User login
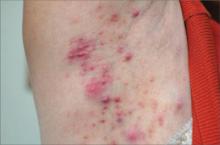
A healthy 29-year-old woman presented to the clinic with pustules and boil-like lesions in the axillary areas, on the left forearm, and on the right thigh (FIGURE). She indicated that she’d had the lesions for 4 weeks. She said that she had not had any recent infections, and there was no evidence of immune compromise in her history.
We incised a pustule in the right axilla and expressed the pus. We sent the sample out for bacterial culture and sensitivity testing.
We immediately considered bacterial folliculitis and hidradenitis suppurativa as part of the differential. The acute nature of the process led us to favor a diagnosis of bacterial folliculitis.
FIGURE
Papules and papulopustules on right axilla
Would you favor this diagnosis?
What would you choose for initial empiric antibiotic treatment?
Diagnosis: MRSA folliculitis
We began empiric systemic antibiotic therapy with doxycycline 100 mg daily and topical treatment with clindamycin 1% lotion twice a day. The bacterial culture came back positive for methicillin-resistant Staphylococcus aureus (MRSA) and the organism proved to be tetracycline sensitive.
Staphylococci species have long been the most common causes of skin and soft tissue infections.1 A recent study in 11 major US cities identified MRSA as the most common cause of acute purulent skin and soft tissue infections in patients presenting to the emergency department.2 This rate, however, varied from 15% to 74% in various cities.2 Today, clinicians must consider MRSA as a potential causative agent whenever they treat a patient with a skin and soft tissue infection.
Community-acquired MRSA: Don’t expect typical risk factors
Community-acquired MRSA (CA-MRSA) cases differ from hospital-acquired MRSA (HA-MRSA) cases demographically, microbiologically, and clinically. CA-MRSA infections typically occur in young, healthy individuals without traditional MRSA risk factors such as recent hospitalization, residence in a long-term care facility, or prior antibiotic use.3,4
CA-MRSA outbreaks have been associated with participation in team sports, living in prison, dormitory, or group home settings, IV drug use, sharing personal items, and men who have sex with men.4,5 However, the prevalence of MRSA infections in patients without any recognized risk factors, like our patient, is increasing.6
CA-MRSA is genetically distinct from HA-MRSA in that it contains the Panton-Valentine leukocidin, an important virulence factor, and carries the type IV or type V SCCmec cassette, which resides at a different methicillin-resistant locus.7,8 CA-MRSA also does not demonstrate the multi-drug resistance typical of HA-MRSA, and these infections tend to be susceptible to most non-beta-lactam antibiotics.6
Is it MRSA or another pathogen?
When considering the differential diagnosis of MRSA folliculitis, consider methicillin-susceptible staphylococci species as equally likely pathogens. Gram-negative bacteria, including Pseudomonas aeruginosa, Malassezia furfur, and Candida species are less common, but notable causes of folliculitis. Other causes include eosinophilic folliculitis, non-bacterial/irritant folliculitis, pseudofolliculitis (chronic in-grown hairs), hidradenitis suppurativa, and acne vulgaris.
Use a low threshold for obtaining cultures CA-MRSA sometimes presents as an edematous, tender abscess, an expanding cellulitis, or both.9,10 These presentations, however, are neither sensitive nor specific. Cutaneous infection may also present as erythematous papules, nodules, pustules, crusted plaques, and infrequently suppurative folliculitis.3,10 Therefore, the clinician should have a low threshold to perform bacterial culture and sensitivity studies whenever a skin and soft tissue infection is suspected.
The potential complications of CA-MRSA infection, including pneumonia, sepsis, and endocarditis, can be avoided or minimized with early diagnosis and initiation of appropriate treatment.4,11,12
Consider community factors
In the past, folliculitis and other localized skin infections were traditionally caused by methicillin-susceptible Staphylococcus aureus and treated with beta-lactam antibiotics. In communities with high prevalence of CA-MRSA skin and soft tissue infections, beta-lactam antibiotics like cephalexin or dicloxacillin may no longer be appropriate.2,5 Therefore, let the prevalence of CA-MRSA in your community help guide your initial antibiotic choice while recognizing that empiric antibiotic therapy may be adjusted after cultures are available.
Good initial choices for CA-MRSA skin and soft tissue infections are trim-ethoprim/sulfamethoxazole with or without rifampin, clindamycin, gentamicin, and tetracycline,2,4,5,7 but inducible clindamycin resistance has been reported.13 if your community has a high prevalence of CA-MRSA, you may decide to begin empiric therapy that provides MRSA coverage.2 In addition, incision and drainage is necessary for adequate treatment of furuncles, carbuncles, and abscesses.5 Healthy, afebrile, immunocompetent patients without cellulitis may not require systemic antibiotics to clear local infections.2,4
Take steps to prevent spread of the disease
Early diagnosis of CA-MRSA infection is important to prevent the spread of this highly virulent disease in the community. It’s important to cover the infected area with sterile gauze and instruct the patient to avoid direct skin-to-skin contact with others, not share sports equipment or personal items, and decontaminate linens and surfaces.4,6
Stress the importance of frequent hand washing and using alcohol-based hand rubs to reduce transmission. Adjuvant topical therapy may be useful: intra-nasal mupirocin 2% ointment to prevent colonization and a persistent carrier state is prudent. Bathing with antimicrobial povidone-iodine or chlorhexidine gluconate is often recommended as an adjunctive treatment for skin and soft tissue infections, colonization, or both.14
Mupirocin 2% for our patient
We instructed our patient to continue the initial medications and to apply mupirocin 2% ointment to the nares twice a day for 1 week to eliminate the possibility of a staphylococcal carrier state.
At follow-up 2 weeks later, only a few excoriated papules and some post-inflammatory erythema remained. The topical clindamycin lotion and the doxycycline were continued for an additional 30 days, at which time the patient was clear and treatment was discontinued.
1. Elston DM. Epidemiology and prevention of skin and soft tissue infections. Cutis 2004;73:S3-7.
2. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. Methicillin-resistant S. aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-674.
3. Cohen PR, Kurzrock R. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: An emerging clinical problem. J Am Acad Dermatol 2004;50:277-280.
4. Romero DV, Treston J, O’Sullivan AL. Hand-to-hand combat: Preventing MRSA infection. Adv Skin Wound Care 2006;19:328-333.
5. Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infec Dis 2005;11:928-930.
6. Cohen PR, Grossman ME. Management of cutaneous lesions associated with an emerging epidemic: Community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol 2004;51:132-135.
7. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976-2984.
8. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978-984.
9. Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: A retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol 2004;50:854-858.
10. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infection presenting as a periumbilical folliculitis. Cutis. 2006;77:229-231.
11. Bahrain M, Vasiliades M, Wolff M, Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant Staphylococcus aureus infections. Scand J Infect Dis 2006;38:702-707.
12. Herold B, Immergluck L, Maranan M, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998;279:593-598.
13. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis 2003;27:1257-1260.
14. Boyce JM. MRSA patient: proven methods to treat colonization and infection. J Hosp Infect 2001;48:S9-14.
A healthy 29-year-old woman presented to the clinic with pustules and boil-like lesions in the axillary areas, on the left forearm, and on the right thigh (FIGURE). She indicated that she’d had the lesions for 4 weeks. She said that she had not had any recent infections, and there was no evidence of immune compromise in her history.
We incised a pustule in the right axilla and expressed the pus. We sent the sample out for bacterial culture and sensitivity testing.
We immediately considered bacterial folliculitis and hidradenitis suppurativa as part of the differential. The acute nature of the process led us to favor a diagnosis of bacterial folliculitis.
FIGURE
Papules and papulopustules on right axilla
Would you favor this diagnosis?
What would you choose for initial empiric antibiotic treatment?
Diagnosis: MRSA folliculitis
We began empiric systemic antibiotic therapy with doxycycline 100 mg daily and topical treatment with clindamycin 1% lotion twice a day. The bacterial culture came back positive for methicillin-resistant Staphylococcus aureus (MRSA) and the organism proved to be tetracycline sensitive.
Staphylococci species have long been the most common causes of skin and soft tissue infections.1 A recent study in 11 major US cities identified MRSA as the most common cause of acute purulent skin and soft tissue infections in patients presenting to the emergency department.2 This rate, however, varied from 15% to 74% in various cities.2 Today, clinicians must consider MRSA as a potential causative agent whenever they treat a patient with a skin and soft tissue infection.
Community-acquired MRSA: Don’t expect typical risk factors
Community-acquired MRSA (CA-MRSA) cases differ from hospital-acquired MRSA (HA-MRSA) cases demographically, microbiologically, and clinically. CA-MRSA infections typically occur in young, healthy individuals without traditional MRSA risk factors such as recent hospitalization, residence in a long-term care facility, or prior antibiotic use.3,4
CA-MRSA outbreaks have been associated with participation in team sports, living in prison, dormitory, or group home settings, IV drug use, sharing personal items, and men who have sex with men.4,5 However, the prevalence of MRSA infections in patients without any recognized risk factors, like our patient, is increasing.6
CA-MRSA is genetically distinct from HA-MRSA in that it contains the Panton-Valentine leukocidin, an important virulence factor, and carries the type IV or type V SCCmec cassette, which resides at a different methicillin-resistant locus.7,8 CA-MRSA also does not demonstrate the multi-drug resistance typical of HA-MRSA, and these infections tend to be susceptible to most non-beta-lactam antibiotics.6
Is it MRSA or another pathogen?
When considering the differential diagnosis of MRSA folliculitis, consider methicillin-susceptible staphylococci species as equally likely pathogens. Gram-negative bacteria, including Pseudomonas aeruginosa, Malassezia furfur, and Candida species are less common, but notable causes of folliculitis. Other causes include eosinophilic folliculitis, non-bacterial/irritant folliculitis, pseudofolliculitis (chronic in-grown hairs), hidradenitis suppurativa, and acne vulgaris.
Use a low threshold for obtaining cultures CA-MRSA sometimes presents as an edematous, tender abscess, an expanding cellulitis, or both.9,10 These presentations, however, are neither sensitive nor specific. Cutaneous infection may also present as erythematous papules, nodules, pustules, crusted plaques, and infrequently suppurative folliculitis.3,10 Therefore, the clinician should have a low threshold to perform bacterial culture and sensitivity studies whenever a skin and soft tissue infection is suspected.
The potential complications of CA-MRSA infection, including pneumonia, sepsis, and endocarditis, can be avoided or minimized with early diagnosis and initiation of appropriate treatment.4,11,12
Consider community factors
In the past, folliculitis and other localized skin infections were traditionally caused by methicillin-susceptible Staphylococcus aureus and treated with beta-lactam antibiotics. In communities with high prevalence of CA-MRSA skin and soft tissue infections, beta-lactam antibiotics like cephalexin or dicloxacillin may no longer be appropriate.2,5 Therefore, let the prevalence of CA-MRSA in your community help guide your initial antibiotic choice while recognizing that empiric antibiotic therapy may be adjusted after cultures are available.
Good initial choices for CA-MRSA skin and soft tissue infections are trim-ethoprim/sulfamethoxazole with or without rifampin, clindamycin, gentamicin, and tetracycline,2,4,5,7 but inducible clindamycin resistance has been reported.13 if your community has a high prevalence of CA-MRSA, you may decide to begin empiric therapy that provides MRSA coverage.2 In addition, incision and drainage is necessary for adequate treatment of furuncles, carbuncles, and abscesses.5 Healthy, afebrile, immunocompetent patients without cellulitis may not require systemic antibiotics to clear local infections.2,4
Take steps to prevent spread of the disease
Early diagnosis of CA-MRSA infection is important to prevent the spread of this highly virulent disease in the community. It’s important to cover the infected area with sterile gauze and instruct the patient to avoid direct skin-to-skin contact with others, not share sports equipment or personal items, and decontaminate linens and surfaces.4,6
Stress the importance of frequent hand washing and using alcohol-based hand rubs to reduce transmission. Adjuvant topical therapy may be useful: intra-nasal mupirocin 2% ointment to prevent colonization and a persistent carrier state is prudent. Bathing with antimicrobial povidone-iodine or chlorhexidine gluconate is often recommended as an adjunctive treatment for skin and soft tissue infections, colonization, or both.14
Mupirocin 2% for our patient
We instructed our patient to continue the initial medications and to apply mupirocin 2% ointment to the nares twice a day for 1 week to eliminate the possibility of a staphylococcal carrier state.
At follow-up 2 weeks later, only a few excoriated papules and some post-inflammatory erythema remained. The topical clindamycin lotion and the doxycycline were continued for an additional 30 days, at which time the patient was clear and treatment was discontinued.
A healthy 29-year-old woman presented to the clinic with pustules and boil-like lesions in the axillary areas, on the left forearm, and on the right thigh (FIGURE). She indicated that she’d had the lesions for 4 weeks. She said that she had not had any recent infections, and there was no evidence of immune compromise in her history.
We incised a pustule in the right axilla and expressed the pus. We sent the sample out for bacterial culture and sensitivity testing.
We immediately considered bacterial folliculitis and hidradenitis suppurativa as part of the differential. The acute nature of the process led us to favor a diagnosis of bacterial folliculitis.
FIGURE
Papules and papulopustules on right axilla
Would you favor this diagnosis?
What would you choose for initial empiric antibiotic treatment?
Diagnosis: MRSA folliculitis
We began empiric systemic antibiotic therapy with doxycycline 100 mg daily and topical treatment with clindamycin 1% lotion twice a day. The bacterial culture came back positive for methicillin-resistant Staphylococcus aureus (MRSA) and the organism proved to be tetracycline sensitive.
Staphylococci species have long been the most common causes of skin and soft tissue infections.1 A recent study in 11 major US cities identified MRSA as the most common cause of acute purulent skin and soft tissue infections in patients presenting to the emergency department.2 This rate, however, varied from 15% to 74% in various cities.2 Today, clinicians must consider MRSA as a potential causative agent whenever they treat a patient with a skin and soft tissue infection.
Community-acquired MRSA: Don’t expect typical risk factors
Community-acquired MRSA (CA-MRSA) cases differ from hospital-acquired MRSA (HA-MRSA) cases demographically, microbiologically, and clinically. CA-MRSA infections typically occur in young, healthy individuals without traditional MRSA risk factors such as recent hospitalization, residence in a long-term care facility, or prior antibiotic use.3,4
CA-MRSA outbreaks have been associated with participation in team sports, living in prison, dormitory, or group home settings, IV drug use, sharing personal items, and men who have sex with men.4,5 However, the prevalence of MRSA infections in patients without any recognized risk factors, like our patient, is increasing.6
CA-MRSA is genetically distinct from HA-MRSA in that it contains the Panton-Valentine leukocidin, an important virulence factor, and carries the type IV or type V SCCmec cassette, which resides at a different methicillin-resistant locus.7,8 CA-MRSA also does not demonstrate the multi-drug resistance typical of HA-MRSA, and these infections tend to be susceptible to most non-beta-lactam antibiotics.6
Is it MRSA or another pathogen?
When considering the differential diagnosis of MRSA folliculitis, consider methicillin-susceptible staphylococci species as equally likely pathogens. Gram-negative bacteria, including Pseudomonas aeruginosa, Malassezia furfur, and Candida species are less common, but notable causes of folliculitis. Other causes include eosinophilic folliculitis, non-bacterial/irritant folliculitis, pseudofolliculitis (chronic in-grown hairs), hidradenitis suppurativa, and acne vulgaris.
Use a low threshold for obtaining cultures CA-MRSA sometimes presents as an edematous, tender abscess, an expanding cellulitis, or both.9,10 These presentations, however, are neither sensitive nor specific. Cutaneous infection may also present as erythematous papules, nodules, pustules, crusted plaques, and infrequently suppurative folliculitis.3,10 Therefore, the clinician should have a low threshold to perform bacterial culture and sensitivity studies whenever a skin and soft tissue infection is suspected.
The potential complications of CA-MRSA infection, including pneumonia, sepsis, and endocarditis, can be avoided or minimized with early diagnosis and initiation of appropriate treatment.4,11,12
Consider community factors
In the past, folliculitis and other localized skin infections were traditionally caused by methicillin-susceptible Staphylococcus aureus and treated with beta-lactam antibiotics. In communities with high prevalence of CA-MRSA skin and soft tissue infections, beta-lactam antibiotics like cephalexin or dicloxacillin may no longer be appropriate.2,5 Therefore, let the prevalence of CA-MRSA in your community help guide your initial antibiotic choice while recognizing that empiric antibiotic therapy may be adjusted after cultures are available.
Good initial choices for CA-MRSA skin and soft tissue infections are trim-ethoprim/sulfamethoxazole with or without rifampin, clindamycin, gentamicin, and tetracycline,2,4,5,7 but inducible clindamycin resistance has been reported.13 if your community has a high prevalence of CA-MRSA, you may decide to begin empiric therapy that provides MRSA coverage.2 In addition, incision and drainage is necessary for adequate treatment of furuncles, carbuncles, and abscesses.5 Healthy, afebrile, immunocompetent patients without cellulitis may not require systemic antibiotics to clear local infections.2,4
Take steps to prevent spread of the disease
Early diagnosis of CA-MRSA infection is important to prevent the spread of this highly virulent disease in the community. It’s important to cover the infected area with sterile gauze and instruct the patient to avoid direct skin-to-skin contact with others, not share sports equipment or personal items, and decontaminate linens and surfaces.4,6
Stress the importance of frequent hand washing and using alcohol-based hand rubs to reduce transmission. Adjuvant topical therapy may be useful: intra-nasal mupirocin 2% ointment to prevent colonization and a persistent carrier state is prudent. Bathing with antimicrobial povidone-iodine or chlorhexidine gluconate is often recommended as an adjunctive treatment for skin and soft tissue infections, colonization, or both.14
Mupirocin 2% for our patient
We instructed our patient to continue the initial medications and to apply mupirocin 2% ointment to the nares twice a day for 1 week to eliminate the possibility of a staphylococcal carrier state.
At follow-up 2 weeks later, only a few excoriated papules and some post-inflammatory erythema remained. The topical clindamycin lotion and the doxycycline were continued for an additional 30 days, at which time the patient was clear and treatment was discontinued.
1. Elston DM. Epidemiology and prevention of skin and soft tissue infections. Cutis 2004;73:S3-7.
2. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. Methicillin-resistant S. aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-674.
3. Cohen PR, Kurzrock R. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: An emerging clinical problem. J Am Acad Dermatol 2004;50:277-280.
4. Romero DV, Treston J, O’Sullivan AL. Hand-to-hand combat: Preventing MRSA infection. Adv Skin Wound Care 2006;19:328-333.
5. Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infec Dis 2005;11:928-930.
6. Cohen PR, Grossman ME. Management of cutaneous lesions associated with an emerging epidemic: Community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol 2004;51:132-135.
7. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976-2984.
8. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978-984.
9. Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: A retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol 2004;50:854-858.
10. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infection presenting as a periumbilical folliculitis. Cutis. 2006;77:229-231.
11. Bahrain M, Vasiliades M, Wolff M, Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant Staphylococcus aureus infections. Scand J Infect Dis 2006;38:702-707.
12. Herold B, Immergluck L, Maranan M, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998;279:593-598.
13. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis 2003;27:1257-1260.
14. Boyce JM. MRSA patient: proven methods to treat colonization and infection. J Hosp Infect 2001;48:S9-14.
1. Elston DM. Epidemiology and prevention of skin and soft tissue infections. Cutis 2004;73:S3-7.
2. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. Methicillin-resistant S. aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-674.
3. Cohen PR, Kurzrock R. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: An emerging clinical problem. J Am Acad Dermatol 2004;50:277-280.
4. Romero DV, Treston J, O’Sullivan AL. Hand-to-hand combat: Preventing MRSA infection. Adv Skin Wound Care 2006;19:328-333.
5. Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infec Dis 2005;11:928-930.
6. Cohen PR, Grossman ME. Management of cutaneous lesions associated with an emerging epidemic: Community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol 2004;51:132-135.
7. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976-2984.
8. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978-984.
9. Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: A retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol 2004;50:854-858.
10. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infection presenting as a periumbilical folliculitis. Cutis. 2006;77:229-231.
11. Bahrain M, Vasiliades M, Wolff M, Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant Staphylococcus aureus infections. Scand J Infect Dis 2006;38:702-707.
12. Herold B, Immergluck L, Maranan M, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998;279:593-598.
13. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis 2003;27:1257-1260.
14. Boyce JM. MRSA patient: proven methods to treat colonization and infection. J Hosp Infect 2001;48:S9-14.