User login
Clostridium difficile Infection
Clostridium difficile, a causative pathogen in antibiotic-associated colitis,1 is a slow-growing, spore-forming, gram-positive anaerobic bacillus,2 named difficile because it was so difficult to culture. Although this pathogenic spore was first identified in the 1930s,3 its vegetative, toxin-producing form was not recognized as a causative organism in diarrhea and colitis until the late 1970s.4,5 Since that time, C difficile has become a growing challenge to health care providers for accurate diagnosis, treatment, and containment of the spread of disease. C difficile infection (CDI) often takes a virulent course with associated morbidity, mortality, and health care costs.6
EPIDEMIOLOGY
In healthy adult patient populations, 2% to 3% are colonized with C difficile.2 The colonization rate among healthy infants is significantly higher, between 60% and 70%, but clinical infection is uncommon.7 As the colon becomes populated with flora, between ages 18 and 24 months, the carrier state disappears.8
In the hospital setting, 20% to 30% of patients become colonized with the organism by the fecal-oral route, which is facilitated when antibiotic therapy disrupts normal flora in the gut, enabling C difficile spores to proliferate; most patients are asymptomatic.5 In 2005, in US acute care hospitals, the incidence of CDI was reported at 84 cases per 100,000 patients.9C difficile remains the leading pathogen associated with inpatient antibiotic-associated diarrhea (AAD). It can be identified as the causative organism in 15% to 25% of cases of AAD in hospitalized patients.2
The mortality rate among hospitalized patients rises significantly in those identified with C difficile: 20.6%, compared with 7.0% of matched inpatient controls.10 ICU admission poses a significant burden of disease: The overall incidence of infection in the ICU population is about 4%. ICU patients who contract CDI have up to a 20% rate of fulminant colitis, a severe form of the disease often necessitating surgery. In this segment of the inpatient population, the mortality rate can approach 60%.8
Data analyzed by Zilberberg11 have demonstrated a significant increase in C difficile–associated infections in US hospitals in recent years. In 2001, the number of discharged patients with documented CDI was approximately 134,000, compared with 291,000 in 2005. The rising incidence of CDI has been attributed to increased antibiotic use, aging of the population, an increasing rate of comorbid conditions, fluoroquinolone resistance, and increased suspicion for the illness, which has led to increased use of testing.12,13
Of Significant Concern
Recurrent disease poses a particular challenge to the health care system. It has been reported that recurrence rates for CDI are between 15% and 35% after a first bout and between 33% and 65% after subsequent episodes of infection.2,14
A hypervirulent strain, the North American pulsed-field gel electrophoresis type 1 (NAP1/B1/027) has been implicated in several C difficile outbreaks.15 This subtype, which is especially resistant to fluoroquinolones,1 produces toxins earlier and in much greater quantities, including toxins A and B at levels 15 to 20 times greater than those seen in less virulent subtypes.4,12 Thus, the NAP1 strain is implicated in more severe disease and is more lethal.16 Affected patients have a 30-day mortality rate twice as high as that among patients with other strains of C difficile.12
The calculated cost of CDI adds between $2,454 and $7,179 in nonreimbursable costs per hospitalized patient, and an additional 3.6 to 7.0 days to their hospital stays.17 Estimates for CDI treatment in the US range from $436 million to $3 billion per year.6,17
Community-Acquired CDI
Community-acquired C difficile infection (CA-CDI) is a subtype that develops in patients who have not been hospitalized in the previous year,18 with incidence recently reported at 11.16 cases per 100,000 person-years.19 Affected patients tend to be younger than those with hospital-acquired infection and to have a less severe disease course. To meet the criteria of this subgroup, according to recent clinical practice guidelines jointly issued by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America (SHEA-IDSA),1 a patient may have developed symptoms no sooner than 12 weeks after hospital discharge (if any hospitalization occurred).
It is always important to perform a thorough history in patients with suspected CA-CDI to assess for recent hospitalizations and antibiotic use. In a retrospective study published in 2011, Kuntz et al19 found that CA-CDI–affected patients were six times as likely as healthy controls to have taken antimicrobials within 180 days before illness (including beta-lactam/beta-lactamase inhibitors, cephalosporins, clindamycin, fluoroquinolones, and penicillin) and twice as likely to have used gastric acid suppressants in that time.
One emerging theory is that CA-CDI is spread through food-borne illness. Unlike the vegetative form that resides in the bowel, C difficile spores are resistant to temperatures at which food is cooked (as they are to alcohol and many other disinfectant agents).5 Several studies have shown that livestock can harbor C difficile.12
PATHOPHYSIOLOGY
Normal gastrointestinal flora resist colonization and proliferation of C difficile; colonization of nontoxigenic strains appears to be protective against toxigenic strains.1 Alteration in the colonic bacterial environment, including the suppression of normal flora proliferation that can occur when a patient receives antibiotics or antineoplastic agents, is thought to allow the overgrowth of C difficile.2
Only C difficile strains that produce exotoxins (the enterotoxin toxin A, and the cytotoxin toxin B) are pathogenic. The organism produces a variety of adhesion proteins (with production accelerated by the presence of antibiotics, such as ampicillin and clindamycin), leading the toxins to bind to specific receptors in the intestinal mucosal cells. C difficile also produces proteases that trigger degradation of the intestinal extracellular matrix and disruption of epithelial cell signaling.16 The toxins activate proinflammatory cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-, and IL-8. The result is an intestinal inflammatory response that is clinically apparent in the form of diarrhea and pseudomembranous colitis (PMC).20
RISK FACTORS
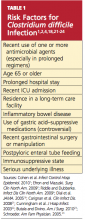
Among several known risk factors for CDI (see Table 11,2,4,18,21-24), perhaps the most widely recognized is the use of nearly any antimicrobial agent. Previous administration of antibiotics has been documented in about 95% of inpatients with CDI. Broad-spectrum antibiotics with anti-anaerobic coverage appear to pose the greatest risk.1,4 These include clindamycin, along with cephalosporins, fluoroquinolones, and beta-lactams.1,13 Fluoroquinolone use has been implicated in the development of the NAP1 subtype of the disease.25 In patients who receive multiple antibiotics or prolonged courses, an even greater risk for CDI is incurred.4 Even single-dose administration of an antibiotic carries a CDI risk of about 1.5%.21
A prolonged hospital stay or residence in a long-term care facility increases the risk for CDI, as does advanced age.1 Patients 65 or older have a 20-fold higher risk for CDI than their younger counterparts.1,2 Patients with inflammatory bowel disease (IBD) have an increased risk for CDI.23
It has been theorized that the use of gastric acid–suppressive medications can increase the risk for CDI to between 2.5 and 2.9 times that among persons who do not take these agents.18,22 Results from other trials have refuted this theory, however.13,26
Additional risk factors include ICU admission, recent gastrointestinal surgery or manipulation, immunosuppression, and serious underlying illnesses.24 Postpyloric enteral tube feeding has also been implicated as a risk factor; this route bypasses the stomach, where a very acidic environment ordinarily helps to kill the organism.4
CLINICAL PRESENTATION
Watery diarrhea, occurring 10 to 15 times in a day, is the most common manifestation of CDI. It may present during administration of an antimicrobial agent or within a few days afterward; rarely, it can develop as long as two months after cessation of such treatment.2 In most studies, median onset of diarrhea is about two days.20 Descriptive characteristics of the stool, including odor and color, may vary. Diarrhea may be accompanied by lower abdominal pain, cramping, low-grade fever, and leukocytosis.
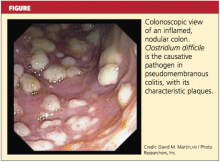
In some patients, particularly those taking narcotics, diarrhea may be absent. This manifestation may signal a more severe disease course, including the possibility of fulminant infection. Endoscopic evaluation of the colon may reveal the classic pseudomembranes (ie, adherent yellow plaques)2; see figure.
Stratifying Disease Severity
The severity of CDI-associated colitis increases as systemic symptoms worsen and the clinical picture deteriorates.4 In the 2010 SHEA-IDSA guidelines,1 CDI is stratified, both in the initial episode and the first recurrence, as mild-to-moderate, severe, or severe complicated. The following data may be used to appropriately stage disease severity in the initial episode:
• Mild-to-moderate disease is characterized by nonbloody diarrhea (fewer than 10 to 12 bowel movements per day), possibly accompanied by mild, crampy abdominal pain. Typically, affected patients do not exhibit significant systemic symptoms or marked abdominal tenderness. Leukocytosis is usually represented by fewer than 15,000 cells/L, and the serum creatinine level is less than 1.5 times the baseline level.1,2,5,8
• Severe disease, which should always be a consideration in older patients,1 includes profuse, watery diarrhea, significant abdominal pain and distension, fever, nausea and vomiting, and clinical volume depletion. Significant leukocytosis (≥ 15,000 cells/L) and serum creatinine ≥ 1.5 times baseline or leukopenia with possible bandemia may occur.1,27 Occult blood may be present, but frank blood is rare. A history of ICU admission alone increases the classification to severe due to the aforementioned poor outcomes associated with CDI in ICU patients.8,24
• Severe, complicated CDI may involve hypotension, shock, or a paralytic ileus1; a paradoxical decrease or absence of diarrhea may occur.2 At the severe end of the disease spectrum, toxic megacolon can develop and progress to colonic perforation.2,5,8,28 Hypoalbuminemia may be present due to the large protein losses in the course of the disease.28
The Most Severe Manifestations
Fulminant CDI can occur in 3% to 8% of patients with CDI.27,29 Fulminant disease carries a mortality rate between 35% and 80%.23 The patient’s clinical picture may resemble that seen in severe disease, with the addition of an acute abdomen indicating peritonitis; lethargy, hypotension, oliguria (including renal failure), and/or tachycardia due to a severe systemic inflammatory response induced by toxin production from C difficile. Up to 20% of patients with fulminant C difficile do not have diarrhea due to reasons explained previously.2,5,23
Bacteremia occurs rarely in patients with CDI.1,30 Risk factors for this severe condition include failure of medical treatment, leukocytosis exceeding 16,000 cells/L, surgery in the previous 30 days, a history of IBD, and previous administration of IV immunoglobulin.23
DIAGNOSIS
According to recommendations from the SHEA-IDSA expert panel,1 only unformed stool from symptomatic patients should be tested for C difficile or its toxins. In spite of slow turnaround time, stool culture (followed by toxigenic culture to identify a toxigenic isolate) is currently considered standard testing for C difficile. Cell cytotoxin assay has 98% sensitivity and 99% specificity, but turnaround time is 24 to 48 hours,28 potentially delaying treatment for patients who test positive.
Enzyme immunoassay (EIA) testing for C difficile toxin A and toxin B yields results within hours but is not as sensitive as the cell cytotoxin assay.1 Because toxin testing lacks sensitivity, a two-step strategy has been proposed and is called an “interim recommendation” by the SHEA-IDSA guideline authors: EIA testing for glutamate dehydrogenase (GDH), an enzyme produced by C difficile; then, in patients with positive results, confirmation by cell cytotoxin assay or toxigenic culture.1,4,28 (EIA testing for GDH can be a rapid, inexpensive method for ruling out CDI.28)
Polymerase chain reaction testing, recently developed for the detection of pathogenic C difficile, is rapid, sensitive, and specific.1 However, this method has not yet gained wide acceptance due to its relatively high cost.6
Imaging Options
Endoscopic visualization confirming the presence of PMC is also considered diagnostic of CDI; although half of patients with CDI lack this finding on endoscopy, CDI is present in 95% of patients with confirmed PMC.1,28 Colonoscopy is advantageous over sigmoidoscopy because up to one-third of patients have only right-sided colonic involvement.28 To its disadvantage, endoscopy carries a risk for perforation (particularly in patients with fulminant disease), as well as the inherent risks of sedation required to perform the procedure.
Though not specific, CT can be used as an adjunct to the diagnosis; features such as colonic wall thickening, pericolic stranding, ascites, pneumatosis, and free air resulting from perforation may suggest CDI and help determine the extent of disease.4,20 The accordion sign (high-attenuation oral contrast in the colon lumen, alternating with low attenuation of inflamed mucosa) and the double halo sign (IV contrast having varying degrees of attenuation due to submucosal inflammation and hyperemia) have also been reported in patients with CDI and may indicate PMC or fulminant CDI.28
The small intestine is typically not involved in CDI except in the setting of ileus and the rare entity of C difficile enteritis.2 Plain radiography is only helpful in cases of ileus or megacolon.28
PHARMACOLOGIC TREATMENT
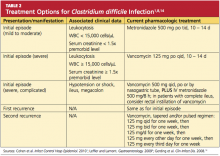
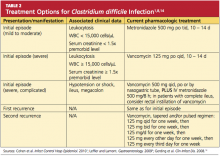
Discontinuation of the offending drug (usually an antibiotic), whenever possible, is the first step; up to 25% of CDI patients recover without any further therapies. Limiting management to antibiotic withdrawal, however, is currently recommended only in patients with the mildest form of CDI due to the risk for subsequent fulminant disease and clinical deterioration.14 In patients with suspected severe (but unconfirmed) CDI, empiric treatment with one of the antimicrobial agents listed below may be appropriate5 (see also Table 21,8,14). For patients with confirmed illness who require continued antimicrobial therapy, an agent not associated with CDI may be substituted (eg, sulfamethoxazole, macrolides, aminoglycosides).14
Metronidazole is the first-line agent for mild-to-moderate initial disease.1 The mechanism of action is through DNA disruption and inhibition of nucleic acid synthesis, a process that induces cell death. Metronidazole also appears to have anti-inflammatory, antioxidant, and immunomodulating properties that assist in overcoming the disease. It should not be used in women who are pregnant.8
Dosage of the drug is 250 mg four times per day or 500 mg three times a day, given orally; or parenterally, if the patient cannot take it orally. The duration is 10 to 14 days (or longer in patients with underlying infection),1 and the cost is much lower than that of other appropriate antimicrobial agents (ie, less than $1/day).2,31
In patients with severe or refractory disease, vancomycin should be used. Vancomycin has shown superiority in severe disease compared with metronidazole, with clinical cure rates of 97% and 76%, respectively.1,4,32 The typical oral dosage is 125 mg four times per day for 10 days.1
Vancomycin is effective only when given enterally; the drug is not absorbed by the gastrointestinal tract, allowing it to achieve high concentrations in the colon. In the patient who cannot receive standard enteral therapy, vancomycin can be instilled directly into the colon by enema or colonic catheter. With this route of administration, there is a small risk for iatrogenic perforation.2 There has been advocacy in very severe or fulminant disease to use vancomycin (orally or rectally) combined with IV metronidazole.1,8 The cost for 10 to 14 days of treatment ranges from $1,000 to $1,500.31
Earlier this year, fidaxomicin, a macrocyclic antibiotic, was approved for treatment of CDI in adults. This agent has minimal systemic absorption and works in the intestinal lumen by inhibiting the bacterial RNA polymerase.33 In a randomized, controlled trial involving 629 patients, fidaxomicin’s effectiveness was found comparable to that of vancomycin for treatment of CDI (clinical cure rates in the intention-to-treat analysis, 88.2% vs 85.8%, respectively), and fidaxomicin-treated patients had a lower rate of recurrence after initial use (15.4% vs 25.3%, respectively; patients with fulminant disease were not included).33 Dosage is 200 mg every 12 hours for 10 days,33,34 at a reported cost of $2,800.31
Neither oral bacitracin nor fusidic acid has been shown to eliminate CDI or reduce recurrence.1,5,33
Clinical resolution reveals adequate response to treatment and need not be confirmed by laboratory testing. Asymptomatic carriers do not require treatment.5
Less Conventional Agents
Several nonantibiotic treatment regimens have been proposed for CDI. Use of probiotics has been controversial. In a systematic review, Dendukuri et al35 found insufficient evidence in the routine use of probiotics to prevent or treat CDI. There have even been reports of Saccharomyces boulardii–associated fungemia and lactobacillus-associated bacteremia resulting from probiotics use.36
An anion-exchange resin, cholestyramine, is thought to help bind toxins; when studied, however, it failed to show promising results in improving patients’ clinical course.1,37 Additionally, it can bind to other drugs, such as vancomycin, resulting in decreased pharmacologic efficacy of this and other agents.1,2 Therefore, it is not recommended.
Intravenous immunoglobulin can provide an option for treatment of severe and/or recurrent disease as a last resort.1,38 Thus far, only results from small observational or retrospective studies have supported its use.
SURGICAL OPTIONS
Early surgical consultation is warranted in severe or refractory disease9 and in patients with specific manifestations detected via abdominal/pelvic CT: ileus, perforation, obstruction, thickening of the colonic wall, toxic megacolon, ascites, necrotizing colitis, or a systemic inflammatory response that could lead to multiorgan system failure.14,27 Colectomy is undertaken in 0.4% to 3.5% of patients with CDI,2 with a goal of resecting the involved bowel and diverting the fecal stream. In the past, near-total colectomy was the treatment of choice.2
There have been published reports of successful segmental resection for fulminant CDI.23 The mortality rate is approximately 50% in patients who undergo colectomy.2 Survival rates are noted to improve with early and prompt surgical management of severe disease,8,29 which can be life threatening.14
A recently developed procedure for fulminant disease involves creating a diverting loop ileostomy. Through the ostomy, 8 L of propylene glycol electrolyte solution is instilled to reduce the colonic C difficile count; the patient is also administered a vancomycin enema. In a literature review of recent studies involving patients who underwent the procedure, Olivas et al29 reported a 30-day mortality rate of 19%, and 93% of surviving patients did not require a colectomy. Further investigation to reduce surgical mortality in patients with fulminant disease is ongoing.
INVESTIGATIVE TREATMENT OPTIONS
Fecal transplantation from healthy donors to those infected with pathogenic C difficile via nasogastric tube or enema have been studied.1,39 The theory is to help reconstitute normal colonic flora with the transplanted stool. This treatment option has only very limited data and acceptance.8
Promising research has been published regarding the infusion of combined monoclonal antibodies against toxin A and toxin B. Among 200 patients who were randomized to receive the study therapy or placebo, the rate of recurrence was 7% versus 25%, respectively; recurrence rates among patients infected with the virulent NAP1/B1/027 strain were 8% and 32%, respectively.40 Length of stay did not improve in patients taking monoclonal antibodies, and adverse events were reported in both patient groups.
In preliminary trials, a parenteral vaccine containing inactivated toxins A and B was reported safe and capable of triggering a “vigorous” serum antitoxin A response in healthy adults.9,14
ADDITIONAL MANAGEMENT RECOMMENDATIONS
Medications that slow gastrointestinal motility should not be used, as the slowing of peristalsis may allow toxins to accumulate in the colon, leading to worsening disease.18,22 The use of opiates and anti-diarrheal medications should be limited.24
Aggressive fluid and electrolyte replacement should be administered until diarrhea has been resolved (usually within three to six days). Patients may require vasoactive medications to support hemodynamics.2,5,14,24
Patients with mild disease can eat as they normally would. Those with severe disease, including those who may require surgery, fare best with bowel rest and possibly enteral nutrition.
Monitoring the patient for signs of improvement during the first 24 to 48 hours is an important component of management. The patient’s white blood cell count and temperature, the number and frequency of bowel movements, and the overall clinical picture should be evaluated daily. Patients who show improvement should complete the current regimen.
TREATMENT FAILURE AND RECURRENT CDI
If a patient’s condition does not improve or worsens at any point during therapy for CDI, a change to another antimicrobial agent is warranted. Also, surgical, gastroenterological, and/or infectious disease consult may be needed if no improvement is evident after five days of seemingly appropriate therapy.14,24
Recurrent CDI, which occurs at least once in 6% to 25% of treated patients, is most likely to occur 7 to 14 days after treatment completion.1,14 Seldom caused by resistant strains of C difficile, it is more likely to result from inadequate adherence to treatment, the presence of residual spores in the colon after treatment, or reinfection—although relapse is considered more common than reinfection.14,24 However, since a patient’s symptoms may have other causes, confirmation of recurrence should be sought through laboratory testing.
Recurrent illness is managed in the same way as successful initial therapy, based on the severity of disease; vancomycin is recommended for the first recurrence in a patient with a rising white blood cell count or serum creatinine level. Otherwise, metronidazole use may be considered.1
In patients who experience a second recurrence of confirmed CDI, a tapered or pulsed-dose regimen of oral vancomycin over a six-week period has been advocated1,8,14 (for details, see Table 2).
Results from small studies of patients with several recurrences of CDI suggest that oral rifaximin therapy can reduce subsequent recurrences if administered immediately after the conclusion of a course of vancomycin.1,41
PREVENTION OF C DIFFICILE INFECTION
Although “research gaps” exist regarding the optimal strategies to prevent CDI,1 decreased prescribing of nonessential antibiotics is key. Without the alteration in colonic flora caused by antimicrobial use, gut colonization cannot occur, and C difficile typically cannot proliferate.2
Preventing transmission of the pathogen is challenging in health care facilities, where C difficile spores have been cultured from staff members’ hands and from beds, floors, windowsills, and other areas; the spores can survive in hospital rooms for as long as 40 days after a patient with CDI has been discharged.24 Appropriate isolation precautions are essential, including single-patient–use equipment (eg, disposable rectal thermometers) and caregivers’ use of gowns, vinyl gloves, and cleaning agents that are effective against the spores, particularly bleach.1 Alcohol-based products are not effective against C difficile spores; diligent handwashing with soap or chlorhexidine is imperative to prevent the spread of CDI.1,2
CONCLUSION
Clinicians and patients alike face the clinical challenge of Clostridium difficile infection. Mild to moderate disease can be treated medically with excellent success rates. Severe disease carries a significant risk to life, and a multidisciplinary approach including early surgical consultation is warranted.
With early recognition and appropriate treatment, along with strict adherence to isolation policies, the health care community can help limit the spread of this insidious illness and its associated morbidity and mortality.
REFERENCES
1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
2. Efron PA, Mazuski JE. Clostridium difficile colitis. Surg Clin North Am. 2009;89(2):483-500.
3. Hall IC, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
4. Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):727-743.
5. Sunenshine RH, McDonald LC. Clostridium difficile–associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006; 73(2):187-197.
6. Currie B. Improved testing methods are improving diagnosis of Clostridium difficile infections. Advance Administrators Laboratory. 2009;21:10. http://laboratory-manager.advance web.com/Article/PCR-for-C-diff.aspx. Accessed November 8, 2011.
7. Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727-733.
8. Leffler DA, Lamont JT. Treatment of Clostridium difficile–associated disease. Gastroenterology. 2009;136(6):1899-1912.
9. Kelly CP, Lamont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359(18): 1932-1940.
10. Pépin J, Valiquette L, Cossette B. Mortality attributed to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173(9):1037-1042.
11. Zilberberg MD. Clostridium difficile–related hospitalizations among US adults, 2006. Emerg Infect Dis. 2009;15(1):122-124.
12. Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in the inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409-416.
13. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260.
14. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46 suppl 1:S32-S42.
15. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23): 2433-2441.
16. Dawson LF, Valiente E, Wren BW. Clostridium difficile: a continually evolving and problematic pathogen. Infect Genet Evol. 2009;9(6):
1410-1417.
17. Dubberke ER, Reske KA, Olsen MA, et al. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical patients. Clin Infect Dis. 2008;46(4):497-504.
18. Dial S, Delaney JAC, Barkun An, et al. Use of gastric acid–suppression agents and the risk of community-acquired Clostridium difficile disease. JAMA. 2005;294(23):2989-2995.
19. Kuntz JL, Chrischilles EA, Pendergast JF, et al. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011 Jul 15;11:194.
20. Kelly CP, Lamont JT. Antibiotic-associated diarrhea, pseudomembranous enterocolitis, and Clostridium difficile–associated diarrhea and colitis. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: WB Saunders; 2010:1889-1902.
21. Carignan A, Allard C, Pépin J, et al. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis. 2008;46(12):1838-1843.
22. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003; 54(3):243-245.
23. Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg. 2010;200(1):131-135.
24. Schroeder MS. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005;71(5): 921-928.
25. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile–associated disease? A review of the evidence. Curr Med Res Opin. 2008;24(2):329-333.
26. Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile–associated disease: a population-based study. Clin Infect Dis. 2006;43(10): 1272-1276.
27. Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433-439.
28. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile. Clin Infect Dis. 2008;46 suppl 1:S12-S18.
29. Olivas AP, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11(3):299-305.
30. Feldman RJ, Kallich M, Weinstein MP. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C difficile infections. Clin Infect Dis. 1995;20(6):1560-1562.
31. BioPharm Physicians. What next for Dificid (fidaxomicin)? Jun 6, 2011. www.biopharmphysi cians.com/what-next-for-dificid-fidaxomicin. Accessed November 7, 2011.
32. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
33. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
34. Sullivan KM, Spooner LM. Fidaxomicin: a macrocyclic antibiotic for the management of Clostridium difficile infection. Ann Pharmacother. 2010;44(2):352-359.
35. Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile–associated diarrhea: a systematic review. CMAJ. 2005;173(2): 167-170.
36. Segarra-Newnham M. Probiotics for Clostridium difficile–associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41(7): 1212-1221.
37. Lagrotteria D, Holmes S, Smieja M, et al. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile–associated diarrhea. Clin Infect Dis. 2006;43(5):547-552.
38. McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49(5): 640-645.
39. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003; 36(5):580-585.
40. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3): 197-205.
41. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44(6):846-848.
Clostridium difficile, a causative pathogen in antibiotic-associated colitis,1 is a slow-growing, spore-forming, gram-positive anaerobic bacillus,2 named difficile because it was so difficult to culture. Although this pathogenic spore was first identified in the 1930s,3 its vegetative, toxin-producing form was not recognized as a causative organism in diarrhea and colitis until the late 1970s.4,5 Since that time, C difficile has become a growing challenge to health care providers for accurate diagnosis, treatment, and containment of the spread of disease. C difficile infection (CDI) often takes a virulent course with associated morbidity, mortality, and health care costs.6
EPIDEMIOLOGY
In healthy adult patient populations, 2% to 3% are colonized with C difficile.2 The colonization rate among healthy infants is significantly higher, between 60% and 70%, but clinical infection is uncommon.7 As the colon becomes populated with flora, between ages 18 and 24 months, the carrier state disappears.8
In the hospital setting, 20% to 30% of patients become colonized with the organism by the fecal-oral route, which is facilitated when antibiotic therapy disrupts normal flora in the gut, enabling C difficile spores to proliferate; most patients are asymptomatic.5 In 2005, in US acute care hospitals, the incidence of CDI was reported at 84 cases per 100,000 patients.9C difficile remains the leading pathogen associated with inpatient antibiotic-associated diarrhea (AAD). It can be identified as the causative organism in 15% to 25% of cases of AAD in hospitalized patients.2
The mortality rate among hospitalized patients rises significantly in those identified with C difficile: 20.6%, compared with 7.0% of matched inpatient controls.10 ICU admission poses a significant burden of disease: The overall incidence of infection in the ICU population is about 4%. ICU patients who contract CDI have up to a 20% rate of fulminant colitis, a severe form of the disease often necessitating surgery. In this segment of the inpatient population, the mortality rate can approach 60%.8
Data analyzed by Zilberberg11 have demonstrated a significant increase in C difficile–associated infections in US hospitals in recent years. In 2001, the number of discharged patients with documented CDI was approximately 134,000, compared with 291,000 in 2005. The rising incidence of CDI has been attributed to increased antibiotic use, aging of the population, an increasing rate of comorbid conditions, fluoroquinolone resistance, and increased suspicion for the illness, which has led to increased use of testing.12,13
Of Significant Concern
Recurrent disease poses a particular challenge to the health care system. It has been reported that recurrence rates for CDI are between 15% and 35% after a first bout and between 33% and 65% after subsequent episodes of infection.2,14
A hypervirulent strain, the North American pulsed-field gel electrophoresis type 1 (NAP1/B1/027) has been implicated in several C difficile outbreaks.15 This subtype, which is especially resistant to fluoroquinolones,1 produces toxins earlier and in much greater quantities, including toxins A and B at levels 15 to 20 times greater than those seen in less virulent subtypes.4,12 Thus, the NAP1 strain is implicated in more severe disease and is more lethal.16 Affected patients have a 30-day mortality rate twice as high as that among patients with other strains of C difficile.12
The calculated cost of CDI adds between $2,454 and $7,179 in nonreimbursable costs per hospitalized patient, and an additional 3.6 to 7.0 days to their hospital stays.17 Estimates for CDI treatment in the US range from $436 million to $3 billion per year.6,17
Community-Acquired CDI
Community-acquired C difficile infection (CA-CDI) is a subtype that develops in patients who have not been hospitalized in the previous year,18 with incidence recently reported at 11.16 cases per 100,000 person-years.19 Affected patients tend to be younger than those with hospital-acquired infection and to have a less severe disease course. To meet the criteria of this subgroup, according to recent clinical practice guidelines jointly issued by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America (SHEA-IDSA),1 a patient may have developed symptoms no sooner than 12 weeks after hospital discharge (if any hospitalization occurred).
It is always important to perform a thorough history in patients with suspected CA-CDI to assess for recent hospitalizations and antibiotic use. In a retrospective study published in 2011, Kuntz et al19 found that CA-CDI–affected patients were six times as likely as healthy controls to have taken antimicrobials within 180 days before illness (including beta-lactam/beta-lactamase inhibitors, cephalosporins, clindamycin, fluoroquinolones, and penicillin) and twice as likely to have used gastric acid suppressants in that time.
One emerging theory is that CA-CDI is spread through food-borne illness. Unlike the vegetative form that resides in the bowel, C difficile spores are resistant to temperatures at which food is cooked (as they are to alcohol and many other disinfectant agents).5 Several studies have shown that livestock can harbor C difficile.12
PATHOPHYSIOLOGY
Normal gastrointestinal flora resist colonization and proliferation of C difficile; colonization of nontoxigenic strains appears to be protective against toxigenic strains.1 Alteration in the colonic bacterial environment, including the suppression of normal flora proliferation that can occur when a patient receives antibiotics or antineoplastic agents, is thought to allow the overgrowth of C difficile.2
Only C difficile strains that produce exotoxins (the enterotoxin toxin A, and the cytotoxin toxin B) are pathogenic. The organism produces a variety of adhesion proteins (with production accelerated by the presence of antibiotics, such as ampicillin and clindamycin), leading the toxins to bind to specific receptors in the intestinal mucosal cells. C difficile also produces proteases that trigger degradation of the intestinal extracellular matrix and disruption of epithelial cell signaling.16 The toxins activate proinflammatory cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-, and IL-8. The result is an intestinal inflammatory response that is clinically apparent in the form of diarrhea and pseudomembranous colitis (PMC).20
RISK FACTORS
Among several known risk factors for CDI (see Table 11,2,4,18,21-24), perhaps the most widely recognized is the use of nearly any antimicrobial agent. Previous administration of antibiotics has been documented in about 95% of inpatients with CDI. Broad-spectrum antibiotics with anti-anaerobic coverage appear to pose the greatest risk.1,4 These include clindamycin, along with cephalosporins, fluoroquinolones, and beta-lactams.1,13 Fluoroquinolone use has been implicated in the development of the NAP1 subtype of the disease.25 In patients who receive multiple antibiotics or prolonged courses, an even greater risk for CDI is incurred.4 Even single-dose administration of an antibiotic carries a CDI risk of about 1.5%.21
A prolonged hospital stay or residence in a long-term care facility increases the risk for CDI, as does advanced age.1 Patients 65 or older have a 20-fold higher risk for CDI than their younger counterparts.1,2 Patients with inflammatory bowel disease (IBD) have an increased risk for CDI.23
It has been theorized that the use of gastric acid–suppressive medications can increase the risk for CDI to between 2.5 and 2.9 times that among persons who do not take these agents.18,22 Results from other trials have refuted this theory, however.13,26
Additional risk factors include ICU admission, recent gastrointestinal surgery or manipulation, immunosuppression, and serious underlying illnesses.24 Postpyloric enteral tube feeding has also been implicated as a risk factor; this route bypasses the stomach, where a very acidic environment ordinarily helps to kill the organism.4
CLINICAL PRESENTATION
Watery diarrhea, occurring 10 to 15 times in a day, is the most common manifestation of CDI. It may present during administration of an antimicrobial agent or within a few days afterward; rarely, it can develop as long as two months after cessation of such treatment.2 In most studies, median onset of diarrhea is about two days.20 Descriptive characteristics of the stool, including odor and color, may vary. Diarrhea may be accompanied by lower abdominal pain, cramping, low-grade fever, and leukocytosis.
In some patients, particularly those taking narcotics, diarrhea may be absent. This manifestation may signal a more severe disease course, including the possibility of fulminant infection. Endoscopic evaluation of the colon may reveal the classic pseudomembranes (ie, adherent yellow plaques)2; see figure.
Stratifying Disease Severity
The severity of CDI-associated colitis increases as systemic symptoms worsen and the clinical picture deteriorates.4 In the 2010 SHEA-IDSA guidelines,1 CDI is stratified, both in the initial episode and the first recurrence, as mild-to-moderate, severe, or severe complicated. The following data may be used to appropriately stage disease severity in the initial episode:
• Mild-to-moderate disease is characterized by nonbloody diarrhea (fewer than 10 to 12 bowel movements per day), possibly accompanied by mild, crampy abdominal pain. Typically, affected patients do not exhibit significant systemic symptoms or marked abdominal tenderness. Leukocytosis is usually represented by fewer than 15,000 cells/L, and the serum creatinine level is less than 1.5 times the baseline level.1,2,5,8
• Severe disease, which should always be a consideration in older patients,1 includes profuse, watery diarrhea, significant abdominal pain and distension, fever, nausea and vomiting, and clinical volume depletion. Significant leukocytosis (≥ 15,000 cells/L) and serum creatinine ≥ 1.5 times baseline or leukopenia with possible bandemia may occur.1,27 Occult blood may be present, but frank blood is rare. A history of ICU admission alone increases the classification to severe due to the aforementioned poor outcomes associated with CDI in ICU patients.8,24
• Severe, complicated CDI may involve hypotension, shock, or a paralytic ileus1; a paradoxical decrease or absence of diarrhea may occur.2 At the severe end of the disease spectrum, toxic megacolon can develop and progress to colonic perforation.2,5,8,28 Hypoalbuminemia may be present due to the large protein losses in the course of the disease.28
The Most Severe Manifestations
Fulminant CDI can occur in 3% to 8% of patients with CDI.27,29 Fulminant disease carries a mortality rate between 35% and 80%.23 The patient’s clinical picture may resemble that seen in severe disease, with the addition of an acute abdomen indicating peritonitis; lethargy, hypotension, oliguria (including renal failure), and/or tachycardia due to a severe systemic inflammatory response induced by toxin production from C difficile. Up to 20% of patients with fulminant C difficile do not have diarrhea due to reasons explained previously.2,5,23
Bacteremia occurs rarely in patients with CDI.1,30 Risk factors for this severe condition include failure of medical treatment, leukocytosis exceeding 16,000 cells/L, surgery in the previous 30 days, a history of IBD, and previous administration of IV immunoglobulin.23
DIAGNOSIS
According to recommendations from the SHEA-IDSA expert panel,1 only unformed stool from symptomatic patients should be tested for C difficile or its toxins. In spite of slow turnaround time, stool culture (followed by toxigenic culture to identify a toxigenic isolate) is currently considered standard testing for C difficile. Cell cytotoxin assay has 98% sensitivity and 99% specificity, but turnaround time is 24 to 48 hours,28 potentially delaying treatment for patients who test positive.
Enzyme immunoassay (EIA) testing for C difficile toxin A and toxin B yields results within hours but is not as sensitive as the cell cytotoxin assay.1 Because toxin testing lacks sensitivity, a two-step strategy has been proposed and is called an “interim recommendation” by the SHEA-IDSA guideline authors: EIA testing for glutamate dehydrogenase (GDH), an enzyme produced by C difficile; then, in patients with positive results, confirmation by cell cytotoxin assay or toxigenic culture.1,4,28 (EIA testing for GDH can be a rapid, inexpensive method for ruling out CDI.28)
Polymerase chain reaction testing, recently developed for the detection of pathogenic C difficile, is rapid, sensitive, and specific.1 However, this method has not yet gained wide acceptance due to its relatively high cost.6
Imaging Options
Endoscopic visualization confirming the presence of PMC is also considered diagnostic of CDI; although half of patients with CDI lack this finding on endoscopy, CDI is present in 95% of patients with confirmed PMC.1,28 Colonoscopy is advantageous over sigmoidoscopy because up to one-third of patients have only right-sided colonic involvement.28 To its disadvantage, endoscopy carries a risk for perforation (particularly in patients with fulminant disease), as well as the inherent risks of sedation required to perform the procedure.
Though not specific, CT can be used as an adjunct to the diagnosis; features such as colonic wall thickening, pericolic stranding, ascites, pneumatosis, and free air resulting from perforation may suggest CDI and help determine the extent of disease.4,20 The accordion sign (high-attenuation oral contrast in the colon lumen, alternating with low attenuation of inflamed mucosa) and the double halo sign (IV contrast having varying degrees of attenuation due to submucosal inflammation and hyperemia) have also been reported in patients with CDI and may indicate PMC or fulminant CDI.28
The small intestine is typically not involved in CDI except in the setting of ileus and the rare entity of C difficile enteritis.2 Plain radiography is only helpful in cases of ileus or megacolon.28
PHARMACOLOGIC TREATMENT
Discontinuation of the offending drug (usually an antibiotic), whenever possible, is the first step; up to 25% of CDI patients recover without any further therapies. Limiting management to antibiotic withdrawal, however, is currently recommended only in patients with the mildest form of CDI due to the risk for subsequent fulminant disease and clinical deterioration.14 In patients with suspected severe (but unconfirmed) CDI, empiric treatment with one of the antimicrobial agents listed below may be appropriate5 (see also Table 21,8,14). For patients with confirmed illness who require continued antimicrobial therapy, an agent not associated with CDI may be substituted (eg, sulfamethoxazole, macrolides, aminoglycosides).14
Metronidazole is the first-line agent for mild-to-moderate initial disease.1 The mechanism of action is through DNA disruption and inhibition of nucleic acid synthesis, a process that induces cell death. Metronidazole also appears to have anti-inflammatory, antioxidant, and immunomodulating properties that assist in overcoming the disease. It should not be used in women who are pregnant.8
Dosage of the drug is 250 mg four times per day or 500 mg three times a day, given orally; or parenterally, if the patient cannot take it orally. The duration is 10 to 14 days (or longer in patients with underlying infection),1 and the cost is much lower than that of other appropriate antimicrobial agents (ie, less than $1/day).2,31
In patients with severe or refractory disease, vancomycin should be used. Vancomycin has shown superiority in severe disease compared with metronidazole, with clinical cure rates of 97% and 76%, respectively.1,4,32 The typical oral dosage is 125 mg four times per day for 10 days.1
Vancomycin is effective only when given enterally; the drug is not absorbed by the gastrointestinal tract, allowing it to achieve high concentrations in the colon. In the patient who cannot receive standard enteral therapy, vancomycin can be instilled directly into the colon by enema or colonic catheter. With this route of administration, there is a small risk for iatrogenic perforation.2 There has been advocacy in very severe or fulminant disease to use vancomycin (orally or rectally) combined with IV metronidazole.1,8 The cost for 10 to 14 days of treatment ranges from $1,000 to $1,500.31
Earlier this year, fidaxomicin, a macrocyclic antibiotic, was approved for treatment of CDI in adults. This agent has minimal systemic absorption and works in the intestinal lumen by inhibiting the bacterial RNA polymerase.33 In a randomized, controlled trial involving 629 patients, fidaxomicin’s effectiveness was found comparable to that of vancomycin for treatment of CDI (clinical cure rates in the intention-to-treat analysis, 88.2% vs 85.8%, respectively), and fidaxomicin-treated patients had a lower rate of recurrence after initial use (15.4% vs 25.3%, respectively; patients with fulminant disease were not included).33 Dosage is 200 mg every 12 hours for 10 days,33,34 at a reported cost of $2,800.31
Neither oral bacitracin nor fusidic acid has been shown to eliminate CDI or reduce recurrence.1,5,33
Clinical resolution reveals adequate response to treatment and need not be confirmed by laboratory testing. Asymptomatic carriers do not require treatment.5
Less Conventional Agents
Several nonantibiotic treatment regimens have been proposed for CDI. Use of probiotics has been controversial. In a systematic review, Dendukuri et al35 found insufficient evidence in the routine use of probiotics to prevent or treat CDI. There have even been reports of Saccharomyces boulardii–associated fungemia and lactobacillus-associated bacteremia resulting from probiotics use.36
An anion-exchange resin, cholestyramine, is thought to help bind toxins; when studied, however, it failed to show promising results in improving patients’ clinical course.1,37 Additionally, it can bind to other drugs, such as vancomycin, resulting in decreased pharmacologic efficacy of this and other agents.1,2 Therefore, it is not recommended.
Intravenous immunoglobulin can provide an option for treatment of severe and/or recurrent disease as a last resort.1,38 Thus far, only results from small observational or retrospective studies have supported its use.
SURGICAL OPTIONS
Early surgical consultation is warranted in severe or refractory disease9 and in patients with specific manifestations detected via abdominal/pelvic CT: ileus, perforation, obstruction, thickening of the colonic wall, toxic megacolon, ascites, necrotizing colitis, or a systemic inflammatory response that could lead to multiorgan system failure.14,27 Colectomy is undertaken in 0.4% to 3.5% of patients with CDI,2 with a goal of resecting the involved bowel and diverting the fecal stream. In the past, near-total colectomy was the treatment of choice.2
There have been published reports of successful segmental resection for fulminant CDI.23 The mortality rate is approximately 50% in patients who undergo colectomy.2 Survival rates are noted to improve with early and prompt surgical management of severe disease,8,29 which can be life threatening.14
A recently developed procedure for fulminant disease involves creating a diverting loop ileostomy. Through the ostomy, 8 L of propylene glycol electrolyte solution is instilled to reduce the colonic C difficile count; the patient is also administered a vancomycin enema. In a literature review of recent studies involving patients who underwent the procedure, Olivas et al29 reported a 30-day mortality rate of 19%, and 93% of surviving patients did not require a colectomy. Further investigation to reduce surgical mortality in patients with fulminant disease is ongoing.
INVESTIGATIVE TREATMENT OPTIONS
Fecal transplantation from healthy donors to those infected with pathogenic C difficile via nasogastric tube or enema have been studied.1,39 The theory is to help reconstitute normal colonic flora with the transplanted stool. This treatment option has only very limited data and acceptance.8
Promising research has been published regarding the infusion of combined monoclonal antibodies against toxin A and toxin B. Among 200 patients who were randomized to receive the study therapy or placebo, the rate of recurrence was 7% versus 25%, respectively; recurrence rates among patients infected with the virulent NAP1/B1/027 strain were 8% and 32%, respectively.40 Length of stay did not improve in patients taking monoclonal antibodies, and adverse events were reported in both patient groups.
In preliminary trials, a parenteral vaccine containing inactivated toxins A and B was reported safe and capable of triggering a “vigorous” serum antitoxin A response in healthy adults.9,14
ADDITIONAL MANAGEMENT RECOMMENDATIONS
Medications that slow gastrointestinal motility should not be used, as the slowing of peristalsis may allow toxins to accumulate in the colon, leading to worsening disease.18,22 The use of opiates and anti-diarrheal medications should be limited.24
Aggressive fluid and electrolyte replacement should be administered until diarrhea has been resolved (usually within three to six days). Patients may require vasoactive medications to support hemodynamics.2,5,14,24
Patients with mild disease can eat as they normally would. Those with severe disease, including those who may require surgery, fare best with bowel rest and possibly enteral nutrition.
Monitoring the patient for signs of improvement during the first 24 to 48 hours is an important component of management. The patient’s white blood cell count and temperature, the number and frequency of bowel movements, and the overall clinical picture should be evaluated daily. Patients who show improvement should complete the current regimen.
TREATMENT FAILURE AND RECURRENT CDI
If a patient’s condition does not improve or worsens at any point during therapy for CDI, a change to another antimicrobial agent is warranted. Also, surgical, gastroenterological, and/or infectious disease consult may be needed if no improvement is evident after five days of seemingly appropriate therapy.14,24
Recurrent CDI, which occurs at least once in 6% to 25% of treated patients, is most likely to occur 7 to 14 days after treatment completion.1,14 Seldom caused by resistant strains of C difficile, it is more likely to result from inadequate adherence to treatment, the presence of residual spores in the colon after treatment, or reinfection—although relapse is considered more common than reinfection.14,24 However, since a patient’s symptoms may have other causes, confirmation of recurrence should be sought through laboratory testing.
Recurrent illness is managed in the same way as successful initial therapy, based on the severity of disease; vancomycin is recommended for the first recurrence in a patient with a rising white blood cell count or serum creatinine level. Otherwise, metronidazole use may be considered.1
In patients who experience a second recurrence of confirmed CDI, a tapered or pulsed-dose regimen of oral vancomycin over a six-week period has been advocated1,8,14 (for details, see Table 2).
Results from small studies of patients with several recurrences of CDI suggest that oral rifaximin therapy can reduce subsequent recurrences if administered immediately after the conclusion of a course of vancomycin.1,41
PREVENTION OF C DIFFICILE INFECTION
Although “research gaps” exist regarding the optimal strategies to prevent CDI,1 decreased prescribing of nonessential antibiotics is key. Without the alteration in colonic flora caused by antimicrobial use, gut colonization cannot occur, and C difficile typically cannot proliferate.2
Preventing transmission of the pathogen is challenging in health care facilities, where C difficile spores have been cultured from staff members’ hands and from beds, floors, windowsills, and other areas; the spores can survive in hospital rooms for as long as 40 days after a patient with CDI has been discharged.24 Appropriate isolation precautions are essential, including single-patient–use equipment (eg, disposable rectal thermometers) and caregivers’ use of gowns, vinyl gloves, and cleaning agents that are effective against the spores, particularly bleach.1 Alcohol-based products are not effective against C difficile spores; diligent handwashing with soap or chlorhexidine is imperative to prevent the spread of CDI.1,2
CONCLUSION
Clinicians and patients alike face the clinical challenge of Clostridium difficile infection. Mild to moderate disease can be treated medically with excellent success rates. Severe disease carries a significant risk to life, and a multidisciplinary approach including early surgical consultation is warranted.
With early recognition and appropriate treatment, along with strict adherence to isolation policies, the health care community can help limit the spread of this insidious illness and its associated morbidity and mortality.
REFERENCES
1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
2. Efron PA, Mazuski JE. Clostridium difficile colitis. Surg Clin North Am. 2009;89(2):483-500.
3. Hall IC, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
4. Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):727-743.
5. Sunenshine RH, McDonald LC. Clostridium difficile–associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006; 73(2):187-197.
6. Currie B. Improved testing methods are improving diagnosis of Clostridium difficile infections. Advance Administrators Laboratory. 2009;21:10. http://laboratory-manager.advance web.com/Article/PCR-for-C-diff.aspx. Accessed November 8, 2011.
7. Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727-733.
8. Leffler DA, Lamont JT. Treatment of Clostridium difficile–associated disease. Gastroenterology. 2009;136(6):1899-1912.
9. Kelly CP, Lamont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359(18): 1932-1940.
10. Pépin J, Valiquette L, Cossette B. Mortality attributed to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173(9):1037-1042.
11. Zilberberg MD. Clostridium difficile–related hospitalizations among US adults, 2006. Emerg Infect Dis. 2009;15(1):122-124.
12. Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in the inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409-416.
13. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260.
14. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46 suppl 1:S32-S42.
15. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23): 2433-2441.
16. Dawson LF, Valiente E, Wren BW. Clostridium difficile: a continually evolving and problematic pathogen. Infect Genet Evol. 2009;9(6):
1410-1417.
17. Dubberke ER, Reske KA, Olsen MA, et al. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical patients. Clin Infect Dis. 2008;46(4):497-504.
18. Dial S, Delaney JAC, Barkun An, et al. Use of gastric acid–suppression agents and the risk of community-acquired Clostridium difficile disease. JAMA. 2005;294(23):2989-2995.
19. Kuntz JL, Chrischilles EA, Pendergast JF, et al. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011 Jul 15;11:194.
20. Kelly CP, Lamont JT. Antibiotic-associated diarrhea, pseudomembranous enterocolitis, and Clostridium difficile–associated diarrhea and colitis. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: WB Saunders; 2010:1889-1902.
21. Carignan A, Allard C, Pépin J, et al. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis. 2008;46(12):1838-1843.
22. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003; 54(3):243-245.
23. Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg. 2010;200(1):131-135.
24. Schroeder MS. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005;71(5): 921-928.
25. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile–associated disease? A review of the evidence. Curr Med Res Opin. 2008;24(2):329-333.
26. Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile–associated disease: a population-based study. Clin Infect Dis. 2006;43(10): 1272-1276.
27. Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433-439.
28. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile. Clin Infect Dis. 2008;46 suppl 1:S12-S18.
29. Olivas AP, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11(3):299-305.
30. Feldman RJ, Kallich M, Weinstein MP. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C difficile infections. Clin Infect Dis. 1995;20(6):1560-1562.
31. BioPharm Physicians. What next for Dificid (fidaxomicin)? Jun 6, 2011. www.biopharmphysi cians.com/what-next-for-dificid-fidaxomicin. Accessed November 7, 2011.
32. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
33. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
34. Sullivan KM, Spooner LM. Fidaxomicin: a macrocyclic antibiotic for the management of Clostridium difficile infection. Ann Pharmacother. 2010;44(2):352-359.
35. Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile–associated diarrhea: a systematic review. CMAJ. 2005;173(2): 167-170.
36. Segarra-Newnham M. Probiotics for Clostridium difficile–associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41(7): 1212-1221.
37. Lagrotteria D, Holmes S, Smieja M, et al. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile–associated diarrhea. Clin Infect Dis. 2006;43(5):547-552.
38. McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49(5): 640-645.
39. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003; 36(5):580-585.
40. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3): 197-205.
41. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44(6):846-848.
Clostridium difficile, a causative pathogen in antibiotic-associated colitis,1 is a slow-growing, spore-forming, gram-positive anaerobic bacillus,2 named difficile because it was so difficult to culture. Although this pathogenic spore was first identified in the 1930s,3 its vegetative, toxin-producing form was not recognized as a causative organism in diarrhea and colitis until the late 1970s.4,5 Since that time, C difficile has become a growing challenge to health care providers for accurate diagnosis, treatment, and containment of the spread of disease. C difficile infection (CDI) often takes a virulent course with associated morbidity, mortality, and health care costs.6
EPIDEMIOLOGY
In healthy adult patient populations, 2% to 3% are colonized with C difficile.2 The colonization rate among healthy infants is significantly higher, between 60% and 70%, but clinical infection is uncommon.7 As the colon becomes populated with flora, between ages 18 and 24 months, the carrier state disappears.8
In the hospital setting, 20% to 30% of patients become colonized with the organism by the fecal-oral route, which is facilitated when antibiotic therapy disrupts normal flora in the gut, enabling C difficile spores to proliferate; most patients are asymptomatic.5 In 2005, in US acute care hospitals, the incidence of CDI was reported at 84 cases per 100,000 patients.9C difficile remains the leading pathogen associated with inpatient antibiotic-associated diarrhea (AAD). It can be identified as the causative organism in 15% to 25% of cases of AAD in hospitalized patients.2
The mortality rate among hospitalized patients rises significantly in those identified with C difficile: 20.6%, compared with 7.0% of matched inpatient controls.10 ICU admission poses a significant burden of disease: The overall incidence of infection in the ICU population is about 4%. ICU patients who contract CDI have up to a 20% rate of fulminant colitis, a severe form of the disease often necessitating surgery. In this segment of the inpatient population, the mortality rate can approach 60%.8
Data analyzed by Zilberberg11 have demonstrated a significant increase in C difficile–associated infections in US hospitals in recent years. In 2001, the number of discharged patients with documented CDI was approximately 134,000, compared with 291,000 in 2005. The rising incidence of CDI has been attributed to increased antibiotic use, aging of the population, an increasing rate of comorbid conditions, fluoroquinolone resistance, and increased suspicion for the illness, which has led to increased use of testing.12,13
Of Significant Concern
Recurrent disease poses a particular challenge to the health care system. It has been reported that recurrence rates for CDI are between 15% and 35% after a first bout and between 33% and 65% after subsequent episodes of infection.2,14
A hypervirulent strain, the North American pulsed-field gel electrophoresis type 1 (NAP1/B1/027) has been implicated in several C difficile outbreaks.15 This subtype, which is especially resistant to fluoroquinolones,1 produces toxins earlier and in much greater quantities, including toxins A and B at levels 15 to 20 times greater than those seen in less virulent subtypes.4,12 Thus, the NAP1 strain is implicated in more severe disease and is more lethal.16 Affected patients have a 30-day mortality rate twice as high as that among patients with other strains of C difficile.12
The calculated cost of CDI adds between $2,454 and $7,179 in nonreimbursable costs per hospitalized patient, and an additional 3.6 to 7.0 days to their hospital stays.17 Estimates for CDI treatment in the US range from $436 million to $3 billion per year.6,17
Community-Acquired CDI
Community-acquired C difficile infection (CA-CDI) is a subtype that develops in patients who have not been hospitalized in the previous year,18 with incidence recently reported at 11.16 cases per 100,000 person-years.19 Affected patients tend to be younger than those with hospital-acquired infection and to have a less severe disease course. To meet the criteria of this subgroup, according to recent clinical practice guidelines jointly issued by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America (SHEA-IDSA),1 a patient may have developed symptoms no sooner than 12 weeks after hospital discharge (if any hospitalization occurred).
It is always important to perform a thorough history in patients with suspected CA-CDI to assess for recent hospitalizations and antibiotic use. In a retrospective study published in 2011, Kuntz et al19 found that CA-CDI–affected patients were six times as likely as healthy controls to have taken antimicrobials within 180 days before illness (including beta-lactam/beta-lactamase inhibitors, cephalosporins, clindamycin, fluoroquinolones, and penicillin) and twice as likely to have used gastric acid suppressants in that time.
One emerging theory is that CA-CDI is spread through food-borne illness. Unlike the vegetative form that resides in the bowel, C difficile spores are resistant to temperatures at which food is cooked (as they are to alcohol and many other disinfectant agents).5 Several studies have shown that livestock can harbor C difficile.12
PATHOPHYSIOLOGY
Normal gastrointestinal flora resist colonization and proliferation of C difficile; colonization of nontoxigenic strains appears to be protective against toxigenic strains.1 Alteration in the colonic bacterial environment, including the suppression of normal flora proliferation that can occur when a patient receives antibiotics or antineoplastic agents, is thought to allow the overgrowth of C difficile.2
Only C difficile strains that produce exotoxins (the enterotoxin toxin A, and the cytotoxin toxin B) are pathogenic. The organism produces a variety of adhesion proteins (with production accelerated by the presence of antibiotics, such as ampicillin and clindamycin), leading the toxins to bind to specific receptors in the intestinal mucosal cells. C difficile also produces proteases that trigger degradation of the intestinal extracellular matrix and disruption of epithelial cell signaling.16 The toxins activate proinflammatory cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-, and IL-8. The result is an intestinal inflammatory response that is clinically apparent in the form of diarrhea and pseudomembranous colitis (PMC).20
RISK FACTORS
Among several known risk factors for CDI (see Table 11,2,4,18,21-24), perhaps the most widely recognized is the use of nearly any antimicrobial agent. Previous administration of antibiotics has been documented in about 95% of inpatients with CDI. Broad-spectrum antibiotics with anti-anaerobic coverage appear to pose the greatest risk.1,4 These include clindamycin, along with cephalosporins, fluoroquinolones, and beta-lactams.1,13 Fluoroquinolone use has been implicated in the development of the NAP1 subtype of the disease.25 In patients who receive multiple antibiotics or prolonged courses, an even greater risk for CDI is incurred.4 Even single-dose administration of an antibiotic carries a CDI risk of about 1.5%.21
A prolonged hospital stay or residence in a long-term care facility increases the risk for CDI, as does advanced age.1 Patients 65 or older have a 20-fold higher risk for CDI than their younger counterparts.1,2 Patients with inflammatory bowel disease (IBD) have an increased risk for CDI.23
It has been theorized that the use of gastric acid–suppressive medications can increase the risk for CDI to between 2.5 and 2.9 times that among persons who do not take these agents.18,22 Results from other trials have refuted this theory, however.13,26
Additional risk factors include ICU admission, recent gastrointestinal surgery or manipulation, immunosuppression, and serious underlying illnesses.24 Postpyloric enteral tube feeding has also been implicated as a risk factor; this route bypasses the stomach, where a very acidic environment ordinarily helps to kill the organism.4
CLINICAL PRESENTATION
Watery diarrhea, occurring 10 to 15 times in a day, is the most common manifestation of CDI. It may present during administration of an antimicrobial agent or within a few days afterward; rarely, it can develop as long as two months after cessation of such treatment.2 In most studies, median onset of diarrhea is about two days.20 Descriptive characteristics of the stool, including odor and color, may vary. Diarrhea may be accompanied by lower abdominal pain, cramping, low-grade fever, and leukocytosis.
In some patients, particularly those taking narcotics, diarrhea may be absent. This manifestation may signal a more severe disease course, including the possibility of fulminant infection. Endoscopic evaluation of the colon may reveal the classic pseudomembranes (ie, adherent yellow plaques)2; see figure.
Stratifying Disease Severity
The severity of CDI-associated colitis increases as systemic symptoms worsen and the clinical picture deteriorates.4 In the 2010 SHEA-IDSA guidelines,1 CDI is stratified, both in the initial episode and the first recurrence, as mild-to-moderate, severe, or severe complicated. The following data may be used to appropriately stage disease severity in the initial episode:
• Mild-to-moderate disease is characterized by nonbloody diarrhea (fewer than 10 to 12 bowel movements per day), possibly accompanied by mild, crampy abdominal pain. Typically, affected patients do not exhibit significant systemic symptoms or marked abdominal tenderness. Leukocytosis is usually represented by fewer than 15,000 cells/L, and the serum creatinine level is less than 1.5 times the baseline level.1,2,5,8
• Severe disease, which should always be a consideration in older patients,1 includes profuse, watery diarrhea, significant abdominal pain and distension, fever, nausea and vomiting, and clinical volume depletion. Significant leukocytosis (≥ 15,000 cells/L) and serum creatinine ≥ 1.5 times baseline or leukopenia with possible bandemia may occur.1,27 Occult blood may be present, but frank blood is rare. A history of ICU admission alone increases the classification to severe due to the aforementioned poor outcomes associated with CDI in ICU patients.8,24
• Severe, complicated CDI may involve hypotension, shock, or a paralytic ileus1; a paradoxical decrease or absence of diarrhea may occur.2 At the severe end of the disease spectrum, toxic megacolon can develop and progress to colonic perforation.2,5,8,28 Hypoalbuminemia may be present due to the large protein losses in the course of the disease.28
The Most Severe Manifestations
Fulminant CDI can occur in 3% to 8% of patients with CDI.27,29 Fulminant disease carries a mortality rate between 35% and 80%.23 The patient’s clinical picture may resemble that seen in severe disease, with the addition of an acute abdomen indicating peritonitis; lethargy, hypotension, oliguria (including renal failure), and/or tachycardia due to a severe systemic inflammatory response induced by toxin production from C difficile. Up to 20% of patients with fulminant C difficile do not have diarrhea due to reasons explained previously.2,5,23
Bacteremia occurs rarely in patients with CDI.1,30 Risk factors for this severe condition include failure of medical treatment, leukocytosis exceeding 16,000 cells/L, surgery in the previous 30 days, a history of IBD, and previous administration of IV immunoglobulin.23
DIAGNOSIS
According to recommendations from the SHEA-IDSA expert panel,1 only unformed stool from symptomatic patients should be tested for C difficile or its toxins. In spite of slow turnaround time, stool culture (followed by toxigenic culture to identify a toxigenic isolate) is currently considered standard testing for C difficile. Cell cytotoxin assay has 98% sensitivity and 99% specificity, but turnaround time is 24 to 48 hours,28 potentially delaying treatment for patients who test positive.
Enzyme immunoassay (EIA) testing for C difficile toxin A and toxin B yields results within hours but is not as sensitive as the cell cytotoxin assay.1 Because toxin testing lacks sensitivity, a two-step strategy has been proposed and is called an “interim recommendation” by the SHEA-IDSA guideline authors: EIA testing for glutamate dehydrogenase (GDH), an enzyme produced by C difficile; then, in patients with positive results, confirmation by cell cytotoxin assay or toxigenic culture.1,4,28 (EIA testing for GDH can be a rapid, inexpensive method for ruling out CDI.28)
Polymerase chain reaction testing, recently developed for the detection of pathogenic C difficile, is rapid, sensitive, and specific.1 However, this method has not yet gained wide acceptance due to its relatively high cost.6
Imaging Options
Endoscopic visualization confirming the presence of PMC is also considered diagnostic of CDI; although half of patients with CDI lack this finding on endoscopy, CDI is present in 95% of patients with confirmed PMC.1,28 Colonoscopy is advantageous over sigmoidoscopy because up to one-third of patients have only right-sided colonic involvement.28 To its disadvantage, endoscopy carries a risk for perforation (particularly in patients with fulminant disease), as well as the inherent risks of sedation required to perform the procedure.
Though not specific, CT can be used as an adjunct to the diagnosis; features such as colonic wall thickening, pericolic stranding, ascites, pneumatosis, and free air resulting from perforation may suggest CDI and help determine the extent of disease.4,20 The accordion sign (high-attenuation oral contrast in the colon lumen, alternating with low attenuation of inflamed mucosa) and the double halo sign (IV contrast having varying degrees of attenuation due to submucosal inflammation and hyperemia) have also been reported in patients with CDI and may indicate PMC or fulminant CDI.28
The small intestine is typically not involved in CDI except in the setting of ileus and the rare entity of C difficile enteritis.2 Plain radiography is only helpful in cases of ileus or megacolon.28
PHARMACOLOGIC TREATMENT
Discontinuation of the offending drug (usually an antibiotic), whenever possible, is the first step; up to 25% of CDI patients recover without any further therapies. Limiting management to antibiotic withdrawal, however, is currently recommended only in patients with the mildest form of CDI due to the risk for subsequent fulminant disease and clinical deterioration.14 In patients with suspected severe (but unconfirmed) CDI, empiric treatment with one of the antimicrobial agents listed below may be appropriate5 (see also Table 21,8,14). For patients with confirmed illness who require continued antimicrobial therapy, an agent not associated with CDI may be substituted (eg, sulfamethoxazole, macrolides, aminoglycosides).14
Metronidazole is the first-line agent for mild-to-moderate initial disease.1 The mechanism of action is through DNA disruption and inhibition of nucleic acid synthesis, a process that induces cell death. Metronidazole also appears to have anti-inflammatory, antioxidant, and immunomodulating properties that assist in overcoming the disease. It should not be used in women who are pregnant.8
Dosage of the drug is 250 mg four times per day or 500 mg three times a day, given orally; or parenterally, if the patient cannot take it orally. The duration is 10 to 14 days (or longer in patients with underlying infection),1 and the cost is much lower than that of other appropriate antimicrobial agents (ie, less than $1/day).2,31
In patients with severe or refractory disease, vancomycin should be used. Vancomycin has shown superiority in severe disease compared with metronidazole, with clinical cure rates of 97% and 76%, respectively.1,4,32 The typical oral dosage is 125 mg four times per day for 10 days.1
Vancomycin is effective only when given enterally; the drug is not absorbed by the gastrointestinal tract, allowing it to achieve high concentrations in the colon. In the patient who cannot receive standard enteral therapy, vancomycin can be instilled directly into the colon by enema or colonic catheter. With this route of administration, there is a small risk for iatrogenic perforation.2 There has been advocacy in very severe or fulminant disease to use vancomycin (orally or rectally) combined with IV metronidazole.1,8 The cost for 10 to 14 days of treatment ranges from $1,000 to $1,500.31
Earlier this year, fidaxomicin, a macrocyclic antibiotic, was approved for treatment of CDI in adults. This agent has minimal systemic absorption and works in the intestinal lumen by inhibiting the bacterial RNA polymerase.33 In a randomized, controlled trial involving 629 patients, fidaxomicin’s effectiveness was found comparable to that of vancomycin for treatment of CDI (clinical cure rates in the intention-to-treat analysis, 88.2% vs 85.8%, respectively), and fidaxomicin-treated patients had a lower rate of recurrence after initial use (15.4% vs 25.3%, respectively; patients with fulminant disease were not included).33 Dosage is 200 mg every 12 hours for 10 days,33,34 at a reported cost of $2,800.31
Neither oral bacitracin nor fusidic acid has been shown to eliminate CDI or reduce recurrence.1,5,33
Clinical resolution reveals adequate response to treatment and need not be confirmed by laboratory testing. Asymptomatic carriers do not require treatment.5
Less Conventional Agents
Several nonantibiotic treatment regimens have been proposed for CDI. Use of probiotics has been controversial. In a systematic review, Dendukuri et al35 found insufficient evidence in the routine use of probiotics to prevent or treat CDI. There have even been reports of Saccharomyces boulardii–associated fungemia and lactobacillus-associated bacteremia resulting from probiotics use.36
An anion-exchange resin, cholestyramine, is thought to help bind toxins; when studied, however, it failed to show promising results in improving patients’ clinical course.1,37 Additionally, it can bind to other drugs, such as vancomycin, resulting in decreased pharmacologic efficacy of this and other agents.1,2 Therefore, it is not recommended.
Intravenous immunoglobulin can provide an option for treatment of severe and/or recurrent disease as a last resort.1,38 Thus far, only results from small observational or retrospective studies have supported its use.
SURGICAL OPTIONS
Early surgical consultation is warranted in severe or refractory disease9 and in patients with specific manifestations detected via abdominal/pelvic CT: ileus, perforation, obstruction, thickening of the colonic wall, toxic megacolon, ascites, necrotizing colitis, or a systemic inflammatory response that could lead to multiorgan system failure.14,27 Colectomy is undertaken in 0.4% to 3.5% of patients with CDI,2 with a goal of resecting the involved bowel and diverting the fecal stream. In the past, near-total colectomy was the treatment of choice.2
There have been published reports of successful segmental resection for fulminant CDI.23 The mortality rate is approximately 50% in patients who undergo colectomy.2 Survival rates are noted to improve with early and prompt surgical management of severe disease,8,29 which can be life threatening.14
A recently developed procedure for fulminant disease involves creating a diverting loop ileostomy. Through the ostomy, 8 L of propylene glycol electrolyte solution is instilled to reduce the colonic C difficile count; the patient is also administered a vancomycin enema. In a literature review of recent studies involving patients who underwent the procedure, Olivas et al29 reported a 30-day mortality rate of 19%, and 93% of surviving patients did not require a colectomy. Further investigation to reduce surgical mortality in patients with fulminant disease is ongoing.
INVESTIGATIVE TREATMENT OPTIONS
Fecal transplantation from healthy donors to those infected with pathogenic C difficile via nasogastric tube or enema have been studied.1,39 The theory is to help reconstitute normal colonic flora with the transplanted stool. This treatment option has only very limited data and acceptance.8
Promising research has been published regarding the infusion of combined monoclonal antibodies against toxin A and toxin B. Among 200 patients who were randomized to receive the study therapy or placebo, the rate of recurrence was 7% versus 25%, respectively; recurrence rates among patients infected with the virulent NAP1/B1/027 strain were 8% and 32%, respectively.40 Length of stay did not improve in patients taking monoclonal antibodies, and adverse events were reported in both patient groups.
In preliminary trials, a parenteral vaccine containing inactivated toxins A and B was reported safe and capable of triggering a “vigorous” serum antitoxin A response in healthy adults.9,14
ADDITIONAL MANAGEMENT RECOMMENDATIONS
Medications that slow gastrointestinal motility should not be used, as the slowing of peristalsis may allow toxins to accumulate in the colon, leading to worsening disease.18,22 The use of opiates and anti-diarrheal medications should be limited.24
Aggressive fluid and electrolyte replacement should be administered until diarrhea has been resolved (usually within three to six days). Patients may require vasoactive medications to support hemodynamics.2,5,14,24
Patients with mild disease can eat as they normally would. Those with severe disease, including those who may require surgery, fare best with bowel rest and possibly enteral nutrition.
Monitoring the patient for signs of improvement during the first 24 to 48 hours is an important component of management. The patient’s white blood cell count and temperature, the number and frequency of bowel movements, and the overall clinical picture should be evaluated daily. Patients who show improvement should complete the current regimen.
TREATMENT FAILURE AND RECURRENT CDI
If a patient’s condition does not improve or worsens at any point during therapy for CDI, a change to another antimicrobial agent is warranted. Also, surgical, gastroenterological, and/or infectious disease consult may be needed if no improvement is evident after five days of seemingly appropriate therapy.14,24
Recurrent CDI, which occurs at least once in 6% to 25% of treated patients, is most likely to occur 7 to 14 days after treatment completion.1,14 Seldom caused by resistant strains of C difficile, it is more likely to result from inadequate adherence to treatment, the presence of residual spores in the colon after treatment, or reinfection—although relapse is considered more common than reinfection.14,24 However, since a patient’s symptoms may have other causes, confirmation of recurrence should be sought through laboratory testing.
Recurrent illness is managed in the same way as successful initial therapy, based on the severity of disease; vancomycin is recommended for the first recurrence in a patient with a rising white blood cell count or serum creatinine level. Otherwise, metronidazole use may be considered.1
In patients who experience a second recurrence of confirmed CDI, a tapered or pulsed-dose regimen of oral vancomycin over a six-week period has been advocated1,8,14 (for details, see Table 2).
Results from small studies of patients with several recurrences of CDI suggest that oral rifaximin therapy can reduce subsequent recurrences if administered immediately after the conclusion of a course of vancomycin.1,41
PREVENTION OF C DIFFICILE INFECTION
Although “research gaps” exist regarding the optimal strategies to prevent CDI,1 decreased prescribing of nonessential antibiotics is key. Without the alteration in colonic flora caused by antimicrobial use, gut colonization cannot occur, and C difficile typically cannot proliferate.2
Preventing transmission of the pathogen is challenging in health care facilities, where C difficile spores have been cultured from staff members’ hands and from beds, floors, windowsills, and other areas; the spores can survive in hospital rooms for as long as 40 days after a patient with CDI has been discharged.24 Appropriate isolation precautions are essential, including single-patient–use equipment (eg, disposable rectal thermometers) and caregivers’ use of gowns, vinyl gloves, and cleaning agents that are effective against the spores, particularly bleach.1 Alcohol-based products are not effective against C difficile spores; diligent handwashing with soap or chlorhexidine is imperative to prevent the spread of CDI.1,2
CONCLUSION
Clinicians and patients alike face the clinical challenge of Clostridium difficile infection. Mild to moderate disease can be treated medically with excellent success rates. Severe disease carries a significant risk to life, and a multidisciplinary approach including early surgical consultation is warranted.
With early recognition and appropriate treatment, along with strict adherence to isolation policies, the health care community can help limit the spread of this insidious illness and its associated morbidity and mortality.
REFERENCES
1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
2. Efron PA, Mazuski JE. Clostridium difficile colitis. Surg Clin North Am. 2009;89(2):483-500.
3. Hall IC, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
4. Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):727-743.
5. Sunenshine RH, McDonald LC. Clostridium difficile–associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006; 73(2):187-197.
6. Currie B. Improved testing methods are improving diagnosis of Clostridium difficile infections. Advance Administrators Laboratory. 2009;21:10. http://laboratory-manager.advance web.com/Article/PCR-for-C-diff.aspx. Accessed November 8, 2011.
7. Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727-733.
8. Leffler DA, Lamont JT. Treatment of Clostridium difficile–associated disease. Gastroenterology. 2009;136(6):1899-1912.
9. Kelly CP, Lamont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008;359(18): 1932-1940.
10. Pépin J, Valiquette L, Cossette B. Mortality attributed to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173(9):1037-1042.
11. Zilberberg MD. Clostridium difficile–related hospitalizations among US adults, 2006. Emerg Infect Dis. 2009;15(1):122-124.
12. Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in the inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409-416.
13. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260.
14. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46 suppl 1:S32-S42.
15. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23): 2433-2441.
16. Dawson LF, Valiente E, Wren BW. Clostridium difficile: a continually evolving and problematic pathogen. Infect Genet Evol. 2009;9(6):
1410-1417.
17. Dubberke ER, Reske KA, Olsen MA, et al. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical patients. Clin Infect Dis. 2008;46(4):497-504.
18. Dial S, Delaney JAC, Barkun An, et al. Use of gastric acid–suppression agents and the risk of community-acquired Clostridium difficile disease. JAMA. 2005;294(23):2989-2995.
19. Kuntz JL, Chrischilles EA, Pendergast JF, et al. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011 Jul 15;11:194.
20. Kelly CP, Lamont JT. Antibiotic-associated diarrhea, pseudomembranous enterocolitis, and Clostridium difficile–associated diarrhea and colitis. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: WB Saunders; 2010:1889-1902.
21. Carignan A, Allard C, Pépin J, et al. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis. 2008;46(12):1838-1843.
22. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003; 54(3):243-245.
23. Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg. 2010;200(1):131-135.
24. Schroeder MS. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005;71(5): 921-928.
25. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile–associated disease? A review of the evidence. Curr Med Res Opin. 2008;24(2):329-333.
26. Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile–associated disease: a population-based study. Clin Infect Dis. 2006;43(10): 1272-1276.
27. Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433-439.
28. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile. Clin Infect Dis. 2008;46 suppl 1:S12-S18.
29. Olivas AP, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11(3):299-305.
30. Feldman RJ, Kallich M, Weinstein MP. Bacteremia due to Clostridium difficile: case report and review of extraintestinal C difficile infections. Clin Infect Dis. 1995;20(6):1560-1562.
31. BioPharm Physicians. What next for Dificid (fidaxomicin)? Jun 6, 2011. www.biopharmphysi cians.com/what-next-for-dificid-fidaxomicin. Accessed November 7, 2011.
32. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
33. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
34. Sullivan KM, Spooner LM. Fidaxomicin: a macrocyclic antibiotic for the management of Clostridium difficile infection. Ann Pharmacother. 2010;44(2):352-359.
35. Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile–associated diarrhea: a systematic review. CMAJ. 2005;173(2): 167-170.
36. Segarra-Newnham M. Probiotics for Clostridium difficile–associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41(7): 1212-1221.
37. Lagrotteria D, Holmes S, Smieja M, et al. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile–associated diarrhea. Clin Infect Dis. 2006;43(5):547-552.
38. McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49(5): 640-645.
39. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003; 36(5):580-585.
40. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3): 197-205.
41. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44(6):846-848.