User login
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
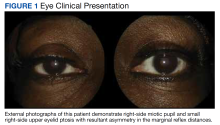
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
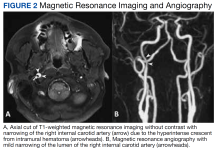
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
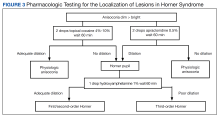
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281