User login
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
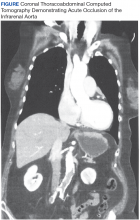
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.