User login
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
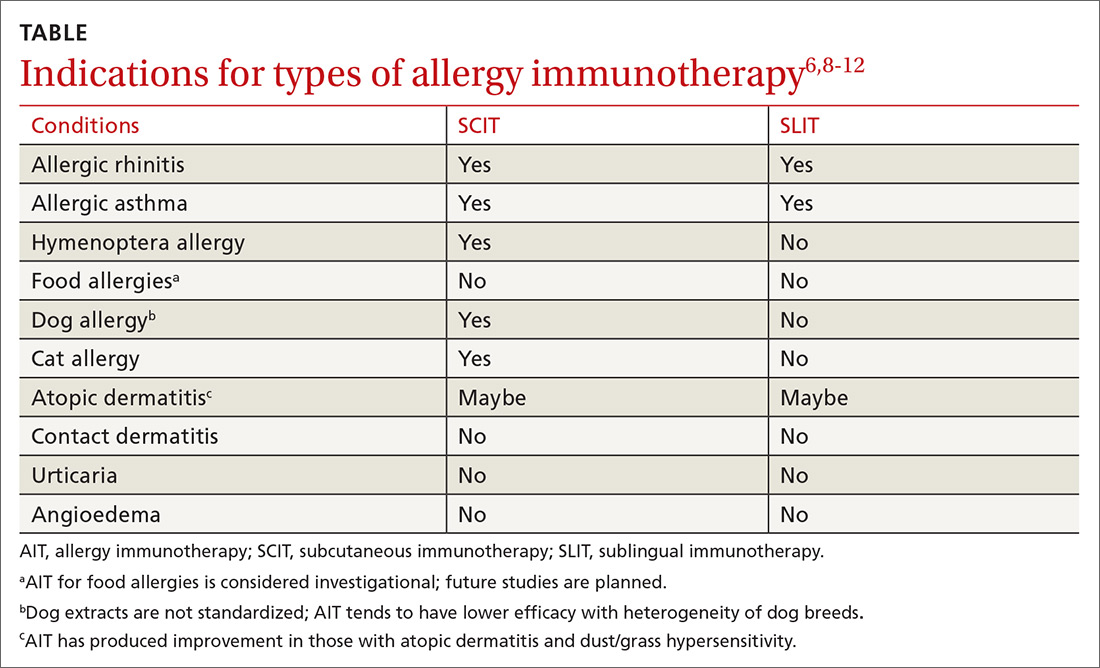
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
PRACTICE RECOMMENDATIONS
› Diagnose allergies that are amenable to allergy immunotherapy (AIT) using skin prick/puncture allergy testing in conjunction with clinical symptoms, triggers, and exposure. A
› Do not use AIT for urticaria, angioedema, drug hypersensitivity, or latex allergy. A
› Do not initiate AIT during pregnancy or in patients with acquired immune deficiency syndrome or severe asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
