User login
Inpatient antibiotic resistance: Everyone’s problem
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17). 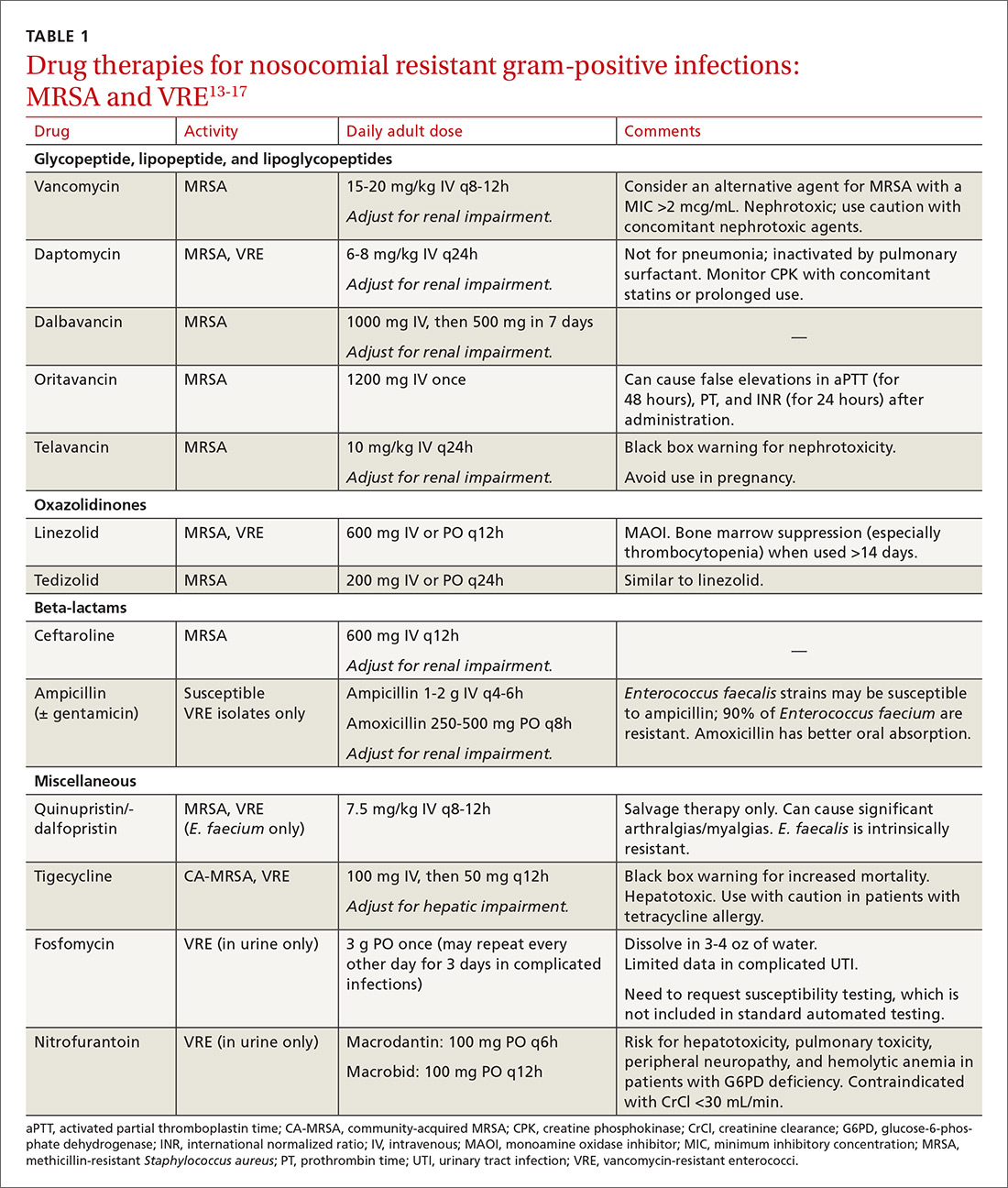
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting. 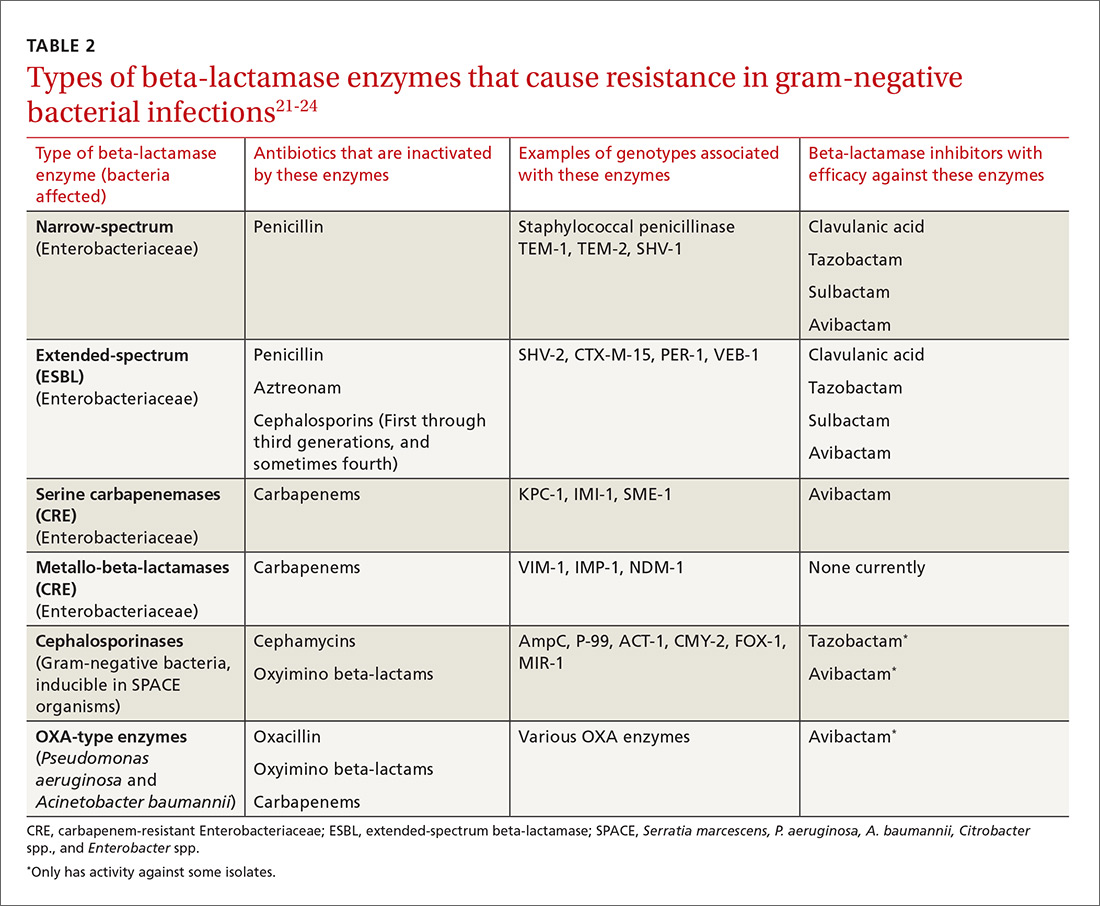
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
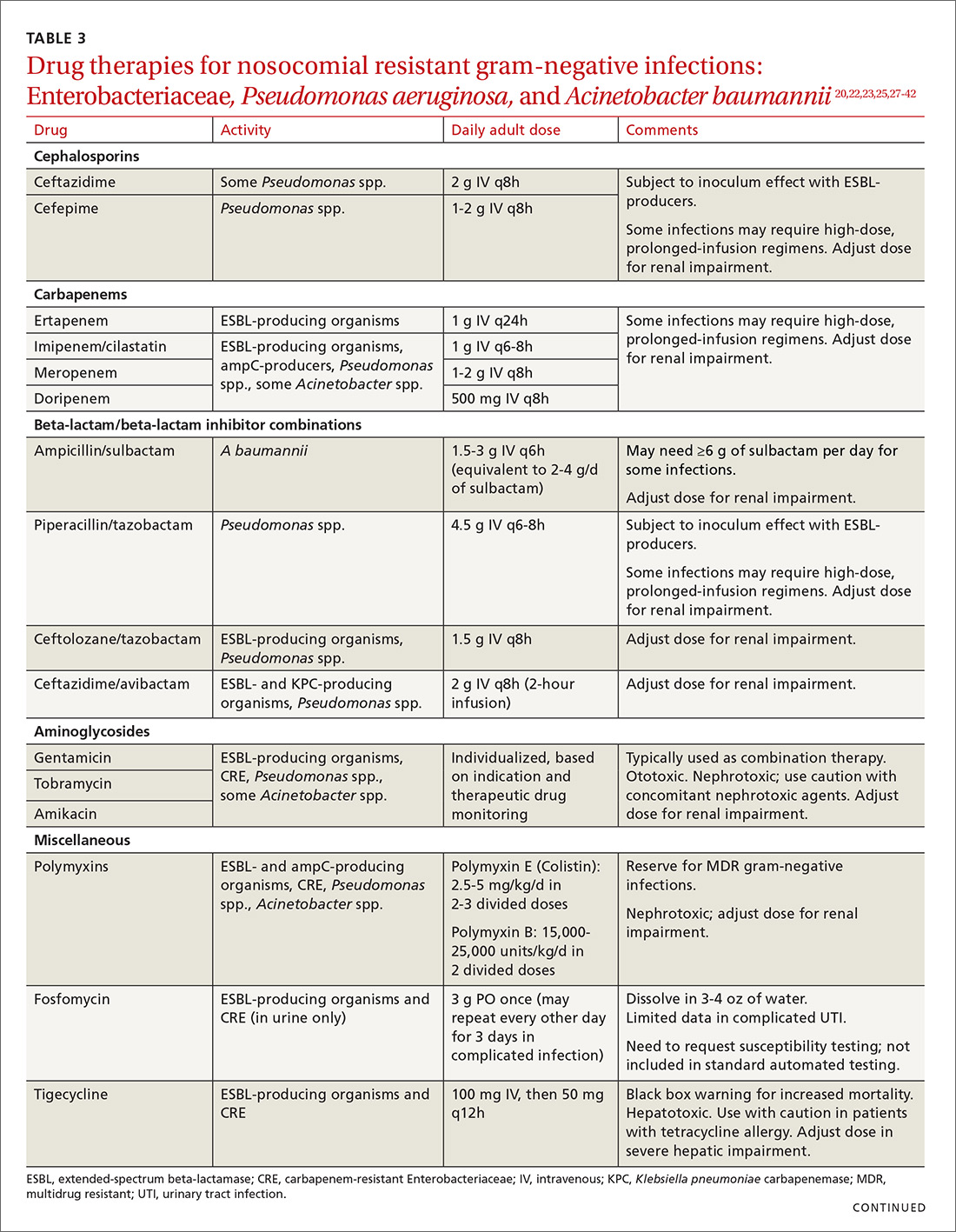
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49 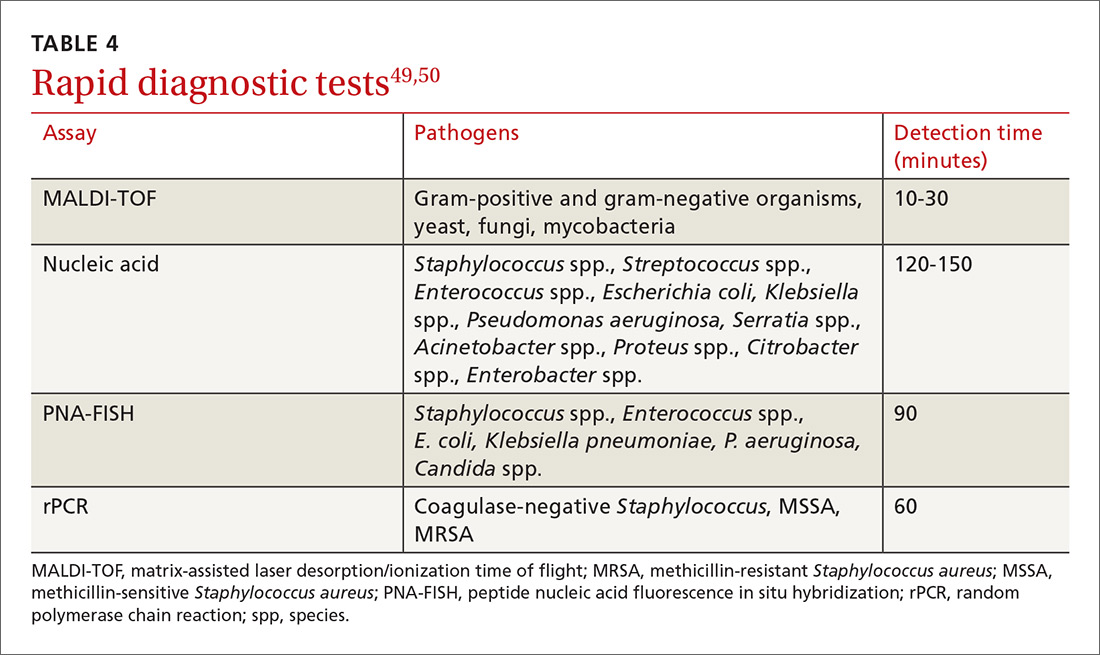
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17). 
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting. 
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22

Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49 
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17). 
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting. 
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22

Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49 
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
From The Journal of Family Practice | 2018;67(2):E1-E11.
PRACTICE RECOMMENDATIONS
› Consider alternatives to vancomycin for health care-associated methicillin-resistant Staphylococcus aureus isolates with a vancomycin minimum inhibitory concentration >2 mcg/mL or in the setting of poor clinical response. A
› Identify colonization vs infection with vancomycin-resistant enterococci (VRE) in the gastrointestinal tract following antibiotic exposure to minimize inappropriate antibiotic prescribing for VRE. C
› Use carbapenems as first-line treatment for severe infections caused by Enterobacteriaceae-producing extended-spectrum beta-lactamases. C
› Treat invasive carbapenem-resistant Enterobacteriaceae infections with combination therapy; site of infection, susceptibility patterns, and patient-specific factors should guide antibiotic selection. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antibiotic stewardship: The FP’s role
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.
CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7
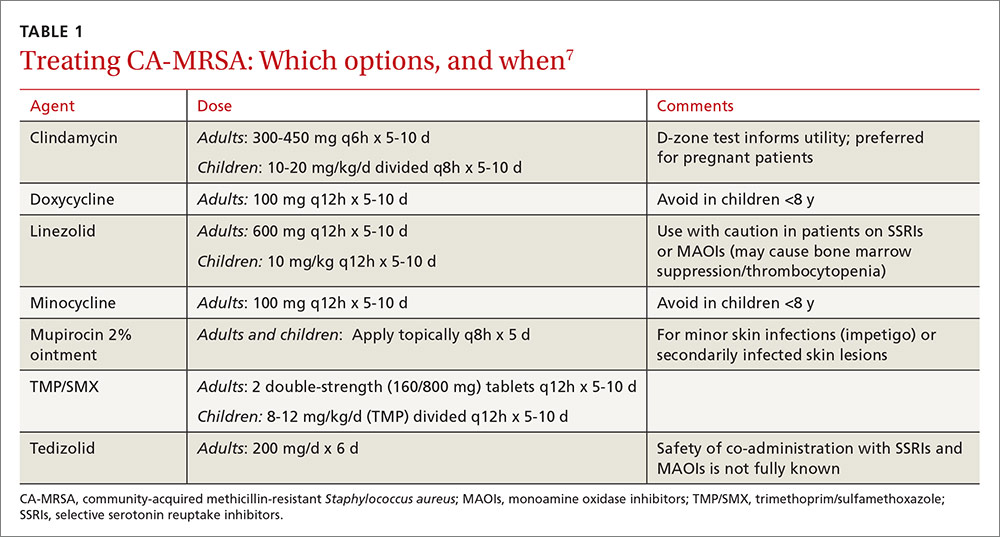
Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13
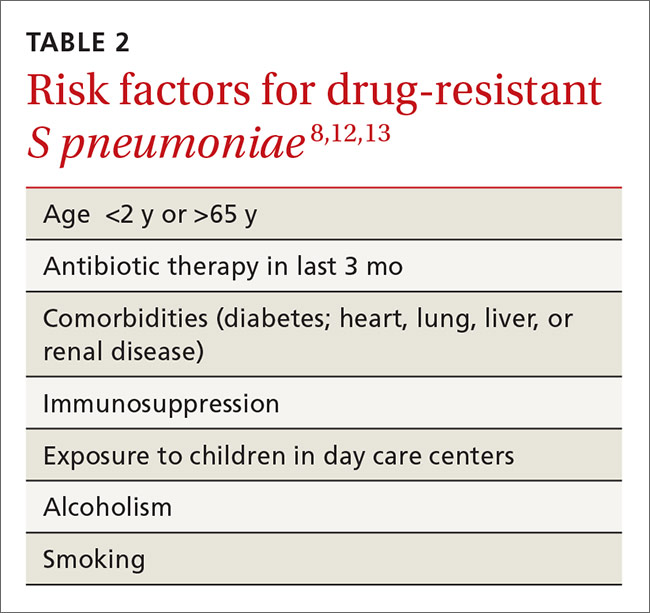
Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19
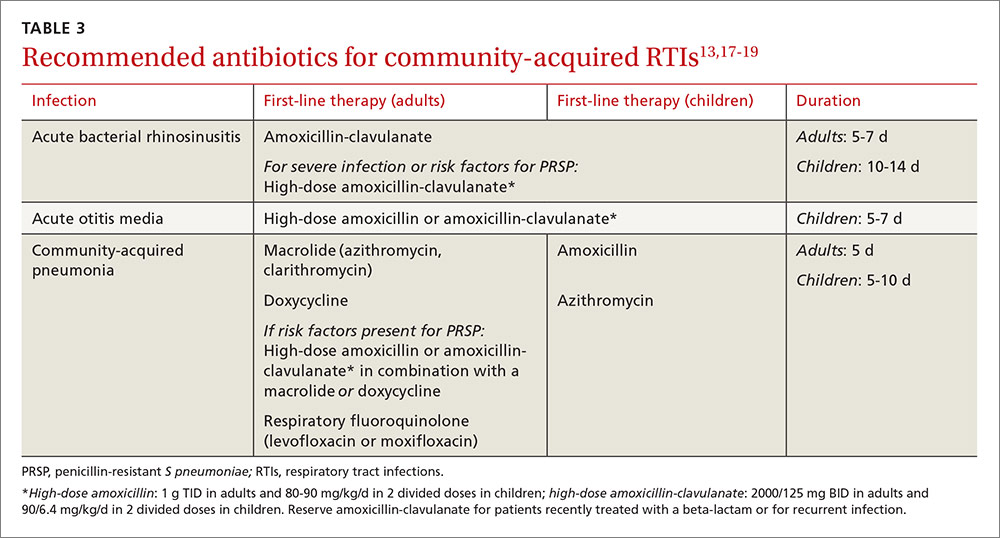
High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1
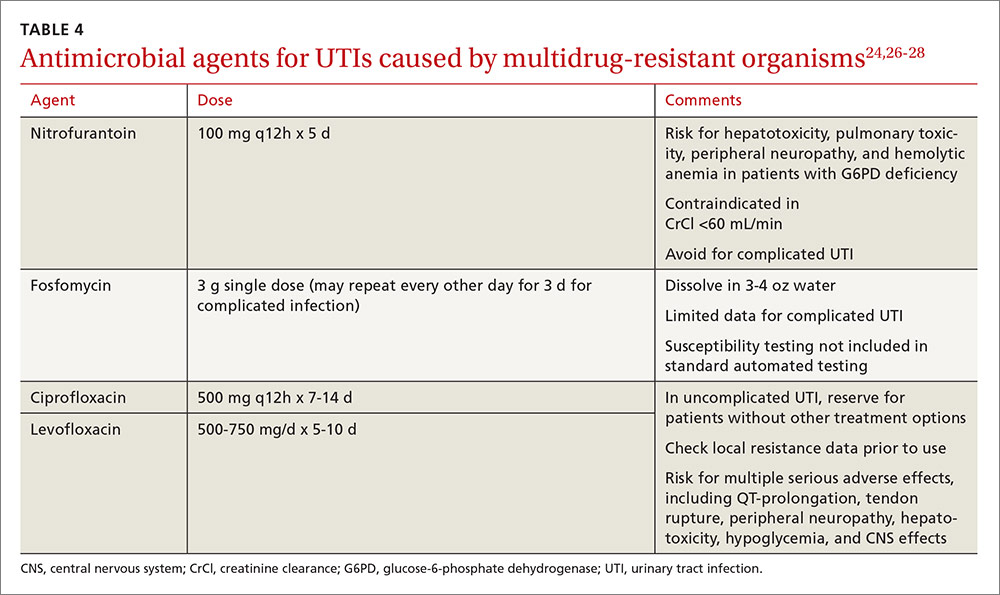
Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.

CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7

Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13

Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19

High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1

Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.

CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7

Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13

Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19

High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1

Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
PRACTICE RECOMMENDATIONS
› Manage uncomplicated cutaneous abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus with incision and drainage alone. A
› Treat upper respiratory infections associated with drug-resistant Streptococcus pneumoniae with high-dose amoxicillin, which has been found to overcome penicillin resistance. A
› Administer dual therapy with ceftriaxone and azithromycin to patients with gonococcal infections. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series