User login
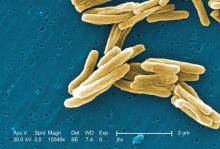
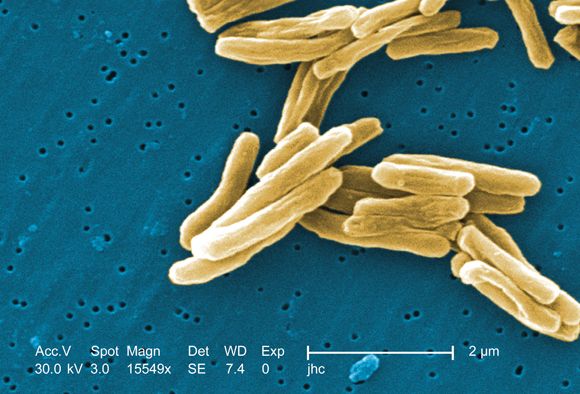
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].