User login
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.
CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7
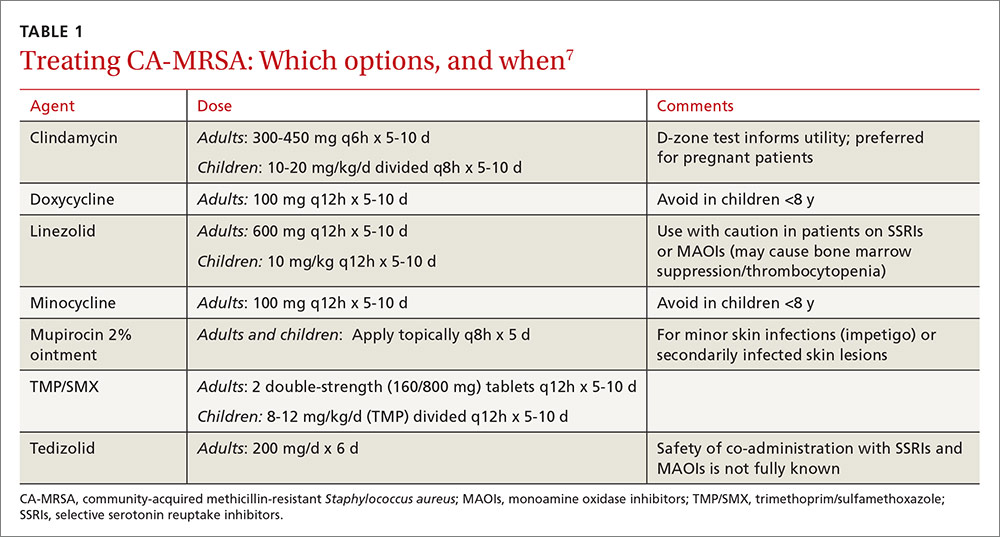
Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
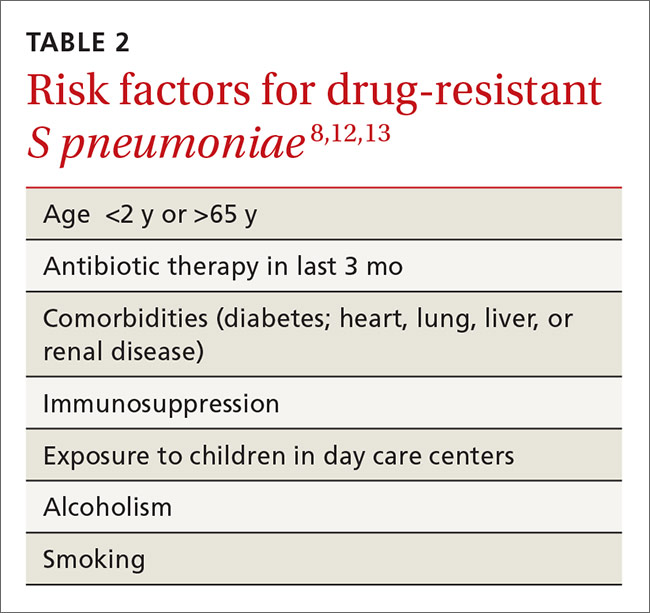
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13
Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19
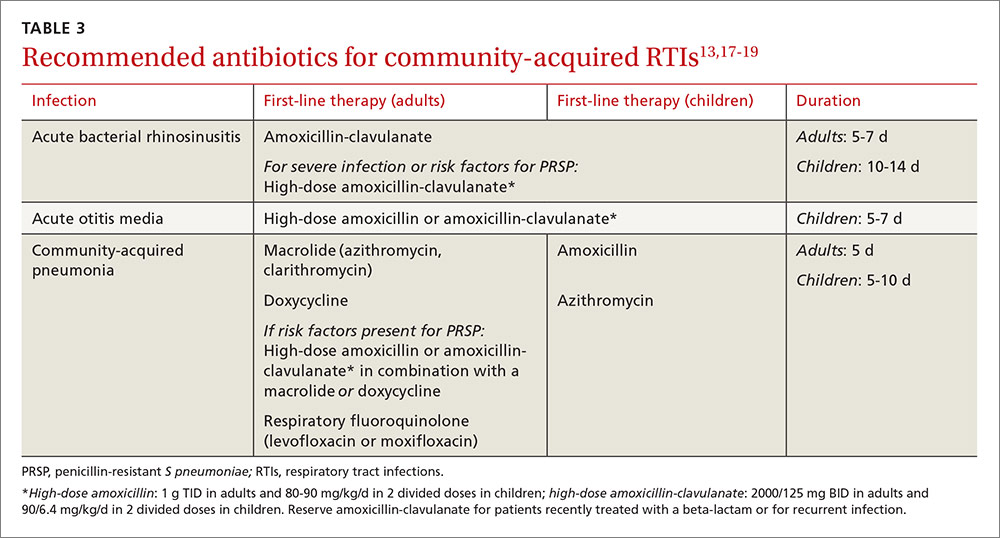
High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
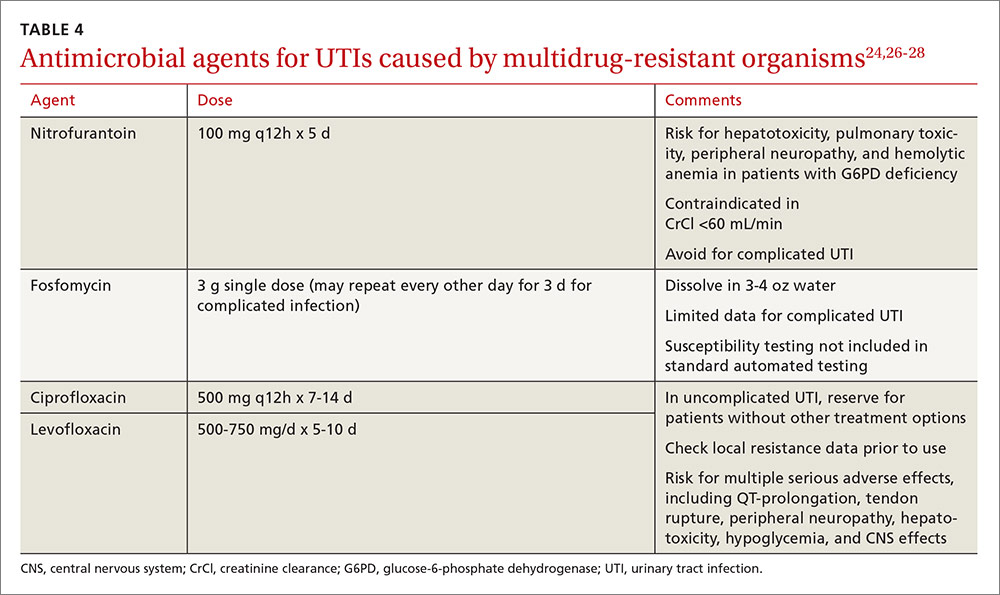
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1
Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.

CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7

Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13

Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19

High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1

Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.

CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7

Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13

Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19

High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1

Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
PRACTICE RECOMMENDATIONS
› Manage uncomplicated cutaneous abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus with incision and drainage alone. A
› Treat upper respiratory infections associated with drug-resistant Streptococcus pneumoniae with high-dose amoxicillin, which has been found to overcome penicillin resistance. A
› Administer dual therapy with ceftriaxone and azithromycin to patients with gonococcal infections. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series