User login
A 19-YEAR-OLD NULLIPAROUS CAUCASIAN WOMAN came into our family practice clinic; she was concerned about redness and swelling that had developed in her left breast 2 weeks earlier. She’d gone to a local emergency department 8 days ago with similar complaints, and was treated with azithromycin and cephalexin for presumptive mastitis and possible cat scratch disease. Despite the antibiotics, she said that her symptoms had worsened; she’d developed a dry cough, dyspnea, general malaise, and a fever of 100.6°F.
The patient was a former smoker and had asthma as a child. She said her mother had been diagnosed with premenopausal breast cancer at age 31. She also indicated that she had a cat and that it might have scratched her arm prior to the onset of symptoms.
On exam, the patient was afebrile but tachycardic at 140 beats per minute. She had left axillary lymphadenopathy. Her left breast was indurated, erythematous, and generally edematous (FIGURE 1). There was no nipple discharge or evidence of trauma to the skin. The patient had decreased breath sounds at the bases.
A breast ultrasound (obtained to evaluate for possible abscess) showed only diffuse edema—but no abscess. A chest x-ray (FIGURE 2). showed an anterior and middle mediastinal soft tissue mass with a left-sided pleural effusion.
The patient was admitted to the hospital for further evaluation.
FIGURE 1
Induration, erythema, and diffuse edema of left breast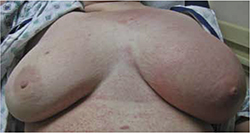
FIGURE 2
Chest x-ray reveals left pleural effusion, mediastinal mass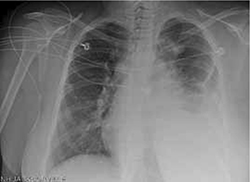
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diffuse large B-cell lymphoma
This patient had Stage IV-B diffuse large B-cell lymphoma (DLBCL), diagnosed by computed tomography (CT)-guided biopsy of the mediastinal mass. DLBCL is the most common histological subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of NHL cases.1,2
Patients with DLBCL typically present with a rapidly enlarging mass in the neck or abdomen. Thirty percent of patients will have systemic “B” symptoms, including fever, weight loss, and night sweats.3 DLBCL can be highly invasive and may cause compression of the airway and lymphatic or circulatory vessels. While dysphagia, hoarseness, breast swelling, chest pain, and cough can be among the presenting symptoms, superior vena cava syndrome is the most common complication and occurs in 30% of patients.3 The lymphatic obstruction seen in lymphoproliferative disease can produce lymphedema.
In this patient, diffuse lymphadenopathy in the subpectoral and axillary region caused lymphatic obstruction and breast edema. Extra-nodal disease occurs in up to one-third of cases.4 DLBCL can be involved in virtually any tissue, including breast, bone, testes, skin, liver, central nervous system, uterus, and gastrointestinal tract.5
The National Cancer Institute reports that between 2004 and 2008, the US age-adjusted incidence rate for non-Hodgkin lymphoma was 19.8 per 100,000 men and women per year.6 Incidence varies by ethnicity (Caucasians have the highest rate) and increases with age (median age at diagnosis is 66 years with a male predominance).6
Consider infection early on in the differential
The differential diagnosis for unilateral breast swelling and lymphadenopathy includes infection and malignancies. Potential infectious etiologies include acute bacterial mastitis, cat scratch disease, and breast abcess. Malignant causes include inflammatory breast cancer and primary malignant lymphoma. Primary malignant lymphoma of the breast is rare, with an estimated prevalence of 0.04% to 1.1% of all breast tumors and 1.7% to 2.2% of all extranodal non-Hodgkin lymphomas.7
Initial evaluation should be directed toward the most likely etiologies of the symptoms and should include breast ultrasound, blood cultures, and serologies—such as Bartonella—as history dictates.
Once more common causes of unilateral breast swelling are excluded, less common etiologies, such as duct ectasia, mammary adenosis or fat necrosis, and blunt force trauma, should be considered.
Due to the age of our patient, mastitis was initially high on the differential; however, when she did not respond to antibiotic therapy, other causes for her symptoms had to be considered. An ultrasound (noted earlier) ruled out a breast abscess. Blood cultures and serologies were negative. Punch skin biopsies of the patient’s breast showed no evidence of carcinoma, making inflammatory breast cancer less likely.
Imaging studies to start, then biopsy
Many imaging modalities can aid in the diagnosis of DLBCL and help determine the extent of involvement for staging of the disease. These modalities include plain radiograph; CT, magnetic resonance imaging, and positron emission tomography (PET) scans; and lymphangiograms. Confirmatory diagnosis of DLBCL is best made by excisional tissue biopsy. Bone marrow biopsy is also used to help determine staging and management after initial diagnosis.8
Survival hinges on chemotherapy
Survival without treatment in patients with aggressive DLBCL can be measured in months. Combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (the CHOP regimen) increases the disease-free survival rate to between 35% and 45% at 4 years.9 The addition of rituximab has further increased survival in adult patients.10 While the optimal number of treatment cycles remains unclear, 6 to 8 cycles are typically given prior to PET imaging to assess for response.
Consider radiation. Some patients benefit from the addition of radiation to their chemotherapy regimen—particularly those with bulky disease.1 After completion of the planned treatment of DLBCL, a one-month follow-up physical exam with labs and a 2-month follow-up PET/CT scan should be obtained to evaluate response.
It took many scans and tests to arrive at a Dx
The patient’s CT scan showed a large anterior mediastinal mass with central necrosis, diffuse lymphadenopathy, a large left-sided pleural effusion, and multiple pulmonary nodules. Cytologic evaluation of pleural fluid did not show evidence of carcinoma. A fine needle aspiration of the substernal mass under radiologic guidance was performed, and histology was consistent with DLBCL. Further testing confirmed that disease was present both above and below the diaphragm, leading to the diagnosis of Stage IV-B DLBCL.
Our patient. The patient was treated with a chemotherapy regimen of rituximab-cyclophosphamide, vincristine, prednisone, and doxorubicin every 2 weeks for a total of 5 cycles.
Vincristine was discontinued after 2 weeks when the patient developed a Grade 2 peripheral neuropathy at her fingertips. The patient is currently in remission.
CORRESPONDENCE
Larissa Buccolo, MD, Naval Hospital Family Practice Clinic, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Nguyen LN, Ha CS, Hess M, et al. The outcome of combined-modality treatments for stage I and II primary large B-cell lymphoma of the mediastinum. Int J Radiat Oncol Biol Phys. 2000;47:1281-1285.
2. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124:151-159.
3. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphoma: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795.
4. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999;22:357-364.
5. Aviles A, Neri N, Huerta-Guzman J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol. 2002;80:111-115.
6. National Cancer Institute. Surveillance epidemiology and end results. SEER stat fact sheets: non-Hodgkin lymphoma. Available at: (http://seer.cancer.gov/statfacts/html/nhl.html.) Accessed September 28, 2011.
7. Gholam D, Bibeau F, El Welshi A, et al. Primary breast lymphoma. Leuk Lymphoma. 2003;44:1173-1178.
8. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-1006.
9. Pond GD, Castellini RA, Horning S, et al. Non-Hodgkin lymphoma: influence of lymphography, CT, and bone marrow biopsy on staging and management. Radiology. 1989;170:159-164.
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027-5033.
A 19-YEAR-OLD NULLIPAROUS CAUCASIAN WOMAN came into our family practice clinic; she was concerned about redness and swelling that had developed in her left breast 2 weeks earlier. She’d gone to a local emergency department 8 days ago with similar complaints, and was treated with azithromycin and cephalexin for presumptive mastitis and possible cat scratch disease. Despite the antibiotics, she said that her symptoms had worsened; she’d developed a dry cough, dyspnea, general malaise, and a fever of 100.6°F.
The patient was a former smoker and had asthma as a child. She said her mother had been diagnosed with premenopausal breast cancer at age 31. She also indicated that she had a cat and that it might have scratched her arm prior to the onset of symptoms.
On exam, the patient was afebrile but tachycardic at 140 beats per minute. She had left axillary lymphadenopathy. Her left breast was indurated, erythematous, and generally edematous (FIGURE 1). There was no nipple discharge or evidence of trauma to the skin. The patient had decreased breath sounds at the bases.
A breast ultrasound (obtained to evaluate for possible abscess) showed only diffuse edema—but no abscess. A chest x-ray (FIGURE 2). showed an anterior and middle mediastinal soft tissue mass with a left-sided pleural effusion.
The patient was admitted to the hospital for further evaluation.
FIGURE 1
Induration, erythema, and diffuse edema of left breast
FIGURE 2
Chest x-ray reveals left pleural effusion, mediastinal mass
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diffuse large B-cell lymphoma
This patient had Stage IV-B diffuse large B-cell lymphoma (DLBCL), diagnosed by computed tomography (CT)-guided biopsy of the mediastinal mass. DLBCL is the most common histological subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of NHL cases.1,2
Patients with DLBCL typically present with a rapidly enlarging mass in the neck or abdomen. Thirty percent of patients will have systemic “B” symptoms, including fever, weight loss, and night sweats.3 DLBCL can be highly invasive and may cause compression of the airway and lymphatic or circulatory vessels. While dysphagia, hoarseness, breast swelling, chest pain, and cough can be among the presenting symptoms, superior vena cava syndrome is the most common complication and occurs in 30% of patients.3 The lymphatic obstruction seen in lymphoproliferative disease can produce lymphedema.
In this patient, diffuse lymphadenopathy in the subpectoral and axillary region caused lymphatic obstruction and breast edema. Extra-nodal disease occurs in up to one-third of cases.4 DLBCL can be involved in virtually any tissue, including breast, bone, testes, skin, liver, central nervous system, uterus, and gastrointestinal tract.5
The National Cancer Institute reports that between 2004 and 2008, the US age-adjusted incidence rate for non-Hodgkin lymphoma was 19.8 per 100,000 men and women per year.6 Incidence varies by ethnicity (Caucasians have the highest rate) and increases with age (median age at diagnosis is 66 years with a male predominance).6
Consider infection early on in the differential
The differential diagnosis for unilateral breast swelling and lymphadenopathy includes infection and malignancies. Potential infectious etiologies include acute bacterial mastitis, cat scratch disease, and breast abcess. Malignant causes include inflammatory breast cancer and primary malignant lymphoma. Primary malignant lymphoma of the breast is rare, with an estimated prevalence of 0.04% to 1.1% of all breast tumors and 1.7% to 2.2% of all extranodal non-Hodgkin lymphomas.7
Initial evaluation should be directed toward the most likely etiologies of the symptoms and should include breast ultrasound, blood cultures, and serologies—such as Bartonella—as history dictates.
Once more common causes of unilateral breast swelling are excluded, less common etiologies, such as duct ectasia, mammary adenosis or fat necrosis, and blunt force trauma, should be considered.
Due to the age of our patient, mastitis was initially high on the differential; however, when she did not respond to antibiotic therapy, other causes for her symptoms had to be considered. An ultrasound (noted earlier) ruled out a breast abscess. Blood cultures and serologies were negative. Punch skin biopsies of the patient’s breast showed no evidence of carcinoma, making inflammatory breast cancer less likely.
Imaging studies to start, then biopsy
Many imaging modalities can aid in the diagnosis of DLBCL and help determine the extent of involvement for staging of the disease. These modalities include plain radiograph; CT, magnetic resonance imaging, and positron emission tomography (PET) scans; and lymphangiograms. Confirmatory diagnosis of DLBCL is best made by excisional tissue biopsy. Bone marrow biopsy is also used to help determine staging and management after initial diagnosis.8
Survival hinges on chemotherapy
Survival without treatment in patients with aggressive DLBCL can be measured in months. Combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (the CHOP regimen) increases the disease-free survival rate to between 35% and 45% at 4 years.9 The addition of rituximab has further increased survival in adult patients.10 While the optimal number of treatment cycles remains unclear, 6 to 8 cycles are typically given prior to PET imaging to assess for response.
Consider radiation. Some patients benefit from the addition of radiation to their chemotherapy regimen—particularly those with bulky disease.1 After completion of the planned treatment of DLBCL, a one-month follow-up physical exam with labs and a 2-month follow-up PET/CT scan should be obtained to evaluate response.
It took many scans and tests to arrive at a Dx
The patient’s CT scan showed a large anterior mediastinal mass with central necrosis, diffuse lymphadenopathy, a large left-sided pleural effusion, and multiple pulmonary nodules. Cytologic evaluation of pleural fluid did not show evidence of carcinoma. A fine needle aspiration of the substernal mass under radiologic guidance was performed, and histology was consistent with DLBCL. Further testing confirmed that disease was present both above and below the diaphragm, leading to the diagnosis of Stage IV-B DLBCL.
Our patient. The patient was treated with a chemotherapy regimen of rituximab-cyclophosphamide, vincristine, prednisone, and doxorubicin every 2 weeks for a total of 5 cycles.
Vincristine was discontinued after 2 weeks when the patient developed a Grade 2 peripheral neuropathy at her fingertips. The patient is currently in remission.
CORRESPONDENCE
Larissa Buccolo, MD, Naval Hospital Family Practice Clinic, 2080 Child Street, Jacksonville, FL 32214; [email protected]
A 19-YEAR-OLD NULLIPAROUS CAUCASIAN WOMAN came into our family practice clinic; she was concerned about redness and swelling that had developed in her left breast 2 weeks earlier. She’d gone to a local emergency department 8 days ago with similar complaints, and was treated with azithromycin and cephalexin for presumptive mastitis and possible cat scratch disease. Despite the antibiotics, she said that her symptoms had worsened; she’d developed a dry cough, dyspnea, general malaise, and a fever of 100.6°F.
The patient was a former smoker and had asthma as a child. She said her mother had been diagnosed with premenopausal breast cancer at age 31. She also indicated that she had a cat and that it might have scratched her arm prior to the onset of symptoms.
On exam, the patient was afebrile but tachycardic at 140 beats per minute. She had left axillary lymphadenopathy. Her left breast was indurated, erythematous, and generally edematous (FIGURE 1). There was no nipple discharge or evidence of trauma to the skin. The patient had decreased breath sounds at the bases.
A breast ultrasound (obtained to evaluate for possible abscess) showed only diffuse edema—but no abscess. A chest x-ray (FIGURE 2). showed an anterior and middle mediastinal soft tissue mass with a left-sided pleural effusion.
The patient was admitted to the hospital for further evaluation.
FIGURE 1
Induration, erythema, and diffuse edema of left breast
FIGURE 2
Chest x-ray reveals left pleural effusion, mediastinal mass
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diffuse large B-cell lymphoma
This patient had Stage IV-B diffuse large B-cell lymphoma (DLBCL), diagnosed by computed tomography (CT)-guided biopsy of the mediastinal mass. DLBCL is the most common histological subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of NHL cases.1,2
Patients with DLBCL typically present with a rapidly enlarging mass in the neck or abdomen. Thirty percent of patients will have systemic “B” symptoms, including fever, weight loss, and night sweats.3 DLBCL can be highly invasive and may cause compression of the airway and lymphatic or circulatory vessels. While dysphagia, hoarseness, breast swelling, chest pain, and cough can be among the presenting symptoms, superior vena cava syndrome is the most common complication and occurs in 30% of patients.3 The lymphatic obstruction seen in lymphoproliferative disease can produce lymphedema.
In this patient, diffuse lymphadenopathy in the subpectoral and axillary region caused lymphatic obstruction and breast edema. Extra-nodal disease occurs in up to one-third of cases.4 DLBCL can be involved in virtually any tissue, including breast, bone, testes, skin, liver, central nervous system, uterus, and gastrointestinal tract.5
The National Cancer Institute reports that between 2004 and 2008, the US age-adjusted incidence rate for non-Hodgkin lymphoma was 19.8 per 100,000 men and women per year.6 Incidence varies by ethnicity (Caucasians have the highest rate) and increases with age (median age at diagnosis is 66 years with a male predominance).6
Consider infection early on in the differential
The differential diagnosis for unilateral breast swelling and lymphadenopathy includes infection and malignancies. Potential infectious etiologies include acute bacterial mastitis, cat scratch disease, and breast abcess. Malignant causes include inflammatory breast cancer and primary malignant lymphoma. Primary malignant lymphoma of the breast is rare, with an estimated prevalence of 0.04% to 1.1% of all breast tumors and 1.7% to 2.2% of all extranodal non-Hodgkin lymphomas.7
Initial evaluation should be directed toward the most likely etiologies of the symptoms and should include breast ultrasound, blood cultures, and serologies—such as Bartonella—as history dictates.
Once more common causes of unilateral breast swelling are excluded, less common etiologies, such as duct ectasia, mammary adenosis or fat necrosis, and blunt force trauma, should be considered.
Due to the age of our patient, mastitis was initially high on the differential; however, when she did not respond to antibiotic therapy, other causes for her symptoms had to be considered. An ultrasound (noted earlier) ruled out a breast abscess. Blood cultures and serologies were negative. Punch skin biopsies of the patient’s breast showed no evidence of carcinoma, making inflammatory breast cancer less likely.
Imaging studies to start, then biopsy
Many imaging modalities can aid in the diagnosis of DLBCL and help determine the extent of involvement for staging of the disease. These modalities include plain radiograph; CT, magnetic resonance imaging, and positron emission tomography (PET) scans; and lymphangiograms. Confirmatory diagnosis of DLBCL is best made by excisional tissue biopsy. Bone marrow biopsy is also used to help determine staging and management after initial diagnosis.8
Survival hinges on chemotherapy
Survival without treatment in patients with aggressive DLBCL can be measured in months. Combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (the CHOP regimen) increases the disease-free survival rate to between 35% and 45% at 4 years.9 The addition of rituximab has further increased survival in adult patients.10 While the optimal number of treatment cycles remains unclear, 6 to 8 cycles are typically given prior to PET imaging to assess for response.
Consider radiation. Some patients benefit from the addition of radiation to their chemotherapy regimen—particularly those with bulky disease.1 After completion of the planned treatment of DLBCL, a one-month follow-up physical exam with labs and a 2-month follow-up PET/CT scan should be obtained to evaluate response.
It took many scans and tests to arrive at a Dx
The patient’s CT scan showed a large anterior mediastinal mass with central necrosis, diffuse lymphadenopathy, a large left-sided pleural effusion, and multiple pulmonary nodules. Cytologic evaluation of pleural fluid did not show evidence of carcinoma. A fine needle aspiration of the substernal mass under radiologic guidance was performed, and histology was consistent with DLBCL. Further testing confirmed that disease was present both above and below the diaphragm, leading to the diagnosis of Stage IV-B DLBCL.
Our patient. The patient was treated with a chemotherapy regimen of rituximab-cyclophosphamide, vincristine, prednisone, and doxorubicin every 2 weeks for a total of 5 cycles.
Vincristine was discontinued after 2 weeks when the patient developed a Grade 2 peripheral neuropathy at her fingertips. The patient is currently in remission.
CORRESPONDENCE
Larissa Buccolo, MD, Naval Hospital Family Practice Clinic, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Nguyen LN, Ha CS, Hess M, et al. The outcome of combined-modality treatments for stage I and II primary large B-cell lymphoma of the mediastinum. Int J Radiat Oncol Biol Phys. 2000;47:1281-1285.
2. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124:151-159.
3. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphoma: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795.
4. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999;22:357-364.
5. Aviles A, Neri N, Huerta-Guzman J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol. 2002;80:111-115.
6. National Cancer Institute. Surveillance epidemiology and end results. SEER stat fact sheets: non-Hodgkin lymphoma. Available at: (http://seer.cancer.gov/statfacts/html/nhl.html.) Accessed September 28, 2011.
7. Gholam D, Bibeau F, El Welshi A, et al. Primary breast lymphoma. Leuk Lymphoma. 2003;44:1173-1178.
8. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-1006.
9. Pond GD, Castellini RA, Horning S, et al. Non-Hodgkin lymphoma: influence of lymphography, CT, and bone marrow biopsy on staging and management. Radiology. 1989;170:159-164.
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027-5033.
1. Nguyen LN, Ha CS, Hess M, et al. The outcome of combined-modality treatments for stage I and II primary large B-cell lymphoma of the mediastinum. Int J Radiat Oncol Biol Phys. 2000;47:1281-1285.
2. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124:151-159.
3. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphoma: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795.
4. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999;22:357-364.
5. Aviles A, Neri N, Huerta-Guzman J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol. 2002;80:111-115.
6. National Cancer Institute. Surveillance epidemiology and end results. SEER stat fact sheets: non-Hodgkin lymphoma. Available at: (http://seer.cancer.gov/statfacts/html/nhl.html.) Accessed September 28, 2011.
7. Gholam D, Bibeau F, El Welshi A, et al. Primary breast lymphoma. Leuk Lymphoma. 2003;44:1173-1178.
8. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-1006.
9. Pond GD, Castellini RA, Horning S, et al. Non-Hodgkin lymphoma: influence of lymphography, CT, and bone marrow biopsy on staging and management. Radiology. 1989;170:159-164.
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027-5033.