User login
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
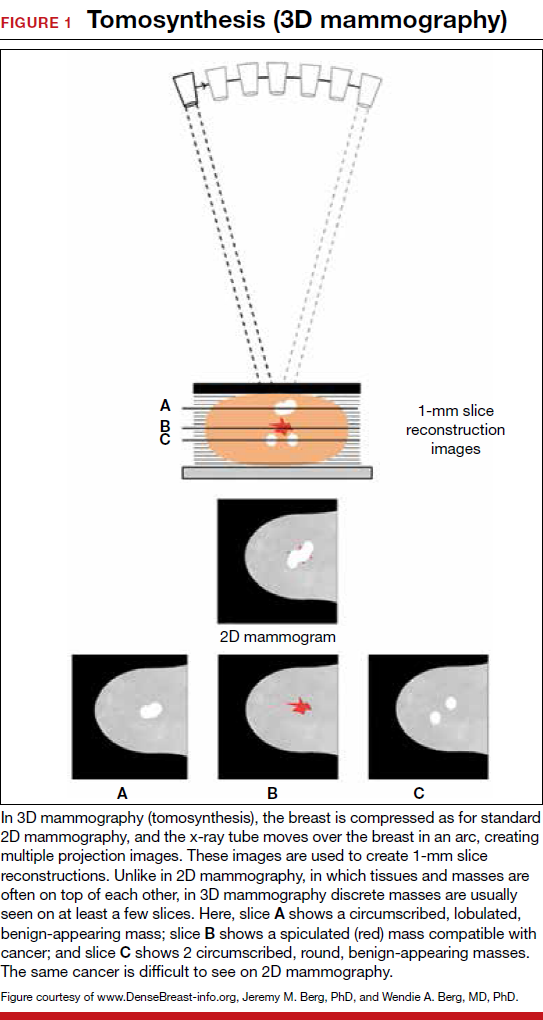
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.
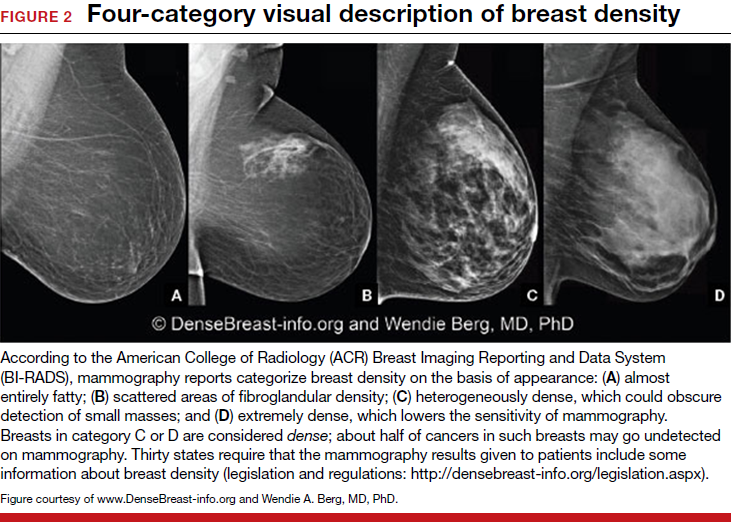
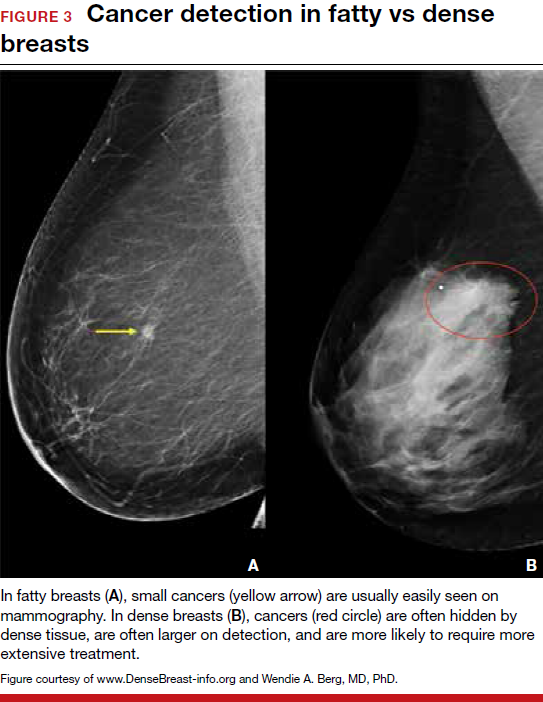
Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).
3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts
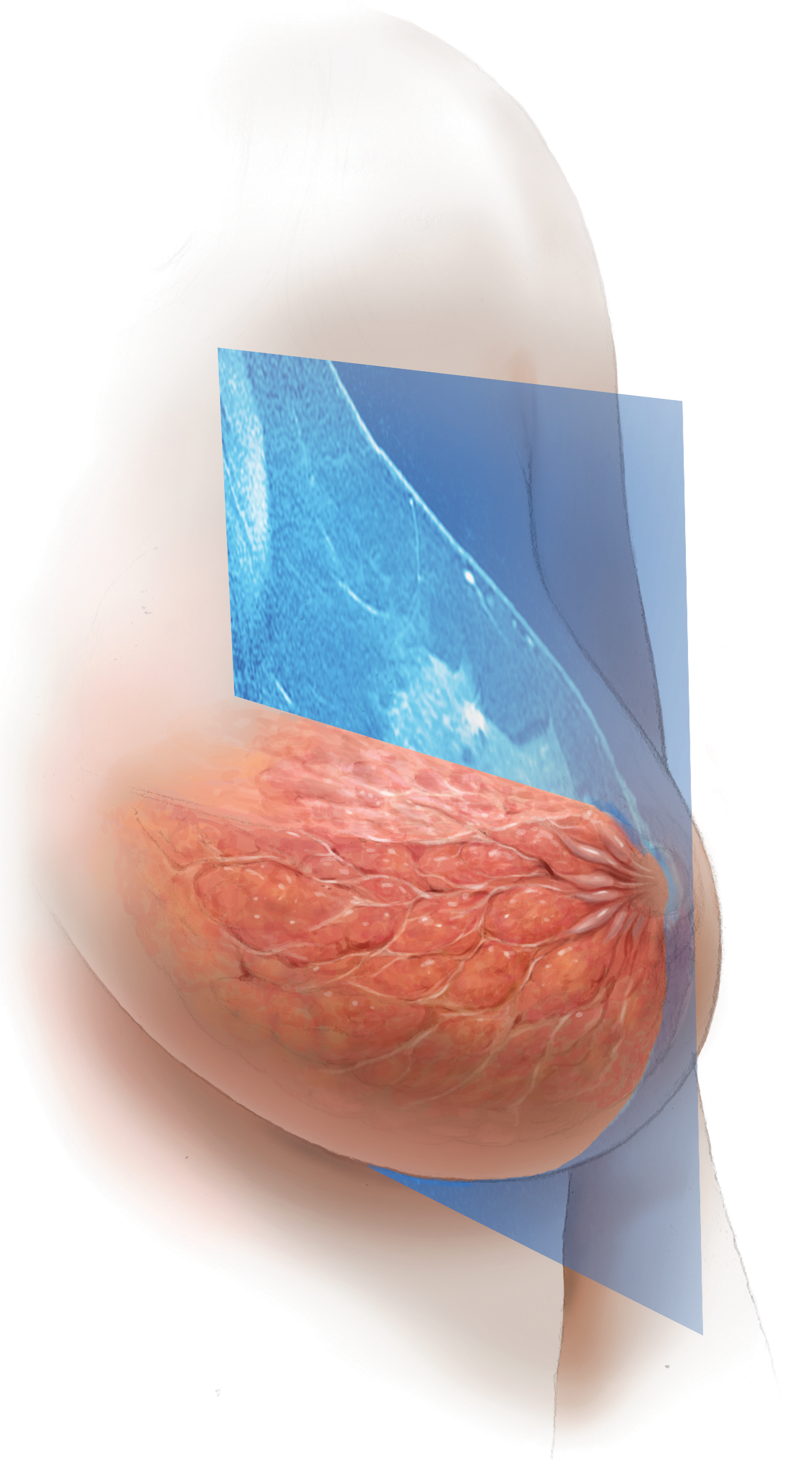
MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.

FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.

Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.

FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.

Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.

FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.

Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.