User login
The Immune Heartache: Pericarditis Following Checkpoint Inhibition
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Brief Immunotherapy Yields Major Survival Benefits in Advanced NSCLC: A Case Report
Background
Lung cancer, primarily non-small cell lung cancer (NSCLC), typically presents at an advanced stage with a five-year survival rate below 5%. Treatment includes platinum-based chemotherapy and targeted therapies for specific mutations, with immunotherapy significantly improving outcomes for patients with high PD-L1 expression.
Case Presentation
A 72-year-old male, diagnosed with advanced lung adenocarcinoma in 2020 after showing symptoms of brain metastases, underwent successful surgical and CyberKnife treatments. Despite no actionable genetic targets and a high PD-L1 expression of 80%, his treatment with 3-cycles of Keytruda was cut short due to a psoriatic arthritis flare-up, though it initially decreased his CEA levels significantly. Over the following years, fluctuating CEA levels and various imaging studies indicated some concerning changes, such as potential radionecrosis or recurrence of cancer in the lung. His refusal of biopsy and a preference for avoiding invasive treatments led to only surveillance. Later, an MRI showed some metastasis, and the patient agreed to a lung biopsy, which showed poorly differentiated carcinoma of pulmonary origin. The patient only agreed to restart treatment with Keytruda 4-years later after his initial treatment with Keytruda, under close rheumatological care, and received only two doses. Afterward, the patient lost follow-ups. 3-months later, Repeated CT scans of the chest, abdomen, and pelvis showed no evidence of mass or pathological lymph nodes, and repeated CEA was 3.4.
Discussion
Managing advanced lung adenocarcinoma, especially with complications like brain metastases and psoriatic arthritis, is challenging. Pembrolizumab treatment showed promise by significantly reducing CEA levels despite early discontinuation due to autoimmune side effects, indicating effective tumor response in patients with high PD-L1 expression. The case underscores the need for balancing cancer treatment with autoimmune management and highlights the importance of patient preferences in treatment plans. Ongoing surveillance and genomic profiling remain crucial for guiding therapy.
Conclusions
This case of a 70-year-old male with advanced lung adenocarcinoma highlights the significant impact of immunotherapy, particularly PD-1/ PD-L1 inhibitors like pembrolizumab, in NSCLC. Despite a brief treatment period, the patient experienced extended disease control, demonstrating the potential of immunotherapy to enhance survival and its broad applicability in oncology.
Background
Lung cancer, primarily non-small cell lung cancer (NSCLC), typically presents at an advanced stage with a five-year survival rate below 5%. Treatment includes platinum-based chemotherapy and targeted therapies for specific mutations, with immunotherapy significantly improving outcomes for patients with high PD-L1 expression.
Case Presentation
A 72-year-old male, diagnosed with advanced lung adenocarcinoma in 2020 after showing symptoms of brain metastases, underwent successful surgical and CyberKnife treatments. Despite no actionable genetic targets and a high PD-L1 expression of 80%, his treatment with 3-cycles of Keytruda was cut short due to a psoriatic arthritis flare-up, though it initially decreased his CEA levels significantly. Over the following years, fluctuating CEA levels and various imaging studies indicated some concerning changes, such as potential radionecrosis or recurrence of cancer in the lung. His refusal of biopsy and a preference for avoiding invasive treatments led to only surveillance. Later, an MRI showed some metastasis, and the patient agreed to a lung biopsy, which showed poorly differentiated carcinoma of pulmonary origin. The patient only agreed to restart treatment with Keytruda 4-years later after his initial treatment with Keytruda, under close rheumatological care, and received only two doses. Afterward, the patient lost follow-ups. 3-months later, Repeated CT scans of the chest, abdomen, and pelvis showed no evidence of mass or pathological lymph nodes, and repeated CEA was 3.4.
Discussion
Managing advanced lung adenocarcinoma, especially with complications like brain metastases and psoriatic arthritis, is challenging. Pembrolizumab treatment showed promise by significantly reducing CEA levels despite early discontinuation due to autoimmune side effects, indicating effective tumor response in patients with high PD-L1 expression. The case underscores the need for balancing cancer treatment with autoimmune management and highlights the importance of patient preferences in treatment plans. Ongoing surveillance and genomic profiling remain crucial for guiding therapy.
Conclusions
This case of a 70-year-old male with advanced lung adenocarcinoma highlights the significant impact of immunotherapy, particularly PD-1/ PD-L1 inhibitors like pembrolizumab, in NSCLC. Despite a brief treatment period, the patient experienced extended disease control, demonstrating the potential of immunotherapy to enhance survival and its broad applicability in oncology.
Background
Lung cancer, primarily non-small cell lung cancer (NSCLC), typically presents at an advanced stage with a five-year survival rate below 5%. Treatment includes platinum-based chemotherapy and targeted therapies for specific mutations, with immunotherapy significantly improving outcomes for patients with high PD-L1 expression.
Case Presentation
A 72-year-old male, diagnosed with advanced lung adenocarcinoma in 2020 after showing symptoms of brain metastases, underwent successful surgical and CyberKnife treatments. Despite no actionable genetic targets and a high PD-L1 expression of 80%, his treatment with 3-cycles of Keytruda was cut short due to a psoriatic arthritis flare-up, though it initially decreased his CEA levels significantly. Over the following years, fluctuating CEA levels and various imaging studies indicated some concerning changes, such as potential radionecrosis or recurrence of cancer in the lung. His refusal of biopsy and a preference for avoiding invasive treatments led to only surveillance. Later, an MRI showed some metastasis, and the patient agreed to a lung biopsy, which showed poorly differentiated carcinoma of pulmonary origin. The patient only agreed to restart treatment with Keytruda 4-years later after his initial treatment with Keytruda, under close rheumatological care, and received only two doses. Afterward, the patient lost follow-ups. 3-months later, Repeated CT scans of the chest, abdomen, and pelvis showed no evidence of mass or pathological lymph nodes, and repeated CEA was 3.4.
Discussion
Managing advanced lung adenocarcinoma, especially with complications like brain metastases and psoriatic arthritis, is challenging. Pembrolizumab treatment showed promise by significantly reducing CEA levels despite early discontinuation due to autoimmune side effects, indicating effective tumor response in patients with high PD-L1 expression. The case underscores the need for balancing cancer treatment with autoimmune management and highlights the importance of patient preferences in treatment plans. Ongoing surveillance and genomic profiling remain crucial for guiding therapy.
Conclusions
This case of a 70-year-old male with advanced lung adenocarcinoma highlights the significant impact of immunotherapy, particularly PD-1/ PD-L1 inhibitors like pembrolizumab, in NSCLC. Despite a brief treatment period, the patient experienced extended disease control, demonstrating the potential of immunotherapy to enhance survival and its broad applicability in oncology.
VA Ann Arbor Immunotherapy Stewardship Program
Purpose
To compare vial utilization and spending between fixed and weight-based dosing of pembrolizumab in Veterans. Promote and assess pembrolizumab extended interval dosing.
Background
FDA approved pembrolizumab label change from weight-based to fixed dosing without evidence of fixed-dosing’s superiority. Retrospective studies demonstrate equivalent outcomes for 2 mg/kg every 3 weeks (Q3W), 200 mg Q3W, 4 mg/kg every 6 weeks (Q6W), and 400 mg Q6W.
Methods
In July 2024 VAAAHS (VA Ann Arbor Healthcare System) initiated an immunotherapy stewardship quality improvement program to deprescribe unnecessary pembrolizumab units and promote extended-interval dosing. Specific interventions included order template modification and targeted outreach to key stakeholders.
Data Analysis
All pembrolizumab doses administered at VAAAHS between July 1, 2024 (launch) and March 31, 2025 (data cutoff) were extracted from EHR. Drug utilization, spending, and healthcare contact hours averted were compared to a fixed-dosing counterfactual.
Results
Sixty-three Veterans received 286 total pembrolizumab doses, of which 107 (37.4%) were Q6W and 179 (62.6%) were Q3W. In total, 741 vials were utilized, against expectation of 786 (5.7% reduction), reflecting approximately $182,000 in savings (annualized, $243,000) and 86.5% of the theoretical maximum savings were captured. Q6W’s share of all doses rose from 27.3% in July 2024 to 53.8% in March 2025. Amongst monotherapy, Q6W’s share rose from 60.0% in July 2024 to 86.7% in March 2025. Q6W adoption saved 381 Veteran-healthcare contact hours, not including travel time.
Conclusions
Stewardship efforts reduced unnecessary pembrolizumab utilization and spending while saving Veterans and VAAAHS providers’ time. Continued provider reinforcement, preparation for Oracle/ Cerner implementation, VISN expansion, refinement of pembrolizumab dose-banding, and development of dose bands for other immunotherapies are underway.
Significance
National implementation would improve Veteran convenience and quality of life, enable reductions in drug and resource costs, and enhance clinic throughput.
Purpose
To compare vial utilization and spending between fixed and weight-based dosing of pembrolizumab in Veterans. Promote and assess pembrolizumab extended interval dosing.
Background
FDA approved pembrolizumab label change from weight-based to fixed dosing without evidence of fixed-dosing’s superiority. Retrospective studies demonstrate equivalent outcomes for 2 mg/kg every 3 weeks (Q3W), 200 mg Q3W, 4 mg/kg every 6 weeks (Q6W), and 400 mg Q6W.
Methods
In July 2024 VAAAHS (VA Ann Arbor Healthcare System) initiated an immunotherapy stewardship quality improvement program to deprescribe unnecessary pembrolizumab units and promote extended-interval dosing. Specific interventions included order template modification and targeted outreach to key stakeholders.
Data Analysis
All pembrolizumab doses administered at VAAAHS between July 1, 2024 (launch) and March 31, 2025 (data cutoff) were extracted from EHR. Drug utilization, spending, and healthcare contact hours averted were compared to a fixed-dosing counterfactual.
Results
Sixty-three Veterans received 286 total pembrolizumab doses, of which 107 (37.4%) were Q6W and 179 (62.6%) were Q3W. In total, 741 vials were utilized, against expectation of 786 (5.7% reduction), reflecting approximately $182,000 in savings (annualized, $243,000) and 86.5% of the theoretical maximum savings were captured. Q6W’s share of all doses rose from 27.3% in July 2024 to 53.8% in March 2025. Amongst monotherapy, Q6W’s share rose from 60.0% in July 2024 to 86.7% in March 2025. Q6W adoption saved 381 Veteran-healthcare contact hours, not including travel time.
Conclusions
Stewardship efforts reduced unnecessary pembrolizumab utilization and spending while saving Veterans and VAAAHS providers’ time. Continued provider reinforcement, preparation for Oracle/ Cerner implementation, VISN expansion, refinement of pembrolizumab dose-banding, and development of dose bands for other immunotherapies are underway.
Significance
National implementation would improve Veteran convenience and quality of life, enable reductions in drug and resource costs, and enhance clinic throughput.
Purpose
To compare vial utilization and spending between fixed and weight-based dosing of pembrolizumab in Veterans. Promote and assess pembrolizumab extended interval dosing.
Background
FDA approved pembrolizumab label change from weight-based to fixed dosing without evidence of fixed-dosing’s superiority. Retrospective studies demonstrate equivalent outcomes for 2 mg/kg every 3 weeks (Q3W), 200 mg Q3W, 4 mg/kg every 6 weeks (Q6W), and 400 mg Q6W.
Methods
In July 2024 VAAAHS (VA Ann Arbor Healthcare System) initiated an immunotherapy stewardship quality improvement program to deprescribe unnecessary pembrolizumab units and promote extended-interval dosing. Specific interventions included order template modification and targeted outreach to key stakeholders.
Data Analysis
All pembrolizumab doses administered at VAAAHS between July 1, 2024 (launch) and March 31, 2025 (data cutoff) were extracted from EHR. Drug utilization, spending, and healthcare contact hours averted were compared to a fixed-dosing counterfactual.
Results
Sixty-three Veterans received 286 total pembrolizumab doses, of which 107 (37.4%) were Q6W and 179 (62.6%) were Q3W. In total, 741 vials were utilized, against expectation of 786 (5.7% reduction), reflecting approximately $182,000 in savings (annualized, $243,000) and 86.5% of the theoretical maximum savings were captured. Q6W’s share of all doses rose from 27.3% in July 2024 to 53.8% in March 2025. Amongst monotherapy, Q6W’s share rose from 60.0% in July 2024 to 86.7% in March 2025. Q6W adoption saved 381 Veteran-healthcare contact hours, not including travel time.
Conclusions
Stewardship efforts reduced unnecessary pembrolizumab utilization and spending while saving Veterans and VAAAHS providers’ time. Continued provider reinforcement, preparation for Oracle/ Cerner implementation, VISN expansion, refinement of pembrolizumab dose-banding, and development of dose bands for other immunotherapies are underway.
Significance
National implementation would improve Veteran convenience and quality of life, enable reductions in drug and resource costs, and enhance clinic throughput.
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
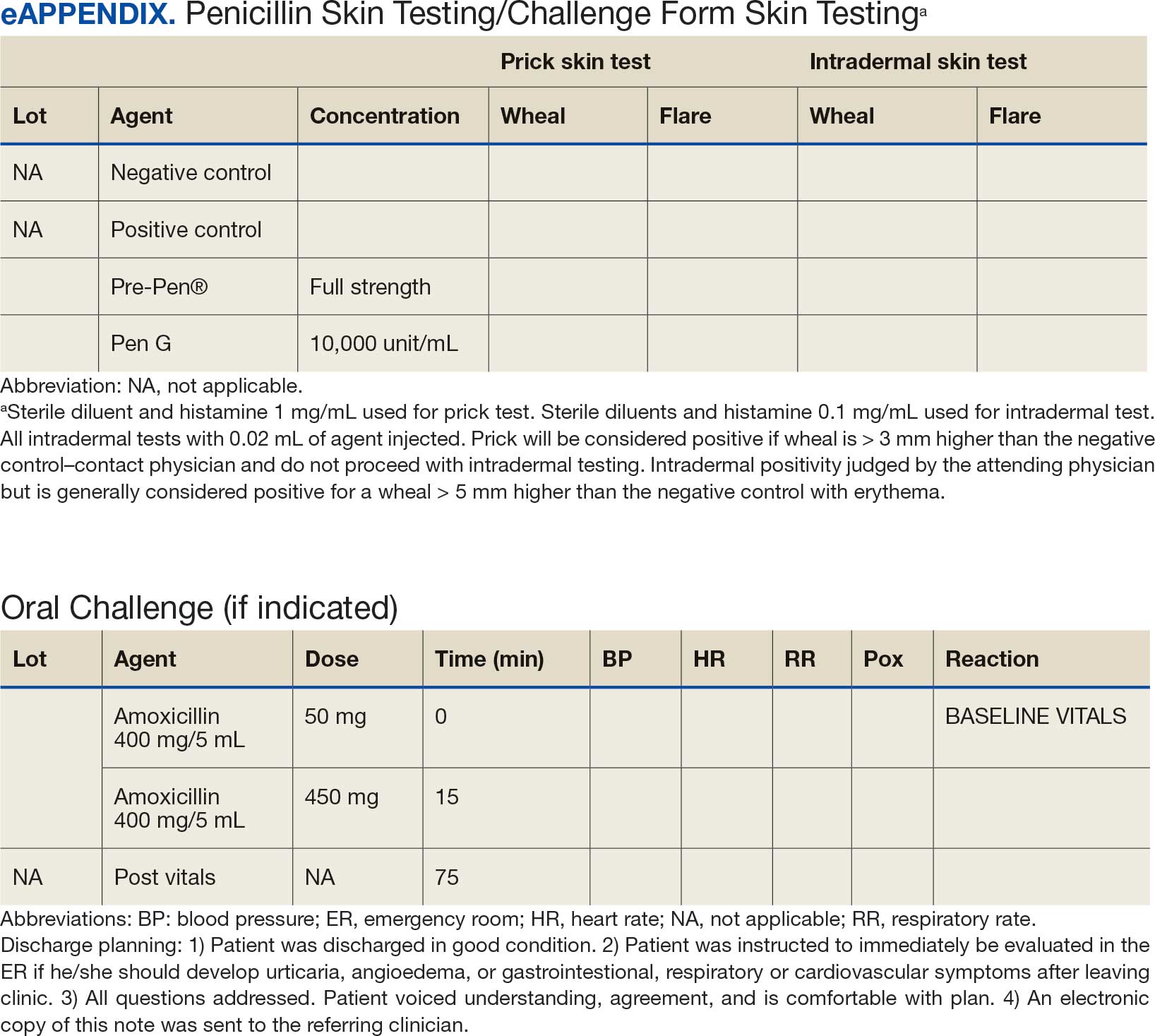
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.
This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).
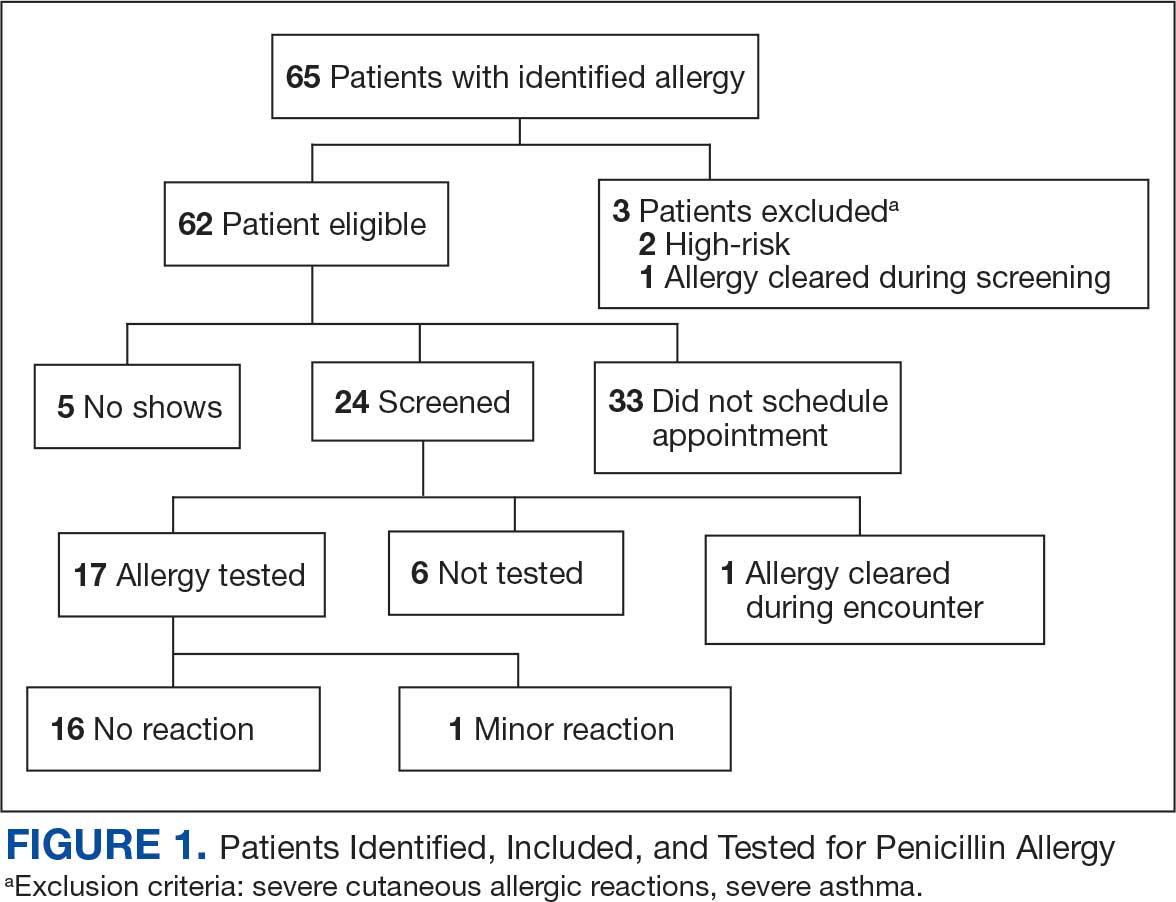
Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
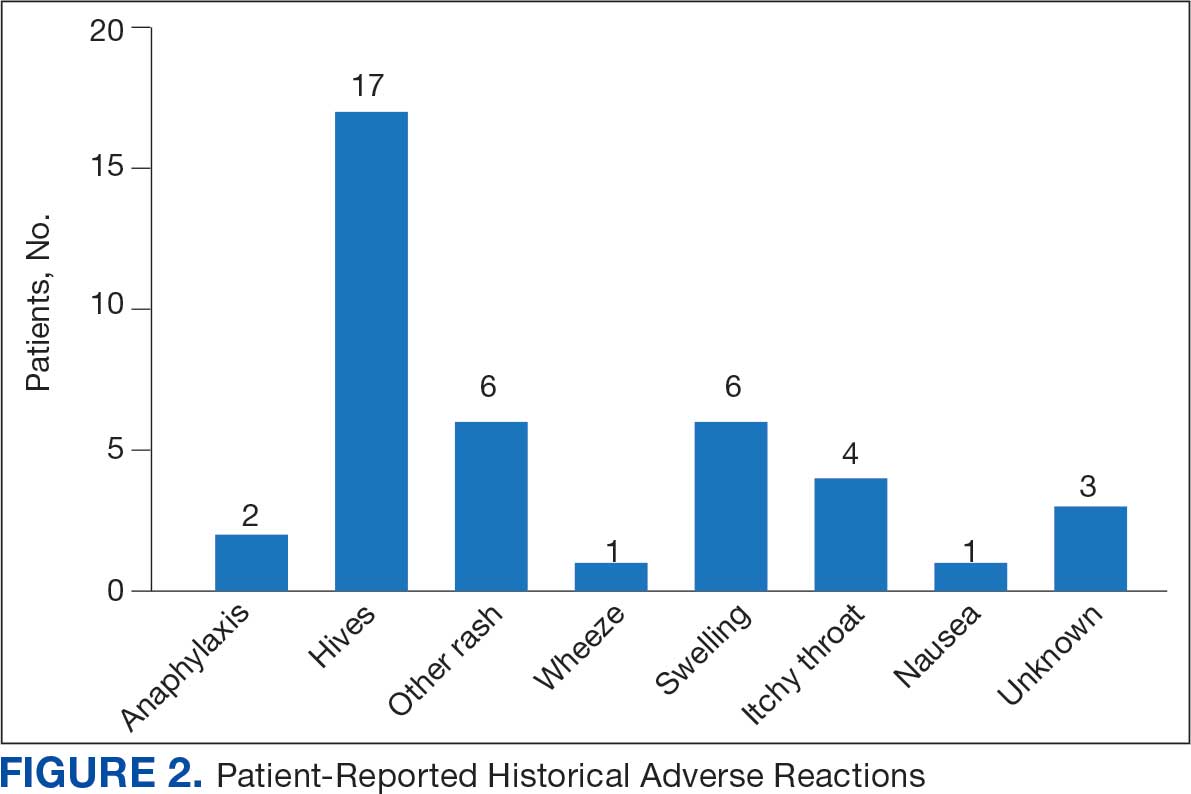
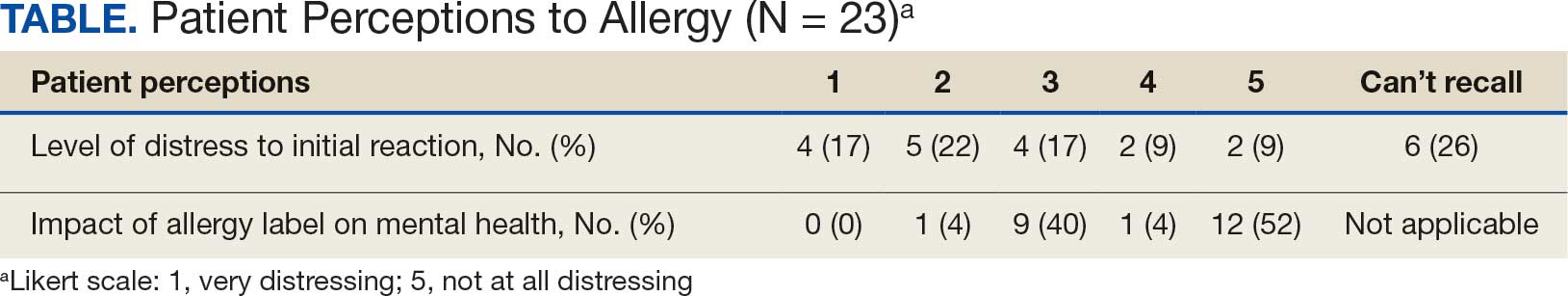
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).
Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.

This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).

Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).


Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.

This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).

Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).


Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Around 5% of US Population Diagnosed With Autoimmune Disease
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
The Protein Problem: The Unsolved Mystery of AI Drug Dev
The question has been lingering for years in medical science circles. Since 2020, when the artificial intelligence (AI) model AlphaFold made it possible to predict protein structures, would the technology open the drug discovery floodgates?
Short answer: No. At least not yet.
The longer answer goes something like this:
A drug target (such as a mutation) is like a lock. The right drug (a protein designed to bind to the mutation, stopping its activity) is the key. But proteins are fidgety and flexible.
“They’re basically molecular springs,” said Gabriel Monteiro da Silva, PhD, a computational chemistry research scientist at Genesis Therapeutics. “Your key can bend and alter the shape of the lock, and if you don’t account for that, your key might fail.”
This is the protein problem in drug development. Another issue making this challenge so vexing is that proteins don’t act in isolation. Their interactions with other proteins, ribonucleic acid, and DNA can affect how they bind to molecules and the shapes they adopt.
Newer versions of AlphaFold, such as AlphaFold Multimer and AlphaFold 3 (the code for which was recently revealed for academic use), can predict many interactions among proteins and between proteins and other molecules. But these tools still have weak points scientists are trying to overcome or work around.
“Those kinds of dynamics and multiple conformations are still quite challenging for the AI models to predict,” said James Zou, PhD, associate professor of biomedical data science at Stanford University in California.
“We’re finding more and more that the only way we can make these structures useful for drug discovery is if we incorporate dynamics, if we incorporate more physics into the model,” said Monteiro da Silva.
Monteiro da Silva spent 3 years during his PhD at Brown University, Providence, Rhode Island, running physics-based simulations in the lab, trying to understand why proteins carrying certain mutations are drug resistant. His results showed how “the changing landscape of shapes that a protein can take” prevented the drug from binding.
It took him 3 years to model just four mutations.
AI can do better — and the struggle is fascinating. By developing models that build on the predictive power of AlphaFold, scientists are uncovering new details about protein activity — insights that can lead to new therapeutics and reveal why existing ones stop working — much faster than they could with traditional methods or AlphaFold alone.
New Windows into Protein Dynamics
A notable step, “but that’s just the starting point,” said Pedro Beltrao, PhD, an associate professor at Institute of Molecular Systems Biology, ETH Zurich in Switzerland. “It’s still very difficult, given a pocket, to actually design the drug or figure out what the pocket binds.”
Going back to the lock-and-key analogy: While he was at Brown, with a team of researchers in the Rubenstein Group, Monteiro da Silva helped create a model to better understand how mutations affect “the shape and dynamics of the lock.” They manipulated the amino acid sequences of proteins, guiding their evolution. This enabled them to use AlphaFold to predict “protein ensembles” and how frequently those ensembles appear. Each ensemble represents the many different shapes a protein can take under given conditions.
“Essentially, it tries to find the most common shapes that a protein will take over an arbitrary amount of time,” Monteiro da Silva said. “If we can predict these ensembles at scale and fast, then we can screen many mutations that cause resistance and develop drugs that will not be affected by that resistance.”
To evaluate their method, the researchers focused on ABL1, a well-studied kinase that causes leukemia. ABL1 can be drugged – unless it carries or develops a mutation that causes drug resistance. Currently there are no drugs that work against proteins carrying those mutations, according to Monteiro da Silva. The researchers used their hybrid AI-meets-physics method to investigate how drugs bind to different ABL1 mutations, screening 100 mutations in just 1 month.
“It’s not going to be perfect for every one of them. But if we have 100 and we get 20 with good accuracy, that’s better than doing four over 3 years,” Monteiro da Silva said.
A forthcoming paper will make their model publicly available in “an easy-to-use graphical interface” that they hope clinicians and medicinal chemists will try out. It can also complement other AI-based tools that dig into protein dynamics, according to Monteiro da Silva.
Complementary Tools to Speed Up Discovery
Another aspect of the protein problem is scale. One protein can interact with hundreds of other proteins, which in turn may interact with hundreds more, all of which comprise the human interactome.
Feixiong Cheng, PhD, helped build PIONEER, a deep learning model that predicts the three-dimensional (3D) structure of interactions between proteins across the interactome.
Most disease mutations disrupt specific interactions between proteins, making their affinity stronger or weaker, explained Cheng. To treat a disease without causing major side effects, scientists need a precise understanding of those interactions.
“From the drug discovery perspective, we cannot just focus on single proteins. We have to understand the protein environment, in particular how the protein interacts with other proteins,” said Cheng, director of Cleveland Clinic Genome Center, Cleveland.
PIONEER helps by blending AlphaFold’s protein structure predictions with next-generation sequencing, a type of genomic research that identifies mutations in the human genome. The model predicts the 3D structure of the places where proteins interact — the binding sites, or interfaces — across the interactome.
“We tell you not only that a binds b, but where on a and where on b the two proteins interact,” said Haiyuan Yu, PhD, director of the Center for Innovative Proteomics, Cornell University, and co-creator of PIONEER.
This can help scientists understand “why a mutation, protein, or even network is a good target for therapeutic discovery,” Cheng said.
The researchers validated PIONEER’s predictions in the lab, testing the impacts of roughly 3000 mutations on 7000 pairs of interacting proteins. Based on their findings, they plan to develop and test treatments for lung and endometrial cancer.
PIONEER can also help scientists home in on how a mutation causes a disease, such as by showing recurrent mutations.
“If you find cancer mutations hitting an interface again and again and again, it means that this is likely to be driving cancer progression,” said Beltrao.
Beltrao’s lab and others have looked for recurrent mutations by using AlphaFold Multimer and AlphaFold 3 to directly model protein interactions. It’s a much slower approach (Pioneer is more than 5000 faster than AlphaFold Multimer, according to Cheng). But it could allow scientists to model interfaces that are not shown by PIONEER.
“You will need many different things to try to come up with a structural modeling of the interactome, and all these will have limitations,” said Beltrao. “Their method is a very good step forward, and there’ll be other approaches that are complementary, to continue to add details.”
And It Wouldn’t be an AI Mission Without ChatGPT
Large language models, such as ChatGPT, are another way that scientists are adding details to protein structure predictions. Zou used GPT-4 to “fine tune” a protein language model, called evolutionary scale modeling (ESM-2), which predicts protein structures directly from a protein sequence.
First, they trained ChatGPT on thousands of papers and studies containing information about the functions, biophysical properties, and disease relevance of different mutations. Next, they used the trained model to “teach” ESM-2, boosting its ability “to predict which mutations are likely to have larger effects or smaller effects,” Zou said. The same could be done for a model like AlphaFold, according to Zou.
“They are quite complementary in that the large language model contains a lot more information about the functions and the biophysics of different mutations and proteins as captured in text,” he said, whereas “you can’t give AlphaFold a piece of paper.”
Exactly how AlphaFold makes its predictions is another mystery. “It will somehow learn protein dynamics phenomenologically,” said Monteiro da Silva. He and others are trying to understand how that happens, in hopes of creating even more accurate predictive models. But for the time being, AI-based methods still need assistance from physics.
“The dream is that we achieve a state where we rely on just the fast methods, and they’re accurate enough,” he said. “But we’re so far from that.”
A version of this article first appeared on Medscape.com.
The question has been lingering for years in medical science circles. Since 2020, when the artificial intelligence (AI) model AlphaFold made it possible to predict protein structures, would the technology open the drug discovery floodgates?
Short answer: No. At least not yet.
The longer answer goes something like this:
A drug target (such as a mutation) is like a lock. The right drug (a protein designed to bind to the mutation, stopping its activity) is the key. But proteins are fidgety and flexible.
“They’re basically molecular springs,” said Gabriel Monteiro da Silva, PhD, a computational chemistry research scientist at Genesis Therapeutics. “Your key can bend and alter the shape of the lock, and if you don’t account for that, your key might fail.”
This is the protein problem in drug development. Another issue making this challenge so vexing is that proteins don’t act in isolation. Their interactions with other proteins, ribonucleic acid, and DNA can affect how they bind to molecules and the shapes they adopt.
Newer versions of AlphaFold, such as AlphaFold Multimer and AlphaFold 3 (the code for which was recently revealed for academic use), can predict many interactions among proteins and between proteins and other molecules. But these tools still have weak points scientists are trying to overcome or work around.
“Those kinds of dynamics and multiple conformations are still quite challenging for the AI models to predict,” said James Zou, PhD, associate professor of biomedical data science at Stanford University in California.
“We’re finding more and more that the only way we can make these structures useful for drug discovery is if we incorporate dynamics, if we incorporate more physics into the model,” said Monteiro da Silva.
Monteiro da Silva spent 3 years during his PhD at Brown University, Providence, Rhode Island, running physics-based simulations in the lab, trying to understand why proteins carrying certain mutations are drug resistant. His results showed how “the changing landscape of shapes that a protein can take” prevented the drug from binding.
It took him 3 years to model just four mutations.
AI can do better — and the struggle is fascinating. By developing models that build on the predictive power of AlphaFold, scientists are uncovering new details about protein activity — insights that can lead to new therapeutics and reveal why existing ones stop working — much faster than they could with traditional methods or AlphaFold alone.
New Windows into Protein Dynamics
A notable step, “but that’s just the starting point,” said Pedro Beltrao, PhD, an associate professor at Institute of Molecular Systems Biology, ETH Zurich in Switzerland. “It’s still very difficult, given a pocket, to actually design the drug or figure out what the pocket binds.”
Going back to the lock-and-key analogy: While he was at Brown, with a team of researchers in the Rubenstein Group, Monteiro da Silva helped create a model to better understand how mutations affect “the shape and dynamics of the lock.” They manipulated the amino acid sequences of proteins, guiding their evolution. This enabled them to use AlphaFold to predict “protein ensembles” and how frequently those ensembles appear. Each ensemble represents the many different shapes a protein can take under given conditions.
“Essentially, it tries to find the most common shapes that a protein will take over an arbitrary amount of time,” Monteiro da Silva said. “If we can predict these ensembles at scale and fast, then we can screen many mutations that cause resistance and develop drugs that will not be affected by that resistance.”
To evaluate their method, the researchers focused on ABL1, a well-studied kinase that causes leukemia. ABL1 can be drugged – unless it carries or develops a mutation that causes drug resistance. Currently there are no drugs that work against proteins carrying those mutations, according to Monteiro da Silva. The researchers used their hybrid AI-meets-physics method to investigate how drugs bind to different ABL1 mutations, screening 100 mutations in just 1 month.
“It’s not going to be perfect for every one of them. But if we have 100 and we get 20 with good accuracy, that’s better than doing four over 3 years,” Monteiro da Silva said.
A forthcoming paper will make their model publicly available in “an easy-to-use graphical interface” that they hope clinicians and medicinal chemists will try out. It can also complement other AI-based tools that dig into protein dynamics, according to Monteiro da Silva.
Complementary Tools to Speed Up Discovery
Another aspect of the protein problem is scale. One protein can interact with hundreds of other proteins, which in turn may interact with hundreds more, all of which comprise the human interactome.
Feixiong Cheng, PhD, helped build PIONEER, a deep learning model that predicts the three-dimensional (3D) structure of interactions between proteins across the interactome.
Most disease mutations disrupt specific interactions between proteins, making their affinity stronger or weaker, explained Cheng. To treat a disease without causing major side effects, scientists need a precise understanding of those interactions.
“From the drug discovery perspective, we cannot just focus on single proteins. We have to understand the protein environment, in particular how the protein interacts with other proteins,” said Cheng, director of Cleveland Clinic Genome Center, Cleveland.
PIONEER helps by blending AlphaFold’s protein structure predictions with next-generation sequencing, a type of genomic research that identifies mutations in the human genome. The model predicts the 3D structure of the places where proteins interact — the binding sites, or interfaces — across the interactome.
“We tell you not only that a binds b, but where on a and where on b the two proteins interact,” said Haiyuan Yu, PhD, director of the Center for Innovative Proteomics, Cornell University, and co-creator of PIONEER.
This can help scientists understand “why a mutation, protein, or even network is a good target for therapeutic discovery,” Cheng said.
The researchers validated PIONEER’s predictions in the lab, testing the impacts of roughly 3000 mutations on 7000 pairs of interacting proteins. Based on their findings, they plan to develop and test treatments for lung and endometrial cancer.
PIONEER can also help scientists home in on how a mutation causes a disease, such as by showing recurrent mutations.
“If you find cancer mutations hitting an interface again and again and again, it means that this is likely to be driving cancer progression,” said Beltrao.
Beltrao’s lab and others have looked for recurrent mutations by using AlphaFold Multimer and AlphaFold 3 to directly model protein interactions. It’s a much slower approach (Pioneer is more than 5000 faster than AlphaFold Multimer, according to Cheng). But it could allow scientists to model interfaces that are not shown by PIONEER.
“You will need many different things to try to come up with a structural modeling of the interactome, and all these will have limitations,” said Beltrao. “Their method is a very good step forward, and there’ll be other approaches that are complementary, to continue to add details.”
And It Wouldn’t be an AI Mission Without ChatGPT
Large language models, such as ChatGPT, are another way that scientists are adding details to protein structure predictions. Zou used GPT-4 to “fine tune” a protein language model, called evolutionary scale modeling (ESM-2), which predicts protein structures directly from a protein sequence.
First, they trained ChatGPT on thousands of papers and studies containing information about the functions, biophysical properties, and disease relevance of different mutations. Next, they used the trained model to “teach” ESM-2, boosting its ability “to predict which mutations are likely to have larger effects or smaller effects,” Zou said. The same could be done for a model like AlphaFold, according to Zou.
“They are quite complementary in that the large language model contains a lot more information about the functions and the biophysics of different mutations and proteins as captured in text,” he said, whereas “you can’t give AlphaFold a piece of paper.”
Exactly how AlphaFold makes its predictions is another mystery. “It will somehow learn protein dynamics phenomenologically,” said Monteiro da Silva. He and others are trying to understand how that happens, in hopes of creating even more accurate predictive models. But for the time being, AI-based methods still need assistance from physics.
“The dream is that we achieve a state where we rely on just the fast methods, and they’re accurate enough,” he said. “But we’re so far from that.”
A version of this article first appeared on Medscape.com.
The question has been lingering for years in medical science circles. Since 2020, when the artificial intelligence (AI) model AlphaFold made it possible to predict protein structures, would the technology open the drug discovery floodgates?
Short answer: No. At least not yet.
The longer answer goes something like this:
A drug target (such as a mutation) is like a lock. The right drug (a protein designed to bind to the mutation, stopping its activity) is the key. But proteins are fidgety and flexible.
“They’re basically molecular springs,” said Gabriel Monteiro da Silva, PhD, a computational chemistry research scientist at Genesis Therapeutics. “Your key can bend and alter the shape of the lock, and if you don’t account for that, your key might fail.”
This is the protein problem in drug development. Another issue making this challenge so vexing is that proteins don’t act in isolation. Their interactions with other proteins, ribonucleic acid, and DNA can affect how they bind to molecules and the shapes they adopt.
Newer versions of AlphaFold, such as AlphaFold Multimer and AlphaFold 3 (the code for which was recently revealed for academic use), can predict many interactions among proteins and between proteins and other molecules. But these tools still have weak points scientists are trying to overcome or work around.
“Those kinds of dynamics and multiple conformations are still quite challenging for the AI models to predict,” said James Zou, PhD, associate professor of biomedical data science at Stanford University in California.
“We’re finding more and more that the only way we can make these structures useful for drug discovery is if we incorporate dynamics, if we incorporate more physics into the model,” said Monteiro da Silva.
Monteiro da Silva spent 3 years during his PhD at Brown University, Providence, Rhode Island, running physics-based simulations in the lab, trying to understand why proteins carrying certain mutations are drug resistant. His results showed how “the changing landscape of shapes that a protein can take” prevented the drug from binding.
It took him 3 years to model just four mutations.
AI can do better — and the struggle is fascinating. By developing models that build on the predictive power of AlphaFold, scientists are uncovering new details about protein activity — insights that can lead to new therapeutics and reveal why existing ones stop working — much faster than they could with traditional methods or AlphaFold alone.
New Windows into Protein Dynamics
A notable step, “but that’s just the starting point,” said Pedro Beltrao, PhD, an associate professor at Institute of Molecular Systems Biology, ETH Zurich in Switzerland. “It’s still very difficult, given a pocket, to actually design the drug or figure out what the pocket binds.”
Going back to the lock-and-key analogy: While he was at Brown, with a team of researchers in the Rubenstein Group, Monteiro da Silva helped create a model to better understand how mutations affect “the shape and dynamics of the lock.” They manipulated the amino acid sequences of proteins, guiding their evolution. This enabled them to use AlphaFold to predict “protein ensembles” and how frequently those ensembles appear. Each ensemble represents the many different shapes a protein can take under given conditions.
“Essentially, it tries to find the most common shapes that a protein will take over an arbitrary amount of time,” Monteiro da Silva said. “If we can predict these ensembles at scale and fast, then we can screen many mutations that cause resistance and develop drugs that will not be affected by that resistance.”
To evaluate their method, the researchers focused on ABL1, a well-studied kinase that causes leukemia. ABL1 can be drugged – unless it carries or develops a mutation that causes drug resistance. Currently there are no drugs that work against proteins carrying those mutations, according to Monteiro da Silva. The researchers used their hybrid AI-meets-physics method to investigate how drugs bind to different ABL1 mutations, screening 100 mutations in just 1 month.
“It’s not going to be perfect for every one of them. But if we have 100 and we get 20 with good accuracy, that’s better than doing four over 3 years,” Monteiro da Silva said.
A forthcoming paper will make their model publicly available in “an easy-to-use graphical interface” that they hope clinicians and medicinal chemists will try out. It can also complement other AI-based tools that dig into protein dynamics, according to Monteiro da Silva.
Complementary Tools to Speed Up Discovery
Another aspect of the protein problem is scale. One protein can interact with hundreds of other proteins, which in turn may interact with hundreds more, all of which comprise the human interactome.
Feixiong Cheng, PhD, helped build PIONEER, a deep learning model that predicts the three-dimensional (3D) structure of interactions between proteins across the interactome.
Most disease mutations disrupt specific interactions between proteins, making their affinity stronger or weaker, explained Cheng. To treat a disease without causing major side effects, scientists need a precise understanding of those interactions.
“From the drug discovery perspective, we cannot just focus on single proteins. We have to understand the protein environment, in particular how the protein interacts with other proteins,” said Cheng, director of Cleveland Clinic Genome Center, Cleveland.
PIONEER helps by blending AlphaFold’s protein structure predictions with next-generation sequencing, a type of genomic research that identifies mutations in the human genome. The model predicts the 3D structure of the places where proteins interact — the binding sites, or interfaces — across the interactome.
“We tell you not only that a binds b, but where on a and where on b the two proteins interact,” said Haiyuan Yu, PhD, director of the Center for Innovative Proteomics, Cornell University, and co-creator of PIONEER.
This can help scientists understand “why a mutation, protein, or even network is a good target for therapeutic discovery,” Cheng said.
The researchers validated PIONEER’s predictions in the lab, testing the impacts of roughly 3000 mutations on 7000 pairs of interacting proteins. Based on their findings, they plan to develop and test treatments for lung and endometrial cancer.
PIONEER can also help scientists home in on how a mutation causes a disease, such as by showing recurrent mutations.
“If you find cancer mutations hitting an interface again and again and again, it means that this is likely to be driving cancer progression,” said Beltrao.
Beltrao’s lab and others have looked for recurrent mutations by using AlphaFold Multimer and AlphaFold 3 to directly model protein interactions. It’s a much slower approach (Pioneer is more than 5000 faster than AlphaFold Multimer, according to Cheng). But it could allow scientists to model interfaces that are not shown by PIONEER.
“You will need many different things to try to come up with a structural modeling of the interactome, and all these will have limitations,” said Beltrao. “Their method is a very good step forward, and there’ll be other approaches that are complementary, to continue to add details.”
And It Wouldn’t be an AI Mission Without ChatGPT
Large language models, such as ChatGPT, are another way that scientists are adding details to protein structure predictions. Zou used GPT-4 to “fine tune” a protein language model, called evolutionary scale modeling (ESM-2), which predicts protein structures directly from a protein sequence.
First, they trained ChatGPT on thousands of papers and studies containing information about the functions, biophysical properties, and disease relevance of different mutations. Next, they used the trained model to “teach” ESM-2, boosting its ability “to predict which mutations are likely to have larger effects or smaller effects,” Zou said. The same could be done for a model like AlphaFold, according to Zou.
“They are quite complementary in that the large language model contains a lot more information about the functions and the biophysics of different mutations and proteins as captured in text,” he said, whereas “you can’t give AlphaFold a piece of paper.”
Exactly how AlphaFold makes its predictions is another mystery. “It will somehow learn protein dynamics phenomenologically,” said Monteiro da Silva. He and others are trying to understand how that happens, in hopes of creating even more accurate predictive models. But for the time being, AI-based methods still need assistance from physics.
“The dream is that we achieve a state where we rely on just the fast methods, and they’re accurate enough,” he said. “But we’re so far from that.”
A version of this article first appeared on Medscape.com.
Alpha-Gal Syndrome: 5 Things to Know
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
The Cause of All That Stress: Tonsillectomy?
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.
That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.
Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Anaphylaxis Treatment Uncertainty Persists for Patients and Professionals
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Just Call It ‘Chronic Rhinitis’ and Reach for These Treatments
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about rhinitis?
Paul N. Williams, MD: I’m excited. It’s always the season to talk about rhinitis.
Watto: We had a great guest for this podcast, Rhinitis and Environmental Allergies with Dr. Olajumoke Fadugba from Penn Medicine. She’s an allergist and immunologist. One of her pet peeves is when people just call everything “allergic rhinitis” because we should be calling it “chronic rhinitis,” if it’s chronic. That’s an umbrella term, and there are many buckets underneath it that people could fall into.
When you’re taking a history, you have to figure out whether it’s perennial (meaning it happens year round) because certain things can cause that. Cat dander is around all the time, so people with cats might have sinus symptoms all year. Dust mites are another one, and it’s pretty hard to avoid those. Those are some perennial allergens.
Then there is allergic vs nonallergic rhinitis, which is something I hadn’t really put too much thought into.
Williams: I didn’t realize exactly how nuanced it got. Nonallergic rhinitis can still be seasonal because changes in temperature and humidity can trigger the rhinitis. And it matters what medications you use for what.
Watto: Here are some ways you can try to figure out if rhinitis is allergic or nonallergic. Ask the patient if they have itchy eyes and are sneezing a lot. That can be more of an allergic rhinitis, but both allergic and nonallergic rhinitis have the congestion, the rhinorrhea, so you can’t figure it out based on that alone.
Dr. Fadugba said that one clue that it might be nonallergic rhinitis is the age of onset. If the symptoms are later in onset (older age), then 30%-40% of rhinitis is nonallergic. If the patient has never had allergies and now all of a sudden they have new chronic sinus symptoms, it’s probably nonallergic rhinitis. It’s a diagnosis of exclusion.
I guess they need allergy testing?
Williams: If you want to make a definitive diagnosis, you need to rule it out. I suspect that you might be able to get away with some empirical treatment. If they get better, you can feel like a winner because getting booked in for allergy testing can be a little bit of a challenge.
Watto: The main treatment difference is that the oral antihistamines do not really seem to work for nonallergic rhinitis, but they can help with allergic rhinitis. Weirdly, the nasal antihistamines and nasal steroids do seem to work for both allergic and nonallergic rhinitis.
I don’t understand the mechanism there, but if you think someone might have nonallergic rhinitis, I wouldn’t go with the oral antihistamines as your first-line treatment. I would go with a nasal spray; you pretty much can’t go wrong with either an antihistamine or a steroid nasal spray.
Williams: We typically start with the nasal sprays. That’s kind of first-line for almost everybody, allergic or nonallergic. You’re probably going to start with an intranasal steroid, and then it’s kind of dealer’s choice what the patient can tolerate and afford. Sometimes you can get them covered by insurance, at least in my experience.
I will say that this is one of the medications — like nicotine patches and other things — where we as doctors don’t really counsel patients on how to use it appropriately. So with our expert, we revisited the idea of the patient pointing the nasal spray laterally, toward their ear basically, and not spraying toward their brain. There should not be a slurping sound afterward, because “if you taste it, you waste it,” as the allergists and immunologists say. It’s supposed to sit up there and not be swallowed immediately.
If your patient is sensitive to the floral flavor of some of the fluticasones (which I don’t mind so much as a user myself), then you can try mometasone or the other formulations. They are all roughly equivalent.
Speaking of medications, which medications can cause rhinitis? Any meds we commonly use in primary care?
Williams: Apparently the combined hormonal oral contraceptives can do it. Also the phosphodiesterase 5 (PDE-5) inhibitors. Drugs that cause vasodilation can also do it. Some of the antihypertensives. I’ve seen beta-blockers and angiotensin-converting enzyme (ACE) inhibitors listed specifically, and some of the medications for benign prostatic hyperplasia (BPH). So there are a couple of medications that you can think about as a potential cause of rhinitis, although my suspicion is not going to be as high as for some of the other causes.
Watto: We mentioned medication treatments for patients who are really bothered by rhinorrhea, and maybe they are already on a steroid or an antihistamine.
You can try nasal ipratropium for people that have really prominent rhinorrhea. Dr. Fadugba said that can work well, and it’s usually taken three or four times a day. I’ve had good success prescribing it for my patients. Another one that I have never prescribed, but that Dr. Fadugba said is available over the counter, is intranasal cromolyn — a mast cell stabilizer. She said it can be beneficial.
Let’s say I had a cat allergy and I was going to visit Paul. I could use the intranasal cromolyn ahead of time to reduce rhinitis when I’m around the cats.
Paul, what about montelukast? I never know what to do with that one.
Williams: I’ve seen it prescribed as a last-ditch attempt to fix chronic rhinitis. Dr. Fadugba said she only ever prescribes it for patients who have rhinitis symptoms and asthma and never just for chronic rhinitis because it doesn’t work. And also, there have been some new black-box warnings from the US Food and Drug Administration (FDA). So unless there’s a solid indication for it, montelukast is not something you should just prescribe to try to see if it will work. That’s probably not the right approach for this.
But if the patient has challenging control asthma, and as a component, challenging nasal symptoms as well, it might be a reasonable medication to try.
Watto: And finally, Paul, how does climate change possibly have anything to do with rhinitis?
Williams: I feel like I’m just seeing more and more of the stuff every year. I don’t know if I’m more sensitive to it or because I’m having more symptoms myself, but it turns out the prevalence actually is going up.
We’re seeing more of it in part because it’s getting hotter outside, which is in turn worsening the production of allergens and increasing the allergen exposure and the severity of the symptoms that go along with it. More people are having more severe disease because the world is changing as a result of the stuff that we do. So fix that. But also be mindful and expect to see even more of these problems as you move forward in your careers.
Watto: Dr. Fadugba gave us so many great tips. You can listen to the full podcast episode here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, disclosed ties with The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about rhinitis?
Paul N. Williams, MD: I’m excited. It’s always the season to talk about rhinitis.
Watto: We had a great guest for this podcast, Rhinitis and Environmental Allergies with Dr. Olajumoke Fadugba from Penn Medicine. She’s an allergist and immunologist. One of her pet peeves is when people just call everything “allergic rhinitis” because we should be calling it “chronic rhinitis,” if it’s chronic. That’s an umbrella term, and there are many buckets underneath it that people could fall into.
When you’re taking a history, you have to figure out whether it’s perennial (meaning it happens year round) because certain things can cause that. Cat dander is around all the time, so people with cats might have sinus symptoms all year. Dust mites are another one, and it’s pretty hard to avoid those. Those are some perennial allergens.
Then there is allergic vs nonallergic rhinitis, which is something I hadn’t really put too much thought into.
Williams: I didn’t realize exactly how nuanced it got. Nonallergic rhinitis can still be seasonal because changes in temperature and humidity can trigger the rhinitis. And it matters what medications you use for what.
Watto: Here are some ways you can try to figure out if rhinitis is allergic or nonallergic. Ask the patient if they have itchy eyes and are sneezing a lot. That can be more of an allergic rhinitis, but both allergic and nonallergic rhinitis have the congestion, the rhinorrhea, so you can’t figure it out based on that alone.
Dr. Fadugba said that one clue that it might be nonallergic rhinitis is the age of onset. If the symptoms are later in onset (older age), then 30%-40% of rhinitis is nonallergic. If the patient has never had allergies and now all of a sudden they have new chronic sinus symptoms, it’s probably nonallergic rhinitis. It’s a diagnosis of exclusion.
I guess they need allergy testing?
Williams: If you want to make a definitive diagnosis, you need to rule it out. I suspect that you might be able to get away with some empirical treatment. If they get better, you can feel like a winner because getting booked in for allergy testing can be a little bit of a challenge.
Watto: The main treatment difference is that the oral antihistamines do not really seem to work for nonallergic rhinitis, but they can help with allergic rhinitis. Weirdly, the nasal antihistamines and nasal steroids do seem to work for both allergic and nonallergic rhinitis.
I don’t understand the mechanism there, but if you think someone might have nonallergic rhinitis, I wouldn’t go with the oral antihistamines as your first-line treatment. I would go with a nasal spray; you pretty much can’t go wrong with either an antihistamine or a steroid nasal spray.
Williams: We typically start with the nasal sprays. That’s kind of first-line for almost everybody, allergic or nonallergic. You’re probably going to start with an intranasal steroid, and then it’s kind of dealer’s choice what the patient can tolerate and afford. Sometimes you can get them covered by insurance, at least in my experience.
I will say that this is one of the medications — like nicotine patches and other things — where we as doctors don’t really counsel patients on how to use it appropriately. So with our expert, we revisited the idea of the patient pointing the nasal spray laterally, toward their ear basically, and not spraying toward their brain. There should not be a slurping sound afterward, because “if you taste it, you waste it,” as the allergists and immunologists say. It’s supposed to sit up there and not be swallowed immediately.
If your patient is sensitive to the floral flavor of some of the fluticasones (which I don’t mind so much as a user myself), then you can try mometasone or the other formulations. They are all roughly equivalent.
Speaking of medications, which medications can cause rhinitis? Any meds we commonly use in primary care?
Williams: Apparently the combined hormonal oral contraceptives can do it. Also the phosphodiesterase 5 (PDE-5) inhibitors. Drugs that cause vasodilation can also do it. Some of the antihypertensives. I’ve seen beta-blockers and angiotensin-converting enzyme (ACE) inhibitors listed specifically, and some of the medications for benign prostatic hyperplasia (BPH). So there are a couple of medications that you can think about as a potential cause of rhinitis, although my suspicion is not going to be as high as for some of the other causes.
Watto: We mentioned medication treatments for patients who are really bothered by rhinorrhea, and maybe they are already on a steroid or an antihistamine.
You can try nasal ipratropium for people that have really prominent rhinorrhea. Dr. Fadugba said that can work well, and it’s usually taken three or four times a day. I’ve had good success prescribing it for my patients. Another one that I have never prescribed, but that Dr. Fadugba said is available over the counter, is intranasal cromolyn — a mast cell stabilizer. She said it can be beneficial.
Let’s say I had a cat allergy and I was going to visit Paul. I could use the intranasal cromolyn ahead of time to reduce rhinitis when I’m around the cats.
Paul, what about montelukast? I never know what to do with that one.
Williams: I’ve seen it prescribed as a last-ditch attempt to fix chronic rhinitis. Dr. Fadugba said she only ever prescribes it for patients who have rhinitis symptoms and asthma and never just for chronic rhinitis because it doesn’t work. And also, there have been some new black-box warnings from the US Food and Drug Administration (FDA). So unless there’s a solid indication for it, montelukast is not something you should just prescribe to try to see if it will work. That’s probably not the right approach for this.
But if the patient has challenging control asthma, and as a component, challenging nasal symptoms as well, it might be a reasonable medication to try.
Watto: And finally, Paul, how does climate change possibly have anything to do with rhinitis?
Williams: I feel like I’m just seeing more and more of the stuff every year. I don’t know if I’m more sensitive to it or because I’m having more symptoms myself, but it turns out the prevalence actually is going up.
We’re seeing more of it in part because it’s getting hotter outside, which is in turn worsening the production of allergens and increasing the allergen exposure and the severity of the symptoms that go along with it. More people are having more severe disease because the world is changing as a result of the stuff that we do. So fix that. But also be mindful and expect to see even more of these problems as you move forward in your careers.
Watto: Dr. Fadugba gave us so many great tips. You can listen to the full podcast episode here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, disclosed ties with The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about rhinitis?
Paul N. Williams, MD: I’m excited. It’s always the season to talk about rhinitis.
Watto: We had a great guest for this podcast, Rhinitis and Environmental Allergies with Dr. Olajumoke Fadugba from Penn Medicine. She’s an allergist and immunologist. One of her pet peeves is when people just call everything “allergic rhinitis” because we should be calling it “chronic rhinitis,” if it’s chronic. That’s an umbrella term, and there are many buckets underneath it that people could fall into.
When you’re taking a history, you have to figure out whether it’s perennial (meaning it happens year round) because certain things can cause that. Cat dander is around all the time, so people with cats might have sinus symptoms all year. Dust mites are another one, and it’s pretty hard to avoid those. Those are some perennial allergens.
Then there is allergic vs nonallergic rhinitis, which is something I hadn’t really put too much thought into.
Williams: I didn’t realize exactly how nuanced it got. Nonallergic rhinitis can still be seasonal because changes in temperature and humidity can trigger the rhinitis. And it matters what medications you use for what.
Watto: Here are some ways you can try to figure out if rhinitis is allergic or nonallergic. Ask the patient if they have itchy eyes and are sneezing a lot. That can be more of an allergic rhinitis, but both allergic and nonallergic rhinitis have the congestion, the rhinorrhea, so you can’t figure it out based on that alone.
Dr. Fadugba said that one clue that it might be nonallergic rhinitis is the age of onset. If the symptoms are later in onset (older age), then 30%-40% of rhinitis is nonallergic. If the patient has never had allergies and now all of a sudden they have new chronic sinus symptoms, it’s probably nonallergic rhinitis. It’s a diagnosis of exclusion.
I guess they need allergy testing?
Williams: If you want to make a definitive diagnosis, you need to rule it out. I suspect that you might be able to get away with some empirical treatment. If they get better, you can feel like a winner because getting booked in for allergy testing can be a little bit of a challenge.
Watto: The main treatment difference is that the oral antihistamines do not really seem to work for nonallergic rhinitis, but they can help with allergic rhinitis. Weirdly, the nasal antihistamines and nasal steroids do seem to work for both allergic and nonallergic rhinitis.
I don’t understand the mechanism there, but if you think someone might have nonallergic rhinitis, I wouldn’t go with the oral antihistamines as your first-line treatment. I would go with a nasal spray; you pretty much can’t go wrong with either an antihistamine or a steroid nasal spray.
Williams: We typically start with the nasal sprays. That’s kind of first-line for almost everybody, allergic or nonallergic. You’re probably going to start with an intranasal steroid, and then it’s kind of dealer’s choice what the patient can tolerate and afford. Sometimes you can get them covered by insurance, at least in my experience.
I will say that this is one of the medications — like nicotine patches and other things — where we as doctors don’t really counsel patients on how to use it appropriately. So with our expert, we revisited the idea of the patient pointing the nasal spray laterally, toward their ear basically, and not spraying toward their brain. There should not be a slurping sound afterward, because “if you taste it, you waste it,” as the allergists and immunologists say. It’s supposed to sit up there and not be swallowed immediately.
If your patient is sensitive to the floral flavor of some of the fluticasones (which I don’t mind so much as a user myself), then you can try mometasone or the other formulations. They are all roughly equivalent.
Speaking of medications, which medications can cause rhinitis? Any meds we commonly use in primary care?
Williams: Apparently the combined hormonal oral contraceptives can do it. Also the phosphodiesterase 5 (PDE-5) inhibitors. Drugs that cause vasodilation can also do it. Some of the antihypertensives. I’ve seen beta-blockers and angiotensin-converting enzyme (ACE) inhibitors listed specifically, and some of the medications for benign prostatic hyperplasia (BPH). So there are a couple of medications that you can think about as a potential cause of rhinitis, although my suspicion is not going to be as high as for some of the other causes.
Watto: We mentioned medication treatments for patients who are really bothered by rhinorrhea, and maybe they are already on a steroid or an antihistamine.
You can try nasal ipratropium for people that have really prominent rhinorrhea. Dr. Fadugba said that can work well, and it’s usually taken three or four times a day. I’ve had good success prescribing it for my patients. Another one that I have never prescribed, but that Dr. Fadugba said is available over the counter, is intranasal cromolyn — a mast cell stabilizer. She said it can be beneficial.
Let’s say I had a cat allergy and I was going to visit Paul. I could use the intranasal cromolyn ahead of time to reduce rhinitis when I’m around the cats.
Paul, what about montelukast? I never know what to do with that one.
Williams: I’ve seen it prescribed as a last-ditch attempt to fix chronic rhinitis. Dr. Fadugba said she only ever prescribes it for patients who have rhinitis symptoms and asthma and never just for chronic rhinitis because it doesn’t work. And also, there have been some new black-box warnings from the US Food and Drug Administration (FDA). So unless there’s a solid indication for it, montelukast is not something you should just prescribe to try to see if it will work. That’s probably not the right approach for this.
But if the patient has challenging control asthma, and as a component, challenging nasal symptoms as well, it might be a reasonable medication to try.
Watto: And finally, Paul, how does climate change possibly have anything to do with rhinitis?
Williams: I feel like I’m just seeing more and more of the stuff every year. I don’t know if I’m more sensitive to it or because I’m having more symptoms myself, but it turns out the prevalence actually is going up.
We’re seeing more of it in part because it’s getting hotter outside, which is in turn worsening the production of allergens and increasing the allergen exposure and the severity of the symptoms that go along with it. More people are having more severe disease because the world is changing as a result of the stuff that we do. So fix that. But also be mindful and expect to see even more of these problems as you move forward in your careers.
Watto: Dr. Fadugba gave us so many great tips. You can listen to the full podcast episode here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, disclosed ties with The Curbsiders.
A version of this article first appeared on Medscape.com.
