User login
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).
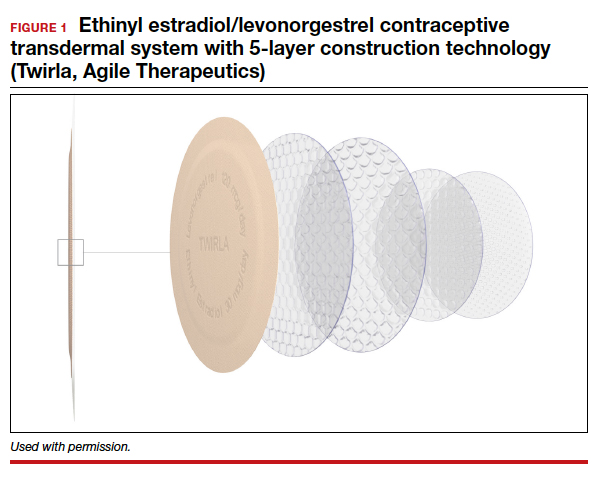
Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4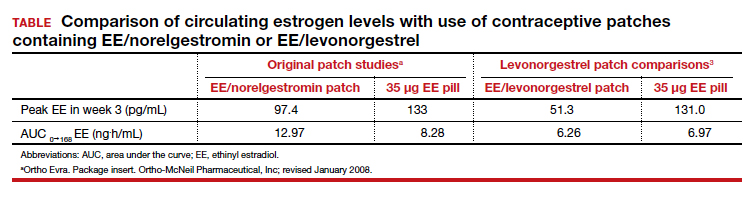
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6
Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.