User login
Case Report
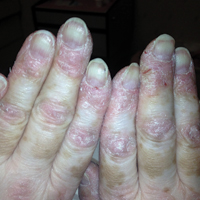
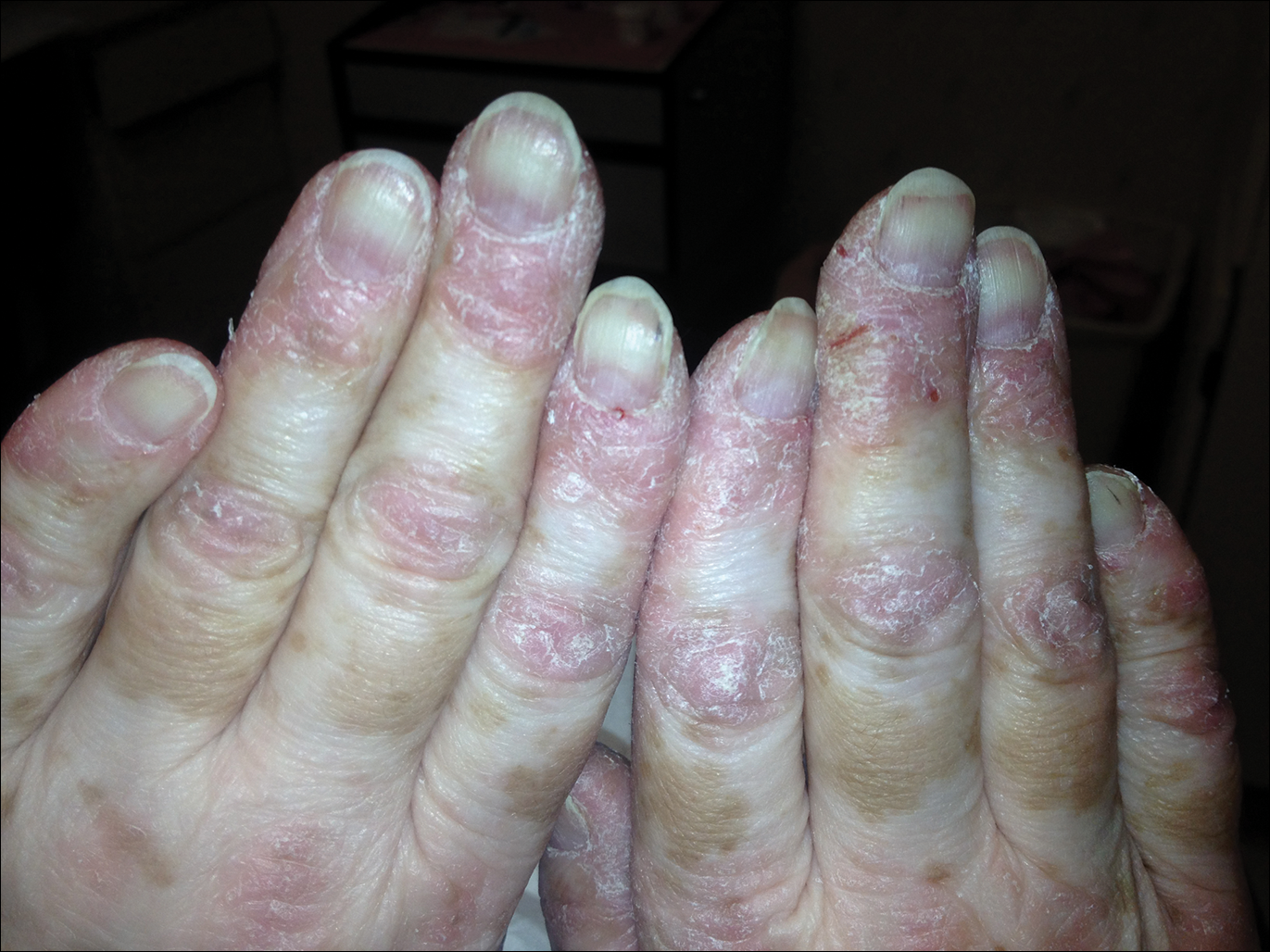
A 54-year-old woman presented with a painful pruritic rash on the hands and feet of 7 years’ duration. She reported intermittent joint pain but denied muscle weakness. Physical examination revealed fissured fingertips and heavy scaling of the palms and lateral fingers (Figure 1). Violaceous scaly papules were seen on the distal and proximal interphalangeal joints (Figure 2). A severe plantar keratoderma also was noted (Figure 3). Pink scaly plaques were present on the bilateral elbows and postauricular skin. Diffuse mat telangiectases covered the malar skin. Extensive poikilodermatous skin changes covered approximately 20% of the total body surface area. Salt-and-pepper patches and papules were noted over the bilateral thighs. She reported an uncertain history of recent radiographs of one or both hands, which showed no joint degeneration characteristic of psoriatic arthritis. She previously had been given a diagnosis of psoriasis by an outside dermatologist but was not responding to topical therapy.

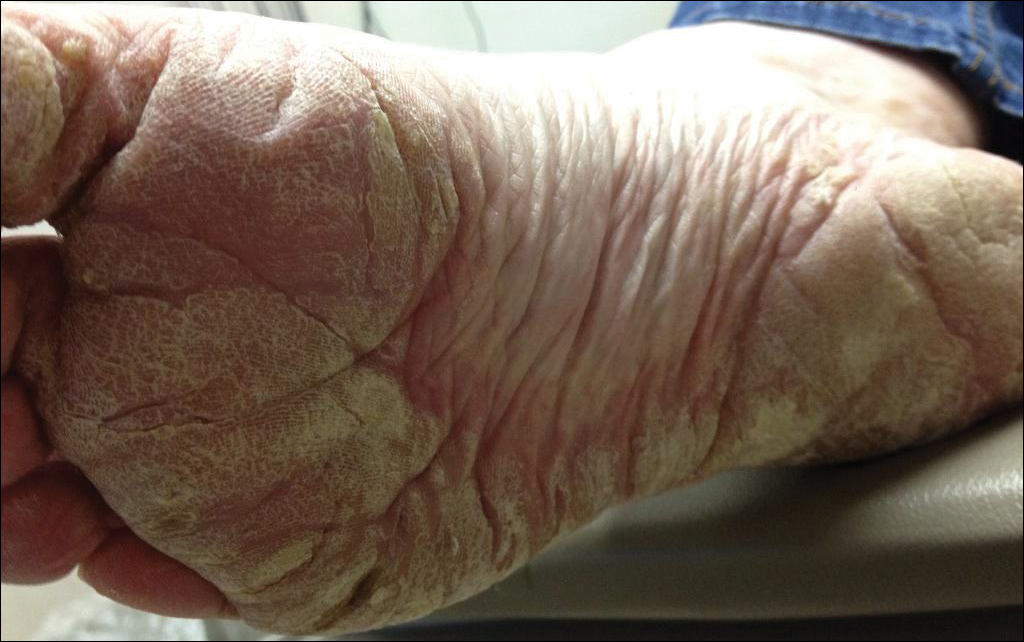
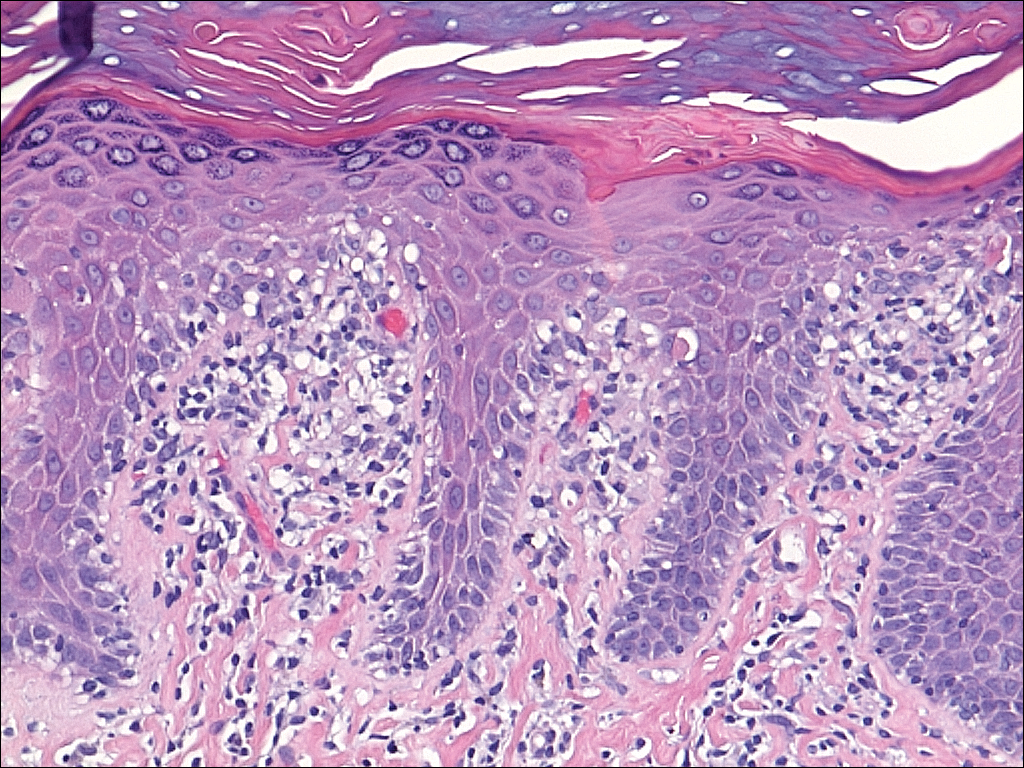
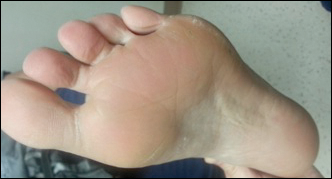
Several skin biopsies showed histologic evidence of dermatomyositis (DM)(Figure 4). Prominent basement thickening also was seen on periodic acid–Schiff staining (not shown). Laboratory workup showed negative antinuclear antibodies and anti–Jo-1, anti-Ku, and anti-Mi2 antibodies. Muscle enzymes including creatinine kinase and aldolase were within reference range. Pelvic ultrasonography and mammography were negative. Pulmonary function tests were unremarkable. High-resolution chest computed tomography (CT) was ordered because of a history of chronic cough; however, no evidence of malignancy or interstitial lung disease was seen. The patient was diagnosed with amyopathic dermatomyositis (ADM). Rheumatology was consulted and initiated oral hydroxychloroquine therapy. After 3 months, the patient’s cutaneous disease did not respond and she reported having headaches associated with this medication; therefore, methotrexate was started. Within 2 months of treatment, full resolution of the plantar keratoderma (Figure 5) and clearance of the scaling/fissuring of the hands as well as the psoriatic-appearing plaques on the elbows was noted.
Comment
Amyopathic DM is a subset of DM that accounts for 10% to 20% of DM cases.1,2 Sontheimer’s3 diagnostic criteria for ADM require histopathologic confirmation of the hallmark skin findings of classic DM and lack of muscle weakness or muscle enzyme (creatine kinase/aldolase) elevation for at least 2 years.
Similar to classic DM, ADM typically presents in the fifth decade of life and has a female predilection.1,4 The term hypomyopathic DM is used to describe patients who exhibit classic skin findings and evidence of muscle involvement on magnetic resonance imaging, electromyography, biopsy, or serum enzymes but have no clinical evidence of muscle weakness for at least 6 months. Together, hypomyopathic DM and ADM are referred to as clinically ADM (CADM). Patients who have met the criteria for hypomyopathic DM or ADM may later develop frank myopathy, progressing to a diagnosis of CADM, which may occur in as many as 10% to 13% of cases of CADM.1,2 Clinical evidence of muscle weakness typically is heralded by elevation of creatine kinase and aldolase; therefore, patients with ADM should have muscle enzymes periodically checked.
Cutaneous findings of ADM are the same as the hallmark skin findings in CADM.3 Poikiloderma appears as thin telangiectatic skin in a background of mottled hyperpigmentation and hypopigmentation. It represents chronic inflammation and often occurs in sun-exposed areas. Poikiloderma located on the posterior neck and shoulders is known as the shawl sign and on the lateral thighs as the holster sign.5 The term mechanic’s hands is used to describe the clinical finding of palmar erythema with scaling and fissuring of the fingertips.6 Scalp findings include erythematous, atrophic, scaly plaques resembling psoriasis and nonscarring alopecia.7 Gottron papules are nearly pathognomonic for DM. These violaceous papules often are pruritic and found over the finger joints, in contrast to the hand rash of lupus erythematosus that involves the skin between finger joints.8 Psoriatic-appearing plaques overlying the elbows and knees are known as Gottron sign and can contribute to misdiagnosis as psoriasis.8 The classic heliotrope rash presents as a violaceous hue in the periorbital area and may be associated with periorbital edema.9 Calcinosis cutis is common in CADM but rarely is reported in ADM.10 Nail findings include periungual hyperemia, cuticular overgrowth, and nail bed changes due to avascular areas and dilated capillaries. The cutaneous histopathologic findings in ADM are the same as with CADM: a smudged dermoepidermal interface, vacuolar alterations of the basal layer, and dermal mucin deposits.
Palmoplantar keratoderma rarely is reported as a cutaneous finding in DM. The finding of keratoderma has mainly been reported in association with Wong-type DM, a rare subtype of DM with features of pityriasis rubra pilaris.11-13 Palmoplantar keratoderma also has been reported in a case of an ADM-like hydroxyurea-induced eruption14 and as an early presenting feature in one patient with CADM and one with juvenile DM.15,16
The autoantibody profile in patients with ADM varies from that of CADM and can be helpful in both diagnosis and prognosis. Similar to CADM, the majority of patients with ADM have positive antinuclear antibodies.2,17 Anti–Jo-1 (an anti–aminoacyl-transfer RNA synthetase) antibody frequently is found in CADM but rarely in ADM.2 Anti–Jo-1 is predictive of interstitial lung disease (ILD) in CADM. Positive anti–Jo-1 in combination with Raynaud phenomenon and mechanic’s hands is referred to as antisynthetase syndrome in patients with CADM.18,19 An antibody uniquely linked with CADM is the anti–CADM-140/MDA5 antibody and can be a marker of rapidly progressing ILD in these patients.20 Anti–Mi-2 is another myositis-specific antibody not commonly found in ADM but is present in 15% to 30% of DM cases.2,21 In CADM, the anti–Mi-2 antibody is associated with the shawl sign, ragged cuticles, and carpal tunnel syndrome and has a favorable prognosis.17,21 Myositis-associated autoantibodies (eg, anti-Ku) are found in patients with symptoms overlapping both DM and scleroderma or other connective tissue diseases.22 More recently described, the anti-p155/140 antibody is highly specific (up to 89%) for occult malignancy in DM.23
Lung disease is an important association in ADM. When it develops, it may be more aggressive compared to lung disease associated with CADM.24-26 In a systematic review of 197 cases of ADM by Gerami et al,2 10% of patients had ILD, and it was fatal in 42% of cases. Most cases of ILD associated with CADM were diagnosed as interstitial pneumonitis or diffuse alveolar disease; bronchiolitis obliterans organizing pneumonia and basilar fibrosis also were recorded.2 Anti–Jo-1 antibodies often accompany lung disease in CADM but are not typically found in lung disease associated with ADM. The anti–CADM-140/MDA5 antibody is associated with an increased risk for rapidly progressing ILD in patients with CADM.20 Recommended baseline screening for lung disease in DM includes chest radiography, pulmonary function tests with diffusion capacity,8 and in some instances high-resolution chest CT.27 Follow-up visits should include screening for symptoms of ILD such as cough, shortness of breath, or dyspnea. Treatment of myopathy-associated ILD is systemic steroids combined with various immunosuppressants including cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine, tacrolimus, and intravenous immunoglobulin.28,29
The risk of malignancy in ADM is thought to be similar to the rate of 20% to 25% found in CADM.1,30-32 The most commonly reported malignancies associated with ADM are nasopharyngeal, breast, lung, ovarian, colorectal, pancreatic, and stomach cancers and lymphoma/leukemia.2,33 Patients with ADM should be screened for malignancy at diagnosis, then yearly for 3 years.8,31,33 In addition to history, physical examination, and age/sex-appropriate screening, a complete blood cell count, chemistry panel, urinalysis, stool guaiac, CA 125, CA 19-9, chest radiograph, and abdominal ultrasound should be performed. For women, mammography and pelvic ultrasonography should be completed.31 Some experts also recommend a full-body CT scan. Because Asian patients have a higher risk for nasopharyngeal carcinoma, referral to an ear, nose, and throat surgeon for direct visualization also can be considered.33 The risk of cancer in patients with DM compared to the general population is increased for at least the first 5 years after diagnosis, but most associated cancers are found within the first 3 years.34
Several therapies have been found useful in ADM. Because lesions often are photoexacerbated, sun protection is essential. Antimalarials such as hydroxychloroquine are considered first-line therapy. Clinicians must be aware of 2 possible hydroxychloroquine side effects that can uniquely confuse the clinical picture in ADM. The first is a rash, most often morbilliform and pruritic, that occurs in DM more frequently than in other diseases.35 The second is a myopathy found in as many as 6.7% of patients using antimalarials for rheumatic disease,36 which can clinically mimic the progression of ADM to CADM.37 Two small retrospective case series found that methotrexate was beneficial in ADM.38,39 Methotrexate also has been reported as an efficacious treatment of ILD in patients with connective tissue diseases.40,41 Intravenous immunoglobulin and other immunosuppressants are additional agents to be considered.42
In summary, ADM is an important subset of DM and is more likely to present to dermatology practices than to other specialists. Amyopathic DM shares cutaneous findings with DM, and both overlap and differ with respect to other key disease characteristics including autoantibody profile, associated lung disease, and malignancy risk. Palmoplantar keratoderma is a rarely reported skin finding in DM. We report a case of ADM with the unique finding of severe plantar keratoderma. The fact that our patient’s keratoderma and other skin findings resolved concomitantly during methotrexate therapy leads us to believe that the keratoderma was a unique skin manifestation of the ADM itself.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30.
- Gerami P, Schope JM, McDonald L, et al. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597-613.
- Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol. 1999;11:475-482.
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27.
- Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57:375-381.
- Stahl NI, Klippel JH, Decker JL. A cutaneous lesion associated with myositis. Ann Intern Med. 1979;91:577-579.
- Kasteler JS, Callen JP. Scalp involvement in dermatomyositis. often overlooked or misdiagnosed. JAMA. 1994;272:1939-1941.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Russo T, Piccolo V, Ruocco E, et al. The heliotrope sign of dermatomyositis: the correct meaning of the term heliotrope. Arch Dermatol. 2012;148:1178.
- Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol. 2009;60:1076-1077.
- Requena L, Grilli R, Soriano L, et al. Dermatomyositis with a pityriasis rubra pilaris-like eruption: a little-known distinctive cutaneous manifestation of dermatomyositis. Br J Dermatol. 1997;136:768-771.
- Lupton JR, Figueroa P, Berberian BJ, et al. An unusual presentation of dermatomyositis: the type Wong variant revisited. J Am Acad Dermatol. 2000;43(5 part 2):908-912.
- Caporali R, Cavagna L, Bellosta M, et al. Inflammatory myopathy in a patient with cutaneous findings of pityriasis rubra pilaris: a case of Wong’s dermatomyositis. Clin Rheumatol. 2004;23:63-65.
- Nofal A, El-Din ES. Hydroxyurea-induced dermatomyositis: true amyopathic dermatomyositis or dermatomyositis-like eruption? Int J Dermatol. 2012;51:535-541.
- See Y, Rooney M, Woo P. Palmar plantar hyperkeratosis—a previously undescribed skin manifestation of juvenile dermatomyositis. Br J Rheumatol. 1997;36(8):917-919.
- Chang LY, Yang LJ, Wu YJJ. Keratoderma plantaris and mechanic’s hands as the initial presentation in a case of dermatomyositis. Dermatol Sinica. 2002;20:329-334.
- Love L, Leff R, Fraser D, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70:360-374.
- Marguerie C, Bunn CC, Beynon HL, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77:1019-1038.
- Marie I, Hatron PY, Hachulla E, et al. Pulmonary involvement in polymyositis and in dermatomyositis. J Rheumatol. 1998;25:1336-1343.
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576.
- Dimachkie MM. Idiopathic inflammatory myopathies. J Neuroimmunol. 2011;231:32-42.
- Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, et al. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22:627-632.
- Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis, and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44:1282-1286.
- Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26:1647-1654.
- Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341-1347.
- Fathi M, Dastmalchi, M, Rasmussen E, et al. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297-301.
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010;31:501-512.
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107:890-896.
- Klein RQ, Teal V, Taylor L, et al. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937-943.
- Sontheimer RD. Clinically amyopathic dermatomyositis: what can we now tell our patients? Arch Dermatol. 2010;146:76-80.
- Azuma K, Yamada H, Ohkubo M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21:178-183.
- Femia AN, Vleugels RA, Callen JP. Cutaneous dermatomyositis: an updated review of treatment options and internal associations. Am J Clin Dermatol. 2013;14:291-313.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095.
- Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138:1231-1233.
- Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390.
- Zieglschmid-Adams ME, Pandya AG, Cohen SB, et al. Treatment of dermatomyositis with methotrexate. J Am Acad Dermatol. 1995;32(5, pt 1):754-757.
- Foulke G, Baccon J, Marks JG, et al. Antimalarial myopathy in amyopathic dermatomyositis. Arch Dermatol. 2012;148:1100-1101.
- Kasteler JS, Callen JP. Low-dose methotrexate administered weekly is an effective corticosteroid-sparing agent for the treatment of the cutaneous manifestations of dermatomyositis. J Am Acad Dermatol. 1997;36:67-71.
- Scott DG, Bacon PA. Response to methotrexate in fibrosing alveolitis associated with connective tissue disease. Thorax. 1980;35:725-731.
- Fink SD, Kremer JM. Successful treatment of interstitial lung disease in systemic lupus erythematosus with methotrexate. J Rheumatol. 1995;22:967-969.
- Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83-105.
Case Report
A 54-year-old woman presented with a painful pruritic rash on the hands and feet of 7 years’ duration. She reported intermittent joint pain but denied muscle weakness. Physical examination revealed fissured fingertips and heavy scaling of the palms and lateral fingers (Figure 1). Violaceous scaly papules were seen on the distal and proximal interphalangeal joints (Figure 2). A severe plantar keratoderma also was noted (Figure 3). Pink scaly plaques were present on the bilateral elbows and postauricular skin. Diffuse mat telangiectases covered the malar skin. Extensive poikilodermatous skin changes covered approximately 20% of the total body surface area. Salt-and-pepper patches and papules were noted over the bilateral thighs. She reported an uncertain history of recent radiographs of one or both hands, which showed no joint degeneration characteristic of psoriatic arthritis. She previously had been given a diagnosis of psoriasis by an outside dermatologist but was not responding to topical therapy.



Several skin biopsies showed histologic evidence of dermatomyositis (DM)(Figure 4). Prominent basement thickening also was seen on periodic acid–Schiff staining (not shown). Laboratory workup showed negative antinuclear antibodies and anti–Jo-1, anti-Ku, and anti-Mi2 antibodies. Muscle enzymes including creatinine kinase and aldolase were within reference range. Pelvic ultrasonography and mammography were negative. Pulmonary function tests were unremarkable. High-resolution chest computed tomography (CT) was ordered because of a history of chronic cough; however, no evidence of malignancy or interstitial lung disease was seen. The patient was diagnosed with amyopathic dermatomyositis (ADM). Rheumatology was consulted and initiated oral hydroxychloroquine therapy. After 3 months, the patient’s cutaneous disease did not respond and she reported having headaches associated with this medication; therefore, methotrexate was started. Within 2 months of treatment, full resolution of the plantar keratoderma (Figure 5) and clearance of the scaling/fissuring of the hands as well as the psoriatic-appearing plaques on the elbows was noted.


Comment
Amyopathic DM is a subset of DM that accounts for 10% to 20% of DM cases.1,2 Sontheimer’s3 diagnostic criteria for ADM require histopathologic confirmation of the hallmark skin findings of classic DM and lack of muscle weakness or muscle enzyme (creatine kinase/aldolase) elevation for at least 2 years.
Similar to classic DM, ADM typically presents in the fifth decade of life and has a female predilection.1,4 The term hypomyopathic DM is used to describe patients who exhibit classic skin findings and evidence of muscle involvement on magnetic resonance imaging, electromyography, biopsy, or serum enzymes but have no clinical evidence of muscle weakness for at least 6 months. Together, hypomyopathic DM and ADM are referred to as clinically ADM (CADM). Patients who have met the criteria for hypomyopathic DM or ADM may later develop frank myopathy, progressing to a diagnosis of CADM, which may occur in as many as 10% to 13% of cases of CADM.1,2 Clinical evidence of muscle weakness typically is heralded by elevation of creatine kinase and aldolase; therefore, patients with ADM should have muscle enzymes periodically checked.
Cutaneous findings of ADM are the same as the hallmark skin findings in CADM.3 Poikiloderma appears as thin telangiectatic skin in a background of mottled hyperpigmentation and hypopigmentation. It represents chronic inflammation and often occurs in sun-exposed areas. Poikiloderma located on the posterior neck and shoulders is known as the shawl sign and on the lateral thighs as the holster sign.5 The term mechanic’s hands is used to describe the clinical finding of palmar erythema with scaling and fissuring of the fingertips.6 Scalp findings include erythematous, atrophic, scaly plaques resembling psoriasis and nonscarring alopecia.7 Gottron papules are nearly pathognomonic for DM. These violaceous papules often are pruritic and found over the finger joints, in contrast to the hand rash of lupus erythematosus that involves the skin between finger joints.8 Psoriatic-appearing plaques overlying the elbows and knees are known as Gottron sign and can contribute to misdiagnosis as psoriasis.8 The classic heliotrope rash presents as a violaceous hue in the periorbital area and may be associated with periorbital edema.9 Calcinosis cutis is common in CADM but rarely is reported in ADM.10 Nail findings include periungual hyperemia, cuticular overgrowth, and nail bed changes due to avascular areas and dilated capillaries. The cutaneous histopathologic findings in ADM are the same as with CADM: a smudged dermoepidermal interface, vacuolar alterations of the basal layer, and dermal mucin deposits.
Palmoplantar keratoderma rarely is reported as a cutaneous finding in DM. The finding of keratoderma has mainly been reported in association with Wong-type DM, a rare subtype of DM with features of pityriasis rubra pilaris.11-13 Palmoplantar keratoderma also has been reported in a case of an ADM-like hydroxyurea-induced eruption14 and as an early presenting feature in one patient with CADM and one with juvenile DM.15,16
The autoantibody profile in patients with ADM varies from that of CADM and can be helpful in both diagnosis and prognosis. Similar to CADM, the majority of patients with ADM have positive antinuclear antibodies.2,17 Anti–Jo-1 (an anti–aminoacyl-transfer RNA synthetase) antibody frequently is found in CADM but rarely in ADM.2 Anti–Jo-1 is predictive of interstitial lung disease (ILD) in CADM. Positive anti–Jo-1 in combination with Raynaud phenomenon and mechanic’s hands is referred to as antisynthetase syndrome in patients with CADM.18,19 An antibody uniquely linked with CADM is the anti–CADM-140/MDA5 antibody and can be a marker of rapidly progressing ILD in these patients.20 Anti–Mi-2 is another myositis-specific antibody not commonly found in ADM but is present in 15% to 30% of DM cases.2,21 In CADM, the anti–Mi-2 antibody is associated with the shawl sign, ragged cuticles, and carpal tunnel syndrome and has a favorable prognosis.17,21 Myositis-associated autoantibodies (eg, anti-Ku) are found in patients with symptoms overlapping both DM and scleroderma or other connective tissue diseases.22 More recently described, the anti-p155/140 antibody is highly specific (up to 89%) for occult malignancy in DM.23
Lung disease is an important association in ADM. When it develops, it may be more aggressive compared to lung disease associated with CADM.24-26 In a systematic review of 197 cases of ADM by Gerami et al,2 10% of patients had ILD, and it was fatal in 42% of cases. Most cases of ILD associated with CADM were diagnosed as interstitial pneumonitis or diffuse alveolar disease; bronchiolitis obliterans organizing pneumonia and basilar fibrosis also were recorded.2 Anti–Jo-1 antibodies often accompany lung disease in CADM but are not typically found in lung disease associated with ADM. The anti–CADM-140/MDA5 antibody is associated with an increased risk for rapidly progressing ILD in patients with CADM.20 Recommended baseline screening for lung disease in DM includes chest radiography, pulmonary function tests with diffusion capacity,8 and in some instances high-resolution chest CT.27 Follow-up visits should include screening for symptoms of ILD such as cough, shortness of breath, or dyspnea. Treatment of myopathy-associated ILD is systemic steroids combined with various immunosuppressants including cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine, tacrolimus, and intravenous immunoglobulin.28,29
The risk of malignancy in ADM is thought to be similar to the rate of 20% to 25% found in CADM.1,30-32 The most commonly reported malignancies associated with ADM are nasopharyngeal, breast, lung, ovarian, colorectal, pancreatic, and stomach cancers and lymphoma/leukemia.2,33 Patients with ADM should be screened for malignancy at diagnosis, then yearly for 3 years.8,31,33 In addition to history, physical examination, and age/sex-appropriate screening, a complete blood cell count, chemistry panel, urinalysis, stool guaiac, CA 125, CA 19-9, chest radiograph, and abdominal ultrasound should be performed. For women, mammography and pelvic ultrasonography should be completed.31 Some experts also recommend a full-body CT scan. Because Asian patients have a higher risk for nasopharyngeal carcinoma, referral to an ear, nose, and throat surgeon for direct visualization also can be considered.33 The risk of cancer in patients with DM compared to the general population is increased for at least the first 5 years after diagnosis, but most associated cancers are found within the first 3 years.34
Several therapies have been found useful in ADM. Because lesions often are photoexacerbated, sun protection is essential. Antimalarials such as hydroxychloroquine are considered first-line therapy. Clinicians must be aware of 2 possible hydroxychloroquine side effects that can uniquely confuse the clinical picture in ADM. The first is a rash, most often morbilliform and pruritic, that occurs in DM more frequently than in other diseases.35 The second is a myopathy found in as many as 6.7% of patients using antimalarials for rheumatic disease,36 which can clinically mimic the progression of ADM to CADM.37 Two small retrospective case series found that methotrexate was beneficial in ADM.38,39 Methotrexate also has been reported as an efficacious treatment of ILD in patients with connective tissue diseases.40,41 Intravenous immunoglobulin and other immunosuppressants are additional agents to be considered.42
In summary, ADM is an important subset of DM and is more likely to present to dermatology practices than to other specialists. Amyopathic DM shares cutaneous findings with DM, and both overlap and differ with respect to other key disease characteristics including autoantibody profile, associated lung disease, and malignancy risk. Palmoplantar keratoderma is a rarely reported skin finding in DM. We report a case of ADM with the unique finding of severe plantar keratoderma. The fact that our patient’s keratoderma and other skin findings resolved concomitantly during methotrexate therapy leads us to believe that the keratoderma was a unique skin manifestation of the ADM itself.
Case Report
A 54-year-old woman presented with a painful pruritic rash on the hands and feet of 7 years’ duration. She reported intermittent joint pain but denied muscle weakness. Physical examination revealed fissured fingertips and heavy scaling of the palms and lateral fingers (Figure 1). Violaceous scaly papules were seen on the distal and proximal interphalangeal joints (Figure 2). A severe plantar keratoderma also was noted (Figure 3). Pink scaly plaques were present on the bilateral elbows and postauricular skin. Diffuse mat telangiectases covered the malar skin. Extensive poikilodermatous skin changes covered approximately 20% of the total body surface area. Salt-and-pepper patches and papules were noted over the bilateral thighs. She reported an uncertain history of recent radiographs of one or both hands, which showed no joint degeneration characteristic of psoriatic arthritis. She previously had been given a diagnosis of psoriasis by an outside dermatologist but was not responding to topical therapy.



Several skin biopsies showed histologic evidence of dermatomyositis (DM)(Figure 4). Prominent basement thickening also was seen on periodic acid–Schiff staining (not shown). Laboratory workup showed negative antinuclear antibodies and anti–Jo-1, anti-Ku, and anti-Mi2 antibodies. Muscle enzymes including creatinine kinase and aldolase were within reference range. Pelvic ultrasonography and mammography were negative. Pulmonary function tests were unremarkable. High-resolution chest computed tomography (CT) was ordered because of a history of chronic cough; however, no evidence of malignancy or interstitial lung disease was seen. The patient was diagnosed with amyopathic dermatomyositis (ADM). Rheumatology was consulted and initiated oral hydroxychloroquine therapy. After 3 months, the patient’s cutaneous disease did not respond and she reported having headaches associated with this medication; therefore, methotrexate was started. Within 2 months of treatment, full resolution of the plantar keratoderma (Figure 5) and clearance of the scaling/fissuring of the hands as well as the psoriatic-appearing plaques on the elbows was noted.


Comment
Amyopathic DM is a subset of DM that accounts for 10% to 20% of DM cases.1,2 Sontheimer’s3 diagnostic criteria for ADM require histopathologic confirmation of the hallmark skin findings of classic DM and lack of muscle weakness or muscle enzyme (creatine kinase/aldolase) elevation for at least 2 years.
Similar to classic DM, ADM typically presents in the fifth decade of life and has a female predilection.1,4 The term hypomyopathic DM is used to describe patients who exhibit classic skin findings and evidence of muscle involvement on magnetic resonance imaging, electromyography, biopsy, or serum enzymes but have no clinical evidence of muscle weakness for at least 6 months. Together, hypomyopathic DM and ADM are referred to as clinically ADM (CADM). Patients who have met the criteria for hypomyopathic DM or ADM may later develop frank myopathy, progressing to a diagnosis of CADM, which may occur in as many as 10% to 13% of cases of CADM.1,2 Clinical evidence of muscle weakness typically is heralded by elevation of creatine kinase and aldolase; therefore, patients with ADM should have muscle enzymes periodically checked.
Cutaneous findings of ADM are the same as the hallmark skin findings in CADM.3 Poikiloderma appears as thin telangiectatic skin in a background of mottled hyperpigmentation and hypopigmentation. It represents chronic inflammation and often occurs in sun-exposed areas. Poikiloderma located on the posterior neck and shoulders is known as the shawl sign and on the lateral thighs as the holster sign.5 The term mechanic’s hands is used to describe the clinical finding of palmar erythema with scaling and fissuring of the fingertips.6 Scalp findings include erythematous, atrophic, scaly plaques resembling psoriasis and nonscarring alopecia.7 Gottron papules are nearly pathognomonic for DM. These violaceous papules often are pruritic and found over the finger joints, in contrast to the hand rash of lupus erythematosus that involves the skin between finger joints.8 Psoriatic-appearing plaques overlying the elbows and knees are known as Gottron sign and can contribute to misdiagnosis as psoriasis.8 The classic heliotrope rash presents as a violaceous hue in the periorbital area and may be associated with periorbital edema.9 Calcinosis cutis is common in CADM but rarely is reported in ADM.10 Nail findings include periungual hyperemia, cuticular overgrowth, and nail bed changes due to avascular areas and dilated capillaries. The cutaneous histopathologic findings in ADM are the same as with CADM: a smudged dermoepidermal interface, vacuolar alterations of the basal layer, and dermal mucin deposits.
Palmoplantar keratoderma rarely is reported as a cutaneous finding in DM. The finding of keratoderma has mainly been reported in association with Wong-type DM, a rare subtype of DM with features of pityriasis rubra pilaris.11-13 Palmoplantar keratoderma also has been reported in a case of an ADM-like hydroxyurea-induced eruption14 and as an early presenting feature in one patient with CADM and one with juvenile DM.15,16
The autoantibody profile in patients with ADM varies from that of CADM and can be helpful in both diagnosis and prognosis. Similar to CADM, the majority of patients with ADM have positive antinuclear antibodies.2,17 Anti–Jo-1 (an anti–aminoacyl-transfer RNA synthetase) antibody frequently is found in CADM but rarely in ADM.2 Anti–Jo-1 is predictive of interstitial lung disease (ILD) in CADM. Positive anti–Jo-1 in combination with Raynaud phenomenon and mechanic’s hands is referred to as antisynthetase syndrome in patients with CADM.18,19 An antibody uniquely linked with CADM is the anti–CADM-140/MDA5 antibody and can be a marker of rapidly progressing ILD in these patients.20 Anti–Mi-2 is another myositis-specific antibody not commonly found in ADM but is present in 15% to 30% of DM cases.2,21 In CADM, the anti–Mi-2 antibody is associated with the shawl sign, ragged cuticles, and carpal tunnel syndrome and has a favorable prognosis.17,21 Myositis-associated autoantibodies (eg, anti-Ku) are found in patients with symptoms overlapping both DM and scleroderma or other connective tissue diseases.22 More recently described, the anti-p155/140 antibody is highly specific (up to 89%) for occult malignancy in DM.23
Lung disease is an important association in ADM. When it develops, it may be more aggressive compared to lung disease associated with CADM.24-26 In a systematic review of 197 cases of ADM by Gerami et al,2 10% of patients had ILD, and it was fatal in 42% of cases. Most cases of ILD associated with CADM were diagnosed as interstitial pneumonitis or diffuse alveolar disease; bronchiolitis obliterans organizing pneumonia and basilar fibrosis also were recorded.2 Anti–Jo-1 antibodies often accompany lung disease in CADM but are not typically found in lung disease associated with ADM. The anti–CADM-140/MDA5 antibody is associated with an increased risk for rapidly progressing ILD in patients with CADM.20 Recommended baseline screening for lung disease in DM includes chest radiography, pulmonary function tests with diffusion capacity,8 and in some instances high-resolution chest CT.27 Follow-up visits should include screening for symptoms of ILD such as cough, shortness of breath, or dyspnea. Treatment of myopathy-associated ILD is systemic steroids combined with various immunosuppressants including cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine, tacrolimus, and intravenous immunoglobulin.28,29
The risk of malignancy in ADM is thought to be similar to the rate of 20% to 25% found in CADM.1,30-32 The most commonly reported malignancies associated with ADM are nasopharyngeal, breast, lung, ovarian, colorectal, pancreatic, and stomach cancers and lymphoma/leukemia.2,33 Patients with ADM should be screened for malignancy at diagnosis, then yearly for 3 years.8,31,33 In addition to history, physical examination, and age/sex-appropriate screening, a complete blood cell count, chemistry panel, urinalysis, stool guaiac, CA 125, CA 19-9, chest radiograph, and abdominal ultrasound should be performed. For women, mammography and pelvic ultrasonography should be completed.31 Some experts also recommend a full-body CT scan. Because Asian patients have a higher risk for nasopharyngeal carcinoma, referral to an ear, nose, and throat surgeon for direct visualization also can be considered.33 The risk of cancer in patients with DM compared to the general population is increased for at least the first 5 years after diagnosis, but most associated cancers are found within the first 3 years.34
Several therapies have been found useful in ADM. Because lesions often are photoexacerbated, sun protection is essential. Antimalarials such as hydroxychloroquine are considered first-line therapy. Clinicians must be aware of 2 possible hydroxychloroquine side effects that can uniquely confuse the clinical picture in ADM. The first is a rash, most often morbilliform and pruritic, that occurs in DM more frequently than in other diseases.35 The second is a myopathy found in as many as 6.7% of patients using antimalarials for rheumatic disease,36 which can clinically mimic the progression of ADM to CADM.37 Two small retrospective case series found that methotrexate was beneficial in ADM.38,39 Methotrexate also has been reported as an efficacious treatment of ILD in patients with connective tissue diseases.40,41 Intravenous immunoglobulin and other immunosuppressants are additional agents to be considered.42
In summary, ADM is an important subset of DM and is more likely to present to dermatology practices than to other specialists. Amyopathic DM shares cutaneous findings with DM, and both overlap and differ with respect to other key disease characteristics including autoantibody profile, associated lung disease, and malignancy risk. Palmoplantar keratoderma is a rarely reported skin finding in DM. We report a case of ADM with the unique finding of severe plantar keratoderma. The fact that our patient’s keratoderma and other skin findings resolved concomitantly during methotrexate therapy leads us to believe that the keratoderma was a unique skin manifestation of the ADM itself.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30.
- Gerami P, Schope JM, McDonald L, et al. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597-613.
- Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol. 1999;11:475-482.
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27.
- Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57:375-381.
- Stahl NI, Klippel JH, Decker JL. A cutaneous lesion associated with myositis. Ann Intern Med. 1979;91:577-579.
- Kasteler JS, Callen JP. Scalp involvement in dermatomyositis. often overlooked or misdiagnosed. JAMA. 1994;272:1939-1941.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Russo T, Piccolo V, Ruocco E, et al. The heliotrope sign of dermatomyositis: the correct meaning of the term heliotrope. Arch Dermatol. 2012;148:1178.
- Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol. 2009;60:1076-1077.
- Requena L, Grilli R, Soriano L, et al. Dermatomyositis with a pityriasis rubra pilaris-like eruption: a little-known distinctive cutaneous manifestation of dermatomyositis. Br J Dermatol. 1997;136:768-771.
- Lupton JR, Figueroa P, Berberian BJ, et al. An unusual presentation of dermatomyositis: the type Wong variant revisited. J Am Acad Dermatol. 2000;43(5 part 2):908-912.
- Caporali R, Cavagna L, Bellosta M, et al. Inflammatory myopathy in a patient with cutaneous findings of pityriasis rubra pilaris: a case of Wong’s dermatomyositis. Clin Rheumatol. 2004;23:63-65.
- Nofal A, El-Din ES. Hydroxyurea-induced dermatomyositis: true amyopathic dermatomyositis or dermatomyositis-like eruption? Int J Dermatol. 2012;51:535-541.
- See Y, Rooney M, Woo P. Palmar plantar hyperkeratosis—a previously undescribed skin manifestation of juvenile dermatomyositis. Br J Rheumatol. 1997;36(8):917-919.
- Chang LY, Yang LJ, Wu YJJ. Keratoderma plantaris and mechanic’s hands as the initial presentation in a case of dermatomyositis. Dermatol Sinica. 2002;20:329-334.
- Love L, Leff R, Fraser D, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70:360-374.
- Marguerie C, Bunn CC, Beynon HL, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77:1019-1038.
- Marie I, Hatron PY, Hachulla E, et al. Pulmonary involvement in polymyositis and in dermatomyositis. J Rheumatol. 1998;25:1336-1343.
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576.
- Dimachkie MM. Idiopathic inflammatory myopathies. J Neuroimmunol. 2011;231:32-42.
- Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, et al. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22:627-632.
- Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis, and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44:1282-1286.
- Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26:1647-1654.
- Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341-1347.
- Fathi M, Dastmalchi, M, Rasmussen E, et al. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297-301.
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010;31:501-512.
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107:890-896.
- Klein RQ, Teal V, Taylor L, et al. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937-943.
- Sontheimer RD. Clinically amyopathic dermatomyositis: what can we now tell our patients? Arch Dermatol. 2010;146:76-80.
- Azuma K, Yamada H, Ohkubo M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21:178-183.
- Femia AN, Vleugels RA, Callen JP. Cutaneous dermatomyositis: an updated review of treatment options and internal associations. Am J Clin Dermatol. 2013;14:291-313.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095.
- Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138:1231-1233.
- Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390.
- Zieglschmid-Adams ME, Pandya AG, Cohen SB, et al. Treatment of dermatomyositis with methotrexate. J Am Acad Dermatol. 1995;32(5, pt 1):754-757.
- Foulke G, Baccon J, Marks JG, et al. Antimalarial myopathy in amyopathic dermatomyositis. Arch Dermatol. 2012;148:1100-1101.
- Kasteler JS, Callen JP. Low-dose methotrexate administered weekly is an effective corticosteroid-sparing agent for the treatment of the cutaneous manifestations of dermatomyositis. J Am Acad Dermatol. 1997;36:67-71.
- Scott DG, Bacon PA. Response to methotrexate in fibrosing alveolitis associated with connective tissue disease. Thorax. 1980;35:725-731.
- Fink SD, Kremer JM. Successful treatment of interstitial lung disease in systemic lupus erythematosus with methotrexate. J Rheumatol. 1995;22:967-969.
- Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83-105.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30.
- Gerami P, Schope JM, McDonald L, et al. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597-613.
- Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol. 1999;11:475-482.
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27.
- Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57:375-381.
- Stahl NI, Klippel JH, Decker JL. A cutaneous lesion associated with myositis. Ann Intern Med. 1979;91:577-579.
- Kasteler JS, Callen JP. Scalp involvement in dermatomyositis. often overlooked or misdiagnosed. JAMA. 1994;272:1939-1941.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Russo T, Piccolo V, Ruocco E, et al. The heliotrope sign of dermatomyositis: the correct meaning of the term heliotrope. Arch Dermatol. 2012;148:1178.
- Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol. 2009;60:1076-1077.
- Requena L, Grilli R, Soriano L, et al. Dermatomyositis with a pityriasis rubra pilaris-like eruption: a little-known distinctive cutaneous manifestation of dermatomyositis. Br J Dermatol. 1997;136:768-771.
- Lupton JR, Figueroa P, Berberian BJ, et al. An unusual presentation of dermatomyositis: the type Wong variant revisited. J Am Acad Dermatol. 2000;43(5 part 2):908-912.
- Caporali R, Cavagna L, Bellosta M, et al. Inflammatory myopathy in a patient with cutaneous findings of pityriasis rubra pilaris: a case of Wong’s dermatomyositis. Clin Rheumatol. 2004;23:63-65.
- Nofal A, El-Din ES. Hydroxyurea-induced dermatomyositis: true amyopathic dermatomyositis or dermatomyositis-like eruption? Int J Dermatol. 2012;51:535-541.
- See Y, Rooney M, Woo P. Palmar plantar hyperkeratosis—a previously undescribed skin manifestation of juvenile dermatomyositis. Br J Rheumatol. 1997;36(8):917-919.
- Chang LY, Yang LJ, Wu YJJ. Keratoderma plantaris and mechanic’s hands as the initial presentation in a case of dermatomyositis. Dermatol Sinica. 2002;20:329-334.
- Love L, Leff R, Fraser D, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70:360-374.
- Marguerie C, Bunn CC, Beynon HL, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77:1019-1038.
- Marie I, Hatron PY, Hachulla E, et al. Pulmonary involvement in polymyositis and in dermatomyositis. J Rheumatol. 1998;25:1336-1343.
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576.
- Dimachkie MM. Idiopathic inflammatory myopathies. J Neuroimmunol. 2011;231:32-42.
- Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, et al. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22:627-632.
- Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis, and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44:1282-1286.
- Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26:1647-1654.
- Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341-1347.
- Fathi M, Dastmalchi, M, Rasmussen E, et al. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297-301.
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010;31:501-512.
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107:890-896.
- Klein RQ, Teal V, Taylor L, et al. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937-943.
- Sontheimer RD. Clinically amyopathic dermatomyositis: what can we now tell our patients? Arch Dermatol. 2010;146:76-80.
- Azuma K, Yamada H, Ohkubo M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21:178-183.
- Femia AN, Vleugels RA, Callen JP. Cutaneous dermatomyositis: an updated review of treatment options and internal associations. Am J Clin Dermatol. 2013;14:291-313.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095.
- Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138:1231-1233.
- Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390.
- Zieglschmid-Adams ME, Pandya AG, Cohen SB, et al. Treatment of dermatomyositis with methotrexate. J Am Acad Dermatol. 1995;32(5, pt 1):754-757.
- Foulke G, Baccon J, Marks JG, et al. Antimalarial myopathy in amyopathic dermatomyositis. Arch Dermatol. 2012;148:1100-1101.
- Kasteler JS, Callen JP. Low-dose methotrexate administered weekly is an effective corticosteroid-sparing agent for the treatment of the cutaneous manifestations of dermatomyositis. J Am Acad Dermatol. 1997;36:67-71.
- Scott DG, Bacon PA. Response to methotrexate in fibrosing alveolitis associated with connective tissue disease. Thorax. 1980;35:725-731.
- Fink SD, Kremer JM. Successful treatment of interstitial lung disease in systemic lupus erythematosus with methotrexate. J Rheumatol. 1995;22:967-969.
- Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83-105.
Practice Points
- Dermatomyositis (DM) can present without muscular weakness as clinically amyopathic dermatomyositis (CADM).
- Clinically amyopathic dermatomyositis has cutaneous findings that can mimic other diseases including psoriasis.
- Clinically amyopathic dermatomyositis may have similar systemic associations as DM in general, such as an increased risk for malignancies.
- Treatments to consider for CADM should include systemic methotrexate.