User login
Surgery involves a certain degree of risk. The risk could be in the form of a complication, or it could be surgical failure. When I was a resident, gynecologic oncologist Gary Johnson, MD, used to say: “If you don’t want complications, don’t do surgery.” I was never sure whether he was trying to make me feel better when complications occurred, or was just stating the facts. Maybe both. It could be argued that one should consider offering medical options before exposing a patient to surgical risks.
In this article, I review three recent studies that shed some light on patient selection for surgical intervention—more specifically, on surgical and counseling failures associated with surgical management of abnormal uterine bleeding (AUB):
- an analysis of perioperative hysterectomy data from 52 hospitals in Michigan that showed that alternatives to hysterectomy were underutilized in women with AUB, fibroids, or pelvic pain
- a retrospective cohort study of 300 patients from two large academic medical centers, which explored risk factors for postablation pain
- another retrospective cohort study of 968 women who underwent endometrial ablation. This study was designed to highlight any association between preoperative bleeding patterns and the risk of ablation failure.
Don’t resort to surgery until alternative treatments have been exhausted
Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative [published online ahead of print December 23, 2014]. Am J Obstet Gynecol. pii: S0002-9378(14)02355-2. doi: 10.1016/j.ajog.2014.11.031.
In this analysis, Corona and colleagues evaluated the use of alternative treatments among women who underwent hysterectomy for uterine fibroids, AUB, endometriosis, or pelvic pain at 52 hospitals participating in the Michigan Surgical Quality Collaborative in 2013. They also determined whether the pathology was “supportive” or “unsupportive” of the surgical indication.
A significant percentage of hysterectomies were performed with an indication of AUB or fibroids, or both (49.1%). A combination of pain, AUB, and/or fibroids was the indication in 48.1% of hysterectomies, and endometriosis and/or pain was listed in 9.2%.
In 37.7% of cases (n = 1,281), no offering of alternative treatments was documented.
Although endometrial ablation was offered to 44.1% of women younger than age 40, to 48.3% of women aged 40 to 50 years, and to 31.6% of women older than age 50, the conservative, nonsurgical option of a levonorgestrel intrauterine system (LNG-IUS) was offered in only 12.7%, 12.4%, and 9.3% of these cases, respectively.
Overall, the rate of unsupportive pathology was 18.3% (n = 621). That rate was higher in women younger than age 40, compared with those aged 40 to 50 and older than age 50 (37.8% vs 12.0% and 7.5%, respectively; P<.001).
These data suggest that a significant number of women with AUB associated with ovulatory dysfunction (AUB-O) undergo hysterectomy. The authors point out that the American College of Obstetricians and Gynecologists (ACOG) recommends medical therapy as a first-line therapy for AUB-O rather than surgical therapy.
What this EVIDENCE means for practice
Although AUB is a common indication for hysterectomy, conservative alternative therapies should be offered when appropriate. A particularly cost-effective and effective conservative therapy—the LNG-IUS—is underutilized and should be considered more often.
How to determine who is most likely to benefit from endometrial ablation
Wishall KM, Price J, Pereira N, et al. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124(5):904–910.
Smithling KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211(5):556.e1–e6.
Endometrial ablation has been around a long time—likely since the 1930s. However, it was not until the 1980s that operative hysteroscopy and endometrial ablation became commonplace. As a result of new, more “automated” technology, five nonresectoscopic endometrial ablation techniques were introduced, starting with FDA approval of the thermal balloon device in 1997.
Initially, information on the feasibility of endometrial ablation was presented in the form of case reports. Efficacy and safety were studied through FDA trials, which yielded variable amenorrhea rates but relatively high satisfaction rates in the range of 85% to 95%. In the interim, we have learned more refined details about endometrial ablation as case reports of unintended consequences have cropped up and as this technology has reached a broader physician base. After almost two decades of experience with nonresectoscopic endometrial ablation devices, information on “failure”—ie, the need for additional treatment—is surfacing.
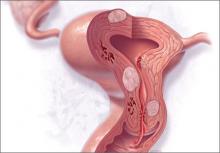
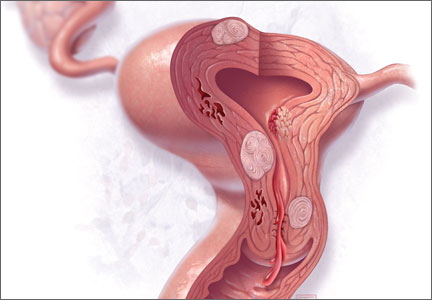
Over the past year, as we have increased adoption of the PALM-COEIN classification system for the causes of AUB in women of reproductive age, we also have gleaned more information about how endometrial ablation works in this context. In general, PALM (polyp, adenomyosis, leiomyoma, and malignancy/hyperplasia) represents lesions that can be seen but may not necessarily be the cause of bleeding. COEIN (coagulopathy, ovulatory dysfunction, endometrial factors, iatrogenic, and not yet classified) represents causes of bleeding that may not be visible.
Although endometrial ablation is ideally suited for women with AUB related to endometrial factors (AUB-E), two studies from 2014 provide insight into endometrial ablation performed when lesions are present within the PALM classification, such as polyps (AUB-P), adenomyosis (AUB-A), and leiomyomas (AUB-L), and in patients with COEIN conditions, such as ovulatory dysfunction (AUB-O) and AUB-E.
Findings of Wishall and colleagues
Three hundred women who underwent endometrial ablation were evaluated in regard to postoperative pain and the need for subsequent hysterectomy. A total of 270 women were available for follow-up in this retrospective cohort (10% lost to follow-up). Wishall and colleagues set out to identify prognostic factors that would put a woman at risk for post-ablation pain. Their secondary outcome was the rate of hysterectomy after ablation.
The study was limited to second-generation endometrial ablation devices, including the thermal balloon, microwave, circulating hot fluid, and bipolar radiofrequency devices.
Wishall and colleagues found that the risk of failure was the highest (a quadrupling) when uterine abnormalities such as leiomyomas, adenomyosis, a thickened endometrial stripe, or polyps were present (adjusted odds ratio [OR], 3.96; 95% confidence interval [CI], 1.25–12.56).
As in other series, 19% of women ultimately required hysterectomy. Twenty-three percent developed new or worsening pain after ablation. Risk factors for postablation pain included a history of dysmenorrhea (OR, 1.74) and tubal sterilization (OR, 2.06).
Findings of Smithling and colleagues
Investigators evaluated the records of 968 women with AUB who had undergone endometrial ablation, categorizing their preoperative bleeding patterns as either regular (presumed AUB-E) or irregular (presumed AUB-O). Of these women, 961 (99.3%) had undergone radiofrequency bipolar endometrial ablation.
Smithling and colleagues hypothesized that women with AUB-O would have a higher failure rate—defined as the need for reablation or subsequent hysterectomy—than women with AUB-E because endometrial ablation does not necessarily address the pathology that underlies AUB-O. However, they found no difference in treatment failure or the need for a subsequent gynecologic procedure between groups during the 3-year period after endometrial ablation. The rate of treatment failure was 16.4% in women with regular bleeding—essentially the same as the rate for women with irregular bleeding (17.6%; P = .7) Risk factors associated with failure included:
- tubal sterilization (16.4% vs 9.0% for women without it)
- pelvic pain or dysmenorrhea (21.8% vs 10.7% for women without it)
- obesity (16.7% vs 9.8%) (P = .003).
Although there was no difference in failure rates between the group with regular bleeding versus the group with irregular bleeding, Smithling and colleagues were careful to avoid interpreting this finding as a recommendation for endometrial ablation in women with AUB-O.
What this EVIDENCE means for practice
When endometrial ablation is performed to treat lesions such as polyps, adenomyosis, and leiomyomata, women are nearly four times more likely to require subsequent hysterectomy.
A history of dysmenorrhea yielded a 74% higher risk of developing postablation pain, and a history of tubal sterilization more than doubled the risk, compared with no history of dysmenorrhea or tubal sterilization.
Women who undergo endometrial ablation for presumed AUB-O and presumed AUB-E have similar failure rates.
Preoperative factors such as dysmenorrhea, prior tubal sterilization, and obesity were identified as risk factors for ablation failure.
The choice between endometrial ablation and hysterectomy for patients with AUB-O depends on an individualized assessment of risks and benefits, including evaluation of medical comorbidities.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Surgery involves a certain degree of risk. The risk could be in the form of a complication, or it could be surgical failure. When I was a resident, gynecologic oncologist Gary Johnson, MD, used to say: “If you don’t want complications, don’t do surgery.” I was never sure whether he was trying to make me feel better when complications occurred, or was just stating the facts. Maybe both. It could be argued that one should consider offering medical options before exposing a patient to surgical risks.
In this article, I review three recent studies that shed some light on patient selection for surgical intervention—more specifically, on surgical and counseling failures associated with surgical management of abnormal uterine bleeding (AUB):
- an analysis of perioperative hysterectomy data from 52 hospitals in Michigan that showed that alternatives to hysterectomy were underutilized in women with AUB, fibroids, or pelvic pain
- a retrospective cohort study of 300 patients from two large academic medical centers, which explored risk factors for postablation pain
- another retrospective cohort study of 968 women who underwent endometrial ablation. This study was designed to highlight any association between preoperative bleeding patterns and the risk of ablation failure.
Don’t resort to surgery until alternative treatments have been exhausted
Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative [published online ahead of print December 23, 2014]. Am J Obstet Gynecol. pii: S0002-9378(14)02355-2. doi: 10.1016/j.ajog.2014.11.031.
In this analysis, Corona and colleagues evaluated the use of alternative treatments among women who underwent hysterectomy for uterine fibroids, AUB, endometriosis, or pelvic pain at 52 hospitals participating in the Michigan Surgical Quality Collaborative in 2013. They also determined whether the pathology was “supportive” or “unsupportive” of the surgical indication.
A significant percentage of hysterectomies were performed with an indication of AUB or fibroids, or both (49.1%). A combination of pain, AUB, and/or fibroids was the indication in 48.1% of hysterectomies, and endometriosis and/or pain was listed in 9.2%.
In 37.7% of cases (n = 1,281), no offering of alternative treatments was documented.
Although endometrial ablation was offered to 44.1% of women younger than age 40, to 48.3% of women aged 40 to 50 years, and to 31.6% of women older than age 50, the conservative, nonsurgical option of a levonorgestrel intrauterine system (LNG-IUS) was offered in only 12.7%, 12.4%, and 9.3% of these cases, respectively.
Overall, the rate of unsupportive pathology was 18.3% (n = 621). That rate was higher in women younger than age 40, compared with those aged 40 to 50 and older than age 50 (37.8% vs 12.0% and 7.5%, respectively; P<.001).
These data suggest that a significant number of women with AUB associated with ovulatory dysfunction (AUB-O) undergo hysterectomy. The authors point out that the American College of Obstetricians and Gynecologists (ACOG) recommends medical therapy as a first-line therapy for AUB-O rather than surgical therapy.
What this EVIDENCE means for practice
Although AUB is a common indication for hysterectomy, conservative alternative therapies should be offered when appropriate. A particularly cost-effective and effective conservative therapy—the LNG-IUS—is underutilized and should be considered more often.
How to determine who is most likely to benefit from endometrial ablation
Wishall KM, Price J, Pereira N, et al. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124(5):904–910.
Smithling KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211(5):556.e1–e6.
Endometrial ablation has been around a long time—likely since the 1930s. However, it was not until the 1980s that operative hysteroscopy and endometrial ablation became commonplace. As a result of new, more “automated” technology, five nonresectoscopic endometrial ablation techniques were introduced, starting with FDA approval of the thermal balloon device in 1997.
Initially, information on the feasibility of endometrial ablation was presented in the form of case reports. Efficacy and safety were studied through FDA trials, which yielded variable amenorrhea rates but relatively high satisfaction rates in the range of 85% to 95%. In the interim, we have learned more refined details about endometrial ablation as case reports of unintended consequences have cropped up and as this technology has reached a broader physician base. After almost two decades of experience with nonresectoscopic endometrial ablation devices, information on “failure”—ie, the need for additional treatment—is surfacing.
Over the past year, as we have increased adoption of the PALM-COEIN classification system for the causes of AUB in women of reproductive age, we also have gleaned more information about how endometrial ablation works in this context. In general, PALM (polyp, adenomyosis, leiomyoma, and malignancy/hyperplasia) represents lesions that can be seen but may not necessarily be the cause of bleeding. COEIN (coagulopathy, ovulatory dysfunction, endometrial factors, iatrogenic, and not yet classified) represents causes of bleeding that may not be visible.
Although endometrial ablation is ideally suited for women with AUB related to endometrial factors (AUB-E), two studies from 2014 provide insight into endometrial ablation performed when lesions are present within the PALM classification, such as polyps (AUB-P), adenomyosis (AUB-A), and leiomyomas (AUB-L), and in patients with COEIN conditions, such as ovulatory dysfunction (AUB-O) and AUB-E.
Findings of Wishall and colleagues
Three hundred women who underwent endometrial ablation were evaluated in regard to postoperative pain and the need for subsequent hysterectomy. A total of 270 women were available for follow-up in this retrospective cohort (10% lost to follow-up). Wishall and colleagues set out to identify prognostic factors that would put a woman at risk for post-ablation pain. Their secondary outcome was the rate of hysterectomy after ablation.
The study was limited to second-generation endometrial ablation devices, including the thermal balloon, microwave, circulating hot fluid, and bipolar radiofrequency devices.
Wishall and colleagues found that the risk of failure was the highest (a quadrupling) when uterine abnormalities such as leiomyomas, adenomyosis, a thickened endometrial stripe, or polyps were present (adjusted odds ratio [OR], 3.96; 95% confidence interval [CI], 1.25–12.56).
As in other series, 19% of women ultimately required hysterectomy. Twenty-three percent developed new or worsening pain after ablation. Risk factors for postablation pain included a history of dysmenorrhea (OR, 1.74) and tubal sterilization (OR, 2.06).
Findings of Smithling and colleagues
Investigators evaluated the records of 968 women with AUB who had undergone endometrial ablation, categorizing their preoperative bleeding patterns as either regular (presumed AUB-E) or irregular (presumed AUB-O). Of these women, 961 (99.3%) had undergone radiofrequency bipolar endometrial ablation.
Smithling and colleagues hypothesized that women with AUB-O would have a higher failure rate—defined as the need for reablation or subsequent hysterectomy—than women with AUB-E because endometrial ablation does not necessarily address the pathology that underlies AUB-O. However, they found no difference in treatment failure or the need for a subsequent gynecologic procedure between groups during the 3-year period after endometrial ablation. The rate of treatment failure was 16.4% in women with regular bleeding—essentially the same as the rate for women with irregular bleeding (17.6%; P = .7) Risk factors associated with failure included:
- tubal sterilization (16.4% vs 9.0% for women without it)
- pelvic pain or dysmenorrhea (21.8% vs 10.7% for women without it)
- obesity (16.7% vs 9.8%) (P = .003).
Although there was no difference in failure rates between the group with regular bleeding versus the group with irregular bleeding, Smithling and colleagues were careful to avoid interpreting this finding as a recommendation for endometrial ablation in women with AUB-O.
What this EVIDENCE means for practice
When endometrial ablation is performed to treat lesions such as polyps, adenomyosis, and leiomyomata, women are nearly four times more likely to require subsequent hysterectomy.
A history of dysmenorrhea yielded a 74% higher risk of developing postablation pain, and a history of tubal sterilization more than doubled the risk, compared with no history of dysmenorrhea or tubal sterilization.
Women who undergo endometrial ablation for presumed AUB-O and presumed AUB-E have similar failure rates.
Preoperative factors such as dysmenorrhea, prior tubal sterilization, and obesity were identified as risk factors for ablation failure.
The choice between endometrial ablation and hysterectomy for patients with AUB-O depends on an individualized assessment of risks and benefits, including evaluation of medical comorbidities.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Surgery involves a certain degree of risk. The risk could be in the form of a complication, or it could be surgical failure. When I was a resident, gynecologic oncologist Gary Johnson, MD, used to say: “If you don’t want complications, don’t do surgery.” I was never sure whether he was trying to make me feel better when complications occurred, or was just stating the facts. Maybe both. It could be argued that one should consider offering medical options before exposing a patient to surgical risks.
In this article, I review three recent studies that shed some light on patient selection for surgical intervention—more specifically, on surgical and counseling failures associated with surgical management of abnormal uterine bleeding (AUB):
- an analysis of perioperative hysterectomy data from 52 hospitals in Michigan that showed that alternatives to hysterectomy were underutilized in women with AUB, fibroids, or pelvic pain
- a retrospective cohort study of 300 patients from two large academic medical centers, which explored risk factors for postablation pain
- another retrospective cohort study of 968 women who underwent endometrial ablation. This study was designed to highlight any association between preoperative bleeding patterns and the risk of ablation failure.
Don’t resort to surgery until alternative treatments have been exhausted
Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative [published online ahead of print December 23, 2014]. Am J Obstet Gynecol. pii: S0002-9378(14)02355-2. doi: 10.1016/j.ajog.2014.11.031.
In this analysis, Corona and colleagues evaluated the use of alternative treatments among women who underwent hysterectomy for uterine fibroids, AUB, endometriosis, or pelvic pain at 52 hospitals participating in the Michigan Surgical Quality Collaborative in 2013. They also determined whether the pathology was “supportive” or “unsupportive” of the surgical indication.
A significant percentage of hysterectomies were performed with an indication of AUB or fibroids, or both (49.1%). A combination of pain, AUB, and/or fibroids was the indication in 48.1% of hysterectomies, and endometriosis and/or pain was listed in 9.2%.
In 37.7% of cases (n = 1,281), no offering of alternative treatments was documented.
Although endometrial ablation was offered to 44.1% of women younger than age 40, to 48.3% of women aged 40 to 50 years, and to 31.6% of women older than age 50, the conservative, nonsurgical option of a levonorgestrel intrauterine system (LNG-IUS) was offered in only 12.7%, 12.4%, and 9.3% of these cases, respectively.
Overall, the rate of unsupportive pathology was 18.3% (n = 621). That rate was higher in women younger than age 40, compared with those aged 40 to 50 and older than age 50 (37.8% vs 12.0% and 7.5%, respectively; P<.001).
These data suggest that a significant number of women with AUB associated with ovulatory dysfunction (AUB-O) undergo hysterectomy. The authors point out that the American College of Obstetricians and Gynecologists (ACOG) recommends medical therapy as a first-line therapy for AUB-O rather than surgical therapy.
What this EVIDENCE means for practice
Although AUB is a common indication for hysterectomy, conservative alternative therapies should be offered when appropriate. A particularly cost-effective and effective conservative therapy—the LNG-IUS—is underutilized and should be considered more often.
How to determine who is most likely to benefit from endometrial ablation
Wishall KM, Price J, Pereira N, et al. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124(5):904–910.
Smithling KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211(5):556.e1–e6.
Endometrial ablation has been around a long time—likely since the 1930s. However, it was not until the 1980s that operative hysteroscopy and endometrial ablation became commonplace. As a result of new, more “automated” technology, five nonresectoscopic endometrial ablation techniques were introduced, starting with FDA approval of the thermal balloon device in 1997.
Initially, information on the feasibility of endometrial ablation was presented in the form of case reports. Efficacy and safety were studied through FDA trials, which yielded variable amenorrhea rates but relatively high satisfaction rates in the range of 85% to 95%. In the interim, we have learned more refined details about endometrial ablation as case reports of unintended consequences have cropped up and as this technology has reached a broader physician base. After almost two decades of experience with nonresectoscopic endometrial ablation devices, information on “failure”—ie, the need for additional treatment—is surfacing.
Over the past year, as we have increased adoption of the PALM-COEIN classification system for the causes of AUB in women of reproductive age, we also have gleaned more information about how endometrial ablation works in this context. In general, PALM (polyp, adenomyosis, leiomyoma, and malignancy/hyperplasia) represents lesions that can be seen but may not necessarily be the cause of bleeding. COEIN (coagulopathy, ovulatory dysfunction, endometrial factors, iatrogenic, and not yet classified) represents causes of bleeding that may not be visible.
Although endometrial ablation is ideally suited for women with AUB related to endometrial factors (AUB-E), two studies from 2014 provide insight into endometrial ablation performed when lesions are present within the PALM classification, such as polyps (AUB-P), adenomyosis (AUB-A), and leiomyomas (AUB-L), and in patients with COEIN conditions, such as ovulatory dysfunction (AUB-O) and AUB-E.
Findings of Wishall and colleagues
Three hundred women who underwent endometrial ablation were evaluated in regard to postoperative pain and the need for subsequent hysterectomy. A total of 270 women were available for follow-up in this retrospective cohort (10% lost to follow-up). Wishall and colleagues set out to identify prognostic factors that would put a woman at risk for post-ablation pain. Their secondary outcome was the rate of hysterectomy after ablation.
The study was limited to second-generation endometrial ablation devices, including the thermal balloon, microwave, circulating hot fluid, and bipolar radiofrequency devices.
Wishall and colleagues found that the risk of failure was the highest (a quadrupling) when uterine abnormalities such as leiomyomas, adenomyosis, a thickened endometrial stripe, or polyps were present (adjusted odds ratio [OR], 3.96; 95% confidence interval [CI], 1.25–12.56).
As in other series, 19% of women ultimately required hysterectomy. Twenty-three percent developed new or worsening pain after ablation. Risk factors for postablation pain included a history of dysmenorrhea (OR, 1.74) and tubal sterilization (OR, 2.06).
Findings of Smithling and colleagues
Investigators evaluated the records of 968 women with AUB who had undergone endometrial ablation, categorizing their preoperative bleeding patterns as either regular (presumed AUB-E) or irregular (presumed AUB-O). Of these women, 961 (99.3%) had undergone radiofrequency bipolar endometrial ablation.
Smithling and colleagues hypothesized that women with AUB-O would have a higher failure rate—defined as the need for reablation or subsequent hysterectomy—than women with AUB-E because endometrial ablation does not necessarily address the pathology that underlies AUB-O. However, they found no difference in treatment failure or the need for a subsequent gynecologic procedure between groups during the 3-year period after endometrial ablation. The rate of treatment failure was 16.4% in women with regular bleeding—essentially the same as the rate for women with irregular bleeding (17.6%; P = .7) Risk factors associated with failure included:
- tubal sterilization (16.4% vs 9.0% for women without it)
- pelvic pain or dysmenorrhea (21.8% vs 10.7% for women without it)
- obesity (16.7% vs 9.8%) (P = .003).
Although there was no difference in failure rates between the group with regular bleeding versus the group with irregular bleeding, Smithling and colleagues were careful to avoid interpreting this finding as a recommendation for endometrial ablation in women with AUB-O.
What this EVIDENCE means for practice
When endometrial ablation is performed to treat lesions such as polyps, adenomyosis, and leiomyomata, women are nearly four times more likely to require subsequent hysterectomy.
A history of dysmenorrhea yielded a 74% higher risk of developing postablation pain, and a history of tubal sterilization more than doubled the risk, compared with no history of dysmenorrhea or tubal sterilization.
Women who undergo endometrial ablation for presumed AUB-O and presumed AUB-E have similar failure rates.
Preoperative factors such as dysmenorrhea, prior tubal sterilization, and obesity were identified as risk factors for ablation failure.
The choice between endometrial ablation and hysterectomy for patients with AUB-O depends on an individualized assessment of risks and benefits, including evaluation of medical comorbidities.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
IN THIS ARTICLE
– When to offer an alternative treatment to patients considering surgery for AUB
– Who is most likely to benefit from endometrial ablation?