User login
In 2015, Cleveland Clinic cardiac and vascular surgeons performed more than 1,000 open or endovascular operations involving the thoracic aorta, the most of any US medical center. Cardioaortic operations account for a large volume of the procedures performed annually in the Department of Thoracic and Cardiovascular Surgery at Cleveland Clinic. Of the approximately 4,000 cardiac procedures performed per year at Cleveland Clinic, nearly 1 in 5 includes thoracic aorta replacement.
Providing optimal care to patients with thoracic aortic disease requires a multidisciplinary approach beginning in the preoperative phase and extending through the life of patients and their families. In the Aortic Center at Cleveland Clinic Heart & Vascular Institute, cardiovascular medicine and imaging specialists, geneticists, and cardioaortic and vascular surgeons work in unison to provide the highest quality care. This involves active analysis of outcomes to continuously improve the quality of care provided.
This paper examines trends in the treatment of thoracic aortic disease, describes the different types of therapeutic procedures, and explores details about their safety and efficacy by summarizing the key research findings on cardioaortic procedures published from our Center during the last 2 years.
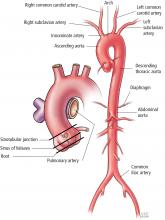
SEGMENTAL PERSPECTIVE
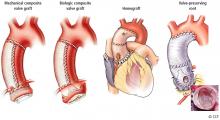
1. Modified Bentall procedure with a mechanical composite valve graft (CVG)
2. Modified Bentall procedure with a biologic CVG
3. Homograft, or allograft, root replacement with a human cadaveric aorta
4. Valve-preserving aortic root replacement with a prosthetic graft but which leaves the patient’s native aortic valve intact with or without accompanying repair of that valve.
A Cleveland Clinic study published in 2016 analyzed 957 elective aortic root replacement procedures performed from 1995 through 2014.1 The number of procedures in this study were evenly distributed across these 4 aortic root replacement strategies.
The perioperative mortality rate was 0.73% and the stroke rate was 1.4%. For 3 of the 4 procedure types, 15-year survival rates were excellent: above 80% for mechanical CVG, allografts, and valve-preservation surgery. The survival rate for biologic CVG was lower (57%), reflecting the difference in population, as these were typically older patients.
This study also demonstrated the durability of these operations, with a reoperation rate of approximately 15% at 15 years. Reoperation rates for patients having undergone these operations should be considered in the light of competing risk of death from other causes. As such, the risk of reoperation after mechanical CVG, biologic CVG, and valve-preserving procedures were similar, ranging from 5% to 15%. Allografts had the highest reoperation rates (approximately 30% at 15 years) because they used to be the biologic root replacement of choice for younger patients but have since been found to wear out at a similar rate as other bioprostheses.2 As a result, they are now used less frequently for elective indications.
Cleveland Clinic practitioners now perform more than 80 valve-preserving root replacement operations per year, approximately 700 overall.
Clinical implications
For patients presenting with aortic root aneurysm, consider the following:
- Valve-preserving aortic root replacement is preferred for patients with root aneurysm and a tricuspid aortic valve without valve stenosis.
- Valve-preserving aortic root replacement with either remodeling or reimplantation is also preferred for patients with a bicuspid aortic valve with a dilated annulus or root aneurysm, but without aortic-associated aortic valve stenosis
- Mechanical CVG is preferred for younger patients with root aneurysm and aortic valve stenosis (usually a bicuspid or unicuspid aortic valve); biomechanical CVG is preferred for older patients with root aneurysm and associated aortic valve stenosis.
- Allografts are now reserved primarily for patients with endocarditis and for older patients with a small aortic root.
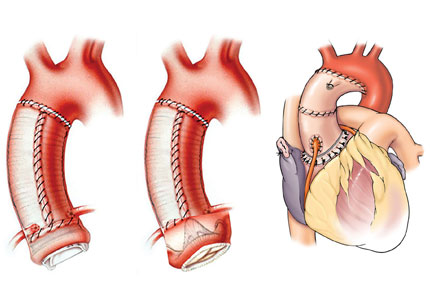
WHAT ARE THE RISKS WITH ASCENDING AORTIC REPAIR?
The condition of the patient at presentation has become the strongest predictor of surgical risk. An improved understanding of these associations can improve our prediction of risks and the decision about when to operate. Patients needing aortic replacement can present with a broad spectrum of pathologies. For example, a patient who presents with acute type A dissection is quite different from a patient with an enlarging ascending aneurysm who had a previous aortic valve replacement for bicuspid aortic valve stenosis as a young adult. Further, both are different from the elderly patient with the complex constellation of coronary disease, multivalve disease, atrial fibrillation, and an ascending aneurysm—an increasingly common presentation.
Guidelines supporting the decision to replace the aorta in patients with chronic asymptomatic aortic disease are limited by a lack of data on surgical risk and long-term effectiveness.
A study from the Society of Thoracic Surgeons database assessed outcomes in patients who had surgical replacement of the ascending aorta, with or without root repair.3 The operative mortality (either in-hospital or within 30 days of surgery) was 8.3% and ranged from 3.5% for elective surgery to 9.1% for urgent surgery, and 21.5% for emergencies. End-stage kidney disease and reoperation were also shown to be independent predictors of risk in that study.
Outcomes at Cleveland Clinic for elective ascending aortic procedures are much better than these national averages. Outcomes data are important to patients when making a decision about prophylactic surgery. In a study analyzing 1,889 patients undergoing elective ascending replacement at Cleveland Clinic between 2006 and 2010, the operative mortality was only 0.5% for those undergoing isolated ascending replacement and 2% for those requiring a multicomponent operation. In the multicomponent group, 87% included aortic valve replacement, 29% coronary bypass, and 25% underwent more than 2 different combined procedures.4
Patient risk factors
A comparison of patient risk factors for the 2 groups showed that the isolated replacement group had larger aortic diameters, more extensive disease with dilated descending aortas, and were more frequently undergoing a reoperation than the multicomponent group.
To further define the risks, we conducted a propensity-matching study of 197 pairs of these patients, comparing 62 variables including aortic morphology data gathered from 3-dimensional analysis of computed tomography scans. Results showed no differences in survival rates between the groups during 4 years of follow-up.4 A comparison of the risk of other perioperative complications—death, stroke, need for dialysis, respiratory failure, and bleeding—also showed no differences between the groups.
Does adding ascending aortic replacement to other cardiac procedures increase the surgical risk?
To answer this question, we collected data on Cleveland Clinic patients between 2006 and 2011 who had aortic surgery in combination with cardiac surgery (N = 1,677) and compared them against a similar cohort who only had cardiac surgery (N = 12,617).5 The objectives were to determine the risk of adding aortic surgery to an elective cardiac operation. A second objective was to determine the impact of circulatory arrest on outcomes.
Comparison 1. We identified 1,284 matched pairs from the 2 groups. Data showed a slightly higher risk of stroke in patients who had cardioaortic surgery (2.4%) compared with those who had cardiac surgery alone (1.7%); however, the mortality rate was not significantly different between the groups.
Does circulatory arrest affect the stroke rate?
From the matched pairs of patients who underwent cardioaortic surgery, we identified a subset of patients who had circulatory arrest and compared them with those who did not have circulatory arrest. The circulatory arrest group had worse outcomes. Mortality rates were 4.1% vs 1.0%, respectively, and stroke rates were 3.9% vs 0.9%.
This raised the question of whether circulatory arrest was the cause of the worse outcomes or a marker of patients with more advanced disease.
The decision to use circulatory arrest is primarily based on 2 factors:
- Patient-specific factors, such as those with advanced aortic disease in whom circulatory arrest is unavoidable.
- Surgeon preference/technical decision. For example, in a patient with a bicuspid valve, the surgeon may choose to use a brief period of circulatory arrest instead of clamping the proximal arch.
Comparison 2. To further define the impact of circulatory arrest, we grouped the patients who underwent cardioaortic surgery (N = 1,677) into those who had circulatory arrest (n = 728) or no arrest (n = 949). From those groups, we identified 324 matched pairs of patients and compared the outcomes.
Our results showed no differences associated with the use of circulatory arrest in rates of mortality (1.2% with and 0.6% without) or stroke (1.5% for both groups) when comparing patients with similar disease characteristics. These results suggest that the need for circulatory arrest was probably not the culprit but more likely a marker of patients with more complex disease. It is their more advanced disease that puts them at higher risk.
Comparison 3. To determine whether circulatory arrest has an overall impact on cardiac surgery, we took the population of matched cardioaortic patients from comparison 2 regardless of whether they had circulatory arrest and compared them to the larger group of 12,617 cardiac surgery-alone patients. Again, results indicated that the addition of aortic surgery had no real impact on outcomes. Both groups had similarly low risks for both mortality (0.9% with aortic replacement vs 0.5% without) and stroke (1.4% with aortic replacement vs 1.1% without).
Clinical implications
This multistepped comparison study found that adding ascending aortic replacement to cardiac surgery had essentially no impact on mortality or stroke. These data provide evidence indicating that cardiac surgeons should be more proactive in deciding whether to add ascending aorta replacement to cardiac surgery when treating a patient with a dilated ascending aorta. It must be noted, however, that patients with more advanced aortic disease are a higher risk population. All of these findings highlight the importance of managing thoracic aortic disease within an experienced multidisciplinary center.
AORTIC DISSECTION RISK IN PATIENTS WITH A BICUSPID AORTIC VALVE AND AORTOPATHY
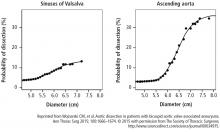
These findings provided important evidence supporting the need to be more proactive in the decision to perform aortic replacement. Furthermore, the data prompted the American Heart Association and the American College of Cardiology to publish a clarification statement providing more detail to its thoracic aorta and aortic valve guidelines. This update indicates that in patients with a bicuspid aortic valve, it is reasonable to recommend surgery when the aorta is 5 cm instead of waiting until 5.5 cm in high-volume centers that have demonstrated excellent surgical outcomes. This clarification statement was based on Cleveland Clinic outcomes showing a mortality rate of 0.25% and a stroke rate of 0.75% in a population that included patients undergoing emergency aortic dissection surgery.6
This study also analyzed data on patients treated with expectant care with optimal medical management and imaging surveillance (ie, to monitor the dilated aorta). Results from this subset showed that the probability of needing an aortic intervention is about 60% during the next 10 years once the aorta is within the 4.5 cm to 5 cm range.
Another study addressing the correlation between risk and aortic size examined 771 patients with a dilated ascending aorta (≥ 4 cm) and a tricuspid aortic valve.7 This study confirmed the use of patient height as an important factor for indexing maximum aortic size to patient body size for predicting risk of late complications. Specifically, this study suggested that the risk of complications from aortic aneurysm rises when the maximum aortic area-to-height ratio exceeds 10. This serves as a follow-up to previously published data demonstrating the value of aortic cross-sectional area-to-height ratio as a predictor of risk in patients with bicuspid valves.8 In general, the results of all 3 studies suggest that we should be more proactive in operating on patients with a dilated ascending aorta to prevent later risk of rupture or dissection when the surgical risk is low.
When making decisions about patients who need aortic replacement, it is important to assess many patient details: their aortic disease, their other nonaortic comorbidities, and the institution’s outcomes. This decision is best made by a dedicated cardioaortic specialist at a dedicated center of excellence.
WHAT IS COMING?
Minimally invasive and endovascular surgery
More ascending aortic surgeries are being done using minimally invasive approaches. At Cleveland Clinic, about 40% of isolated ascending aortic operations are performed through a mini-sternotomy J incision approach. A Cleveland Clinic study published in 2017 evaluated outcomes from this less-invasive technique for proximal aortic surgery compared with full median sternotomy.9 Results showed it was an effective approach with fewer complications, shorter hospital stays, and lower costs.
Stent grafts
The role for stent-graft devices has continued to expand.10 At Cleveland Clinic, we have performed more than 40 ascending aortic stent-graft procedures, one of the largest numbers in the world. Having this stent-graft option has enabled us to provide treatment for the patients at exceedingly high risk who previously had few or no options. Industry partners are working to develop dedicated devices for these indications, and we are working with them to bring new device trials to this underserved population of patients.
- Svensson LG, Pillai ST, Rajeswaran J, Desai MY, Griffin B, Grimm R, Hammer DF, Thamilarasan M, Roselli EE, Pettersson GB, Gillinov AM, Navia JL, Smedira NG, Sabik JF III, Lytle BW, Blackstone EH. Long-term survival, valve durability, and reoperation for 4 aortic root procedures combined with ascending aorta replacement. J Thorac Cardiovasc Surg 2016; 151:764–771.
- Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg 2006; 131:558–564.
- Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012; 60:1156–1162.
- Idrees JJ, Roselli EE, Lowry AM, Reside JM, Javadikasgari H, Johnson DJ, Soltesz EG, Johnston DR, Pettersson GB, Blackstone EH, Sabik JF III, Svensson LG. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101:2185–2192.
- Idrees JJ, Roselli ER, Blackstone EH, Lowry AM, Johnston DR, Soltesz EG, Tong MA, Pettersson GB, Gillinov MA, Griffin B, Svensson LG. Risk of adding aortic replacement to a multi-component cardiac operation . J Thorac Cardiovasc Surg 2017; in press.
- Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, Pettersson GB, Gillinov AM, Johnston DR, Soltesz EG, Navia JL, Hammer DF, Griffin B, Thamilarasan M, Kalahasti V, Sabik JF III, Blackstone EH, Lytle BW. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg 2015; 100:1666–1673.
- Masri A, Kalahasti V, Svensson LG, Roselli EE, Johnston D, Hammer D, Schoenhagen P, Griffin BP, Desai MY. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134:1724–1737.
- Masri A, Kalahasti V, Svensson LG, Alashi A, Schoenhagen P, Roselli EE, Johnston DR, Rodriguez LL, Griffin BP, Desai MY. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging 2017; 10:e006249.
- Levack MM, Aftab M, Roselli EE, Johnston DR, Soltesz EG, Gillinov AM, Pettersson GB, Griffin B, Grimm R, Hammer DF, Al Kindi AH, Albacker TB, Sepulveda E, Thuita L, Blackstone EH, Sabik JF III, Svensson LG. Outcomes of a less-invasive approach for proximal aortic operations. Ann Thorac Surg 2017; 103:533–540.
- Roselli EE, Hasan SM, Idrees JJ, Aftab M, Eagleton MJ, Menon V, Svensson LG. Inoperable patients with acute type A dissection: are they candidates for endovascular repair? Interact Cardiovasc Thorac Surg 2017:1–7. https://doi.org/10.1093/icvts/ivx193.
In 2015, Cleveland Clinic cardiac and vascular surgeons performed more than 1,000 open or endovascular operations involving the thoracic aorta, the most of any US medical center. Cardioaortic operations account for a large volume of the procedures performed annually in the Department of Thoracic and Cardiovascular Surgery at Cleveland Clinic. Of the approximately 4,000 cardiac procedures performed per year at Cleveland Clinic, nearly 1 in 5 includes thoracic aorta replacement.
Providing optimal care to patients with thoracic aortic disease requires a multidisciplinary approach beginning in the preoperative phase and extending through the life of patients and their families. In the Aortic Center at Cleveland Clinic Heart & Vascular Institute, cardiovascular medicine and imaging specialists, geneticists, and cardioaortic and vascular surgeons work in unison to provide the highest quality care. This involves active analysis of outcomes to continuously improve the quality of care provided.
This paper examines trends in the treatment of thoracic aortic disease, describes the different types of therapeutic procedures, and explores details about their safety and efficacy by summarizing the key research findings on cardioaortic procedures published from our Center during the last 2 years.
SEGMENTAL PERSPECTIVE
1. Modified Bentall procedure with a mechanical composite valve graft (CVG)
2. Modified Bentall procedure with a biologic CVG
3. Homograft, or allograft, root replacement with a human cadaveric aorta
4. Valve-preserving aortic root replacement with a prosthetic graft but which leaves the patient’s native aortic valve intact with or without accompanying repair of that valve.
A Cleveland Clinic study published in 2016 analyzed 957 elective aortic root replacement procedures performed from 1995 through 2014.1 The number of procedures in this study were evenly distributed across these 4 aortic root replacement strategies.
The perioperative mortality rate was 0.73% and the stroke rate was 1.4%. For 3 of the 4 procedure types, 15-year survival rates were excellent: above 80% for mechanical CVG, allografts, and valve-preservation surgery. The survival rate for biologic CVG was lower (57%), reflecting the difference in population, as these were typically older patients.
This study also demonstrated the durability of these operations, with a reoperation rate of approximately 15% at 15 years. Reoperation rates for patients having undergone these operations should be considered in the light of competing risk of death from other causes. As such, the risk of reoperation after mechanical CVG, biologic CVG, and valve-preserving procedures were similar, ranging from 5% to 15%. Allografts had the highest reoperation rates (approximately 30% at 15 years) because they used to be the biologic root replacement of choice for younger patients but have since been found to wear out at a similar rate as other bioprostheses.2 As a result, they are now used less frequently for elective indications.
Cleveland Clinic practitioners now perform more than 80 valve-preserving root replacement operations per year, approximately 700 overall.
Clinical implications
For patients presenting with aortic root aneurysm, consider the following:
- Valve-preserving aortic root replacement is preferred for patients with root aneurysm and a tricuspid aortic valve without valve stenosis.
- Valve-preserving aortic root replacement with either remodeling or reimplantation is also preferred for patients with a bicuspid aortic valve with a dilated annulus or root aneurysm, but without aortic-associated aortic valve stenosis
- Mechanical CVG is preferred for younger patients with root aneurysm and aortic valve stenosis (usually a bicuspid or unicuspid aortic valve); biomechanical CVG is preferred for older patients with root aneurysm and associated aortic valve stenosis.
- Allografts are now reserved primarily for patients with endocarditis and for older patients with a small aortic root.
WHAT ARE THE RISKS WITH ASCENDING AORTIC REPAIR?
The condition of the patient at presentation has become the strongest predictor of surgical risk. An improved understanding of these associations can improve our prediction of risks and the decision about when to operate. Patients needing aortic replacement can present with a broad spectrum of pathologies. For example, a patient who presents with acute type A dissection is quite different from a patient with an enlarging ascending aneurysm who had a previous aortic valve replacement for bicuspid aortic valve stenosis as a young adult. Further, both are different from the elderly patient with the complex constellation of coronary disease, multivalve disease, atrial fibrillation, and an ascending aneurysm—an increasingly common presentation.
Guidelines supporting the decision to replace the aorta in patients with chronic asymptomatic aortic disease are limited by a lack of data on surgical risk and long-term effectiveness.
A study from the Society of Thoracic Surgeons database assessed outcomes in patients who had surgical replacement of the ascending aorta, with or without root repair.3 The operative mortality (either in-hospital or within 30 days of surgery) was 8.3% and ranged from 3.5% for elective surgery to 9.1% for urgent surgery, and 21.5% for emergencies. End-stage kidney disease and reoperation were also shown to be independent predictors of risk in that study.
Outcomes at Cleveland Clinic for elective ascending aortic procedures are much better than these national averages. Outcomes data are important to patients when making a decision about prophylactic surgery. In a study analyzing 1,889 patients undergoing elective ascending replacement at Cleveland Clinic between 2006 and 2010, the operative mortality was only 0.5% for those undergoing isolated ascending replacement and 2% for those requiring a multicomponent operation. In the multicomponent group, 87% included aortic valve replacement, 29% coronary bypass, and 25% underwent more than 2 different combined procedures.4
Patient risk factors
A comparison of patient risk factors for the 2 groups showed that the isolated replacement group had larger aortic diameters, more extensive disease with dilated descending aortas, and were more frequently undergoing a reoperation than the multicomponent group.
To further define the risks, we conducted a propensity-matching study of 197 pairs of these patients, comparing 62 variables including aortic morphology data gathered from 3-dimensional analysis of computed tomography scans. Results showed no differences in survival rates between the groups during 4 years of follow-up.4 A comparison of the risk of other perioperative complications—death, stroke, need for dialysis, respiratory failure, and bleeding—also showed no differences between the groups.
Does adding ascending aortic replacement to other cardiac procedures increase the surgical risk?
To answer this question, we collected data on Cleveland Clinic patients between 2006 and 2011 who had aortic surgery in combination with cardiac surgery (N = 1,677) and compared them against a similar cohort who only had cardiac surgery (N = 12,617).5 The objectives were to determine the risk of adding aortic surgery to an elective cardiac operation. A second objective was to determine the impact of circulatory arrest on outcomes.
Comparison 1. We identified 1,284 matched pairs from the 2 groups. Data showed a slightly higher risk of stroke in patients who had cardioaortic surgery (2.4%) compared with those who had cardiac surgery alone (1.7%); however, the mortality rate was not significantly different between the groups.
Does circulatory arrest affect the stroke rate?
From the matched pairs of patients who underwent cardioaortic surgery, we identified a subset of patients who had circulatory arrest and compared them with those who did not have circulatory arrest. The circulatory arrest group had worse outcomes. Mortality rates were 4.1% vs 1.0%, respectively, and stroke rates were 3.9% vs 0.9%.
This raised the question of whether circulatory arrest was the cause of the worse outcomes or a marker of patients with more advanced disease.
The decision to use circulatory arrest is primarily based on 2 factors:
- Patient-specific factors, such as those with advanced aortic disease in whom circulatory arrest is unavoidable.
- Surgeon preference/technical decision. For example, in a patient with a bicuspid valve, the surgeon may choose to use a brief period of circulatory arrest instead of clamping the proximal arch.
Comparison 2. To further define the impact of circulatory arrest, we grouped the patients who underwent cardioaortic surgery (N = 1,677) into those who had circulatory arrest (n = 728) or no arrest (n = 949). From those groups, we identified 324 matched pairs of patients and compared the outcomes.
Our results showed no differences associated with the use of circulatory arrest in rates of mortality (1.2% with and 0.6% without) or stroke (1.5% for both groups) when comparing patients with similar disease characteristics. These results suggest that the need for circulatory arrest was probably not the culprit but more likely a marker of patients with more complex disease. It is their more advanced disease that puts them at higher risk.
Comparison 3. To determine whether circulatory arrest has an overall impact on cardiac surgery, we took the population of matched cardioaortic patients from comparison 2 regardless of whether they had circulatory arrest and compared them to the larger group of 12,617 cardiac surgery-alone patients. Again, results indicated that the addition of aortic surgery had no real impact on outcomes. Both groups had similarly low risks for both mortality (0.9% with aortic replacement vs 0.5% without) and stroke (1.4% with aortic replacement vs 1.1% without).
Clinical implications
This multistepped comparison study found that adding ascending aortic replacement to cardiac surgery had essentially no impact on mortality or stroke. These data provide evidence indicating that cardiac surgeons should be more proactive in deciding whether to add ascending aorta replacement to cardiac surgery when treating a patient with a dilated ascending aorta. It must be noted, however, that patients with more advanced aortic disease are a higher risk population. All of these findings highlight the importance of managing thoracic aortic disease within an experienced multidisciplinary center.
AORTIC DISSECTION RISK IN PATIENTS WITH A BICUSPID AORTIC VALVE AND AORTOPATHY
These findings provided important evidence supporting the need to be more proactive in the decision to perform aortic replacement. Furthermore, the data prompted the American Heart Association and the American College of Cardiology to publish a clarification statement providing more detail to its thoracic aorta and aortic valve guidelines. This update indicates that in patients with a bicuspid aortic valve, it is reasonable to recommend surgery when the aorta is 5 cm instead of waiting until 5.5 cm in high-volume centers that have demonstrated excellent surgical outcomes. This clarification statement was based on Cleveland Clinic outcomes showing a mortality rate of 0.25% and a stroke rate of 0.75% in a population that included patients undergoing emergency aortic dissection surgery.6
This study also analyzed data on patients treated with expectant care with optimal medical management and imaging surveillance (ie, to monitor the dilated aorta). Results from this subset showed that the probability of needing an aortic intervention is about 60% during the next 10 years once the aorta is within the 4.5 cm to 5 cm range.
Another study addressing the correlation between risk and aortic size examined 771 patients with a dilated ascending aorta (≥ 4 cm) and a tricuspid aortic valve.7 This study confirmed the use of patient height as an important factor for indexing maximum aortic size to patient body size for predicting risk of late complications. Specifically, this study suggested that the risk of complications from aortic aneurysm rises when the maximum aortic area-to-height ratio exceeds 10. This serves as a follow-up to previously published data demonstrating the value of aortic cross-sectional area-to-height ratio as a predictor of risk in patients with bicuspid valves.8 In general, the results of all 3 studies suggest that we should be more proactive in operating on patients with a dilated ascending aorta to prevent later risk of rupture or dissection when the surgical risk is low.
When making decisions about patients who need aortic replacement, it is important to assess many patient details: their aortic disease, their other nonaortic comorbidities, and the institution’s outcomes. This decision is best made by a dedicated cardioaortic specialist at a dedicated center of excellence.
WHAT IS COMING?
Minimally invasive and endovascular surgery
More ascending aortic surgeries are being done using minimally invasive approaches. At Cleveland Clinic, about 40% of isolated ascending aortic operations are performed through a mini-sternotomy J incision approach. A Cleveland Clinic study published in 2017 evaluated outcomes from this less-invasive technique for proximal aortic surgery compared with full median sternotomy.9 Results showed it was an effective approach with fewer complications, shorter hospital stays, and lower costs.
Stent grafts
The role for stent-graft devices has continued to expand.10 At Cleveland Clinic, we have performed more than 40 ascending aortic stent-graft procedures, one of the largest numbers in the world. Having this stent-graft option has enabled us to provide treatment for the patients at exceedingly high risk who previously had few or no options. Industry partners are working to develop dedicated devices for these indications, and we are working with them to bring new device trials to this underserved population of patients.
In 2015, Cleveland Clinic cardiac and vascular surgeons performed more than 1,000 open or endovascular operations involving the thoracic aorta, the most of any US medical center. Cardioaortic operations account for a large volume of the procedures performed annually in the Department of Thoracic and Cardiovascular Surgery at Cleveland Clinic. Of the approximately 4,000 cardiac procedures performed per year at Cleveland Clinic, nearly 1 in 5 includes thoracic aorta replacement.
Providing optimal care to patients with thoracic aortic disease requires a multidisciplinary approach beginning in the preoperative phase and extending through the life of patients and their families. In the Aortic Center at Cleveland Clinic Heart & Vascular Institute, cardiovascular medicine and imaging specialists, geneticists, and cardioaortic and vascular surgeons work in unison to provide the highest quality care. This involves active analysis of outcomes to continuously improve the quality of care provided.
This paper examines trends in the treatment of thoracic aortic disease, describes the different types of therapeutic procedures, and explores details about their safety and efficacy by summarizing the key research findings on cardioaortic procedures published from our Center during the last 2 years.
SEGMENTAL PERSPECTIVE
1. Modified Bentall procedure with a mechanical composite valve graft (CVG)
2. Modified Bentall procedure with a biologic CVG
3. Homograft, or allograft, root replacement with a human cadaveric aorta
4. Valve-preserving aortic root replacement with a prosthetic graft but which leaves the patient’s native aortic valve intact with or without accompanying repair of that valve.
A Cleveland Clinic study published in 2016 analyzed 957 elective aortic root replacement procedures performed from 1995 through 2014.1 The number of procedures in this study were evenly distributed across these 4 aortic root replacement strategies.
The perioperative mortality rate was 0.73% and the stroke rate was 1.4%. For 3 of the 4 procedure types, 15-year survival rates were excellent: above 80% for mechanical CVG, allografts, and valve-preservation surgery. The survival rate for biologic CVG was lower (57%), reflecting the difference in population, as these were typically older patients.
This study also demonstrated the durability of these operations, with a reoperation rate of approximately 15% at 15 years. Reoperation rates for patients having undergone these operations should be considered in the light of competing risk of death from other causes. As such, the risk of reoperation after mechanical CVG, biologic CVG, and valve-preserving procedures were similar, ranging from 5% to 15%. Allografts had the highest reoperation rates (approximately 30% at 15 years) because they used to be the biologic root replacement of choice for younger patients but have since been found to wear out at a similar rate as other bioprostheses.2 As a result, they are now used less frequently for elective indications.
Cleveland Clinic practitioners now perform more than 80 valve-preserving root replacement operations per year, approximately 700 overall.
Clinical implications
For patients presenting with aortic root aneurysm, consider the following:
- Valve-preserving aortic root replacement is preferred for patients with root aneurysm and a tricuspid aortic valve without valve stenosis.
- Valve-preserving aortic root replacement with either remodeling or reimplantation is also preferred for patients with a bicuspid aortic valve with a dilated annulus or root aneurysm, but without aortic-associated aortic valve stenosis
- Mechanical CVG is preferred for younger patients with root aneurysm and aortic valve stenosis (usually a bicuspid or unicuspid aortic valve); biomechanical CVG is preferred for older patients with root aneurysm and associated aortic valve stenosis.
- Allografts are now reserved primarily for patients with endocarditis and for older patients with a small aortic root.
WHAT ARE THE RISKS WITH ASCENDING AORTIC REPAIR?
The condition of the patient at presentation has become the strongest predictor of surgical risk. An improved understanding of these associations can improve our prediction of risks and the decision about when to operate. Patients needing aortic replacement can present with a broad spectrum of pathologies. For example, a patient who presents with acute type A dissection is quite different from a patient with an enlarging ascending aneurysm who had a previous aortic valve replacement for bicuspid aortic valve stenosis as a young adult. Further, both are different from the elderly patient with the complex constellation of coronary disease, multivalve disease, atrial fibrillation, and an ascending aneurysm—an increasingly common presentation.
Guidelines supporting the decision to replace the aorta in patients with chronic asymptomatic aortic disease are limited by a lack of data on surgical risk and long-term effectiveness.
A study from the Society of Thoracic Surgeons database assessed outcomes in patients who had surgical replacement of the ascending aorta, with or without root repair.3 The operative mortality (either in-hospital or within 30 days of surgery) was 8.3% and ranged from 3.5% for elective surgery to 9.1% for urgent surgery, and 21.5% for emergencies. End-stage kidney disease and reoperation were also shown to be independent predictors of risk in that study.
Outcomes at Cleveland Clinic for elective ascending aortic procedures are much better than these national averages. Outcomes data are important to patients when making a decision about prophylactic surgery. In a study analyzing 1,889 patients undergoing elective ascending replacement at Cleveland Clinic between 2006 and 2010, the operative mortality was only 0.5% for those undergoing isolated ascending replacement and 2% for those requiring a multicomponent operation. In the multicomponent group, 87% included aortic valve replacement, 29% coronary bypass, and 25% underwent more than 2 different combined procedures.4
Patient risk factors
A comparison of patient risk factors for the 2 groups showed that the isolated replacement group had larger aortic diameters, more extensive disease with dilated descending aortas, and were more frequently undergoing a reoperation than the multicomponent group.
To further define the risks, we conducted a propensity-matching study of 197 pairs of these patients, comparing 62 variables including aortic morphology data gathered from 3-dimensional analysis of computed tomography scans. Results showed no differences in survival rates between the groups during 4 years of follow-up.4 A comparison of the risk of other perioperative complications—death, stroke, need for dialysis, respiratory failure, and bleeding—also showed no differences between the groups.
Does adding ascending aortic replacement to other cardiac procedures increase the surgical risk?
To answer this question, we collected data on Cleveland Clinic patients between 2006 and 2011 who had aortic surgery in combination with cardiac surgery (N = 1,677) and compared them against a similar cohort who only had cardiac surgery (N = 12,617).5 The objectives were to determine the risk of adding aortic surgery to an elective cardiac operation. A second objective was to determine the impact of circulatory arrest on outcomes.
Comparison 1. We identified 1,284 matched pairs from the 2 groups. Data showed a slightly higher risk of stroke in patients who had cardioaortic surgery (2.4%) compared with those who had cardiac surgery alone (1.7%); however, the mortality rate was not significantly different between the groups.
Does circulatory arrest affect the stroke rate?
From the matched pairs of patients who underwent cardioaortic surgery, we identified a subset of patients who had circulatory arrest and compared them with those who did not have circulatory arrest. The circulatory arrest group had worse outcomes. Mortality rates were 4.1% vs 1.0%, respectively, and stroke rates were 3.9% vs 0.9%.
This raised the question of whether circulatory arrest was the cause of the worse outcomes or a marker of patients with more advanced disease.
The decision to use circulatory arrest is primarily based on 2 factors:
- Patient-specific factors, such as those with advanced aortic disease in whom circulatory arrest is unavoidable.
- Surgeon preference/technical decision. For example, in a patient with a bicuspid valve, the surgeon may choose to use a brief period of circulatory arrest instead of clamping the proximal arch.
Comparison 2. To further define the impact of circulatory arrest, we grouped the patients who underwent cardioaortic surgery (N = 1,677) into those who had circulatory arrest (n = 728) or no arrest (n = 949). From those groups, we identified 324 matched pairs of patients and compared the outcomes.
Our results showed no differences associated with the use of circulatory arrest in rates of mortality (1.2% with and 0.6% without) or stroke (1.5% for both groups) when comparing patients with similar disease characteristics. These results suggest that the need for circulatory arrest was probably not the culprit but more likely a marker of patients with more complex disease. It is their more advanced disease that puts them at higher risk.
Comparison 3. To determine whether circulatory arrest has an overall impact on cardiac surgery, we took the population of matched cardioaortic patients from comparison 2 regardless of whether they had circulatory arrest and compared them to the larger group of 12,617 cardiac surgery-alone patients. Again, results indicated that the addition of aortic surgery had no real impact on outcomes. Both groups had similarly low risks for both mortality (0.9% with aortic replacement vs 0.5% without) and stroke (1.4% with aortic replacement vs 1.1% without).
Clinical implications
This multistepped comparison study found that adding ascending aortic replacement to cardiac surgery had essentially no impact on mortality or stroke. These data provide evidence indicating that cardiac surgeons should be more proactive in deciding whether to add ascending aorta replacement to cardiac surgery when treating a patient with a dilated ascending aorta. It must be noted, however, that patients with more advanced aortic disease are a higher risk population. All of these findings highlight the importance of managing thoracic aortic disease within an experienced multidisciplinary center.
AORTIC DISSECTION RISK IN PATIENTS WITH A BICUSPID AORTIC VALVE AND AORTOPATHY
These findings provided important evidence supporting the need to be more proactive in the decision to perform aortic replacement. Furthermore, the data prompted the American Heart Association and the American College of Cardiology to publish a clarification statement providing more detail to its thoracic aorta and aortic valve guidelines. This update indicates that in patients with a bicuspid aortic valve, it is reasonable to recommend surgery when the aorta is 5 cm instead of waiting until 5.5 cm in high-volume centers that have demonstrated excellent surgical outcomes. This clarification statement was based on Cleveland Clinic outcomes showing a mortality rate of 0.25% and a stroke rate of 0.75% in a population that included patients undergoing emergency aortic dissection surgery.6
This study also analyzed data on patients treated with expectant care with optimal medical management and imaging surveillance (ie, to monitor the dilated aorta). Results from this subset showed that the probability of needing an aortic intervention is about 60% during the next 10 years once the aorta is within the 4.5 cm to 5 cm range.
Another study addressing the correlation between risk and aortic size examined 771 patients with a dilated ascending aorta (≥ 4 cm) and a tricuspid aortic valve.7 This study confirmed the use of patient height as an important factor for indexing maximum aortic size to patient body size for predicting risk of late complications. Specifically, this study suggested that the risk of complications from aortic aneurysm rises when the maximum aortic area-to-height ratio exceeds 10. This serves as a follow-up to previously published data demonstrating the value of aortic cross-sectional area-to-height ratio as a predictor of risk in patients with bicuspid valves.8 In general, the results of all 3 studies suggest that we should be more proactive in operating on patients with a dilated ascending aorta to prevent later risk of rupture or dissection when the surgical risk is low.
When making decisions about patients who need aortic replacement, it is important to assess many patient details: their aortic disease, their other nonaortic comorbidities, and the institution’s outcomes. This decision is best made by a dedicated cardioaortic specialist at a dedicated center of excellence.
WHAT IS COMING?
Minimally invasive and endovascular surgery
More ascending aortic surgeries are being done using minimally invasive approaches. At Cleveland Clinic, about 40% of isolated ascending aortic operations are performed through a mini-sternotomy J incision approach. A Cleveland Clinic study published in 2017 evaluated outcomes from this less-invasive technique for proximal aortic surgery compared with full median sternotomy.9 Results showed it was an effective approach with fewer complications, shorter hospital stays, and lower costs.
Stent grafts
The role for stent-graft devices has continued to expand.10 At Cleveland Clinic, we have performed more than 40 ascending aortic stent-graft procedures, one of the largest numbers in the world. Having this stent-graft option has enabled us to provide treatment for the patients at exceedingly high risk who previously had few or no options. Industry partners are working to develop dedicated devices for these indications, and we are working with them to bring new device trials to this underserved population of patients.
- Svensson LG, Pillai ST, Rajeswaran J, Desai MY, Griffin B, Grimm R, Hammer DF, Thamilarasan M, Roselli EE, Pettersson GB, Gillinov AM, Navia JL, Smedira NG, Sabik JF III, Lytle BW, Blackstone EH. Long-term survival, valve durability, and reoperation for 4 aortic root procedures combined with ascending aorta replacement. J Thorac Cardiovasc Surg 2016; 151:764–771.
- Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg 2006; 131:558–564.
- Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012; 60:1156–1162.
- Idrees JJ, Roselli EE, Lowry AM, Reside JM, Javadikasgari H, Johnson DJ, Soltesz EG, Johnston DR, Pettersson GB, Blackstone EH, Sabik JF III, Svensson LG. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101:2185–2192.
- Idrees JJ, Roselli ER, Blackstone EH, Lowry AM, Johnston DR, Soltesz EG, Tong MA, Pettersson GB, Gillinov MA, Griffin B, Svensson LG. Risk of adding aortic replacement to a multi-component cardiac operation . J Thorac Cardiovasc Surg 2017; in press.
- Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, Pettersson GB, Gillinov AM, Johnston DR, Soltesz EG, Navia JL, Hammer DF, Griffin B, Thamilarasan M, Kalahasti V, Sabik JF III, Blackstone EH, Lytle BW. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg 2015; 100:1666–1673.
- Masri A, Kalahasti V, Svensson LG, Roselli EE, Johnston D, Hammer D, Schoenhagen P, Griffin BP, Desai MY. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134:1724–1737.
- Masri A, Kalahasti V, Svensson LG, Alashi A, Schoenhagen P, Roselli EE, Johnston DR, Rodriguez LL, Griffin BP, Desai MY. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging 2017; 10:e006249.
- Levack MM, Aftab M, Roselli EE, Johnston DR, Soltesz EG, Gillinov AM, Pettersson GB, Griffin B, Grimm R, Hammer DF, Al Kindi AH, Albacker TB, Sepulveda E, Thuita L, Blackstone EH, Sabik JF III, Svensson LG. Outcomes of a less-invasive approach for proximal aortic operations. Ann Thorac Surg 2017; 103:533–540.
- Roselli EE, Hasan SM, Idrees JJ, Aftab M, Eagleton MJ, Menon V, Svensson LG. Inoperable patients with acute type A dissection: are they candidates for endovascular repair? Interact Cardiovasc Thorac Surg 2017:1–7. https://doi.org/10.1093/icvts/ivx193.
- Svensson LG, Pillai ST, Rajeswaran J, Desai MY, Griffin B, Grimm R, Hammer DF, Thamilarasan M, Roselli EE, Pettersson GB, Gillinov AM, Navia JL, Smedira NG, Sabik JF III, Lytle BW, Blackstone EH. Long-term survival, valve durability, and reoperation for 4 aortic root procedures combined with ascending aorta replacement. J Thorac Cardiovasc Surg 2016; 151:764–771.
- Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg 2006; 131:558–564.
- Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012; 60:1156–1162.
- Idrees JJ, Roselli EE, Lowry AM, Reside JM, Javadikasgari H, Johnson DJ, Soltesz EG, Johnston DR, Pettersson GB, Blackstone EH, Sabik JF III, Svensson LG. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101:2185–2192.
- Idrees JJ, Roselli ER, Blackstone EH, Lowry AM, Johnston DR, Soltesz EG, Tong MA, Pettersson GB, Gillinov MA, Griffin B, Svensson LG. Risk of adding aortic replacement to a multi-component cardiac operation . J Thorac Cardiovasc Surg 2017; in press.
- Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, Pettersson GB, Gillinov AM, Johnston DR, Soltesz EG, Navia JL, Hammer DF, Griffin B, Thamilarasan M, Kalahasti V, Sabik JF III, Blackstone EH, Lytle BW. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg 2015; 100:1666–1673.
- Masri A, Kalahasti V, Svensson LG, Roselli EE, Johnston D, Hammer D, Schoenhagen P, Griffin BP, Desai MY. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134:1724–1737.
- Masri A, Kalahasti V, Svensson LG, Alashi A, Schoenhagen P, Roselli EE, Johnston DR, Rodriguez LL, Griffin BP, Desai MY. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging 2017; 10:e006249.
- Levack MM, Aftab M, Roselli EE, Johnston DR, Soltesz EG, Gillinov AM, Pettersson GB, Griffin B, Grimm R, Hammer DF, Al Kindi AH, Albacker TB, Sepulveda E, Thuita L, Blackstone EH, Sabik JF III, Svensson LG. Outcomes of a less-invasive approach for proximal aortic operations. Ann Thorac Surg 2017; 103:533–540.
- Roselli EE, Hasan SM, Idrees JJ, Aftab M, Eagleton MJ, Menon V, Svensson LG. Inoperable patients with acute type A dissection: are they candidates for endovascular repair? Interact Cardiovasc Thorac Surg 2017:1–7. https://doi.org/10.1093/icvts/ivx193.
KEY POINTS
- Adding a proximal thoracic aortic procedure to cardiac surgery does not adversely affect safety and efficacy.
- Presence of a bicuspid aortic valve does not significantly affect outcomes of aortic root procedures.
- Data support aortic replacement in patients when the aortic root vessels reach 5.5 cm in diameter.
- Use of circulatory arrest does not directly affect the stroke risk associated with ascending aortic replacement surgery, but it may be a marker for more serious pathology.