User login
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
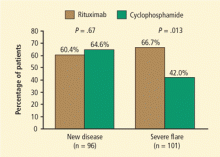
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
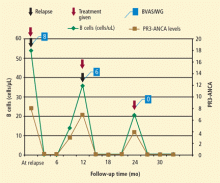
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584