User login
The first human birth from a frozen oocyte was reported in 1986.1 Nearly 3 decades later, mature oocyte cryopreservation has emerged as a meaningful technology to preserve reproductive potential in women of reproductive age. In 2013, the American Society for Reproductive Medicine (ASRM) removed the “experimental” label from egg freezing but cautioned that more data on safety and efficacy were needed prior to widespread adoption of the technique.2
In this Update, we present the current protocols for oocyte cryopreservation, how we arrived at them, and the questions regarding outcomes that still remain. In addition, we discuss the ethical dilemmas egg freezing presents, according to the varying rhetoric within the media and our own profession. Finally, we consider what preliminary data suggest as to the live-birth rate using frozen eggs from women of varying ages and what the costs are associated with using oocyte cryopreservation as the approach to fertility treatment.
Vitrification and slow freezing: How did we get here and how effective are they?
Fertility preservation is a rapidly advancing area of reproductive medicine. Cryopreservation is the cooling of cells to subzero temperatures to halt biologic activity and preserve the cells for future use. Clinically, oocyte cryopreservation requires a patient to undergo in vitro fertilization (IVF). After egg retrieval, the oocytes are cryopreserved for use at a later time.
The prefix “cryo” originated from the Greek word “kryos,” meaning icy cold or frost. Cryopreservation is not a new science. In 1776, the Italian priest and scientist Lazzaro Spallanzani reported that sperm became motionless when cooled by snow. A pivotal discovery in the field came in 1949, when Christopher Polge, an English scientist, showed that glycerol, a permeating solute, could provide protection to cells at low temperatures.3 Progress in sperm cryopreservation advanced quickly, partly due to the ease of observing sperm motility as a marker of postthaw function.4
The ongoing evolution of cryopreservation science led to landmark achievements, including the first birth using human cryopreserved sperm in the 1950s, and the first human birth after embryo thaw in 1983. Since that time cryopreservation has become a cornerstone in the field of reproductive medicine.
Initial problems encountered with egg freezing
Although the first birth after thaw of a human oocyte occurred in 1986, oocyte cryopreservation was fraught with technical difficulties. Oocytes (vs sperm and embryos) proved challenging to successfully cryopreserve. The problem lay in the damage caused by water crystals forming ice and rising concentrations of intracellular solutes as cells were cooled to freezing temperatures.5 The large size and high water content of the human oocyte made it particularly vulnerable to the detrimental effects of freezing. In addition, freeze−thaw hardening of the zone pellucida led to decreased postthaw fertilization. The delicate meiotic spindle within the oocyte was prone to injury from ice crystals.6
Use of cryoprotectants, such as ethylene glycol, glycerol, and dimethylsulfoxide (DMSO), can prevent ice crystal formation, but high concentrations are theoretically toxic. The fine balance between protection and toxicity led to the development of diverse egg freezing protocols using various types and concentrations of cryoprotectants. Inconsistent results and lack of reproducibility among labs, together with concerns about postthaw oocyte function and safety, slowed the progression of oocyte freezing. By the end of the 1980s, clinical oocyte cryopreservation had been effectively halted and the field was confined to small groups of researchers who continued laboratory experiments with limited success.5
In 1997, clinical work with frozen oocytes resumed with a Bologna team reporting postthawing oocyte survival rates of up to 80% using propanediol as the primary cryoprotectant, and viable pregnancies with the use of intracytoplasmic sperm injection (ICSI) for fertilization.7,8 Since the late 1990s, further modifications in freezing technologies have resulted in greater success. And currently, both slow freezing and vitrification methods are used to preserve oocytes.
Slow freezing
Slow freezing involves a low rate of oocyte temperature decline with a simultaneous gradual increase in the concentration of cryoprotectants. As the metabolic activity of the oocyte decreases, the concentration of cryoprotectant can be increased to prevent ice crystal formation. Once solidification of the oocyte is achieved, the oocyte can be exposed to freezing at colder temperatures. Results of a meta-analysis of 26 studies revealed that, compared with using fresh oocytes, eggs thawed after slow-freezing yielded significantly lower rates of fertilization (61.0% [1,346/2,217] vs 76.7% [2,788/3,637]), clinical pregnancy rate per transfer (27.1% [95/351] vs 68.5% [272/397]), and live birth per transfer (21.6% [76/351] vs 32.4% [24/72]).9
Vitrification
Vitrification involves the rapid cooling of cells to extremely low temperatures. During vitrification, oocytes are exposed to high concentrations of cryoprotectants and, after a short equilibration time, rapidly cooled. The rate of cooling is dramatic, up to 20,000°C per minute—so fast that ice does not have time to form and a glass-like state is achieved within the oocyte. Studies suggest that the use of vitrification improves oocyte survival and function compared with slow freezing.9-11 A prospective randomized controlled trial comparing frozen/thawed with vitrified/warmed oocytes demonstrated superior oocyte function in the vitrification group, with higher oocyte survival (81% for vitrification/warming vs 67% for slow freezing/thawing); higher rates of fertilization, cleavage, and embryo morphology; as well as higher clinical pregnancy rates (38% for vitrified/warmed vs 13% for frozen/thawed).10
The Practice Committee of ASRM published a guideline for mature cryopreservation in 2013.2 The committee reviewed the literature on efficacy and safety of mature oocyte cryopreservation. Although data are limited, studies comparing outcomes of IVF using cryopreserved versus fresh oocytes, including four randomized controlled trials and a meta-analysis, provide evidence that previously vitrified/thawed eggs result in similar fertilization and pregnancy rates as IVF/ICSI with fresh oocytes. Similar to data from fresh IVF cycles, decreased success with oocyte vitrification is seen in women with advanced age, and delivery rates, not unexpectedly, are inversely correlated with maternal age.12
Safety outcomes data are limited but reassuring
Two major factors limit our current understanding of egg cryopreservation outcomes. First, many women who have cryopreserved their eggs have not yet used them and, second, babies born after use of cryopreserved oocytes have not reached ages in which safety of the technique can be fully evaluated. Despite this important gap in our knowledge, to date, results of studies examining safety outcomes of the procedure have been reassuring.
For instance, chromosomal analysis via fluorescence in-situ hybridization of embryos created with thawed oocytes versus controls revealed no difference in the incidence of chromosomal abnormalities, decreasing concerns about damage to the oocyte spindle secondary to freezing.13
Data from a review of 900 live births resulting from embryos created from thawed oocytes frozen via the slow freeze technique revealed no increase in the risk of congenital anomalies.14 Similarly, no increased risk of congenital anomalies or difference in birth weights was noted in a study of 200 live births after transfers with embryos derived from vitrified oocytes compared with fresh oocytes.15
In a study of 954 clinical pregnancies occurring in 855 couples with cryopreserved oocytes after assisted reproductive technology, the outcomes of 197 pregnancies from frozen/thawed oocytes were compared with 757 obtained from fresh sibling oocyte cycles. A significantly higher rate of spontaneous abortions at 12 weeks or less was observed in the frozen/thawed oocyte group. No statistically significant differences were noted in gestational age at delivery or in the incidence of major congenital anomalies at birth, but mean birth weights were significantly lower in fresh oocyte pregnancies. Interestingly, in the group of 63 women who had pregnancies derived from both fresh and thawed oocytes, no differences were noted in the abortion rate or mean birth weight.16
We can freeze eggs, but when should we?
Based on media presentations and professional perspectives, it appears that many people differentiate between “medical” and “social” egg freezing.
Medical versus social freezing
Medical egg freezing is done when there is an immediate medical need to preserve fertility, such as before cancer treatment when the woman can’t reproduce now and will have reduced or no capacity later. Social freezing, on the other hand, occurs when there is no immediate need, such as when there is a desire to delay parenthood so that educational, professional, or other goals can be met. The difference is important because medical freezing is usually seen as a “need” and is therefore acceptable, whereas social freezing is elective or a “wish” and therefore is questionable.17
The labels are important for both ethical and political reasons because most people would consider medical freezing to be ethically acceptable and also worthy of societal support, perhaps even financial coverage, while some might consider social freezing to be neither ethically acceptable nor worthy of coverage.
What’s the difference?
But is the difference really all that clear? If a woman has a mother and a sister who have undergone premature menopause in their 30s and she now has signs of diminishing ovarian reserve in her late 20s, would a desire to freeze eggs be medical or social? She has no immediate need for treatment but a reasonable expectation of need later. One could argue that she should go to a sperm bank now if she has no partner, or change her life plans—but is this a reasonable expectation? If a woman is perfectly healthy but her husband has severe sperm problems and she elects IVF to treat male-factor infertility, is it medical or social? There are many situations in which it is unclear whether the reason for egg freezing would be medical or social.
Does it matter?
In any event, are social reasons to freeze eggs not legitimate? Many would argue that medical services should be used to treat diseases, not social causes. Yet we use medicine all the time to treat problems caused by social factors (obesity, depression, anxiety).
Some would argue that it is a personal decision to delay reproduction, and that health problems caused by personal decisions do not merit medical intervention. However, it is common to provide medical services to people who require the services only because of personal decisions—for instance, professional and amateur athletes who injure themselves pursuing activities for compensation or pleasure, or smokers or persons with alcoholism.
Others have argued that social freezing is inappropriate because it is only being done to avoid the consequences of aging, and that its need could be avoided by not waiting too long to get pregnant. But we treat many conditions that occur primarily as a result of aging (hypertension, dementia, poor eyesight).
Because it has become generally accepted to treat older women with diminished ovarian reserve and infertility, why is it inappropriate to treat women—when they are younger—with egg freezing to mitigate the impact of aging on reproductive performance that we know will occur later? If we could prevent or limit the impact of aging on the cardiovascular or neurologic systems by interventions earlier in a person’s life, would we not provide that medical service? Do we not provide statins and other medications to delay or limit the sequelae of aging? What is the difference with egg freezing?
Therefore, could it be discriminatory not to consider egg freezing ethical and acceptable, even if the reason for the procedure is considered social? Why should egg freezing for social reasons not be acceptable and widely available?
Who should pay for egg freezing?
Even if egg freezing performed because of social reasons is considered ethical and is supported by society, it does not necessarily follow that it will or should be paid for by society. The creation of policies determining coverage for health-care services is a complex process and is based on overall societal needs, economic capabilities, and relative social value of the services. Because infertility carries such a large personal burden and childbearing is so essential to any society, one can argue that infertility, per se, should be covered by society and, in the United States, its surrogate employers and insurance companies. This is often not the case, however. So, while it can be argued that egg freezing should be covered by insurance for both medical and social reasons, even the success of that argument does not mean it will be so in the current US health-care system.
Because egg freezing involves two major steps: (1) ovarian stimulation, egg retrieval, egg freezing, and egg storage followed at a later date by (2) egg thawing, fertilization, embryo culture, and embryo replacement in the uterus, what would be socially justified coverage of egg freezing? Society could cover just the first step or just the second, or both. Such decisions would depend on an assessment of the social benefit from coverage of these services. Such analysis is not yet available because of limited experience.
Is the cost worth it?
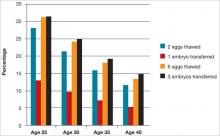
A major issue for women considering egg freezing for social reasons is whether a sufficient number of eggs will be retrieved to provide a reasonable chance for pregnancy later when they are used. The FIGURE illustrates the probability of a live birth after egg freezing. It should be noted that while most, but not all, eggs survive thawing after vitrification, not all eggs will become fertilized. Only about half of the fertilized eggs will grow to a day 3 embryo, and not all of those embryos will be viable. Therefore, constant reproductive loss occurs after the eggs are retrieved.
Furthermore, even after embryo replacement, pregnancy does not occur in every case, and some pregnancies are lost to miscarriage as well as other complications of pregnancy and childbirth. The FIGURE shows that a 25-year-old woman with 12 eggs frozen would have an estimated pregnancy rate much greater than 50%. However, the numbers also indicate that egg freezing is not very successful for older women who, at this time, constitute many of those considering the procedure.18
Another consideration is that a significant, but currently unknown, number of women who freeze their eggs will never use them for a variety of reasons. This is especially true of younger women, for whom many of the factors determining their eventual reproductive life might well change. They may eventually decide not to have children or they might become pregnant naturally or after fertility treatments that are cheaper than using the frozen eggs.
A $200,000 price tag?
Let’s consider the near 20% estimated pregnancy rate for age 35 in the FIGURE. If only half of the women aged 35 who freeze 6 eggs eventually use them (but, again, only about 20% have a baby), it means that only one of every 10 women who freeze their eggs eventually will have a baby as a result of the procedure. The number needed to treat (NNT) is therefore 10, and if the cost is $20,000 for the egg freezing procedure and storage over 5 to 10 years, the overall cost per baby born is about $200,000. If 12 eggs are frozen, the cost is $100,000. This clearly is a significant cost, and a greater cost than most other fertility treatments to achieve a baby, even in the older population. Therefore, the cost-effectiveness of social egg freezing is yet to be determined.
What should we do as we move forward?
Abandon the medical versus social rhetoric
It is difficult to argue against egg freezing for medical reasons, and the distinction between medical and social freezing is largely an artificial construct. In general, therefore, the differentiation between medical and social egg freezing should be abandoned, and egg freezing to preserve future fertility should be considered ethical for whatever reasons.
Consider the ideal time frame for health insurance coverage of egg freezing
That does not mean that egg freezing should always be reimbursed. The decision for coverage by employers, insurers, and other payers should be based on a cost–benefit analysis of the social benefit, individual benefit, biological chances of success, probability that the frozen eggs will be used, medical risks/sequelae, and the financial costs. Therefore, whether or not egg freezing for fertility preservation is covered will vary among countries and even within countries and among different individuals. Such an approach to coverage should apply to all medical interventions, including both medical and social egg freezing.
This approach could possibly result in findings and resulting policies that do not cover egg freezing before age 30 because too few women will return to use their eggs, or after age 38 because the chances of success are too low. Other instances of freezing should not be forbidden but would not be reimbursed by public or payer money.17
Many considerations must go into the development of social, professional, and payment policies. Policies that are seen as family-friendly that promote childbearing, especially at an earlier age, can be seen as limiting women’s academic and career opportunities and therefore women-unfriendly. Policies supporting women’s reproductive autonomy and ability to delay childbearing can be seen as women- but not family-friendly. Therefore, reproductive policies affect not only the individual woman but also society, its demographics, politics, and economics.17
The future
The new technology of egg freezing is a wonderful advance for many people. We are learning innovative ways to apply this technology for both infertile and noninfertile people. Research, better evidence, public education, informed consent, ethical practice of medicine, societal support for reproductive rights, and consideration of patient autonomy and social justice will enable us to optimize egg freezing as a treatment intervention.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886.
2. Practice Committees of the American Society of Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43.
3. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666.
4. Gook D. History of oocyte cryopreservation. Reprod Biomed Online. 2011;23(3):281−289.
5. Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96(2):264−268.
6. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update. 2003;9(5):463–470.
7. Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–726.
8. Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–416.
9. Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80.
10. Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservationwith slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095.
11. Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod Update. 2007;13(6):591–605.
12. Rienzi L, Cobo A, Paffoni A, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612.
13. Cobo A, Rubio C, Gerli S, Ruiz A, Pellicer A, Remohi J. Use of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytes. Fertil Steril. 2001;75(2):354–360.
14. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–776.
15. Chian RC, Huang JY, Tan SL, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16(5):608–610.
16. Levi Setti P, Albani E, Morenghi E, et al. Comparative analysis of fetal and neonatal outcomes of pregnancies from fresh and cryopreserved/thawed oocytes in the same group of patients. Fertil Steril. 2013;100(2):396–401.
17. Pennings G. Ethical aspects of social freezing. Gynecol Obstet Fertil. 2013;41(9):521–523.
18. Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):492–499.
The first human birth from a frozen oocyte was reported in 1986.1 Nearly 3 decades later, mature oocyte cryopreservation has emerged as a meaningful technology to preserve reproductive potential in women of reproductive age. In 2013, the American Society for Reproductive Medicine (ASRM) removed the “experimental” label from egg freezing but cautioned that more data on safety and efficacy were needed prior to widespread adoption of the technique.2
In this Update, we present the current protocols for oocyte cryopreservation, how we arrived at them, and the questions regarding outcomes that still remain. In addition, we discuss the ethical dilemmas egg freezing presents, according to the varying rhetoric within the media and our own profession. Finally, we consider what preliminary data suggest as to the live-birth rate using frozen eggs from women of varying ages and what the costs are associated with using oocyte cryopreservation as the approach to fertility treatment.
Vitrification and slow freezing: How did we get here and how effective are they?
Fertility preservation is a rapidly advancing area of reproductive medicine. Cryopreservation is the cooling of cells to subzero temperatures to halt biologic activity and preserve the cells for future use. Clinically, oocyte cryopreservation requires a patient to undergo in vitro fertilization (IVF). After egg retrieval, the oocytes are cryopreserved for use at a later time.
The prefix “cryo” originated from the Greek word “kryos,” meaning icy cold or frost. Cryopreservation is not a new science. In 1776, the Italian priest and scientist Lazzaro Spallanzani reported that sperm became motionless when cooled by snow. A pivotal discovery in the field came in 1949, when Christopher Polge, an English scientist, showed that glycerol, a permeating solute, could provide protection to cells at low temperatures.3 Progress in sperm cryopreservation advanced quickly, partly due to the ease of observing sperm motility as a marker of postthaw function.4
The ongoing evolution of cryopreservation science led to landmark achievements, including the first birth using human cryopreserved sperm in the 1950s, and the first human birth after embryo thaw in 1983. Since that time cryopreservation has become a cornerstone in the field of reproductive medicine.
Initial problems encountered with egg freezing
Although the first birth after thaw of a human oocyte occurred in 1986, oocyte cryopreservation was fraught with technical difficulties. Oocytes (vs sperm and embryos) proved challenging to successfully cryopreserve. The problem lay in the damage caused by water crystals forming ice and rising concentrations of intracellular solutes as cells were cooled to freezing temperatures.5 The large size and high water content of the human oocyte made it particularly vulnerable to the detrimental effects of freezing. In addition, freeze−thaw hardening of the zone pellucida led to decreased postthaw fertilization. The delicate meiotic spindle within the oocyte was prone to injury from ice crystals.6
Use of cryoprotectants, such as ethylene glycol, glycerol, and dimethylsulfoxide (DMSO), can prevent ice crystal formation, but high concentrations are theoretically toxic. The fine balance between protection and toxicity led to the development of diverse egg freezing protocols using various types and concentrations of cryoprotectants. Inconsistent results and lack of reproducibility among labs, together with concerns about postthaw oocyte function and safety, slowed the progression of oocyte freezing. By the end of the 1980s, clinical oocyte cryopreservation had been effectively halted and the field was confined to small groups of researchers who continued laboratory experiments with limited success.5
In 1997, clinical work with frozen oocytes resumed with a Bologna team reporting postthawing oocyte survival rates of up to 80% using propanediol as the primary cryoprotectant, and viable pregnancies with the use of intracytoplasmic sperm injection (ICSI) for fertilization.7,8 Since the late 1990s, further modifications in freezing technologies have resulted in greater success. And currently, both slow freezing and vitrification methods are used to preserve oocytes.
Slow freezing
Slow freezing involves a low rate of oocyte temperature decline with a simultaneous gradual increase in the concentration of cryoprotectants. As the metabolic activity of the oocyte decreases, the concentration of cryoprotectant can be increased to prevent ice crystal formation. Once solidification of the oocyte is achieved, the oocyte can be exposed to freezing at colder temperatures. Results of a meta-analysis of 26 studies revealed that, compared with using fresh oocytes, eggs thawed after slow-freezing yielded significantly lower rates of fertilization (61.0% [1,346/2,217] vs 76.7% [2,788/3,637]), clinical pregnancy rate per transfer (27.1% [95/351] vs 68.5% [272/397]), and live birth per transfer (21.6% [76/351] vs 32.4% [24/72]).9
Vitrification
Vitrification involves the rapid cooling of cells to extremely low temperatures. During vitrification, oocytes are exposed to high concentrations of cryoprotectants and, after a short equilibration time, rapidly cooled. The rate of cooling is dramatic, up to 20,000°C per minute—so fast that ice does not have time to form and a glass-like state is achieved within the oocyte. Studies suggest that the use of vitrification improves oocyte survival and function compared with slow freezing.9-11 A prospective randomized controlled trial comparing frozen/thawed with vitrified/warmed oocytes demonstrated superior oocyte function in the vitrification group, with higher oocyte survival (81% for vitrification/warming vs 67% for slow freezing/thawing); higher rates of fertilization, cleavage, and embryo morphology; as well as higher clinical pregnancy rates (38% for vitrified/warmed vs 13% for frozen/thawed).10
The Practice Committee of ASRM published a guideline for mature cryopreservation in 2013.2 The committee reviewed the literature on efficacy and safety of mature oocyte cryopreservation. Although data are limited, studies comparing outcomes of IVF using cryopreserved versus fresh oocytes, including four randomized controlled trials and a meta-analysis, provide evidence that previously vitrified/thawed eggs result in similar fertilization and pregnancy rates as IVF/ICSI with fresh oocytes. Similar to data from fresh IVF cycles, decreased success with oocyte vitrification is seen in women with advanced age, and delivery rates, not unexpectedly, are inversely correlated with maternal age.12
Safety outcomes data are limited but reassuring
Two major factors limit our current understanding of egg cryopreservation outcomes. First, many women who have cryopreserved their eggs have not yet used them and, second, babies born after use of cryopreserved oocytes have not reached ages in which safety of the technique can be fully evaluated. Despite this important gap in our knowledge, to date, results of studies examining safety outcomes of the procedure have been reassuring.
For instance, chromosomal analysis via fluorescence in-situ hybridization of embryos created with thawed oocytes versus controls revealed no difference in the incidence of chromosomal abnormalities, decreasing concerns about damage to the oocyte spindle secondary to freezing.13
Data from a review of 900 live births resulting from embryos created from thawed oocytes frozen via the slow freeze technique revealed no increase in the risk of congenital anomalies.14 Similarly, no increased risk of congenital anomalies or difference in birth weights was noted in a study of 200 live births after transfers with embryos derived from vitrified oocytes compared with fresh oocytes.15
In a study of 954 clinical pregnancies occurring in 855 couples with cryopreserved oocytes after assisted reproductive technology, the outcomes of 197 pregnancies from frozen/thawed oocytes were compared with 757 obtained from fresh sibling oocyte cycles. A significantly higher rate of spontaneous abortions at 12 weeks or less was observed in the frozen/thawed oocyte group. No statistically significant differences were noted in gestational age at delivery or in the incidence of major congenital anomalies at birth, but mean birth weights were significantly lower in fresh oocyte pregnancies. Interestingly, in the group of 63 women who had pregnancies derived from both fresh and thawed oocytes, no differences were noted in the abortion rate or mean birth weight.16
We can freeze eggs, but when should we?
Based on media presentations and professional perspectives, it appears that many people differentiate between “medical” and “social” egg freezing.
Medical versus social freezing
Medical egg freezing is done when there is an immediate medical need to preserve fertility, such as before cancer treatment when the woman can’t reproduce now and will have reduced or no capacity later. Social freezing, on the other hand, occurs when there is no immediate need, such as when there is a desire to delay parenthood so that educational, professional, or other goals can be met. The difference is important because medical freezing is usually seen as a “need” and is therefore acceptable, whereas social freezing is elective or a “wish” and therefore is questionable.17
The labels are important for both ethical and political reasons because most people would consider medical freezing to be ethically acceptable and also worthy of societal support, perhaps even financial coverage, while some might consider social freezing to be neither ethically acceptable nor worthy of coverage.
What’s the difference?
But is the difference really all that clear? If a woman has a mother and a sister who have undergone premature menopause in their 30s and she now has signs of diminishing ovarian reserve in her late 20s, would a desire to freeze eggs be medical or social? She has no immediate need for treatment but a reasonable expectation of need later. One could argue that she should go to a sperm bank now if she has no partner, or change her life plans—but is this a reasonable expectation? If a woman is perfectly healthy but her husband has severe sperm problems and she elects IVF to treat male-factor infertility, is it medical or social? There are many situations in which it is unclear whether the reason for egg freezing would be medical or social.
Does it matter?
In any event, are social reasons to freeze eggs not legitimate? Many would argue that medical services should be used to treat diseases, not social causes. Yet we use medicine all the time to treat problems caused by social factors (obesity, depression, anxiety).
Some would argue that it is a personal decision to delay reproduction, and that health problems caused by personal decisions do not merit medical intervention. However, it is common to provide medical services to people who require the services only because of personal decisions—for instance, professional and amateur athletes who injure themselves pursuing activities for compensation or pleasure, or smokers or persons with alcoholism.
Others have argued that social freezing is inappropriate because it is only being done to avoid the consequences of aging, and that its need could be avoided by not waiting too long to get pregnant. But we treat many conditions that occur primarily as a result of aging (hypertension, dementia, poor eyesight).
Because it has become generally accepted to treat older women with diminished ovarian reserve and infertility, why is it inappropriate to treat women—when they are younger—with egg freezing to mitigate the impact of aging on reproductive performance that we know will occur later? If we could prevent or limit the impact of aging on the cardiovascular or neurologic systems by interventions earlier in a person’s life, would we not provide that medical service? Do we not provide statins and other medications to delay or limit the sequelae of aging? What is the difference with egg freezing?
Therefore, could it be discriminatory not to consider egg freezing ethical and acceptable, even if the reason for the procedure is considered social? Why should egg freezing for social reasons not be acceptable and widely available?
Who should pay for egg freezing?
Even if egg freezing performed because of social reasons is considered ethical and is supported by society, it does not necessarily follow that it will or should be paid for by society. The creation of policies determining coverage for health-care services is a complex process and is based on overall societal needs, economic capabilities, and relative social value of the services. Because infertility carries such a large personal burden and childbearing is so essential to any society, one can argue that infertility, per se, should be covered by society and, in the United States, its surrogate employers and insurance companies. This is often not the case, however. So, while it can be argued that egg freezing should be covered by insurance for both medical and social reasons, even the success of that argument does not mean it will be so in the current US health-care system.
Because egg freezing involves two major steps: (1) ovarian stimulation, egg retrieval, egg freezing, and egg storage followed at a later date by (2) egg thawing, fertilization, embryo culture, and embryo replacement in the uterus, what would be socially justified coverage of egg freezing? Society could cover just the first step or just the second, or both. Such decisions would depend on an assessment of the social benefit from coverage of these services. Such analysis is not yet available because of limited experience.
Is the cost worth it?
A major issue for women considering egg freezing for social reasons is whether a sufficient number of eggs will be retrieved to provide a reasonable chance for pregnancy later when they are used. The FIGURE illustrates the probability of a live birth after egg freezing. It should be noted that while most, but not all, eggs survive thawing after vitrification, not all eggs will become fertilized. Only about half of the fertilized eggs will grow to a day 3 embryo, and not all of those embryos will be viable. Therefore, constant reproductive loss occurs after the eggs are retrieved.
Furthermore, even after embryo replacement, pregnancy does not occur in every case, and some pregnancies are lost to miscarriage as well as other complications of pregnancy and childbirth. The FIGURE shows that a 25-year-old woman with 12 eggs frozen would have an estimated pregnancy rate much greater than 50%. However, the numbers also indicate that egg freezing is not very successful for older women who, at this time, constitute many of those considering the procedure.18
Another consideration is that a significant, but currently unknown, number of women who freeze their eggs will never use them for a variety of reasons. This is especially true of younger women, for whom many of the factors determining their eventual reproductive life might well change. They may eventually decide not to have children or they might become pregnant naturally or after fertility treatments that are cheaper than using the frozen eggs.
A $200,000 price tag?
Let’s consider the near 20% estimated pregnancy rate for age 35 in the FIGURE. If only half of the women aged 35 who freeze 6 eggs eventually use them (but, again, only about 20% have a baby), it means that only one of every 10 women who freeze their eggs eventually will have a baby as a result of the procedure. The number needed to treat (NNT) is therefore 10, and if the cost is $20,000 for the egg freezing procedure and storage over 5 to 10 years, the overall cost per baby born is about $200,000. If 12 eggs are frozen, the cost is $100,000. This clearly is a significant cost, and a greater cost than most other fertility treatments to achieve a baby, even in the older population. Therefore, the cost-effectiveness of social egg freezing is yet to be determined.
What should we do as we move forward?
Abandon the medical versus social rhetoric
It is difficult to argue against egg freezing for medical reasons, and the distinction between medical and social freezing is largely an artificial construct. In general, therefore, the differentiation between medical and social egg freezing should be abandoned, and egg freezing to preserve future fertility should be considered ethical for whatever reasons.
Consider the ideal time frame for health insurance coverage of egg freezing
That does not mean that egg freezing should always be reimbursed. The decision for coverage by employers, insurers, and other payers should be based on a cost–benefit analysis of the social benefit, individual benefit, biological chances of success, probability that the frozen eggs will be used, medical risks/sequelae, and the financial costs. Therefore, whether or not egg freezing for fertility preservation is covered will vary among countries and even within countries and among different individuals. Such an approach to coverage should apply to all medical interventions, including both medical and social egg freezing.
This approach could possibly result in findings and resulting policies that do not cover egg freezing before age 30 because too few women will return to use their eggs, or after age 38 because the chances of success are too low. Other instances of freezing should not be forbidden but would not be reimbursed by public or payer money.17
Many considerations must go into the development of social, professional, and payment policies. Policies that are seen as family-friendly that promote childbearing, especially at an earlier age, can be seen as limiting women’s academic and career opportunities and therefore women-unfriendly. Policies supporting women’s reproductive autonomy and ability to delay childbearing can be seen as women- but not family-friendly. Therefore, reproductive policies affect not only the individual woman but also society, its demographics, politics, and economics.17
The future
The new technology of egg freezing is a wonderful advance for many people. We are learning innovative ways to apply this technology for both infertile and noninfertile people. Research, better evidence, public education, informed consent, ethical practice of medicine, societal support for reproductive rights, and consideration of patient autonomy and social justice will enable us to optimize egg freezing as a treatment intervention.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The first human birth from a frozen oocyte was reported in 1986.1 Nearly 3 decades later, mature oocyte cryopreservation has emerged as a meaningful technology to preserve reproductive potential in women of reproductive age. In 2013, the American Society for Reproductive Medicine (ASRM) removed the “experimental” label from egg freezing but cautioned that more data on safety and efficacy were needed prior to widespread adoption of the technique.2
In this Update, we present the current protocols for oocyte cryopreservation, how we arrived at them, and the questions regarding outcomes that still remain. In addition, we discuss the ethical dilemmas egg freezing presents, according to the varying rhetoric within the media and our own profession. Finally, we consider what preliminary data suggest as to the live-birth rate using frozen eggs from women of varying ages and what the costs are associated with using oocyte cryopreservation as the approach to fertility treatment.
Vitrification and slow freezing: How did we get here and how effective are they?
Fertility preservation is a rapidly advancing area of reproductive medicine. Cryopreservation is the cooling of cells to subzero temperatures to halt biologic activity and preserve the cells for future use. Clinically, oocyte cryopreservation requires a patient to undergo in vitro fertilization (IVF). After egg retrieval, the oocytes are cryopreserved for use at a later time.
The prefix “cryo” originated from the Greek word “kryos,” meaning icy cold or frost. Cryopreservation is not a new science. In 1776, the Italian priest and scientist Lazzaro Spallanzani reported that sperm became motionless when cooled by snow. A pivotal discovery in the field came in 1949, when Christopher Polge, an English scientist, showed that glycerol, a permeating solute, could provide protection to cells at low temperatures.3 Progress in sperm cryopreservation advanced quickly, partly due to the ease of observing sperm motility as a marker of postthaw function.4
The ongoing evolution of cryopreservation science led to landmark achievements, including the first birth using human cryopreserved sperm in the 1950s, and the first human birth after embryo thaw in 1983. Since that time cryopreservation has become a cornerstone in the field of reproductive medicine.
Initial problems encountered with egg freezing
Although the first birth after thaw of a human oocyte occurred in 1986, oocyte cryopreservation was fraught with technical difficulties. Oocytes (vs sperm and embryos) proved challenging to successfully cryopreserve. The problem lay in the damage caused by water crystals forming ice and rising concentrations of intracellular solutes as cells were cooled to freezing temperatures.5 The large size and high water content of the human oocyte made it particularly vulnerable to the detrimental effects of freezing. In addition, freeze−thaw hardening of the zone pellucida led to decreased postthaw fertilization. The delicate meiotic spindle within the oocyte was prone to injury from ice crystals.6
Use of cryoprotectants, such as ethylene glycol, glycerol, and dimethylsulfoxide (DMSO), can prevent ice crystal formation, but high concentrations are theoretically toxic. The fine balance between protection and toxicity led to the development of diverse egg freezing protocols using various types and concentrations of cryoprotectants. Inconsistent results and lack of reproducibility among labs, together with concerns about postthaw oocyte function and safety, slowed the progression of oocyte freezing. By the end of the 1980s, clinical oocyte cryopreservation had been effectively halted and the field was confined to small groups of researchers who continued laboratory experiments with limited success.5
In 1997, clinical work with frozen oocytes resumed with a Bologna team reporting postthawing oocyte survival rates of up to 80% using propanediol as the primary cryoprotectant, and viable pregnancies with the use of intracytoplasmic sperm injection (ICSI) for fertilization.7,8 Since the late 1990s, further modifications in freezing technologies have resulted in greater success. And currently, both slow freezing and vitrification methods are used to preserve oocytes.
Slow freezing
Slow freezing involves a low rate of oocyte temperature decline with a simultaneous gradual increase in the concentration of cryoprotectants. As the metabolic activity of the oocyte decreases, the concentration of cryoprotectant can be increased to prevent ice crystal formation. Once solidification of the oocyte is achieved, the oocyte can be exposed to freezing at colder temperatures. Results of a meta-analysis of 26 studies revealed that, compared with using fresh oocytes, eggs thawed after slow-freezing yielded significantly lower rates of fertilization (61.0% [1,346/2,217] vs 76.7% [2,788/3,637]), clinical pregnancy rate per transfer (27.1% [95/351] vs 68.5% [272/397]), and live birth per transfer (21.6% [76/351] vs 32.4% [24/72]).9
Vitrification
Vitrification involves the rapid cooling of cells to extremely low temperatures. During vitrification, oocytes are exposed to high concentrations of cryoprotectants and, after a short equilibration time, rapidly cooled. The rate of cooling is dramatic, up to 20,000°C per minute—so fast that ice does not have time to form and a glass-like state is achieved within the oocyte. Studies suggest that the use of vitrification improves oocyte survival and function compared with slow freezing.9-11 A prospective randomized controlled trial comparing frozen/thawed with vitrified/warmed oocytes demonstrated superior oocyte function in the vitrification group, with higher oocyte survival (81% for vitrification/warming vs 67% for slow freezing/thawing); higher rates of fertilization, cleavage, and embryo morphology; as well as higher clinical pregnancy rates (38% for vitrified/warmed vs 13% for frozen/thawed).10
The Practice Committee of ASRM published a guideline for mature cryopreservation in 2013.2 The committee reviewed the literature on efficacy and safety of mature oocyte cryopreservation. Although data are limited, studies comparing outcomes of IVF using cryopreserved versus fresh oocytes, including four randomized controlled trials and a meta-analysis, provide evidence that previously vitrified/thawed eggs result in similar fertilization and pregnancy rates as IVF/ICSI with fresh oocytes. Similar to data from fresh IVF cycles, decreased success with oocyte vitrification is seen in women with advanced age, and delivery rates, not unexpectedly, are inversely correlated with maternal age.12
Safety outcomes data are limited but reassuring
Two major factors limit our current understanding of egg cryopreservation outcomes. First, many women who have cryopreserved their eggs have not yet used them and, second, babies born after use of cryopreserved oocytes have not reached ages in which safety of the technique can be fully evaluated. Despite this important gap in our knowledge, to date, results of studies examining safety outcomes of the procedure have been reassuring.
For instance, chromosomal analysis via fluorescence in-situ hybridization of embryos created with thawed oocytes versus controls revealed no difference in the incidence of chromosomal abnormalities, decreasing concerns about damage to the oocyte spindle secondary to freezing.13
Data from a review of 900 live births resulting from embryos created from thawed oocytes frozen via the slow freeze technique revealed no increase in the risk of congenital anomalies.14 Similarly, no increased risk of congenital anomalies or difference in birth weights was noted in a study of 200 live births after transfers with embryos derived from vitrified oocytes compared with fresh oocytes.15
In a study of 954 clinical pregnancies occurring in 855 couples with cryopreserved oocytes after assisted reproductive technology, the outcomes of 197 pregnancies from frozen/thawed oocytes were compared with 757 obtained from fresh sibling oocyte cycles. A significantly higher rate of spontaneous abortions at 12 weeks or less was observed in the frozen/thawed oocyte group. No statistically significant differences were noted in gestational age at delivery or in the incidence of major congenital anomalies at birth, but mean birth weights were significantly lower in fresh oocyte pregnancies. Interestingly, in the group of 63 women who had pregnancies derived from both fresh and thawed oocytes, no differences were noted in the abortion rate or mean birth weight.16
We can freeze eggs, but when should we?
Based on media presentations and professional perspectives, it appears that many people differentiate between “medical” and “social” egg freezing.
Medical versus social freezing
Medical egg freezing is done when there is an immediate medical need to preserve fertility, such as before cancer treatment when the woman can’t reproduce now and will have reduced or no capacity later. Social freezing, on the other hand, occurs when there is no immediate need, such as when there is a desire to delay parenthood so that educational, professional, or other goals can be met. The difference is important because medical freezing is usually seen as a “need” and is therefore acceptable, whereas social freezing is elective or a “wish” and therefore is questionable.17
The labels are important for both ethical and political reasons because most people would consider medical freezing to be ethically acceptable and also worthy of societal support, perhaps even financial coverage, while some might consider social freezing to be neither ethically acceptable nor worthy of coverage.
What’s the difference?
But is the difference really all that clear? If a woman has a mother and a sister who have undergone premature menopause in their 30s and she now has signs of diminishing ovarian reserve in her late 20s, would a desire to freeze eggs be medical or social? She has no immediate need for treatment but a reasonable expectation of need later. One could argue that she should go to a sperm bank now if she has no partner, or change her life plans—but is this a reasonable expectation? If a woman is perfectly healthy but her husband has severe sperm problems and she elects IVF to treat male-factor infertility, is it medical or social? There are many situations in which it is unclear whether the reason for egg freezing would be medical or social.
Does it matter?
In any event, are social reasons to freeze eggs not legitimate? Many would argue that medical services should be used to treat diseases, not social causes. Yet we use medicine all the time to treat problems caused by social factors (obesity, depression, anxiety).
Some would argue that it is a personal decision to delay reproduction, and that health problems caused by personal decisions do not merit medical intervention. However, it is common to provide medical services to people who require the services only because of personal decisions—for instance, professional and amateur athletes who injure themselves pursuing activities for compensation or pleasure, or smokers or persons with alcoholism.
Others have argued that social freezing is inappropriate because it is only being done to avoid the consequences of aging, and that its need could be avoided by not waiting too long to get pregnant. But we treat many conditions that occur primarily as a result of aging (hypertension, dementia, poor eyesight).
Because it has become generally accepted to treat older women with diminished ovarian reserve and infertility, why is it inappropriate to treat women—when they are younger—with egg freezing to mitigate the impact of aging on reproductive performance that we know will occur later? If we could prevent or limit the impact of aging on the cardiovascular or neurologic systems by interventions earlier in a person’s life, would we not provide that medical service? Do we not provide statins and other medications to delay or limit the sequelae of aging? What is the difference with egg freezing?
Therefore, could it be discriminatory not to consider egg freezing ethical and acceptable, even if the reason for the procedure is considered social? Why should egg freezing for social reasons not be acceptable and widely available?
Who should pay for egg freezing?
Even if egg freezing performed because of social reasons is considered ethical and is supported by society, it does not necessarily follow that it will or should be paid for by society. The creation of policies determining coverage for health-care services is a complex process and is based on overall societal needs, economic capabilities, and relative social value of the services. Because infertility carries such a large personal burden and childbearing is so essential to any society, one can argue that infertility, per se, should be covered by society and, in the United States, its surrogate employers and insurance companies. This is often not the case, however. So, while it can be argued that egg freezing should be covered by insurance for both medical and social reasons, even the success of that argument does not mean it will be so in the current US health-care system.
Because egg freezing involves two major steps: (1) ovarian stimulation, egg retrieval, egg freezing, and egg storage followed at a later date by (2) egg thawing, fertilization, embryo culture, and embryo replacement in the uterus, what would be socially justified coverage of egg freezing? Society could cover just the first step or just the second, or both. Such decisions would depend on an assessment of the social benefit from coverage of these services. Such analysis is not yet available because of limited experience.
Is the cost worth it?
A major issue for women considering egg freezing for social reasons is whether a sufficient number of eggs will be retrieved to provide a reasonable chance for pregnancy later when they are used. The FIGURE illustrates the probability of a live birth after egg freezing. It should be noted that while most, but not all, eggs survive thawing after vitrification, not all eggs will become fertilized. Only about half of the fertilized eggs will grow to a day 3 embryo, and not all of those embryos will be viable. Therefore, constant reproductive loss occurs after the eggs are retrieved.
Furthermore, even after embryo replacement, pregnancy does not occur in every case, and some pregnancies are lost to miscarriage as well as other complications of pregnancy and childbirth. The FIGURE shows that a 25-year-old woman with 12 eggs frozen would have an estimated pregnancy rate much greater than 50%. However, the numbers also indicate that egg freezing is not very successful for older women who, at this time, constitute many of those considering the procedure.18
Another consideration is that a significant, but currently unknown, number of women who freeze their eggs will never use them for a variety of reasons. This is especially true of younger women, for whom many of the factors determining their eventual reproductive life might well change. They may eventually decide not to have children or they might become pregnant naturally or after fertility treatments that are cheaper than using the frozen eggs.
A $200,000 price tag?
Let’s consider the near 20% estimated pregnancy rate for age 35 in the FIGURE. If only half of the women aged 35 who freeze 6 eggs eventually use them (but, again, only about 20% have a baby), it means that only one of every 10 women who freeze their eggs eventually will have a baby as a result of the procedure. The number needed to treat (NNT) is therefore 10, and if the cost is $20,000 for the egg freezing procedure and storage over 5 to 10 years, the overall cost per baby born is about $200,000. If 12 eggs are frozen, the cost is $100,000. This clearly is a significant cost, and a greater cost than most other fertility treatments to achieve a baby, even in the older population. Therefore, the cost-effectiveness of social egg freezing is yet to be determined.
What should we do as we move forward?
Abandon the medical versus social rhetoric
It is difficult to argue against egg freezing for medical reasons, and the distinction between medical and social freezing is largely an artificial construct. In general, therefore, the differentiation between medical and social egg freezing should be abandoned, and egg freezing to preserve future fertility should be considered ethical for whatever reasons.
Consider the ideal time frame for health insurance coverage of egg freezing
That does not mean that egg freezing should always be reimbursed. The decision for coverage by employers, insurers, and other payers should be based on a cost–benefit analysis of the social benefit, individual benefit, biological chances of success, probability that the frozen eggs will be used, medical risks/sequelae, and the financial costs. Therefore, whether or not egg freezing for fertility preservation is covered will vary among countries and even within countries and among different individuals. Such an approach to coverage should apply to all medical interventions, including both medical and social egg freezing.
This approach could possibly result in findings and resulting policies that do not cover egg freezing before age 30 because too few women will return to use their eggs, or after age 38 because the chances of success are too low. Other instances of freezing should not be forbidden but would not be reimbursed by public or payer money.17
Many considerations must go into the development of social, professional, and payment policies. Policies that are seen as family-friendly that promote childbearing, especially at an earlier age, can be seen as limiting women’s academic and career opportunities and therefore women-unfriendly. Policies supporting women’s reproductive autonomy and ability to delay childbearing can be seen as women- but not family-friendly. Therefore, reproductive policies affect not only the individual woman but also society, its demographics, politics, and economics.17
The future
The new technology of egg freezing is a wonderful advance for many people. We are learning innovative ways to apply this technology for both infertile and noninfertile people. Research, better evidence, public education, informed consent, ethical practice of medicine, societal support for reproductive rights, and consideration of patient autonomy and social justice will enable us to optimize egg freezing as a treatment intervention.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886.
2. Practice Committees of the American Society of Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43.
3. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666.
4. Gook D. History of oocyte cryopreservation. Reprod Biomed Online. 2011;23(3):281−289.
5. Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96(2):264−268.
6. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update. 2003;9(5):463–470.
7. Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–726.
8. Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–416.
9. Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80.
10. Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservationwith slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095.
11. Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod Update. 2007;13(6):591–605.
12. Rienzi L, Cobo A, Paffoni A, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612.
13. Cobo A, Rubio C, Gerli S, Ruiz A, Pellicer A, Remohi J. Use of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytes. Fertil Steril. 2001;75(2):354–360.
14. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–776.
15. Chian RC, Huang JY, Tan SL, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16(5):608–610.
16. Levi Setti P, Albani E, Morenghi E, et al. Comparative analysis of fetal and neonatal outcomes of pregnancies from fresh and cryopreserved/thawed oocytes in the same group of patients. Fertil Steril. 2013;100(2):396–401.
17. Pennings G. Ethical aspects of social freezing. Gynecol Obstet Fertil. 2013;41(9):521–523.
18. Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):492–499.
1. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886.
2. Practice Committees of the American Society of Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43.
3. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666.
4. Gook D. History of oocyte cryopreservation. Reprod Biomed Online. 2011;23(3):281−289.
5. Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96(2):264−268.
6. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update. 2003;9(5):463–470.
7. Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–726.
8. Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–416.
9. Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80.
10. Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservationwith slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095.
11. Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod Update. 2007;13(6):591–605.
12. Rienzi L, Cobo A, Paffoni A, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612.
13. Cobo A, Rubio C, Gerli S, Ruiz A, Pellicer A, Remohi J. Use of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytes. Fertil Steril. 2001;75(2):354–360.
14. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–776.
15. Chian RC, Huang JY, Tan SL, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16(5):608–610.
16. Levi Setti P, Albani E, Morenghi E, et al. Comparative analysis of fetal and neonatal outcomes of pregnancies from fresh and cryopreserved/thawed oocytes in the same group of patients. Fertil Steril. 2013;100(2):396–401.
17. Pennings G. Ethical aspects of social freezing. Gynecol Obstet Fertil. 2013;41(9):521–523.
18. Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):492–499.
IN THIS ARTICLE
-Vitrification and slow freezing: How did we get here and how effective are they?
-Safety outcomes data are limited but reassuring
-We can freeze eggs, but when should we?
-Who should pay for egg freezing?
-What should we do as we move forward?