. Patients who previously experienced NMS after taking an FGA have developed quetiapine-induced NMS, as have some with a history of Lewy body disease. Two patients treated with quetiapine developed CPK elevations to almost 9,000 U/L (normal <171 U/L)—without other NMS features—that improved after discontinuing the medication.
Risperidone. NMS among patients taking risperidone occurs more frequently in those with history of NMS or who restart risperidone after discontinuation. Time to NMS occurrence after starting risperidone varies from hours to months. Atypical presentations include delayed fever, delayed muscle rigidity, massive intestinal bleeding, massive CPK elevation (such as 46,420 U/L), and hyponatremia instead of hypernatremia.
Ziprasidone. Administering IM ziprasidone or combining any form of the drug with other psychotropics increases NMS risk. Although most cases featured typical presentations, 1 case reported absence of muscle rigidity, which is present in >90% of patients with NMS associated with FGAs.
NMS sequelae related to SGAs
Brain injury following NMS can cause truncal ataxia, limb ataxia, athetosis, hemiballismus, dysmetria, dysarthria, sensory function problems, balance problems, persistent amnesia, difficulties comprehending commands, attention problems, and electroencephalograph or MRI abnormalities.23,24 Postmortem studies of patients with NMS have revealed cerebellar degeneration, reduction of Purkinje and granule cells, and gliosis in the dentate nucleus.25,26
Why some patients develop sequelae after NMS while others recover is unknown. Sustained hyperpyrexia, preexisting medical or neurologic disorders, polypharmacy, prolonged courses, and delayed diagnosis may play a role.25-27
CASE CONTINUED: A complicated illness
Mrs. Z was diagnosed with NMS. Ziprasidone was discontinued, and supportive treatment, bromocriptine (2.5 mg po qid), and lorazepam (2 mg IV qid) were started. Temperatures of 101° to 103° F (38.3° to 39.4° C) persisted for the next 2 days. This hyperthermia was difficult to control because of suspected meningitis.
The team started ceftriaxone (2 gm IV q12h) while awaiting lumbar puncture results. CSF showed mild white blood cell elevation of 20/cu mm (normal 0 to 5/cu mm) with 62% neutrophils (normal 0 to 6%), normal protein, normal glucose, and negative cultures. After 2 days of antibiotic therapy, the patient developed diarrhea and was diagnosed with Clostridium difficile-associated colitis, a side effect of the antibiotic.
Treatment is mainly supportive
Recognizing NMS signs is the first and most important step to quick diagnosis and early medical intervention. Recommendations for medical treatment of NMS vary widely, but most stress stopping the triggering drug and initiating supportive care (Table 3).27-29
Several medications have been used off-label to treat NMS based on anecdotal clinical reports. Benzodiazepines such as parenteral lorazepam, 1 to 2 mg every 6 to 8 hours, have been used to treat catatonic symptoms.30 Dopamine agonists—including bromocriptine, 2.5 mg every 8 hours—have reduced the duration and mortality of NMS but have the potential to worsen psychotic symptoms and cause hypotension and emesis.30
Table 3
Treating NMS: Where to start
| Stop offending agent(s) |
Provide intensive hemodynamic and supportive care:
|
CASE CONTINUED: Resuming antipsychotic Tx
Five days after intubation, Mrs. Z started to improve and was extubated successfully. However, she developed severe truncal ataxia, upper extremity tremors (resting and intentional), athetosis, hemiballismus, dysmetria, and dystonia. She continued to experience hallucinations after transfer back to the psychiatric floor.
Oral olanzapine challenge was started at 2.5 mg/d and titrated up to 10 mg/d over the next 7 days. Her psychotic symptoms showed mild improvement but her ataxic movements worsened and she fell frequently. Benztropine, 1 mg po bid, was added to her regimen and helped with the tremor. She was transferred for rehabilitation and eventually discharged home.
If a patient needs antipsychotics
If a patient who has experienced NMS continues to need pharmacotherapy for psychosis, wait 1 or 2 weeks after NMS symptoms resolve before restarting any antipsychotic.31 Although most patients can be treated safely with an antipsychotic after having NMS, clearly document the indications and your discussions with the patients and their families.
No conclusive evidence indicates which antipsychotic might lower a patient’s risk of recurrent NMS. Using an FGA in patients who recover from NMS carries a 30% risk of recurrent episodes.3 Data on the recurrence of NMS with SGAs are inconclusive. No relationship was found between relapse rate and patients’ age or sex.32
Regardless of which drug you choose, start with a low dosage and titrate slowly. You also can protect patients by reducing risk factors for NMS, such as dehydration, and considering alternate therapies such as electroconvulsive therapy, when appropriate.
This paper was among those entered in the 2007 Promising New Investigators competition sponsored by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition was “New insights on psychotropic drug safety and side effects.”
Current Psychiatry is honored to publish this peer-reviewed, evidenced-based article on a clinically important topic for practicing psychiatrists.
NMSIS is dedicated to reducing morbidity and mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing support information for medical professionals, patients, and families; and improving scientific understanding of these conditions through research.
Related resources
- Neuroleptic Malignant Syndrome Information Service. http://nmsis.org.
- National Institute of Neurological Disorders and Stroke. Neuroleptic malignant syndrome information page www.ninds.nih.gov/disorders/neuroleptic_syndrome/neuroleptic_syndrome.htm.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Ceftriaxone • Rocephin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
When second-generation antipsychotics (SGAs) were introduced, clinicians hoped the drugs would not have the potential to cause neuroleptic malignant syndrome (NMS).1 Since then, however, case reports have made it clear that SGAs—like first-generation antipsychotics (FGAs)—can precipitate this life-threatening neurologic emergency.
To help you protect your patients receiving SGAs, this article explains how to:
- identify those at risk
- recognize the different NMS presentations associated with each SGA
- continue antipsychotic treatment for a patient with a history of NMS.
CASE STUDY: A drug-induced disorder
Mrs. Z, age 39, has a history of multiple hospitalizations for schizoaffective disorder complicated by poor compliance and a history of benzodiazepine abuse. This time she was admitted with increased auditory hallucinations and paranoid delusions of her family trying to poison her. Despite multiple haloperidol injections (5 mg IM q4h prn), Mrs. Z continued to have hallucinations and remained agitated.
Haloperidol was discontinued and ziprasidone (20 mg IM q4h prn) was started. After 3 days, Mrs. Z became less agitated and had fewer hallucinations. The IM route was discontinued and oral ziprasidone was started at 40 mg bid, then titrated to 80 mg bid after 2 days. On the third day after titration, Mrs. Z fell twice. She hit her head in one fall, but a brain CT to rule out bleeding was normal.
The next day, Mrs. Z became more confused and developed fever, tremor, urinary incontinence, and a severe headache. She became obtunded, was intubated, and was transferred to the intensive care unit of a tertiary care center.
On admission, her temperature was 103° F (39.4° C); she had severe muscle rigidity and blood pressure of 85/60 mm Hg. Creatine phosphokinase (CPK) was 2,559 U/L (normal 24 to 170 U/L). Liver enzymes were elevated: alanine transaminase was 202 U/L (normal 13 to 50 U/L), and aspartate transaminase (AST) was 190 U/L (normal 15 to 46 U/L). At 140 μg/dL, Mrs. Z’s serum iron was within normal limits (40 to 150 μg/dL).
Neuroleptic malignant syndrome
Clinical manifestations of NMS range from typical—as defined by the DSM-IV-TR (Table 1)2,3—to atypical, without:
Table 1
DSM-IV-TR definition of NMS*
| Hyperthermia (>38° C) and |
| Muscle rigidity and |
At least 2 of the following:
|
| * Symptoms must be associated with the use of neuroleptic medication, and other central and systemic causes of hyperthermia must be excluded. |
| CPK: creatine phosphokinase; NMS: neuroleptic malignant syndrome |
| Source: DSM-IV-TR |
Many conditions resemble NMS (Table 2). Because NMS can be fatal without emergent diagnosis and treatment, maintain a high index of suspicion for this condition whenever you prescribe antipsychotics.
Table 2
NMS differential diagnosis
| Primary CNS disorders |
| CNS vasculitis |
| Infarctions |
| Infections |
| Parkinson’s disease |
| Status epilepticus |
| Trauma |
| Tumors |
| Systemic disorders |
| Acute porphyria |
| Autoimmune disorders |
| Dehydration |
| Heat stroke |
| Hyperthyroidism |
| Infections |
| Pheochromocytoma |
| Tetanus |
| Psychiatric disorders |
| Idiopathic lethal catatonia |
| Medication-related disorders |
| Anticholinergic syndrome |
| Drug intoxication |
| Levodopa syndrome |
| Malignant hyperthermia |
| Serotonin syndrome |
NMS is believed to be caused by reduced dopamine activity in the brain associated with dopamine antagonists, interruptions in nigrostriatal dopamine pathways, or withdrawal of dopaminergic medications.3 However, dopamine D2 receptor blocking potential is not directly linked to the occurrence of NMS.6 Other mechanisms include genetic susceptibility and different CNS neurotransmitter disturbances.7
NMS develops in an estimated 0.02% to 2.5% of patients treated with antipsychotics.8-10 The syndrome appears to occur slightly less frequently with SGAs than with FGAs.6,10
Risk factors. NMS can develop at any age, in men and women, and in patients with psychiatric or medical illness.11,12 In addition to antipsychotics, other medications—including antiemetics and sedatives—can cause NMS. The syndrome has been triggered when Parkinson’s disease patients stop taking or reduce the dose of a dopamine agonist or switch from 1 dopamine agonist to another.13,14
Symptoms usually develop during the first 2 weeks of pharmacotherapy but may start after the initial dose or during long-term stable therapy.15 Although some studies found NMS development to be dose-independent, multiple cases have demonstrated an association with dose changes. Death occurs from dysautonomic manifestations and systemic complications.
An elevated risk for NMS may exist in patients with:
- mood disorders
- preexisting catatonia16
- complicated medical and neurologic disorders, such as encephalitis or mental retardation17
- poor functional and physiologic status3
- concurrent lithium treatment
- IM injection of an antipsychotic
- use of a high-potency antipsychotic, such as haloperidol
- psychomotor agitation.
Other potential risk factors include dehydration, adolescent age, male gender, low serum iron concentrations, relatively high antipsychotic dosages, and mental retardation or prior structural brain injury.18-20
NMS and SGAs
We reviewed 88 reports of NMS cases associated with 6 SGAs: olanzapine, clozapine, risperidone, ziprasidone, quetiapine, and aripiprazole. In this article, we cite representative cases only; readers interested in the full literature search can find this evidence and its references in the Case Reports Table.
NMS cases were fairly evenly distributed across all age groups (Figure 1). SGAs were implicated in NMS when used as monotherapy in 9 cases (10%) and in combination with other psychotropics in 41 cases (47%). We could not find medication regimen data for 38 cases (43%).
Figure 1
NMS incidence across age groups
Incidence is dispersed fairly evenly; elderly patients may be less likely to be prescribed an antipsychotic than other age groups.
Source: Reference 5Our review suggests that a history of NMS is a risk factor for developing another episode. Twenty cases showed a clear history of NMS, and 2 cases reported 3 different NMS episodes in each patient.19,21
In the cases we reviewed, NMS developed more often among men than women (Figure 2). The reason is not clear. One hypothesis suggests that men are more likely to present with severe agitation that requires aggressive antipsychotic treatment.14,22
Figure 2
NMS: More common in men
Men may be at higher risk because they are more likely to present with severe agitation and receive larger doses of potent antipsychotics.
Source: References 14,22Previous reports suggested that parenteral antipsychotic administration might increase NMS risk. Most NMS cases in our review involved oral administration, perhaps because parenteral SGAs have become available only recently. In the future, increased use of parenteral SGAs might increase the incidence of NMS.
The NMS mortality rate associated with SGAs was lower than that linked to FGAs.6 This finding, however, may be influenced by increasing awareness of NMS among physicians, resulting in earlier diagnosis and treatment.6
Findings for specific SGAs
Aripiprazole. Because aripiprazole is the newest SGA, data on its association with NMS are limited. Our review looked at 2 cases. Both patients had atypical NMS features, including absence of fever and mild CPK elevation. In 1 case, aripiprazole was used to treat agitation in a 13-year-old girl with history of NMS. This resulted in a mild increase in tachycardia and brief worsening of serum CPK but did not significantly affect temperature, respiratory rate, or blood pressure.
Clozapine. Several NMS cases have been connected to clozapine monotherapy (6 cases) or combination therapy (22 cases). Compared with NMS caused by other antipsychotics, clozapine-induced NMS occurred sooner after patients started the drug or restarted it after discontinuation. NMS has developed in patients receiving chronic steady doses of clozapine, after dosage increases, and after other medications have been added.
Clozapine-treated patients need to be closely monitored for agranulocytosis symptoms, so any other adverse effects—such as initial symptoms of NMS—likely will be detected early. Some reports suggested that clozapine-induced NMS may feature fewer extrapyramidal side effects and a lower-than-typical increase in CPK. In the cases we reviewed, however, NMS presentations ranged from typical—with a highly elevated CPK—to mild with no rigidity and mild or no CPK elevation. Two of 28 cases reported neurologic sequelae, including severe truncal ataxia and dysmetria.
Clozapine has been used to treat patients with a history of NMS who experience psychotic relapse. In several cases, however, NMS recurred after clozapine was started. In 1 case, a third rechallenge with slow titration of clozapine was successful.
Olanzapine. Some studies have found olanzapine-induced NMS to be rare (rate ≤0.01%), but our review found 36 such cases. Ten patients (30%) had a history of NMS. Olanzapine dosing did not correlate with NMS—in 11 cases NMS occurred with daily doses ≤10 mg.
As with clozapine, the presentation of olanzapine-induced NMS varies widely. Onset from within 8 hours of starting olanzapine to after 2½ years of stable olanzapine dosing has been reported. Some cases have featured a typical NMS presentation. Atypical presentations have included:
- extremely elevated serum sodium
- absence of rigidity
- normal CPK
- generalized tonic-clonic seizures preceding NMS onset
- anterograde amnesia
- deficits in learning verbal information.
Olanzapine challenge for patients with a history of NMS often has triggered recurring NMS.
Quetiapine. NMS has been reported in patients receiving quetiapine monotherapy and combination therapy. Patients who previously experienced NMS after taking an FGA have developed quetiapine-induced NMS, as have some with a history of Lewy body disease. Two patients treated with quetiapine developed CPK elevations to almost 9,000 U/L (normal <171 U/L)—without other NMS features—that improved after discontinuing the medication.
Risperidone. NMS among patients taking risperidone occurs more frequently in those with history of NMS or who restart risperidone after discontinuation. Time to NMS occurrence after starting risperidone varies from hours to months. Atypical presentations include delayed fever, delayed muscle rigidity, massive intestinal bleeding, massive CPK elevation (such as 46,420 U/L), and hyponatremia instead of hypernatremia.
Ziprasidone. Administering IM ziprasidone or combining any form of the drug with other psychotropics increases NMS risk. Although most cases featured typical presentations, 1 case reported absence of muscle rigidity, which is present in >90% of patients with NMS associated with FGAs.
NMS sequelae related to SGAs
Brain injury following NMS can cause truncal ataxia, limb ataxia, athetosis, hemiballismus, dysmetria, dysarthria, sensory function problems, balance problems, persistent amnesia, difficulties comprehending commands, attention problems, and electroencephalograph or MRI abnormalities.23,24 Postmortem studies of patients with NMS have revealed cerebellar degeneration, reduction of Purkinje and granule cells, and gliosis in the dentate nucleus.25,26
Why some patients develop sequelae after NMS while others recover is unknown. Sustained hyperpyrexia, preexisting medical or neurologic disorders, polypharmacy, prolonged courses, and delayed diagnosis may play a role.25-27
CASE CONTINUED: A complicated illness
Mrs. Z was diagnosed with NMS. Ziprasidone was discontinued, and supportive treatment, bromocriptine (2.5 mg po qid), and lorazepam (2 mg IV qid) were started. Temperatures of 101° to 103° F (38.3° to 39.4° C) persisted for the next 2 days. This hyperthermia was difficult to control because of suspected meningitis.
The team started ceftriaxone (2 gm IV q12h) while awaiting lumbar puncture results. CSF showed mild white blood cell elevation of 20/cu mm (normal 0 to 5/cu mm) with 62% neutrophils (normal 0 to 6%), normal protein, normal glucose, and negative cultures. After 2 days of antibiotic therapy, the patient developed diarrhea and was diagnosed with Clostridium difficile-associated colitis, a side effect of the antibiotic.
Treatment is mainly supportive
Recognizing NMS signs is the first and most important step to quick diagnosis and early medical intervention. Recommendations for medical treatment of NMS vary widely, but most stress stopping the triggering drug and initiating supportive care (Table 3).27-29
Several medications have been used off-label to treat NMS based on anecdotal clinical reports. Benzodiazepines such as parenteral lorazepam, 1 to 2 mg every 6 to 8 hours, have been used to treat catatonic symptoms.30 Dopamine agonists—including bromocriptine, 2.5 mg every 8 hours—have reduced the duration and mortality of NMS but have the potential to worsen psychotic symptoms and cause hypotension and emesis.30
Table 3
Treating NMS: Where to start
| Stop offending agent(s) |
Provide intensive hemodynamic and supportive care:
|
CASE CONTINUED: Resuming antipsychotic Tx
Five days after intubation, Mrs. Z started to improve and was extubated successfully. However, she developed severe truncal ataxia, upper extremity tremors (resting and intentional), athetosis, hemiballismus, dysmetria, and dystonia. She continued to experience hallucinations after transfer back to the psychiatric floor.
Oral olanzapine challenge was started at 2.5 mg/d and titrated up to 10 mg/d over the next 7 days. Her psychotic symptoms showed mild improvement but her ataxic movements worsened and she fell frequently. Benztropine, 1 mg po bid, was added to her regimen and helped with the tremor. She was transferred for rehabilitation and eventually discharged home.
If a patient needs antipsychotics
If a patient who has experienced NMS continues to need pharmacotherapy for psychosis, wait 1 or 2 weeks after NMS symptoms resolve before restarting any antipsychotic.31 Although most patients can be treated safely with an antipsychotic after having NMS, clearly document the indications and your discussions with the patients and their families.
No conclusive evidence indicates which antipsychotic might lower a patient’s risk of recurrent NMS. Using an FGA in patients who recover from NMS carries a 30% risk of recurrent episodes.3 Data on the recurrence of NMS with SGAs are inconclusive. No relationship was found between relapse rate and patients’ age or sex.32
Regardless of which drug you choose, start with a low dosage and titrate slowly. You also can protect patients by reducing risk factors for NMS, such as dehydration, and considering alternate therapies such as electroconvulsive therapy, when appropriate.
This paper was among those entered in the 2007 Promising New Investigators competition sponsored by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition was “New insights on psychotropic drug safety and side effects.”
Current Psychiatry is honored to publish this peer-reviewed, evidenced-based article on a clinically important topic for practicing psychiatrists.
NMSIS is dedicated to reducing morbidity and mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing support information for medical professionals, patients, and families; and improving scientific understanding of these conditions through research.
Related resources
- Neuroleptic Malignant Syndrome Information Service. http://nmsis.org.
- National Institute of Neurological Disorders and Stroke. Neuroleptic malignant syndrome information page www.ninds.nih.gov/disorders/neuroleptic_syndrome/neuroleptic_syndrome.htm.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Ceftriaxone • Rocephin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118:145-52.
2. Thornberg SA, Ereshefsky L. Neuroleptic malignant syndrome associated with clozapine monotherapy. Pharmacotherapy 1993;13:510-4.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
4. Rodriguez OP, Dowell MS. A case report of neuroleptic malignant syndrome without fever in a patient given aripiprazole. J Okla State Med Assoc 2006;9(7):435-8.
5. Kogoj A, Velikonja I. Olanzapine induced neuroleptic malignant syndrome—a case review. Hum Psychopharmacol 2003;18(4):301-9.
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65(4):464-70.
7. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30:314-21.
8. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985;142:1137.-
9. Addonizio G, Susman VL, Roth SD. Symptoms of neuroleptic malignant syndrome in 82 consecutive inpatients. Am J Psychiatry 1986;143:1587-90.
10. Pope HG, Keck PE, McElroy SL. Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry 1986;143:1227-33.
11. Chungh DS, Kim BN, Cho SC. Neuroleptic malignant syndrome due to three atypical antipsychotics in a child. J Psychopharmacol 2005;19(4):422-5.
12. Suh H, Bronson B, Martin R. Neuroleptic malignant syndrome and low-dose olanzapine. Am J Psychiatry 2003;160(4):796.-
13. Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry 1989;50:18.-
14. Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf 1998;19:73.-
15. Pope HG, Jr, Aizley HG, Keck PE, Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry 1991;52:208.-
16. White DAC, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry 1991;158:419-21.
17. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18:349-51.
18. Apple JE, Van Hauer G. Neuroleptic malignant syndrome associated with olanzapine therapy. Psychosomatics 1999;40(3):267-8.
19. Margolese HC, Chouinard G. Olanzapine-induced neuroleptic malignant syndrome with mental retardation. Am J Psychiatry 1999;156(7):1115-6.
20. Boyd RD. Neuroleptic malignant syndrome and mental retardation: review and analysis of 29 cases. Am J Ment Retard 1993;98:143-55.
21. Malyuk R, Gibson B, Procyshyn RM, Kang N. Olanzapine associated weight gain, hyperglycemia and neuroleptic malignant syndrome: case report. Int J Geriatr Psychiatry 2002;17(4):326-8.
22. Zun LS. A prospective study of the complication rate of use of patient restraint in the emergency department. J Emerg Med 2003;24(2):119-24.
23. Labuda A, Cullen N. Brain injury following neuroleptic malignant syndrome: case report and review of the literature. Brain Inj 2006;20(7):775-8.
24. Manto M, Goldman S, Hildebrand J. Cerebellar gait ataxia following neuroleptic malignant syndrome. J Neurol 1996;243(1):101-2.
25. Lee S, Merriam A, Kim TS, et al. Cerebellar degeneration in neuroleptic malignant syndrome: neuropathologic findings and review of the literature concerning heat-related nervous system injury. J Neurol Neurosurg Psychiatry 1989;52(3):387-91.
26. Naramoto A, Koizumi N, Itoh N, Shigematsu H. An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol Jpn 1993;43(1-2):55-8.
27. Gratz SS, Levinson DF, Simpson GM. The treatment and management of neuroleptic malignant syndrome. Prog Neuropsychopharmacol Biol Psychiatry 1992;16(4):425-43.
28. Scheftner WA, Shulman RB. Treatment choice in neuroleptic malignant syndrome. Convuls Ther 1992;8:267-79.
29. Harsch HH. Neuroleptic malignant syndrome: physiological and laboratory findings in a series of nine cases. J Clin Psychiatry 1987;48:328-33.
30. Caroff SN. Neuroleptic malignant syndrome: still a risk, but which patients may be in danger? Current Psychiatry 2003;2:36-42.
31. Wells AJ, Sommi RW, Crismon ML. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm 1988;22:475-80.
32. Susman VL, Addonizio G. Recurrence of neuroleptic malignant syndrome. J Nerv Ment Dis 1988;176:234-41.
1. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118:145-52.
2. Thornberg SA, Ereshefsky L. Neuroleptic malignant syndrome associated with clozapine monotherapy. Pharmacotherapy 1993;13:510-4.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
4. Rodriguez OP, Dowell MS. A case report of neuroleptic malignant syndrome without fever in a patient given aripiprazole. J Okla State Med Assoc 2006;9(7):435-8.
5. Kogoj A, Velikonja I. Olanzapine induced neuroleptic malignant syndrome—a case review. Hum Psychopharmacol 2003;18(4):301-9.
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65(4):464-70.
7. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30:314-21.
8. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985;142:1137.-
9. Addonizio G, Susman VL, Roth SD. Symptoms of neuroleptic malignant syndrome in 82 consecutive inpatients. Am J Psychiatry 1986;143:1587-90.
10. Pope HG, Keck PE, McElroy SL. Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry 1986;143:1227-33.
11. Chungh DS, Kim BN, Cho SC. Neuroleptic malignant syndrome due to three atypical antipsychotics in a child. J Psychopharmacol 2005;19(4):422-5.
12. Suh H, Bronson B, Martin R. Neuroleptic malignant syndrome and low-dose olanzapine. Am J Psychiatry 2003;160(4):796.-
13. Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry 1989;50:18.-
14. Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf 1998;19:73.-
15. Pope HG, Jr, Aizley HG, Keck PE, Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry 1991;52:208.-
16. White DAC, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry 1991;158:419-21.
17. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18:349-51.
18. Apple JE, Van Hauer G. Neuroleptic malignant syndrome associated with olanzapine therapy. Psychosomatics 1999;40(3):267-8.
19. Margolese HC, Chouinard G. Olanzapine-induced neuroleptic malignant syndrome with mental retardation. Am J Psychiatry 1999;156(7):1115-6.
20. Boyd RD. Neuroleptic malignant syndrome and mental retardation: review and analysis of 29 cases. Am J Ment Retard 1993;98:143-55.
21. Malyuk R, Gibson B, Procyshyn RM, Kang N. Olanzapine associated weight gain, hyperglycemia and neuroleptic malignant syndrome: case report. Int J Geriatr Psychiatry 2002;17(4):326-8.
22. Zun LS. A prospective study of the complication rate of use of patient restraint in the emergency department. J Emerg Med 2003;24(2):119-24.
23. Labuda A, Cullen N. Brain injury following neuroleptic malignant syndrome: case report and review of the literature. Brain Inj 2006;20(7):775-8.
24. Manto M, Goldman S, Hildebrand J. Cerebellar gait ataxia following neuroleptic malignant syndrome. J Neurol 1996;243(1):101-2.
25. Lee S, Merriam A, Kim TS, et al. Cerebellar degeneration in neuroleptic malignant syndrome: neuropathologic findings and review of the literature concerning heat-related nervous system injury. J Neurol Neurosurg Psychiatry 1989;52(3):387-91.
26. Naramoto A, Koizumi N, Itoh N, Shigematsu H. An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol Jpn 1993;43(1-2):55-8.
27. Gratz SS, Levinson DF, Simpson GM. The treatment and management of neuroleptic malignant syndrome. Prog Neuropsychopharmacol Biol Psychiatry 1992;16(4):425-43.
28. Scheftner WA, Shulman RB. Treatment choice in neuroleptic malignant syndrome. Convuls Ther 1992;8:267-79.
29. Harsch HH. Neuroleptic malignant syndrome: physiological and laboratory findings in a series of nine cases. J Clin Psychiatry 1987;48:328-33.
30. Caroff SN. Neuroleptic malignant syndrome: still a risk, but which patients may be in danger? Current Psychiatry 2003;2:36-42.
31. Wells AJ, Sommi RW, Crismon ML. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm 1988;22:475-80.
32. Susman VL, Addonizio G. Recurrence of neuroleptic malignant syndrome. J Nerv Ment Dis 1988;176:234-41.