User login
Controversies in ANCA testing
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
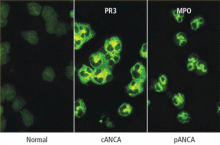
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
Biologic agents in the treatment of granulomatosis with polyangiitis
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
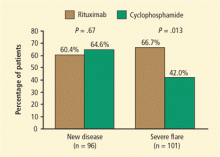
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
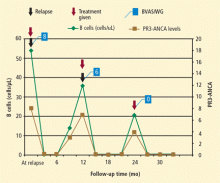
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584