User login
Monoclonal gammopathy of undetermined significance: Using risk stratification to guide follow-up
› For monoclonal gammopathy of undetermined significance (MGUS) patients at low risk, repeat serum protein electrophoresis (SPE) in 6 months. If no significant elevation of M-protein is found, repeat SPE every 2 to 3 years. A
› For patients with smoldering multiple myeloma, order SPE every 2 to 3 months in the first year following diagnosis; repeat every 4 to 6 months in the following year and every 6 to 12 months thereafter. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 54-year-old man’s lab results following a routine annual examination reveal a level of IgM M-protein just under 1.5 g/dL. All other lab values, including free light chain (FLC) ratio and bone marrow exam, are normal. No clinical evidence of a related disorder is found. What is the risk that this patient’s condition could progress toward multiple myeloma, and how would you follow up?
The patient with a monoclonal gammopathy has an abnormal proliferation of monoclonal plasma cells that secrete an immunoglobulin, M-protein. This proliferation occurs most often in the bone marrow but can also be found in extra-medullary body tissue. The condition can begin insidiously, remain stable, or progress to frank malignancy causing bone and end-organ destruction. The major challenge is to separate stable, asymptomatic patients who require no treatment from patients with progressive, symptomatic myeloma who require immediate treatment.
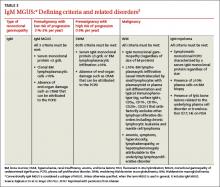
An increased, measurable level of serum monoclonal immunoglobulins or FLCs is called monoclonal gammopathy of undetermined significance (MGUS) when there is <3 g/dL monoclonal protein in the serum, <10% monoclonal plasma cells in the bone marrow, and an absence of beta-cell proliferative disorders, lytic bone lesions, anemia, hypercalcemia, or renal insufficiency (TABLE 1).1,2 Serum and marrow measurements exceeding these values indicate progression of disease to a premalignancy stage. Continued proliferation of plasma cells in the bone marrow results in anemia and bone destruction, while the increase in M-protein leads to end-organ destruction. This final malignant state is multiple myeloma (MM).
Detailed classification of MGUS: A roadmap for monitoring patients
Extensive epidemiologic and clinical studies have refined the classification of MGUS3-5 and related disorders (TABLES 2-4),3 providing physicians with guidance on how to monitor patients. There are 3 kinds of monoclonal gammopathies, each reflecting a particular type of immunoglobulin involvement—non-IgM, IgM, or light chain. Additionally, within each type of gammopathy, patient-specific characteristics determine 3 categories of clinical significance: premalignancy with low risk of progression (1%-2% per year3); premalignancy with high risk of progression (10% per year3); and malignancy.
Non-IgM MGUS with a high risk of progression is designated smoldering multiple myeloma (SMM) (TABLE 2).3 IgM MGUS with a high risk of progression is defined as smoldering Waldenström macroglobulinemia (SWM), with a predisposition to progress to Waldenström macroglobulinemia (WM) and, rarely, to IgM MM (TABLE 3).3
More recently, it has been reported that approximately 20% of the cases of MM belong to a new entity called light-chain MM that features an absence of heavy chain (IgG, IgA, IgM, IgD, or IgE) secretion in serum.6 The premalignant precursor is light-chain MGUS (LC-MGUS). The criteria for LC-MGUS and idiopathic Bence Jones proteinuria are found in TABLE 4.3 Idiopathic Bence Jones proteinuria is equivalent to SMM and SWM due to its higher risk of progression (10%/year)3 to light-chain MM.
Prevalence of MGUS
In general, the prevalence of all types of MGUS increases with age and is affected by race, sex, family history, immunosuppression, and pesticide exposure. The Caucasian American population >50 years exhibits a prevalence of MGUS of approximately 3.2%;7 the African American population exhibits a significantly higher prevalence of 5.9% to 8.4%.7 Native Asians have a lower rate of MM, and, as expected, a lower MGUS prevalence than is seen in the Western population (Thailand ≈2.3%;8 Korea ≈3.3%;9 Japan ≈2.1%;10 China ≈0.8%11). The overall prevalence of the 3 types of MGUS is 4.2% in Caucasians.6
Distinguishing stable from progressive disease
The Mayo Clinic’s risk stratification model12 further specifies risk of disease progression based on 3 indicators: serum M-protein concentration, Ig isotype of M-protein, and serum FLC ratio.
MGUS. A marked increase in risk for disease progression is associated with a serum M-protein concentration ≥1.5 g/dL, a non-IgG isotype, or an abnormal serum FLC ratio (<0.26 or >1.65, reflecting an increase in either the kappa or lambda light chain).12 An MGUS patient exhibiting all 3 of these features has a 58% absolute risk of developing MM after 20 years of follow-up. A patient with 2 of the 3 abnormalities has a 37% risk of progressing to MM, and one who has just one abnormality has a 21% risk. In contrast, an MGUS patient who has an M-protein level <1.5 g/dL, an IgG isotype, and normal FLC range has only a 5% risk of progression to MM in the same 20 years.12
The Spanish Group risk stratification model13 is based on 2 risk factors: a high proportion of abnormal plasma cells (aPC) within the bone marrow plasma cell (BMPC) compartment (ie, ≥95% CD56+/CD19-); and an evolving subtype of the disease (defined as an increase in the level of serum M-protein by at least 10% during the first 6 months of follow-up, or a progressive and constant increase of the M-protein until overt MM develops). The 7-year cumulative probability of progression of MGUS to MM: 2% for patients with neither risk factor, 16% with one risk factor, and 72% with both risk factors.13
SMM. Classification of this progressive state is defined by a serum level of monoclonal protein (IgG, IgA, IgD, or IgE) ≥3 g/dL or a concentration of clonal bone marrow plasma cells ≥10%; and by an absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, and bone lesions (CRAB) that can be attributed to a plasma cell proliferative disorder (TABLE 2).3 Both laboratory and clinical criteria must be met.
According to the Mayo Clinic risk stratification model, likelihood of progression reflects combinations of 3 factors: bone marrow plasmacytosis ≥10%, a serum M-protein level ≥3 g/dL, and a serum FLC ratio ≤0.125 or ≥8.14 Using this stratification scheme, the risk over 10 years of progressing from SMM to MM is 84% for those with all 3 risk factors, 65% with 2 factors, and 52% with one factor.14 As SMM is defined, there is no upper limit of bone marrow involvement. However, Rajkumar et al15 found that progression time was significantly shorter (P<.001) among patients with ≥60% bone marrow involvement, compared with those having <60% involvement.
The Spanish Group risk stratification model13 uses the same model applied to MGUS: a proportion of abnormal plasma cells in the BMPC compartment ≥95% CD56+/CD19-; and an evolving subtype of disease. The 3-year cumulative probability of progression of SMM to MM is 46% for those with both risk factors, 12% for those with one factor, and <1% for those with no risk factors.13
LC-MGUS. The classification of LC-MGUS (TABLE 4)3 is primarily from a Mayo Clinic study6 and research on risk stratification is underway at 2 other institutions. False-positive results are possible in patients with renal16 and inflammatory17 disorders.
Applying risk stratification to patient management
The current approach to a patient with clearly defined MGUS is a prudent “watch and wait” strategy that specifies monitoring details based on risk category (ALGORITHM).1,18
MGUS. In the low-risk MGUS group (IgG subtype, M-protein <1.5 g/dL, and normal FLC ratio)3 there is no need for bone marrow examination or skeletal radiography. Repeat the serum protein electrophoresis (SPE) in 6 months, and if there is no significant elevation of M-protein, repeat the SPE every 2 to 3 years.1,19,20 However, if other findings are suggestive of plasma cell malignancy (anemia, renal insufficiency, hypercalcemia, or bone lesions), bone marrow examination and computed tomographic (CT) scan are advised. Further evaluation of an incidental detection of MGUS is also important since it is occasionally associated with bone diseases,21 arterial and venous thrombosis,22 and an increased risk (P<.05) of developing bacterial (pneumonia, osteomyelitis, septicemia, pyelonephritis, cellulitis, endocarditis, and meningitis) and viral (influenza and herpes zoster) infections.23
Patients in the intermediate- and high-risk MGUS groups with serum monoclonal protein ≥1.5 g/dL, IgA or IgM subtype or an abnormal FLC ratio should undergo tests for CRAB and have bone marrow aspirate and biopsy with cytogenetics, flow cytometry, and fluorescence in situ hybridization (FISH). Patients with IgM MGUS should also undergo a CT scan of the abdomen to rule out the presence of asymptomatic retroperitoneal lymph nodes.1,19 If the BM examination and CT scan yield negative results, repeat SPE and complete blood count (CBC) after 6 months and annually thereafter for life. IgD or IgE MGUS is rare, and patients exhibit a progression similar to the 20-year risk seen with MGUS generally.
SMM. Given the increased risk of progression from SMM to MM compared with MGUS (all risk groups), the 2010 International Myeloma Working Group (IMWG) has suggested monitoring SMM patients more frequently—ie, SPE every 2 to 3 months in the first year following diagnosis.1 Repeat SPE in the second year every 4 to 6 months, and, if results are clinically stable, every 6 to 12 months thereafter. In addition to a baseline bone marrow examination (including cytogenetics, flow cytometry, and FISH studies), consider ordering magnetic resonance imaging of the spine and pelvis to detect occult lesions, as their presence predicts a more rapid progression to MM.24 During the course of the follow-up, evaluate any unexplained anemia or renal function impairment for its origin. A report of MGUS progression over more than a decade to SMM and then to MM illustrates prudent monitoring of a patient.25
LC-MGUS. Once LC-MGUS is detected, first rule out AL-amyloidosis, light-chain deposition disease, or cast nephropathy. If no malignant state is present, repeat the FLC serum assay every 6 months with renal function tests. Idiopathic Bence Jones proteinuria and LC-MGUS have some overlap and both entities put patients at risk for developing MM or amyloidosis. It is not uncommon for MGUS to be accompanied by Bence Jones proteinuria.
In addition to a thorough history and physical examination, recommended followup for both of these entities includes CBC, creatinine, serum FLC, and 24-hour urine protein electrophoresis.6 With idiopathic Bence Jones proteinuria, a monoclonal protein evident on urine protein electrophoresis at >500 mg/24 hr must be followed up with tests for other signs of malignancy (CRAB) and BM examination to exclude the possibility of MM.6
Treatment of MGUS to prevent progression
Multiple myeloma is still an incurable disease. Since MGUS is a precursor of MM, attempts have been made to either slow its progression or eradicate it. Several independent intervention studies26 for the precursor diseases MGUS and SMM have been conducted or are ongoing. Thus far, no conclusive preventive treatment has been found and the 2010 IMWG guidelines do not recommend preventive therapy for MGUS and SMM patients by means of any drug, unless it is a part of a clinical trial.1
CASE › The patient profiled at the start of this article has one abnormal risk factor (IgM isotype) and has a low risk of progression to MM. Management should follow the steps outlined in the ALGORITHM1,18 for low-risk IgM MGUS: repeat SPE, CBC, and CT scan in 6 months and annually thereafter. If any abnormality is observed, rule out the possibilities of IgM SWM, IgM WM, or rapid progression to MM, and consider referral to an oncologist.
CORRESPONDENCE
John M. Boltri, MD, Department of Family and Community Medicine, Northeast Ohio Medical University, College of Medicine, 4209 St. Rt. 44, PO Box 95, Rootstown, Ohio 44272; [email protected].
ACKNOWLEDGEMENTS
The authors thank Kenneth F. Tucker, MD (Webber Cancer Center, St John Macomb-Oakland Hospital, Warren, Mich) and Elizabeth Sykes, MD (Professor, Oakland University, William Beaumont School of Medicine, Rochester, Mich) for their review of this article.
1. Kyle RA, Durie BG, Rajkumar SV, et al; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121-1127.
2. Swerdlow SH, Campro E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IRAC Press; 2008.
3. Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc. 2010;85:945-948.
4. Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood. 2011;117:5573-5581.
5. Landgren O, Kyle RA, Rajkumar SV. From myeloma precursor disease to multiple myeloma: new diagnostic concepts and opportunities for early intervention. Clin Cancer Res. 2011;17:1243-1252.
6. Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721-1728.
7. Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: a systematic review. Mayo Clin Proc. 2010;85:933-942.
8. Watanaboonyongcharoen P, Nakorn TN, Rojnuckarin P. Prevalence of monoclonal gammopathy of undetermined significance in Thailand. Int J Hematol. 2012;95:176-181.
9. Park HK, Lee KR, Kim YJ, et al. Prevalence of monoclonal gammopathy of undetermined significance in an elderly urban Korean population. Am J Hematol. 2011;86:752-755.
10. Iwanaga M, Tagawa M, Tsukasaki K, et al. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin Proc. 2007;82:1474-1479.
11. Wu SP, Minter A, Costello R, et al. MGUS prevalence in an ethnically Chinese population in Hong Kong. Blood. 2013;121:2363-2364.
12. Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812-817.
13. Pérez-Persona E, Mateo G, García-Sanz R, et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol. 2010;148:110-114.
14. Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785-789.
15. Rajkumar SV, Larson D, Kyle RA. Diagnosis of smoldering multiple myeloma. N Engl J Med. 2011;365:474-475.
16. Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684-1690.
17. Gottenberg JE, Aucouturier F, Goetz J, et al. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjögren’s syndrome. Ann Rheum Dis. 2007;66:23-27.
18. Kyle RA, Buadi F, Rajkumar SV. Management of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Oncology. 2011;25:578-586.
19. Landgren O, Waxman AJ. Multiple myeloma precursor disease. JAMA. 2010;304:2397-2404.
20. Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood. 2010;116:2019-2025.
21. Minter AR, Simpson H, Weiss BM, et al. Bone disease from monoclonal gammopathy of undetermined significance to multiple myeloma: pathogenesis, interventions, and future opportunities. Semin Hematol. 2011;48:55-65.
22. Za T, De Stefano V, Rossi E, et al; Multiple Myeloma GIMEMALatium Region Working Group. Arterial and venous thrombosis in patients with monoclonal gammopathy of undetermined significance: incidence and risk factors in a cohort of 1491 patients. Br J Haematol. 2013;160:673-679.
23. Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population based study. Haematologica. 2012;97:854-858.
24. Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606-1610.
25. Yancey MA, Waxman AJ, Landgren O. A case study progression to multiple myeloma. Clin J Oncol Nurs. 2010;14:419-422.
26. ClinicalTrials.gov. Available at: http://www.clinicaltrials.gov/ct2/results?term=MGUS and http://www.clinicaltrials.gov/ct2/results?term=SMM. Accessed June 23, 2015.
› For monoclonal gammopathy of undetermined significance (MGUS) patients at low risk, repeat serum protein electrophoresis (SPE) in 6 months. If no significant elevation of M-protein is found, repeat SPE every 2 to 3 years. A
› For patients with smoldering multiple myeloma, order SPE every 2 to 3 months in the first year following diagnosis; repeat every 4 to 6 months in the following year and every 6 to 12 months thereafter. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 54-year-old man’s lab results following a routine annual examination reveal a level of IgM M-protein just under 1.5 g/dL. All other lab values, including free light chain (FLC) ratio and bone marrow exam, are normal. No clinical evidence of a related disorder is found. What is the risk that this patient’s condition could progress toward multiple myeloma, and how would you follow up?
The patient with a monoclonal gammopathy has an abnormal proliferation of monoclonal plasma cells that secrete an immunoglobulin, M-protein. This proliferation occurs most often in the bone marrow but can also be found in extra-medullary body tissue. The condition can begin insidiously, remain stable, or progress to frank malignancy causing bone and end-organ destruction. The major challenge is to separate stable, asymptomatic patients who require no treatment from patients with progressive, symptomatic myeloma who require immediate treatment.
An increased, measurable level of serum monoclonal immunoglobulins or FLCs is called monoclonal gammopathy of undetermined significance (MGUS) when there is <3 g/dL monoclonal protein in the serum, <10% monoclonal plasma cells in the bone marrow, and an absence of beta-cell proliferative disorders, lytic bone lesions, anemia, hypercalcemia, or renal insufficiency (TABLE 1).1,2 Serum and marrow measurements exceeding these values indicate progression of disease to a premalignancy stage. Continued proliferation of plasma cells in the bone marrow results in anemia and bone destruction, while the increase in M-protein leads to end-organ destruction. This final malignant state is multiple myeloma (MM).
Detailed classification of MGUS: A roadmap for monitoring patients
Extensive epidemiologic and clinical studies have refined the classification of MGUS3-5 and related disorders (TABLES 2-4),3 providing physicians with guidance on how to monitor patients. There are 3 kinds of monoclonal gammopathies, each reflecting a particular type of immunoglobulin involvement—non-IgM, IgM, or light chain. Additionally, within each type of gammopathy, patient-specific characteristics determine 3 categories of clinical significance: premalignancy with low risk of progression (1%-2% per year3); premalignancy with high risk of progression (10% per year3); and malignancy.
Non-IgM MGUS with a high risk of progression is designated smoldering multiple myeloma (SMM) (TABLE 2).3 IgM MGUS with a high risk of progression is defined as smoldering Waldenström macroglobulinemia (SWM), with a predisposition to progress to Waldenström macroglobulinemia (WM) and, rarely, to IgM MM (TABLE 3).3
More recently, it has been reported that approximately 20% of the cases of MM belong to a new entity called light-chain MM that features an absence of heavy chain (IgG, IgA, IgM, IgD, or IgE) secretion in serum.6 The premalignant precursor is light-chain MGUS (LC-MGUS). The criteria for LC-MGUS and idiopathic Bence Jones proteinuria are found in TABLE 4.3 Idiopathic Bence Jones proteinuria is equivalent to SMM and SWM due to its higher risk of progression (10%/year)3 to light-chain MM.
Prevalence of MGUS
In general, the prevalence of all types of MGUS increases with age and is affected by race, sex, family history, immunosuppression, and pesticide exposure. The Caucasian American population >50 years exhibits a prevalence of MGUS of approximately 3.2%;7 the African American population exhibits a significantly higher prevalence of 5.9% to 8.4%.7 Native Asians have a lower rate of MM, and, as expected, a lower MGUS prevalence than is seen in the Western population (Thailand ≈2.3%;8 Korea ≈3.3%;9 Japan ≈2.1%;10 China ≈0.8%11). The overall prevalence of the 3 types of MGUS is 4.2% in Caucasians.6
Distinguishing stable from progressive disease
The Mayo Clinic’s risk stratification model12 further specifies risk of disease progression based on 3 indicators: serum M-protein concentration, Ig isotype of M-protein, and serum FLC ratio.
MGUS. A marked increase in risk for disease progression is associated with a serum M-protein concentration ≥1.5 g/dL, a non-IgG isotype, or an abnormal serum FLC ratio (<0.26 or >1.65, reflecting an increase in either the kappa or lambda light chain).12 An MGUS patient exhibiting all 3 of these features has a 58% absolute risk of developing MM after 20 years of follow-up. A patient with 2 of the 3 abnormalities has a 37% risk of progressing to MM, and one who has just one abnormality has a 21% risk. In contrast, an MGUS patient who has an M-protein level <1.5 g/dL, an IgG isotype, and normal FLC range has only a 5% risk of progression to MM in the same 20 years.12
The Spanish Group risk stratification model13 is based on 2 risk factors: a high proportion of abnormal plasma cells (aPC) within the bone marrow plasma cell (BMPC) compartment (ie, ≥95% CD56+/CD19-); and an evolving subtype of the disease (defined as an increase in the level of serum M-protein by at least 10% during the first 6 months of follow-up, or a progressive and constant increase of the M-protein until overt MM develops). The 7-year cumulative probability of progression of MGUS to MM: 2% for patients with neither risk factor, 16% with one risk factor, and 72% with both risk factors.13
SMM. Classification of this progressive state is defined by a serum level of monoclonal protein (IgG, IgA, IgD, or IgE) ≥3 g/dL or a concentration of clonal bone marrow plasma cells ≥10%; and by an absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, and bone lesions (CRAB) that can be attributed to a plasma cell proliferative disorder (TABLE 2).3 Both laboratory and clinical criteria must be met.
According to the Mayo Clinic risk stratification model, likelihood of progression reflects combinations of 3 factors: bone marrow plasmacytosis ≥10%, a serum M-protein level ≥3 g/dL, and a serum FLC ratio ≤0.125 or ≥8.14 Using this stratification scheme, the risk over 10 years of progressing from SMM to MM is 84% for those with all 3 risk factors, 65% with 2 factors, and 52% with one factor.14 As SMM is defined, there is no upper limit of bone marrow involvement. However, Rajkumar et al15 found that progression time was significantly shorter (P<.001) among patients with ≥60% bone marrow involvement, compared with those having <60% involvement.
The Spanish Group risk stratification model13 uses the same model applied to MGUS: a proportion of abnormal plasma cells in the BMPC compartment ≥95% CD56+/CD19-; and an evolving subtype of disease. The 3-year cumulative probability of progression of SMM to MM is 46% for those with both risk factors, 12% for those with one factor, and <1% for those with no risk factors.13
LC-MGUS. The classification of LC-MGUS (TABLE 4)3 is primarily from a Mayo Clinic study6 and research on risk stratification is underway at 2 other institutions. False-positive results are possible in patients with renal16 and inflammatory17 disorders.
Applying risk stratification to patient management
The current approach to a patient with clearly defined MGUS is a prudent “watch and wait” strategy that specifies monitoring details based on risk category (ALGORITHM).1,18
MGUS. In the low-risk MGUS group (IgG subtype, M-protein <1.5 g/dL, and normal FLC ratio)3 there is no need for bone marrow examination or skeletal radiography. Repeat the serum protein electrophoresis (SPE) in 6 months, and if there is no significant elevation of M-protein, repeat the SPE every 2 to 3 years.1,19,20 However, if other findings are suggestive of plasma cell malignancy (anemia, renal insufficiency, hypercalcemia, or bone lesions), bone marrow examination and computed tomographic (CT) scan are advised. Further evaluation of an incidental detection of MGUS is also important since it is occasionally associated with bone diseases,21 arterial and venous thrombosis,22 and an increased risk (P<.05) of developing bacterial (pneumonia, osteomyelitis, septicemia, pyelonephritis, cellulitis, endocarditis, and meningitis) and viral (influenza and herpes zoster) infections.23
Patients in the intermediate- and high-risk MGUS groups with serum monoclonal protein ≥1.5 g/dL, IgA or IgM subtype or an abnormal FLC ratio should undergo tests for CRAB and have bone marrow aspirate and biopsy with cytogenetics, flow cytometry, and fluorescence in situ hybridization (FISH). Patients with IgM MGUS should also undergo a CT scan of the abdomen to rule out the presence of asymptomatic retroperitoneal lymph nodes.1,19 If the BM examination and CT scan yield negative results, repeat SPE and complete blood count (CBC) after 6 months and annually thereafter for life. IgD or IgE MGUS is rare, and patients exhibit a progression similar to the 20-year risk seen with MGUS generally.
SMM. Given the increased risk of progression from SMM to MM compared with MGUS (all risk groups), the 2010 International Myeloma Working Group (IMWG) has suggested monitoring SMM patients more frequently—ie, SPE every 2 to 3 months in the first year following diagnosis.1 Repeat SPE in the second year every 4 to 6 months, and, if results are clinically stable, every 6 to 12 months thereafter. In addition to a baseline bone marrow examination (including cytogenetics, flow cytometry, and FISH studies), consider ordering magnetic resonance imaging of the spine and pelvis to detect occult lesions, as their presence predicts a more rapid progression to MM.24 During the course of the follow-up, evaluate any unexplained anemia or renal function impairment for its origin. A report of MGUS progression over more than a decade to SMM and then to MM illustrates prudent monitoring of a patient.25
LC-MGUS. Once LC-MGUS is detected, first rule out AL-amyloidosis, light-chain deposition disease, or cast nephropathy. If no malignant state is present, repeat the FLC serum assay every 6 months with renal function tests. Idiopathic Bence Jones proteinuria and LC-MGUS have some overlap and both entities put patients at risk for developing MM or amyloidosis. It is not uncommon for MGUS to be accompanied by Bence Jones proteinuria.
In addition to a thorough history and physical examination, recommended followup for both of these entities includes CBC, creatinine, serum FLC, and 24-hour urine protein electrophoresis.6 With idiopathic Bence Jones proteinuria, a monoclonal protein evident on urine protein electrophoresis at >500 mg/24 hr must be followed up with tests for other signs of malignancy (CRAB) and BM examination to exclude the possibility of MM.6
Treatment of MGUS to prevent progression
Multiple myeloma is still an incurable disease. Since MGUS is a precursor of MM, attempts have been made to either slow its progression or eradicate it. Several independent intervention studies26 for the precursor diseases MGUS and SMM have been conducted or are ongoing. Thus far, no conclusive preventive treatment has been found and the 2010 IMWG guidelines do not recommend preventive therapy for MGUS and SMM patients by means of any drug, unless it is a part of a clinical trial.1
CASE › The patient profiled at the start of this article has one abnormal risk factor (IgM isotype) and has a low risk of progression to MM. Management should follow the steps outlined in the ALGORITHM1,18 for low-risk IgM MGUS: repeat SPE, CBC, and CT scan in 6 months and annually thereafter. If any abnormality is observed, rule out the possibilities of IgM SWM, IgM WM, or rapid progression to MM, and consider referral to an oncologist.
CORRESPONDENCE
John M. Boltri, MD, Department of Family and Community Medicine, Northeast Ohio Medical University, College of Medicine, 4209 St. Rt. 44, PO Box 95, Rootstown, Ohio 44272; [email protected].
ACKNOWLEDGEMENTS
The authors thank Kenneth F. Tucker, MD (Webber Cancer Center, St John Macomb-Oakland Hospital, Warren, Mich) and Elizabeth Sykes, MD (Professor, Oakland University, William Beaumont School of Medicine, Rochester, Mich) for their review of this article.
› For monoclonal gammopathy of undetermined significance (MGUS) patients at low risk, repeat serum protein electrophoresis (SPE) in 6 months. If no significant elevation of M-protein is found, repeat SPE every 2 to 3 years. A
› For patients with smoldering multiple myeloma, order SPE every 2 to 3 months in the first year following diagnosis; repeat every 4 to 6 months in the following year and every 6 to 12 months thereafter. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 54-year-old man’s lab results following a routine annual examination reveal a level of IgM M-protein just under 1.5 g/dL. All other lab values, including free light chain (FLC) ratio and bone marrow exam, are normal. No clinical evidence of a related disorder is found. What is the risk that this patient’s condition could progress toward multiple myeloma, and how would you follow up?
The patient with a monoclonal gammopathy has an abnormal proliferation of monoclonal plasma cells that secrete an immunoglobulin, M-protein. This proliferation occurs most often in the bone marrow but can also be found in extra-medullary body tissue. The condition can begin insidiously, remain stable, or progress to frank malignancy causing bone and end-organ destruction. The major challenge is to separate stable, asymptomatic patients who require no treatment from patients with progressive, symptomatic myeloma who require immediate treatment.
An increased, measurable level of serum monoclonal immunoglobulins or FLCs is called monoclonal gammopathy of undetermined significance (MGUS) when there is <3 g/dL monoclonal protein in the serum, <10% monoclonal plasma cells in the bone marrow, and an absence of beta-cell proliferative disorders, lytic bone lesions, anemia, hypercalcemia, or renal insufficiency (TABLE 1).1,2 Serum and marrow measurements exceeding these values indicate progression of disease to a premalignancy stage. Continued proliferation of plasma cells in the bone marrow results in anemia and bone destruction, while the increase in M-protein leads to end-organ destruction. This final malignant state is multiple myeloma (MM).
Detailed classification of MGUS: A roadmap for monitoring patients
Extensive epidemiologic and clinical studies have refined the classification of MGUS3-5 and related disorders (TABLES 2-4),3 providing physicians with guidance on how to monitor patients. There are 3 kinds of monoclonal gammopathies, each reflecting a particular type of immunoglobulin involvement—non-IgM, IgM, or light chain. Additionally, within each type of gammopathy, patient-specific characteristics determine 3 categories of clinical significance: premalignancy with low risk of progression (1%-2% per year3); premalignancy with high risk of progression (10% per year3); and malignancy.
Non-IgM MGUS with a high risk of progression is designated smoldering multiple myeloma (SMM) (TABLE 2).3 IgM MGUS with a high risk of progression is defined as smoldering Waldenström macroglobulinemia (SWM), with a predisposition to progress to Waldenström macroglobulinemia (WM) and, rarely, to IgM MM (TABLE 3).3
More recently, it has been reported that approximately 20% of the cases of MM belong to a new entity called light-chain MM that features an absence of heavy chain (IgG, IgA, IgM, IgD, or IgE) secretion in serum.6 The premalignant precursor is light-chain MGUS (LC-MGUS). The criteria for LC-MGUS and idiopathic Bence Jones proteinuria are found in TABLE 4.3 Idiopathic Bence Jones proteinuria is equivalent to SMM and SWM due to its higher risk of progression (10%/year)3 to light-chain MM.
Prevalence of MGUS
In general, the prevalence of all types of MGUS increases with age and is affected by race, sex, family history, immunosuppression, and pesticide exposure. The Caucasian American population >50 years exhibits a prevalence of MGUS of approximately 3.2%;7 the African American population exhibits a significantly higher prevalence of 5.9% to 8.4%.7 Native Asians have a lower rate of MM, and, as expected, a lower MGUS prevalence than is seen in the Western population (Thailand ≈2.3%;8 Korea ≈3.3%;9 Japan ≈2.1%;10 China ≈0.8%11). The overall prevalence of the 3 types of MGUS is 4.2% in Caucasians.6
Distinguishing stable from progressive disease
The Mayo Clinic’s risk stratification model12 further specifies risk of disease progression based on 3 indicators: serum M-protein concentration, Ig isotype of M-protein, and serum FLC ratio.
MGUS. A marked increase in risk for disease progression is associated with a serum M-protein concentration ≥1.5 g/dL, a non-IgG isotype, or an abnormal serum FLC ratio (<0.26 or >1.65, reflecting an increase in either the kappa or lambda light chain).12 An MGUS patient exhibiting all 3 of these features has a 58% absolute risk of developing MM after 20 years of follow-up. A patient with 2 of the 3 abnormalities has a 37% risk of progressing to MM, and one who has just one abnormality has a 21% risk. In contrast, an MGUS patient who has an M-protein level <1.5 g/dL, an IgG isotype, and normal FLC range has only a 5% risk of progression to MM in the same 20 years.12
The Spanish Group risk stratification model13 is based on 2 risk factors: a high proportion of abnormal plasma cells (aPC) within the bone marrow plasma cell (BMPC) compartment (ie, ≥95% CD56+/CD19-); and an evolving subtype of the disease (defined as an increase in the level of serum M-protein by at least 10% during the first 6 months of follow-up, or a progressive and constant increase of the M-protein until overt MM develops). The 7-year cumulative probability of progression of MGUS to MM: 2% for patients with neither risk factor, 16% with one risk factor, and 72% with both risk factors.13
SMM. Classification of this progressive state is defined by a serum level of monoclonal protein (IgG, IgA, IgD, or IgE) ≥3 g/dL or a concentration of clonal bone marrow plasma cells ≥10%; and by an absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, and bone lesions (CRAB) that can be attributed to a plasma cell proliferative disorder (TABLE 2).3 Both laboratory and clinical criteria must be met.
According to the Mayo Clinic risk stratification model, likelihood of progression reflects combinations of 3 factors: bone marrow plasmacytosis ≥10%, a serum M-protein level ≥3 g/dL, and a serum FLC ratio ≤0.125 or ≥8.14 Using this stratification scheme, the risk over 10 years of progressing from SMM to MM is 84% for those with all 3 risk factors, 65% with 2 factors, and 52% with one factor.14 As SMM is defined, there is no upper limit of bone marrow involvement. However, Rajkumar et al15 found that progression time was significantly shorter (P<.001) among patients with ≥60% bone marrow involvement, compared with those having <60% involvement.
The Spanish Group risk stratification model13 uses the same model applied to MGUS: a proportion of abnormal plasma cells in the BMPC compartment ≥95% CD56+/CD19-; and an evolving subtype of disease. The 3-year cumulative probability of progression of SMM to MM is 46% for those with both risk factors, 12% for those with one factor, and <1% for those with no risk factors.13
LC-MGUS. The classification of LC-MGUS (TABLE 4)3 is primarily from a Mayo Clinic study6 and research on risk stratification is underway at 2 other institutions. False-positive results are possible in patients with renal16 and inflammatory17 disorders.
Applying risk stratification to patient management
The current approach to a patient with clearly defined MGUS is a prudent “watch and wait” strategy that specifies monitoring details based on risk category (ALGORITHM).1,18
MGUS. In the low-risk MGUS group (IgG subtype, M-protein <1.5 g/dL, and normal FLC ratio)3 there is no need for bone marrow examination or skeletal radiography. Repeat the serum protein electrophoresis (SPE) in 6 months, and if there is no significant elevation of M-protein, repeat the SPE every 2 to 3 years.1,19,20 However, if other findings are suggestive of plasma cell malignancy (anemia, renal insufficiency, hypercalcemia, or bone lesions), bone marrow examination and computed tomographic (CT) scan are advised. Further evaluation of an incidental detection of MGUS is also important since it is occasionally associated with bone diseases,21 arterial and venous thrombosis,22 and an increased risk (P<.05) of developing bacterial (pneumonia, osteomyelitis, septicemia, pyelonephritis, cellulitis, endocarditis, and meningitis) and viral (influenza and herpes zoster) infections.23
Patients in the intermediate- and high-risk MGUS groups with serum monoclonal protein ≥1.5 g/dL, IgA or IgM subtype or an abnormal FLC ratio should undergo tests for CRAB and have bone marrow aspirate and biopsy with cytogenetics, flow cytometry, and fluorescence in situ hybridization (FISH). Patients with IgM MGUS should also undergo a CT scan of the abdomen to rule out the presence of asymptomatic retroperitoneal lymph nodes.1,19 If the BM examination and CT scan yield negative results, repeat SPE and complete blood count (CBC) after 6 months and annually thereafter for life. IgD or IgE MGUS is rare, and patients exhibit a progression similar to the 20-year risk seen with MGUS generally.
SMM. Given the increased risk of progression from SMM to MM compared with MGUS (all risk groups), the 2010 International Myeloma Working Group (IMWG) has suggested monitoring SMM patients more frequently—ie, SPE every 2 to 3 months in the first year following diagnosis.1 Repeat SPE in the second year every 4 to 6 months, and, if results are clinically stable, every 6 to 12 months thereafter. In addition to a baseline bone marrow examination (including cytogenetics, flow cytometry, and FISH studies), consider ordering magnetic resonance imaging of the spine and pelvis to detect occult lesions, as their presence predicts a more rapid progression to MM.24 During the course of the follow-up, evaluate any unexplained anemia or renal function impairment for its origin. A report of MGUS progression over more than a decade to SMM and then to MM illustrates prudent monitoring of a patient.25
LC-MGUS. Once LC-MGUS is detected, first rule out AL-amyloidosis, light-chain deposition disease, or cast nephropathy. If no malignant state is present, repeat the FLC serum assay every 6 months with renal function tests. Idiopathic Bence Jones proteinuria and LC-MGUS have some overlap and both entities put patients at risk for developing MM or amyloidosis. It is not uncommon for MGUS to be accompanied by Bence Jones proteinuria.
In addition to a thorough history and physical examination, recommended followup for both of these entities includes CBC, creatinine, serum FLC, and 24-hour urine protein electrophoresis.6 With idiopathic Bence Jones proteinuria, a monoclonal protein evident on urine protein electrophoresis at >500 mg/24 hr must be followed up with tests for other signs of malignancy (CRAB) and BM examination to exclude the possibility of MM.6
Treatment of MGUS to prevent progression
Multiple myeloma is still an incurable disease. Since MGUS is a precursor of MM, attempts have been made to either slow its progression or eradicate it. Several independent intervention studies26 for the precursor diseases MGUS and SMM have been conducted or are ongoing. Thus far, no conclusive preventive treatment has been found and the 2010 IMWG guidelines do not recommend preventive therapy for MGUS and SMM patients by means of any drug, unless it is a part of a clinical trial.1
CASE › The patient profiled at the start of this article has one abnormal risk factor (IgM isotype) and has a low risk of progression to MM. Management should follow the steps outlined in the ALGORITHM1,18 for low-risk IgM MGUS: repeat SPE, CBC, and CT scan in 6 months and annually thereafter. If any abnormality is observed, rule out the possibilities of IgM SWM, IgM WM, or rapid progression to MM, and consider referral to an oncologist.
CORRESPONDENCE
John M. Boltri, MD, Department of Family and Community Medicine, Northeast Ohio Medical University, College of Medicine, 4209 St. Rt. 44, PO Box 95, Rootstown, Ohio 44272; [email protected].
ACKNOWLEDGEMENTS
The authors thank Kenneth F. Tucker, MD (Webber Cancer Center, St John Macomb-Oakland Hospital, Warren, Mich) and Elizabeth Sykes, MD (Professor, Oakland University, William Beaumont School of Medicine, Rochester, Mich) for their review of this article.
1. Kyle RA, Durie BG, Rajkumar SV, et al; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121-1127.
2. Swerdlow SH, Campro E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IRAC Press; 2008.
3. Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc. 2010;85:945-948.
4. Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood. 2011;117:5573-5581.
5. Landgren O, Kyle RA, Rajkumar SV. From myeloma precursor disease to multiple myeloma: new diagnostic concepts and opportunities for early intervention. Clin Cancer Res. 2011;17:1243-1252.
6. Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721-1728.
7. Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: a systematic review. Mayo Clin Proc. 2010;85:933-942.
8. Watanaboonyongcharoen P, Nakorn TN, Rojnuckarin P. Prevalence of monoclonal gammopathy of undetermined significance in Thailand. Int J Hematol. 2012;95:176-181.
9. Park HK, Lee KR, Kim YJ, et al. Prevalence of monoclonal gammopathy of undetermined significance in an elderly urban Korean population. Am J Hematol. 2011;86:752-755.
10. Iwanaga M, Tagawa M, Tsukasaki K, et al. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin Proc. 2007;82:1474-1479.
11. Wu SP, Minter A, Costello R, et al. MGUS prevalence in an ethnically Chinese population in Hong Kong. Blood. 2013;121:2363-2364.
12. Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812-817.
13. Pérez-Persona E, Mateo G, García-Sanz R, et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol. 2010;148:110-114.
14. Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785-789.
15. Rajkumar SV, Larson D, Kyle RA. Diagnosis of smoldering multiple myeloma. N Engl J Med. 2011;365:474-475.
16. Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684-1690.
17. Gottenberg JE, Aucouturier F, Goetz J, et al. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjögren’s syndrome. Ann Rheum Dis. 2007;66:23-27.
18. Kyle RA, Buadi F, Rajkumar SV. Management of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Oncology. 2011;25:578-586.
19. Landgren O, Waxman AJ. Multiple myeloma precursor disease. JAMA. 2010;304:2397-2404.
20. Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood. 2010;116:2019-2025.
21. Minter AR, Simpson H, Weiss BM, et al. Bone disease from monoclonal gammopathy of undetermined significance to multiple myeloma: pathogenesis, interventions, and future opportunities. Semin Hematol. 2011;48:55-65.
22. Za T, De Stefano V, Rossi E, et al; Multiple Myeloma GIMEMALatium Region Working Group. Arterial and venous thrombosis in patients with monoclonal gammopathy of undetermined significance: incidence and risk factors in a cohort of 1491 patients. Br J Haematol. 2013;160:673-679.
23. Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population based study. Haematologica. 2012;97:854-858.
24. Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606-1610.
25. Yancey MA, Waxman AJ, Landgren O. A case study progression to multiple myeloma. Clin J Oncol Nurs. 2010;14:419-422.
26. ClinicalTrials.gov. Available at: http://www.clinicaltrials.gov/ct2/results?term=MGUS and http://www.clinicaltrials.gov/ct2/results?term=SMM. Accessed June 23, 2015.
1. Kyle RA, Durie BG, Rajkumar SV, et al; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121-1127.
2. Swerdlow SH, Campro E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IRAC Press; 2008.
3. Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc. 2010;85:945-948.
4. Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood. 2011;117:5573-5581.
5. Landgren O, Kyle RA, Rajkumar SV. From myeloma precursor disease to multiple myeloma: new diagnostic concepts and opportunities for early intervention. Clin Cancer Res. 2011;17:1243-1252.
6. Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721-1728.
7. Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: a systematic review. Mayo Clin Proc. 2010;85:933-942.
8. Watanaboonyongcharoen P, Nakorn TN, Rojnuckarin P. Prevalence of monoclonal gammopathy of undetermined significance in Thailand. Int J Hematol. 2012;95:176-181.
9. Park HK, Lee KR, Kim YJ, et al. Prevalence of monoclonal gammopathy of undetermined significance in an elderly urban Korean population. Am J Hematol. 2011;86:752-755.
10. Iwanaga M, Tagawa M, Tsukasaki K, et al. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin Proc. 2007;82:1474-1479.
11. Wu SP, Minter A, Costello R, et al. MGUS prevalence in an ethnically Chinese population in Hong Kong. Blood. 2013;121:2363-2364.
12. Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812-817.
13. Pérez-Persona E, Mateo G, García-Sanz R, et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol. 2010;148:110-114.
14. Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785-789.
15. Rajkumar SV, Larson D, Kyle RA. Diagnosis of smoldering multiple myeloma. N Engl J Med. 2011;365:474-475.
16. Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684-1690.
17. Gottenberg JE, Aucouturier F, Goetz J, et al. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjögren’s syndrome. Ann Rheum Dis. 2007;66:23-27.
18. Kyle RA, Buadi F, Rajkumar SV. Management of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Oncology. 2011;25:578-586.
19. Landgren O, Waxman AJ. Multiple myeloma precursor disease. JAMA. 2010;304:2397-2404.
20. Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood. 2010;116:2019-2025.
21. Minter AR, Simpson H, Weiss BM, et al. Bone disease from monoclonal gammopathy of undetermined significance to multiple myeloma: pathogenesis, interventions, and future opportunities. Semin Hematol. 2011;48:55-65.
22. Za T, De Stefano V, Rossi E, et al; Multiple Myeloma GIMEMALatium Region Working Group. Arterial and venous thrombosis in patients with monoclonal gammopathy of undetermined significance: incidence and risk factors in a cohort of 1491 patients. Br J Haematol. 2013;160:673-679.
23. Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population based study. Haematologica. 2012;97:854-858.
24. Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606-1610.
25. Yancey MA, Waxman AJ, Landgren O. A case study progression to multiple myeloma. Clin J Oncol Nurs. 2010;14:419-422.
26. ClinicalTrials.gov. Available at: http://www.clinicaltrials.gov/ct2/results?term=MGUS and http://www.clinicaltrials.gov/ct2/results?term=SMM. Accessed June 23, 2015.