User login
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
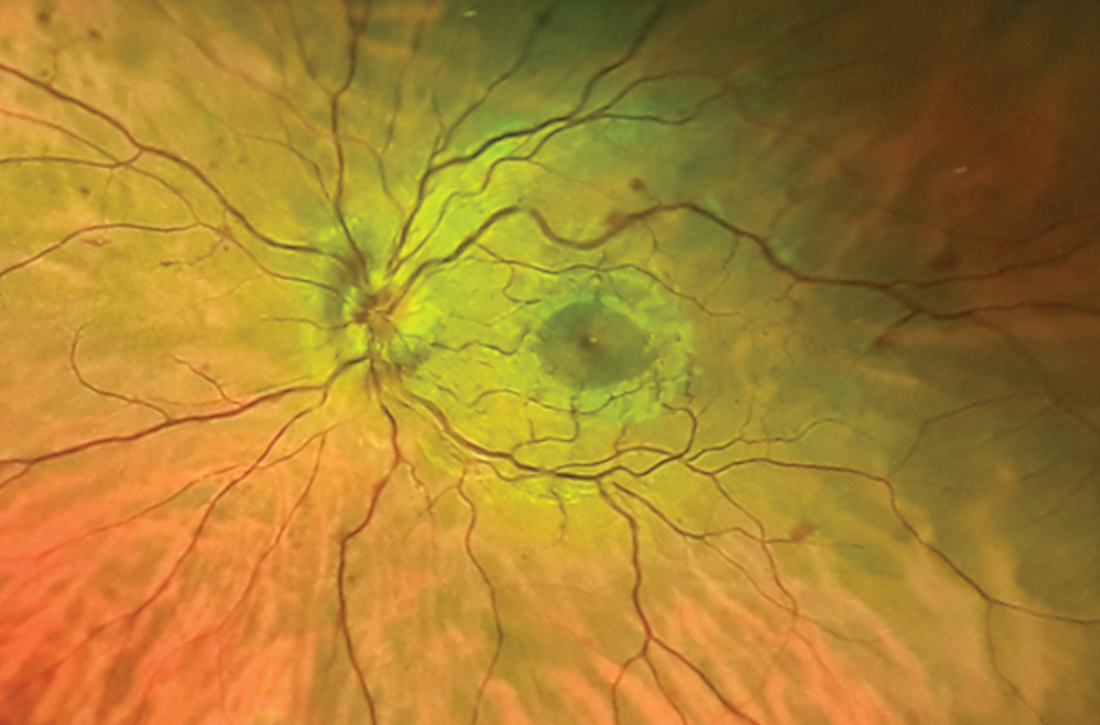
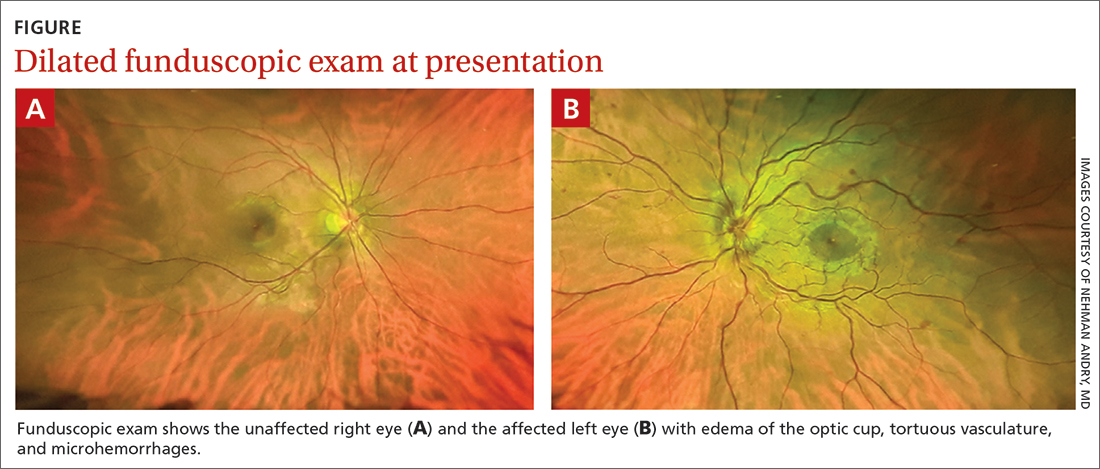
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.