User login
When treating patients who abuse substances, it is important to watch for underlying clinical conditions that have been suppressed, relieved, or muted by alcohol or drugs. Many of these conditions can be mistaken for signs of withdrawal, drug-seeking, or new conditions arising from loss of euphoria from the drug. Prompt recognition of these disorders and use of appropriate non-addictive treatments can prevent “against medical advice” discharges, relapses, and unneeded suffering in many cases.
Because the brain is the target organ, these conditions are either neurologic or psychiatric in nosology. Although psychiatric clinicians might not be familiar with neurologic conditions, quick recognition and treatment is necessary.
Restless legs syndrome and periodic limb movements of sleep
Restless legs syndrome (RLS) has 2 key components: paresthesia and akathisia. Although primarily involving the lower extremities, involvement also can include the upper extremities, torso, and head.
Paresthesia differs from typical neuropathies in that it usually is not painful; rather, patients describe an odd sensation using terms such as ticklish, “creepy-crawly,” and other uncomfortable sensations.
Akathisia is a motor restlessness and need to move. The patient might feel momentary relief by moving or rubbing the extremities, only to have the paresthesia return quickly followed by the akathisia. Generally, reclining is the most prominent position that produces symptoms, but they can occur while sitting.
The cause of RLS is an abnormality of central dopamine or iron, or both, in the substantia nigra; iron is a cofactor in dopamine synthesis. All RLS patients should have a serum ferritin level drawn and if <50 μg/dL, be treated with iron supplementation. Dopamine agonists, such as ropinirole, pramipexole, and carbidopa/levodopa, are effective (Table 1); other useful agents include benzodiazepines such as clonazepam and opioids such as hydrocodone.
When a patient withdraws from benzodiazepines or narcotics, RLS can emerge and cause suffering until it is diagnosed and treated. Typical myalgia in opioid withdrawal can confound the diagnosis. The immediate-release (IR) and extended-release (ER) formulations of gabapentin often are a good choice when treating benzodiazepine or narcotic withdrawal. The side effect profile of gabapentin is relatively benign, with somnolence often reported by non-substance abusers, but it is unlikely that addicts, who have grown tolerant to more potent agents such as benzodiazepines and opioids, will complain of sleepiness. Studies have shown that gabapentin is useful in managing withdrawal as well as anxiety and insomnia.1,2 A randomized trial showed that gabapentin increases abstinence rates and decreases heavy drinking.2 The agent has a short half-life (5 to 7 hours); the IR form needs to be dosed at least 3 times a day to be effective. An ER formulation of gabapentin was released in 2013 with the sole indication for RLS.
Gabapentin is not significantly metabolized by the liver, has a 3% rate of protein binding, and is excreted by the kidneys—making it safe for patients who abuse alcohol or opioids and have impaired hepatic function. Typical starting dosages of IR gabapentin are 100 to 300 mg, 3 times daily, if symptoms are present in the daytime. Asymmetric dosing can be helpful, with larger or single dosages given at bedtime (eg, 100 mg in morning, 100 mg in afternoon, 300 mg at bedtime). Dosing varies from patient to patient, from 300 mg to 3,600 mg/d. Increasing dosages produce lower bioavailability because of saturation in absorption or at the blood-brain barrier. At 100 mg every 8 hours, bioavailability is 80% but at 1,600 mg every 8 hours it drops to 27%.3
Periodic limb movements of sleep (PLMS) essentially is akathisia during sleep, and occurs in most patients with RLS. The patient feels tired in the morning because of lack of deep stage-N3 sleep. Because of the inverse relationship between serotonin and dopamine, most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can exacerbate RLS and PLMS.4,5 Other culprits include antipsychotics, antiemetics, and antihistamines. The differential diagnosis includes withdrawal from opioids and attention-deficit/hyperactivity disorder (ADHD), which may be comorbid with RLS. There are many causes of secondary RLS including renal failure, pregnancy, varicose veins, and neuropathy.
Tremor
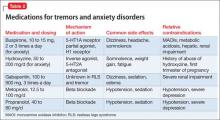
Benign familial, or essential, tremor is a fine intention tremor that can be suppressed by alcohol or benzodiazepines. After detoxification from either of these substances, persistent tremor can re-emerge; often, it is benign, although cerebellar and parkinsonian tremors must be ruled out. Essential tremor can be treated with gabapentin or beta blockers such as propranolol or metoprolol (Table 2).
Anxiety and panic disorder
Social anxiety often presents in addiction treatment centers in the context of group therapy, speaking in 12-step meetings, and having the patient describe his (her) autobiography and history of addiction. Because social anxiety disorder is the third most common psychiatric disorder after simple phobia and major depressive disorder,6 it is not surprising that it emerges after withdrawal.
Patients with social anxiety disorder might self-medicate with alcohol or drugs, especially benzodiazepines (Box). Residential treatment presents an excellent environment for desensitization to fears of public speaking; early recognition is key. Apprehension about group therapy, presenting a substance abuse history, or speaking at a 12-step meeting can lead to premature or “against medical advice” discharge.
Panic disorder commonly is comorbid with substance abuse. Many patients will arrive at treatment with a prescription for benzodiazepines. Because the risk of cross-addiction is high among recovering addicts, benzodiazepines should be avoided. Treating underlying anxiety is crucial for fostering sobriety. Generalized anxiety disorder is common among patients with an addiction, and can lead to relapse if not addressed. Use of non-addictive medications and cognitive therapy is useful in addressing this condition.
A quandary might arise in states where medical marijuana is legal, because Cannabis can be prescribed for anxiety disorders and posttraumatic stress disorder (PTSD). Promoting abstinence from all substances can present a challenge in patients with anxiety disorders who live in these states.
Medications for anxiety and panic disorder include gabapentin, buspirone, hydroxyzine, beta blockers, and atypical antipsychotics (Table 2). Only buspirone and hydroxyzine are FDA-approved for anxiety; buspirone monotherapy generally is ineffective for panic disorder.
Explaining to patients how anxiety arises, such as how classical conditioning leads to specific phobias, can be therapeutic. Describing Klein’s false suffocation alarm theory of panic attacks can illustrate the importance of practicing slow, deep breathing to prevent hyperventilation.7 Also, relabeling a panic attack with self-talk statements such as “I know what this is. It’s just a panic attack” can be helpful. Smartphone apps are available to help patients cope with anxiety and acute panic.8
Mood disorders
Many patients with bipolar disorder experience substance abuse at some point; estimates are that up to 57% of patients have a comorbid addiction.6,9 Persons with a mood disorder are at high risk of substance abuse because of genetic factors; patients also might self-medicate their mood symptoms.
After alcohol or drugs are withdrawn, mood disorders can emerge or resurge. Often, patients enter treatment taking antidepressants and mood stabilizers and usually haven’t been truthful with their treatment provider about their substance abuse. Care must be taken to ascertain whether mood symptoms are secondary to substance abuse. Asking “What’s the longest period of abstinence you’ve had in 2 years and how did you feel emotionally?” often will help you identify a secondary mood disorder. For example, a response of “6 months and I felt really depressed the entire time” would indicate a primary depressive disorder.
Because CNS depressants, such as alcohol and benzodiazepines, can exacerbate a mood disorder, consider continuing or resuming a mood stabilizer or antidepressant during substance abuse treatment. When meeting a new patient, perform an independent evaluation, because substance use can mimic bipolar and depressive disorders. Careful assessment of suicidal ideation is necessary for all patients.
Sleep disorders
Insomnia—as a primary or secondary disorder—is common among patients with a substance use disorder. Insomnia always needs to be addressed. Not sleeping well interferes with cognition and energy and makes depression and bipolar disorder worse. Some experts recommend “waiting out” the insomnia, hoping that sobriety will resolve it—but it might not.
Initial insomnia can be treated with melatonin, 3 to 6 mg at bedtime or earlier in the evening.10-12 Melatonin acts by regulating circadian rhythms, but can cause increased dreaming and nightmares; therefore, it should be avoided in patients who struggle with nightmares. Trazodone, 50 to 150 mg at bedtime, is an inexpensive sleep aid for initial insomnia and doesn’t cause weight gain, which many drugs with antihistaminic properties can. Prazosin, 1 to 2 mg initially, for nightmares in PTSD is effective.13
Antipsychotics might be necessary if nothing else works; quetiapine is effective for sleep and the ER form is FDA-approved as an add-on agent in major depression. Low-dose doxepin (≤10 mg) is effective for middle insomnia.14 At these low dosages, troublesome side effects of tricyclic antidepressants can be avoided.
As many as 40% of adults with ADHD have a delayed sleep-phase disorder. Ask your patient if she is a “night owl,” how chronic the condition is, and when her best sleep occurs.15-17 Morning light and evening melatonin can help, but often are insufficient. Many patients present with undiagnosed or untreated sleep apnea, which can cause excessive daytime sleepiness. Referral to a sleep center is prudent; use of the Epworth Sleepiness Scale is a quick way to assess excessive daytime sleepiness.18
ADHD
ADHD commonly is comorbid with a substance use disorder. Patients might present with an earlier diagnosis, including treatment. Several drugs of abuse can alleviate ADHD symptoms, including amphetamines, opioids, cocaine, and Cannabis; self-medicating is common. Because opioids increase dopamine release, a report of improved work and school performance while taking opioids early in addiction can be a clue to an ADHD diagnosis.
Explaining ADHD as a syndrome of “interest-based attention” helps. If a residential treatment program uses reading and writing assignments, a patient with ADHD might struggle and will need extra help and time and a quiet place to do assignments.19,20 A non-addictive medication, such as atomoxetine, can help, but has an antidepressant-like delay of 3 to 5 weeks until onset of symptom relief. Using a long-acting stimulant can be effective and quick, with an effect size 3 to 4 times higher than atomoxetine; such agents should be avoided in patients who abuse amphetamines.
Studies show that treating ADHD, even with stimulants, neither helps nor hurts outcomes in substance use. Lisdexamfetamine is difficult to abuse and is an inactive prodrug (a bond of lysine and dextroamphetamine) that requires enzymatic cleavage and activation by red blood cells; these characteristics creates a long-acting medication that has a lower abuse liability than other drugs for ADHD. However, abuse can occur and the drug must be used cautiously. Earley’s medication guide referenced below recommends that lisdexamfetamine and other stimulants should be avoided if possible in patients in recovery. However, it adds that specialists in treating ADHD in substance-abusing patients should weigh the potential benefits of stimulant use against the risk of relapse.17 Many patients enter treatment with a diagnosis of bipolar disorder that might, in fact, be comorbid with ADHD.
Chronic pain
Many substance abuse patients began taking opioids for acute, then chronic, pain before their use escalated to addiction. These are challenging patients; often, they are referred for treatment without true addiction.
Keep in mind that dependence is not addiction. Pseudo-addiction is a condition in which pain is undertreated and the patient takes more medication to obtain relief, calls for early refills, and displays drug-seeking behavior but is not using drugs to achieve euphoria. A thorough history and physical and referrals to specialists such as orthopedic surgeons and pain specialists are necessary. Explaining opioid-induced hyperalgesia is important to help the patient understand that (1) pain can be made worse by increasing the dosage of an opioid because of supersensitivity and (2) many patients who are weaned off these drugs will experience a decrease or complete relief of pain.21
Gabapentin, duloxetine, or amitriptyline can be beneficial for chronic pain, as well as mindfulness techniques, physical therapy, and complementary and alternative medicine. Pregabalin can produce euphoria and often should be avoided.
A medication guide for recovery
Paul Earley, MD, former medical director at Talbott Recovery in Atlanta, Georgia, publishes an online guide that classifies medications into categories:
• A: safe
• B: use only under the supervision of an addiction medicine specialist or doctor
• C: completely avoid if the patient is in recovery.17
The Talbott guide lists all stimulants in category C, (except for atomoxetine, which is category A). Hydroxyzine is listed under category B. Many programs for impaired professionals and state medical boards use the Guide, and will question the prescribing of any medication from categories B and C.17
Related Resources
• Spiegel DR, Kumari N, Petri JD. Safer use of benzodiazepines for alcohol detoxification. Current Psychiatry. 2012;11(10):10-15.
• Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
Drug Brand Names
Amitriptyline • Elavil Hydrocodone • Vicodin
Atenolol • Tenormin Hydroxyzine • Vistaril, Atarax
Atomoxetine • Strattera Lisdexamfetamine • Vyvanse
Buprenorphine/ naloxone • Suboxone Metoprolol • Lopressor, Toprol
Buspirone • BuSpar Paroxetine • Paxil
Carbidopa-levodopa • Sinemet Pramipexole • Mirapex
Clonazepam • Klonopin Prazosin • Minipress
Diazepam • Valium Pregabalin • Lyrica
Doxepin • Silenor, Adapin, Sinequan Propranolol • Inderal
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Ropinirole • Requip
Gabapentin • Neurontin, Horizant Sertraline • Zoloft
Trazodone • Desyrel
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
2. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
3. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49(10):661-669.
4. Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510-514.
5. Hoque R, Chesson AL Jr. Pharmacologically induced/ exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/ REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010; 6(1):79-83.
6. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
7. Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50(4):306-317.
8. Holland K. The 17 best anxiety iPhone & Android apps of 2014. http://www.healthline.com/health-slideshow/top-anxiety-iphone-android-apps. Accessed October 28, 2014.
9. Chengappa KN, Levine J, Gershon S, et al. Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord. 2000;2(3 Pt 1):191-195.
10. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders [published online May 17, 2013]. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773.
11. Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. 2007;23(10):2597-2605.
12. Srinivasan V, Brzezinski A, Pandi-Perumal SR, et al. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):913-923.
13. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
14. Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69(10):1557-1564.
15. Baird AL, Coogan AN, Siddiqui A, et al. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17(10):988-995.
16. Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/ hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371-388.
17. Earley PH, Merkin B, Skipper G. The medication guide for safe recovery. Revision 1.7. http://paulearley.net/index. php?option=com_docman&Itemid=239. Published March 2014. Accessed October 28, 2014.
18. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545.
19. Dodson W. Secrets of the ADHD brain. ADDitude. http:// www.additudemag.com/adhd/article/10117.html. Accessed October 28, 2014.
20. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
21. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
When treating patients who abuse substances, it is important to watch for underlying clinical conditions that have been suppressed, relieved, or muted by alcohol or drugs. Many of these conditions can be mistaken for signs of withdrawal, drug-seeking, or new conditions arising from loss of euphoria from the drug. Prompt recognition of these disorders and use of appropriate non-addictive treatments can prevent “against medical advice” discharges, relapses, and unneeded suffering in many cases.
Because the brain is the target organ, these conditions are either neurologic or psychiatric in nosology. Although psychiatric clinicians might not be familiar with neurologic conditions, quick recognition and treatment is necessary.
Restless legs syndrome and periodic limb movements of sleep
Restless legs syndrome (RLS) has 2 key components: paresthesia and akathisia. Although primarily involving the lower extremities, involvement also can include the upper extremities, torso, and head.
Paresthesia differs from typical neuropathies in that it usually is not painful; rather, patients describe an odd sensation using terms such as ticklish, “creepy-crawly,” and other uncomfortable sensations.
Akathisia is a motor restlessness and need to move. The patient might feel momentary relief by moving or rubbing the extremities, only to have the paresthesia return quickly followed by the akathisia. Generally, reclining is the most prominent position that produces symptoms, but they can occur while sitting.
The cause of RLS is an abnormality of central dopamine or iron, or both, in the substantia nigra; iron is a cofactor in dopamine synthesis. All RLS patients should have a serum ferritin level drawn and if <50 μg/dL, be treated with iron supplementation. Dopamine agonists, such as ropinirole, pramipexole, and carbidopa/levodopa, are effective (Table 1); other useful agents include benzodiazepines such as clonazepam and opioids such as hydrocodone.
When a patient withdraws from benzodiazepines or narcotics, RLS can emerge and cause suffering until it is diagnosed and treated. Typical myalgia in opioid withdrawal can confound the diagnosis. The immediate-release (IR) and extended-release (ER) formulations of gabapentin often are a good choice when treating benzodiazepine or narcotic withdrawal. The side effect profile of gabapentin is relatively benign, with somnolence often reported by non-substance abusers, but it is unlikely that addicts, who have grown tolerant to more potent agents such as benzodiazepines and opioids, will complain of sleepiness. Studies have shown that gabapentin is useful in managing withdrawal as well as anxiety and insomnia.1,2 A randomized trial showed that gabapentin increases abstinence rates and decreases heavy drinking.2 The agent has a short half-life (5 to 7 hours); the IR form needs to be dosed at least 3 times a day to be effective. An ER formulation of gabapentin was released in 2013 with the sole indication for RLS.
Gabapentin is not significantly metabolized by the liver, has a 3% rate of protein binding, and is excreted by the kidneys—making it safe for patients who abuse alcohol or opioids and have impaired hepatic function. Typical starting dosages of IR gabapentin are 100 to 300 mg, 3 times daily, if symptoms are present in the daytime. Asymmetric dosing can be helpful, with larger or single dosages given at bedtime (eg, 100 mg in morning, 100 mg in afternoon, 300 mg at bedtime). Dosing varies from patient to patient, from 300 mg to 3,600 mg/d. Increasing dosages produce lower bioavailability because of saturation in absorption or at the blood-brain barrier. At 100 mg every 8 hours, bioavailability is 80% but at 1,600 mg every 8 hours it drops to 27%.3
Periodic limb movements of sleep (PLMS) essentially is akathisia during sleep, and occurs in most patients with RLS. The patient feels tired in the morning because of lack of deep stage-N3 sleep. Because of the inverse relationship between serotonin and dopamine, most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can exacerbate RLS and PLMS.4,5 Other culprits include antipsychotics, antiemetics, and antihistamines. The differential diagnosis includes withdrawal from opioids and attention-deficit/hyperactivity disorder (ADHD), which may be comorbid with RLS. There are many causes of secondary RLS including renal failure, pregnancy, varicose veins, and neuropathy.
Tremor
Benign familial, or essential, tremor is a fine intention tremor that can be suppressed by alcohol or benzodiazepines. After detoxification from either of these substances, persistent tremor can re-emerge; often, it is benign, although cerebellar and parkinsonian tremors must be ruled out. Essential tremor can be treated with gabapentin or beta blockers such as propranolol or metoprolol (Table 2).
Anxiety and panic disorder
Social anxiety often presents in addiction treatment centers in the context of group therapy, speaking in 12-step meetings, and having the patient describe his (her) autobiography and history of addiction. Because social anxiety disorder is the third most common psychiatric disorder after simple phobia and major depressive disorder,6 it is not surprising that it emerges after withdrawal.
Patients with social anxiety disorder might self-medicate with alcohol or drugs, especially benzodiazepines (Box). Residential treatment presents an excellent environment for desensitization to fears of public speaking; early recognition is key. Apprehension about group therapy, presenting a substance abuse history, or speaking at a 12-step meeting can lead to premature or “against medical advice” discharge.
Panic disorder commonly is comorbid with substance abuse. Many patients will arrive at treatment with a prescription for benzodiazepines. Because the risk of cross-addiction is high among recovering addicts, benzodiazepines should be avoided. Treating underlying anxiety is crucial for fostering sobriety. Generalized anxiety disorder is common among patients with an addiction, and can lead to relapse if not addressed. Use of non-addictive medications and cognitive therapy is useful in addressing this condition.
A quandary might arise in states where medical marijuana is legal, because Cannabis can be prescribed for anxiety disorders and posttraumatic stress disorder (PTSD). Promoting abstinence from all substances can present a challenge in patients with anxiety disorders who live in these states.
Medications for anxiety and panic disorder include gabapentin, buspirone, hydroxyzine, beta blockers, and atypical antipsychotics (Table 2). Only buspirone and hydroxyzine are FDA-approved for anxiety; buspirone monotherapy generally is ineffective for panic disorder.
Explaining to patients how anxiety arises, such as how classical conditioning leads to specific phobias, can be therapeutic. Describing Klein’s false suffocation alarm theory of panic attacks can illustrate the importance of practicing slow, deep breathing to prevent hyperventilation.7 Also, relabeling a panic attack with self-talk statements such as “I know what this is. It’s just a panic attack” can be helpful. Smartphone apps are available to help patients cope with anxiety and acute panic.8
Mood disorders
Many patients with bipolar disorder experience substance abuse at some point; estimates are that up to 57% of patients have a comorbid addiction.6,9 Persons with a mood disorder are at high risk of substance abuse because of genetic factors; patients also might self-medicate their mood symptoms.
After alcohol or drugs are withdrawn, mood disorders can emerge or resurge. Often, patients enter treatment taking antidepressants and mood stabilizers and usually haven’t been truthful with their treatment provider about their substance abuse. Care must be taken to ascertain whether mood symptoms are secondary to substance abuse. Asking “What’s the longest period of abstinence you’ve had in 2 years and how did you feel emotionally?” often will help you identify a secondary mood disorder. For example, a response of “6 months and I felt really depressed the entire time” would indicate a primary depressive disorder.
Because CNS depressants, such as alcohol and benzodiazepines, can exacerbate a mood disorder, consider continuing or resuming a mood stabilizer or antidepressant during substance abuse treatment. When meeting a new patient, perform an independent evaluation, because substance use can mimic bipolar and depressive disorders. Careful assessment of suicidal ideation is necessary for all patients.
Sleep disorders
Insomnia—as a primary or secondary disorder—is common among patients with a substance use disorder. Insomnia always needs to be addressed. Not sleeping well interferes with cognition and energy and makes depression and bipolar disorder worse. Some experts recommend “waiting out” the insomnia, hoping that sobriety will resolve it—but it might not.
Initial insomnia can be treated with melatonin, 3 to 6 mg at bedtime or earlier in the evening.10-12 Melatonin acts by regulating circadian rhythms, but can cause increased dreaming and nightmares; therefore, it should be avoided in patients who struggle with nightmares. Trazodone, 50 to 150 mg at bedtime, is an inexpensive sleep aid for initial insomnia and doesn’t cause weight gain, which many drugs with antihistaminic properties can. Prazosin, 1 to 2 mg initially, for nightmares in PTSD is effective.13
Antipsychotics might be necessary if nothing else works; quetiapine is effective for sleep and the ER form is FDA-approved as an add-on agent in major depression. Low-dose doxepin (≤10 mg) is effective for middle insomnia.14 At these low dosages, troublesome side effects of tricyclic antidepressants can be avoided.
As many as 40% of adults with ADHD have a delayed sleep-phase disorder. Ask your patient if she is a “night owl,” how chronic the condition is, and when her best sleep occurs.15-17 Morning light and evening melatonin can help, but often are insufficient. Many patients present with undiagnosed or untreated sleep apnea, which can cause excessive daytime sleepiness. Referral to a sleep center is prudent; use of the Epworth Sleepiness Scale is a quick way to assess excessive daytime sleepiness.18
ADHD
ADHD commonly is comorbid with a substance use disorder. Patients might present with an earlier diagnosis, including treatment. Several drugs of abuse can alleviate ADHD symptoms, including amphetamines, opioids, cocaine, and Cannabis; self-medicating is common. Because opioids increase dopamine release, a report of improved work and school performance while taking opioids early in addiction can be a clue to an ADHD diagnosis.
Explaining ADHD as a syndrome of “interest-based attention” helps. If a residential treatment program uses reading and writing assignments, a patient with ADHD might struggle and will need extra help and time and a quiet place to do assignments.19,20 A non-addictive medication, such as atomoxetine, can help, but has an antidepressant-like delay of 3 to 5 weeks until onset of symptom relief. Using a long-acting stimulant can be effective and quick, with an effect size 3 to 4 times higher than atomoxetine; such agents should be avoided in patients who abuse amphetamines.
Studies show that treating ADHD, even with stimulants, neither helps nor hurts outcomes in substance use. Lisdexamfetamine is difficult to abuse and is an inactive prodrug (a bond of lysine and dextroamphetamine) that requires enzymatic cleavage and activation by red blood cells; these characteristics creates a long-acting medication that has a lower abuse liability than other drugs for ADHD. However, abuse can occur and the drug must be used cautiously. Earley’s medication guide referenced below recommends that lisdexamfetamine and other stimulants should be avoided if possible in patients in recovery. However, it adds that specialists in treating ADHD in substance-abusing patients should weigh the potential benefits of stimulant use against the risk of relapse.17 Many patients enter treatment with a diagnosis of bipolar disorder that might, in fact, be comorbid with ADHD.
Chronic pain
Many substance abuse patients began taking opioids for acute, then chronic, pain before their use escalated to addiction. These are challenging patients; often, they are referred for treatment without true addiction.
Keep in mind that dependence is not addiction. Pseudo-addiction is a condition in which pain is undertreated and the patient takes more medication to obtain relief, calls for early refills, and displays drug-seeking behavior but is not using drugs to achieve euphoria. A thorough history and physical and referrals to specialists such as orthopedic surgeons and pain specialists are necessary. Explaining opioid-induced hyperalgesia is important to help the patient understand that (1) pain can be made worse by increasing the dosage of an opioid because of supersensitivity and (2) many patients who are weaned off these drugs will experience a decrease or complete relief of pain.21
Gabapentin, duloxetine, or amitriptyline can be beneficial for chronic pain, as well as mindfulness techniques, physical therapy, and complementary and alternative medicine. Pregabalin can produce euphoria and often should be avoided.
A medication guide for recovery
Paul Earley, MD, former medical director at Talbott Recovery in Atlanta, Georgia, publishes an online guide that classifies medications into categories:
• A: safe
• B: use only under the supervision of an addiction medicine specialist or doctor
• C: completely avoid if the patient is in recovery.17
The Talbott guide lists all stimulants in category C, (except for atomoxetine, which is category A). Hydroxyzine is listed under category B. Many programs for impaired professionals and state medical boards use the Guide, and will question the prescribing of any medication from categories B and C.17
Related Resources
• Spiegel DR, Kumari N, Petri JD. Safer use of benzodiazepines for alcohol detoxification. Current Psychiatry. 2012;11(10):10-15.
• Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
Drug Brand Names
Amitriptyline • Elavil Hydrocodone • Vicodin
Atenolol • Tenormin Hydroxyzine • Vistaril, Atarax
Atomoxetine • Strattera Lisdexamfetamine • Vyvanse
Buprenorphine/ naloxone • Suboxone Metoprolol • Lopressor, Toprol
Buspirone • BuSpar Paroxetine • Paxil
Carbidopa-levodopa • Sinemet Pramipexole • Mirapex
Clonazepam • Klonopin Prazosin • Minipress
Diazepam • Valium Pregabalin • Lyrica
Doxepin • Silenor, Adapin, Sinequan Propranolol • Inderal
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Ropinirole • Requip
Gabapentin • Neurontin, Horizant Sertraline • Zoloft
Trazodone • Desyrel
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When treating patients who abuse substances, it is important to watch for underlying clinical conditions that have been suppressed, relieved, or muted by alcohol or drugs. Many of these conditions can be mistaken for signs of withdrawal, drug-seeking, or new conditions arising from loss of euphoria from the drug. Prompt recognition of these disorders and use of appropriate non-addictive treatments can prevent “against medical advice” discharges, relapses, and unneeded suffering in many cases.
Because the brain is the target organ, these conditions are either neurologic or psychiatric in nosology. Although psychiatric clinicians might not be familiar with neurologic conditions, quick recognition and treatment is necessary.
Restless legs syndrome and periodic limb movements of sleep
Restless legs syndrome (RLS) has 2 key components: paresthesia and akathisia. Although primarily involving the lower extremities, involvement also can include the upper extremities, torso, and head.
Paresthesia differs from typical neuropathies in that it usually is not painful; rather, patients describe an odd sensation using terms such as ticklish, “creepy-crawly,” and other uncomfortable sensations.
Akathisia is a motor restlessness and need to move. The patient might feel momentary relief by moving or rubbing the extremities, only to have the paresthesia return quickly followed by the akathisia. Generally, reclining is the most prominent position that produces symptoms, but they can occur while sitting.
The cause of RLS is an abnormality of central dopamine or iron, or both, in the substantia nigra; iron is a cofactor in dopamine synthesis. All RLS patients should have a serum ferritin level drawn and if <50 μg/dL, be treated with iron supplementation. Dopamine agonists, such as ropinirole, pramipexole, and carbidopa/levodopa, are effective (Table 1); other useful agents include benzodiazepines such as clonazepam and opioids such as hydrocodone.
When a patient withdraws from benzodiazepines or narcotics, RLS can emerge and cause suffering until it is diagnosed and treated. Typical myalgia in opioid withdrawal can confound the diagnosis. The immediate-release (IR) and extended-release (ER) formulations of gabapentin often are a good choice when treating benzodiazepine or narcotic withdrawal. The side effect profile of gabapentin is relatively benign, with somnolence often reported by non-substance abusers, but it is unlikely that addicts, who have grown tolerant to more potent agents such as benzodiazepines and opioids, will complain of sleepiness. Studies have shown that gabapentin is useful in managing withdrawal as well as anxiety and insomnia.1,2 A randomized trial showed that gabapentin increases abstinence rates and decreases heavy drinking.2 The agent has a short half-life (5 to 7 hours); the IR form needs to be dosed at least 3 times a day to be effective. An ER formulation of gabapentin was released in 2013 with the sole indication for RLS.
Gabapentin is not significantly metabolized by the liver, has a 3% rate of protein binding, and is excreted by the kidneys—making it safe for patients who abuse alcohol or opioids and have impaired hepatic function. Typical starting dosages of IR gabapentin are 100 to 300 mg, 3 times daily, if symptoms are present in the daytime. Asymmetric dosing can be helpful, with larger or single dosages given at bedtime (eg, 100 mg in morning, 100 mg in afternoon, 300 mg at bedtime). Dosing varies from patient to patient, from 300 mg to 3,600 mg/d. Increasing dosages produce lower bioavailability because of saturation in absorption or at the blood-brain barrier. At 100 mg every 8 hours, bioavailability is 80% but at 1,600 mg every 8 hours it drops to 27%.3
Periodic limb movements of sleep (PLMS) essentially is akathisia during sleep, and occurs in most patients with RLS. The patient feels tired in the morning because of lack of deep stage-N3 sleep. Because of the inverse relationship between serotonin and dopamine, most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can exacerbate RLS and PLMS.4,5 Other culprits include antipsychotics, antiemetics, and antihistamines. The differential diagnosis includes withdrawal from opioids and attention-deficit/hyperactivity disorder (ADHD), which may be comorbid with RLS. There are many causes of secondary RLS including renal failure, pregnancy, varicose veins, and neuropathy.
Tremor
Benign familial, or essential, tremor is a fine intention tremor that can be suppressed by alcohol or benzodiazepines. After detoxification from either of these substances, persistent tremor can re-emerge; often, it is benign, although cerebellar and parkinsonian tremors must be ruled out. Essential tremor can be treated with gabapentin or beta blockers such as propranolol or metoprolol (Table 2).
Anxiety and panic disorder
Social anxiety often presents in addiction treatment centers in the context of group therapy, speaking in 12-step meetings, and having the patient describe his (her) autobiography and history of addiction. Because social anxiety disorder is the third most common psychiatric disorder after simple phobia and major depressive disorder,6 it is not surprising that it emerges after withdrawal.
Patients with social anxiety disorder might self-medicate with alcohol or drugs, especially benzodiazepines (Box). Residential treatment presents an excellent environment for desensitization to fears of public speaking; early recognition is key. Apprehension about group therapy, presenting a substance abuse history, or speaking at a 12-step meeting can lead to premature or “against medical advice” discharge.
Panic disorder commonly is comorbid with substance abuse. Many patients will arrive at treatment with a prescription for benzodiazepines. Because the risk of cross-addiction is high among recovering addicts, benzodiazepines should be avoided. Treating underlying anxiety is crucial for fostering sobriety. Generalized anxiety disorder is common among patients with an addiction, and can lead to relapse if not addressed. Use of non-addictive medications and cognitive therapy is useful in addressing this condition.
A quandary might arise in states where medical marijuana is legal, because Cannabis can be prescribed for anxiety disorders and posttraumatic stress disorder (PTSD). Promoting abstinence from all substances can present a challenge in patients with anxiety disorders who live in these states.
Medications for anxiety and panic disorder include gabapentin, buspirone, hydroxyzine, beta blockers, and atypical antipsychotics (Table 2). Only buspirone and hydroxyzine are FDA-approved for anxiety; buspirone monotherapy generally is ineffective for panic disorder.
Explaining to patients how anxiety arises, such as how classical conditioning leads to specific phobias, can be therapeutic. Describing Klein’s false suffocation alarm theory of panic attacks can illustrate the importance of practicing slow, deep breathing to prevent hyperventilation.7 Also, relabeling a panic attack with self-talk statements such as “I know what this is. It’s just a panic attack” can be helpful. Smartphone apps are available to help patients cope with anxiety and acute panic.8
Mood disorders
Many patients with bipolar disorder experience substance abuse at some point; estimates are that up to 57% of patients have a comorbid addiction.6,9 Persons with a mood disorder are at high risk of substance abuse because of genetic factors; patients also might self-medicate their mood symptoms.
After alcohol or drugs are withdrawn, mood disorders can emerge or resurge. Often, patients enter treatment taking antidepressants and mood stabilizers and usually haven’t been truthful with their treatment provider about their substance abuse. Care must be taken to ascertain whether mood symptoms are secondary to substance abuse. Asking “What’s the longest period of abstinence you’ve had in 2 years and how did you feel emotionally?” often will help you identify a secondary mood disorder. For example, a response of “6 months and I felt really depressed the entire time” would indicate a primary depressive disorder.
Because CNS depressants, such as alcohol and benzodiazepines, can exacerbate a mood disorder, consider continuing or resuming a mood stabilizer or antidepressant during substance abuse treatment. When meeting a new patient, perform an independent evaluation, because substance use can mimic bipolar and depressive disorders. Careful assessment of suicidal ideation is necessary for all patients.
Sleep disorders
Insomnia—as a primary or secondary disorder—is common among patients with a substance use disorder. Insomnia always needs to be addressed. Not sleeping well interferes with cognition and energy and makes depression and bipolar disorder worse. Some experts recommend “waiting out” the insomnia, hoping that sobriety will resolve it—but it might not.
Initial insomnia can be treated with melatonin, 3 to 6 mg at bedtime or earlier in the evening.10-12 Melatonin acts by regulating circadian rhythms, but can cause increased dreaming and nightmares; therefore, it should be avoided in patients who struggle with nightmares. Trazodone, 50 to 150 mg at bedtime, is an inexpensive sleep aid for initial insomnia and doesn’t cause weight gain, which many drugs with antihistaminic properties can. Prazosin, 1 to 2 mg initially, for nightmares in PTSD is effective.13
Antipsychotics might be necessary if nothing else works; quetiapine is effective for sleep and the ER form is FDA-approved as an add-on agent in major depression. Low-dose doxepin (≤10 mg) is effective for middle insomnia.14 At these low dosages, troublesome side effects of tricyclic antidepressants can be avoided.
As many as 40% of adults with ADHD have a delayed sleep-phase disorder. Ask your patient if she is a “night owl,” how chronic the condition is, and when her best sleep occurs.15-17 Morning light and evening melatonin can help, but often are insufficient. Many patients present with undiagnosed or untreated sleep apnea, which can cause excessive daytime sleepiness. Referral to a sleep center is prudent; use of the Epworth Sleepiness Scale is a quick way to assess excessive daytime sleepiness.18
ADHD
ADHD commonly is comorbid with a substance use disorder. Patients might present with an earlier diagnosis, including treatment. Several drugs of abuse can alleviate ADHD symptoms, including amphetamines, opioids, cocaine, and Cannabis; self-medicating is common. Because opioids increase dopamine release, a report of improved work and school performance while taking opioids early in addiction can be a clue to an ADHD diagnosis.
Explaining ADHD as a syndrome of “interest-based attention” helps. If a residential treatment program uses reading and writing assignments, a patient with ADHD might struggle and will need extra help and time and a quiet place to do assignments.19,20 A non-addictive medication, such as atomoxetine, can help, but has an antidepressant-like delay of 3 to 5 weeks until onset of symptom relief. Using a long-acting stimulant can be effective and quick, with an effect size 3 to 4 times higher than atomoxetine; such agents should be avoided in patients who abuse amphetamines.
Studies show that treating ADHD, even with stimulants, neither helps nor hurts outcomes in substance use. Lisdexamfetamine is difficult to abuse and is an inactive prodrug (a bond of lysine and dextroamphetamine) that requires enzymatic cleavage and activation by red blood cells; these characteristics creates a long-acting medication that has a lower abuse liability than other drugs for ADHD. However, abuse can occur and the drug must be used cautiously. Earley’s medication guide referenced below recommends that lisdexamfetamine and other stimulants should be avoided if possible in patients in recovery. However, it adds that specialists in treating ADHD in substance-abusing patients should weigh the potential benefits of stimulant use against the risk of relapse.17 Many patients enter treatment with a diagnosis of bipolar disorder that might, in fact, be comorbid with ADHD.
Chronic pain
Many substance abuse patients began taking opioids for acute, then chronic, pain before their use escalated to addiction. These are challenging patients; often, they are referred for treatment without true addiction.
Keep in mind that dependence is not addiction. Pseudo-addiction is a condition in which pain is undertreated and the patient takes more medication to obtain relief, calls for early refills, and displays drug-seeking behavior but is not using drugs to achieve euphoria. A thorough history and physical and referrals to specialists such as orthopedic surgeons and pain specialists are necessary. Explaining opioid-induced hyperalgesia is important to help the patient understand that (1) pain can be made worse by increasing the dosage of an opioid because of supersensitivity and (2) many patients who are weaned off these drugs will experience a decrease or complete relief of pain.21
Gabapentin, duloxetine, or amitriptyline can be beneficial for chronic pain, as well as mindfulness techniques, physical therapy, and complementary and alternative medicine. Pregabalin can produce euphoria and often should be avoided.
A medication guide for recovery
Paul Earley, MD, former medical director at Talbott Recovery in Atlanta, Georgia, publishes an online guide that classifies medications into categories:
• A: safe
• B: use only under the supervision of an addiction medicine specialist or doctor
• C: completely avoid if the patient is in recovery.17
The Talbott guide lists all stimulants in category C, (except for atomoxetine, which is category A). Hydroxyzine is listed under category B. Many programs for impaired professionals and state medical boards use the Guide, and will question the prescribing of any medication from categories B and C.17
Related Resources
• Spiegel DR, Kumari N, Petri JD. Safer use of benzodiazepines for alcohol detoxification. Current Psychiatry. 2012;11(10):10-15.
• Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
Drug Brand Names
Amitriptyline • Elavil Hydrocodone • Vicodin
Atenolol • Tenormin Hydroxyzine • Vistaril, Atarax
Atomoxetine • Strattera Lisdexamfetamine • Vyvanse
Buprenorphine/ naloxone • Suboxone Metoprolol • Lopressor, Toprol
Buspirone • BuSpar Paroxetine • Paxil
Carbidopa-levodopa • Sinemet Pramipexole • Mirapex
Clonazepam • Klonopin Prazosin • Minipress
Diazepam • Valium Pregabalin • Lyrica
Doxepin • Silenor, Adapin, Sinequan Propranolol • Inderal
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Ropinirole • Requip
Gabapentin • Neurontin, Horizant Sertraline • Zoloft
Trazodone • Desyrel
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
2. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
3. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49(10):661-669.
4. Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510-514.
5. Hoque R, Chesson AL Jr. Pharmacologically induced/ exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/ REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010; 6(1):79-83.
6. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
7. Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50(4):306-317.
8. Holland K. The 17 best anxiety iPhone & Android apps of 2014. http://www.healthline.com/health-slideshow/top-anxiety-iphone-android-apps. Accessed October 28, 2014.
9. Chengappa KN, Levine J, Gershon S, et al. Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord. 2000;2(3 Pt 1):191-195.
10. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders [published online May 17, 2013]. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773.
11. Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. 2007;23(10):2597-2605.
12. Srinivasan V, Brzezinski A, Pandi-Perumal SR, et al. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):913-923.
13. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
14. Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69(10):1557-1564.
15. Baird AL, Coogan AN, Siddiqui A, et al. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17(10):988-995.
16. Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/ hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371-388.
17. Earley PH, Merkin B, Skipper G. The medication guide for safe recovery. Revision 1.7. http://paulearley.net/index. php?option=com_docman&Itemid=239. Published March 2014. Accessed October 28, 2014.
18. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545.
19. Dodson W. Secrets of the ADHD brain. ADDitude. http:// www.additudemag.com/adhd/article/10117.html. Accessed October 28, 2014.
20. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
21. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
1. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
2. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
3. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49(10):661-669.
4. Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510-514.
5. Hoque R, Chesson AL Jr. Pharmacologically induced/ exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/ REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010; 6(1):79-83.
6. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
7. Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50(4):306-317.
8. Holland K. The 17 best anxiety iPhone & Android apps of 2014. http://www.healthline.com/health-slideshow/top-anxiety-iphone-android-apps. Accessed October 28, 2014.
9. Chengappa KN, Levine J, Gershon S, et al. Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord. 2000;2(3 Pt 1):191-195.
10. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders [published online May 17, 2013]. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773.
11. Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. 2007;23(10):2597-2605.
12. Srinivasan V, Brzezinski A, Pandi-Perumal SR, et al. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):913-923.
13. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
14. Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69(10):1557-1564.
15. Baird AL, Coogan AN, Siddiqui A, et al. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17(10):988-995.
16. Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/ hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371-388.
17. Earley PH, Merkin B, Skipper G. The medication guide for safe recovery. Revision 1.7. http://paulearley.net/index. php?option=com_docman&Itemid=239. Published March 2014. Accessed October 28, 2014.
18. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545.
19. Dodson W. Secrets of the ADHD brain. ADDitude. http:// www.additudemag.com/adhd/article/10117.html. Accessed October 28, 2014.
20. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
21. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.