User login
Weight Loss • Diarrhea • Mild Eosinophilia • Dx?
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
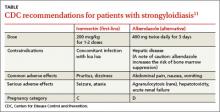
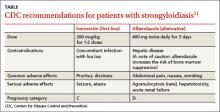
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.
Weight loss • diarrhea • mild eosinophilia • Dx?
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.