User login
Breast swelling and erythema in a teen
A 19-YEAR-OLD NULLIPAROUS CAUCASIAN WOMAN came into our family practice clinic; she was concerned about redness and swelling that had developed in her left breast 2 weeks earlier. She’d gone to a local emergency department 8 days ago with similar complaints, and was treated with azithromycin and cephalexin for presumptive mastitis and possible cat scratch disease. Despite the antibiotics, she said that her symptoms had worsened; she’d developed a dry cough, dyspnea, general malaise, and a fever of 100.6°F.
The patient was a former smoker and had asthma as a child. She said her mother had been diagnosed with premenopausal breast cancer at age 31. She also indicated that she had a cat and that it might have scratched her arm prior to the onset of symptoms.
On exam, the patient was afebrile but tachycardic at 140 beats per minute. She had left axillary lymphadenopathy. Her left breast was indurated, erythematous, and generally edematous (FIGURE 1). There was no nipple discharge or evidence of trauma to the skin. The patient had decreased breath sounds at the bases.
A breast ultrasound (obtained to evaluate for possible abscess) showed only diffuse edema—but no abscess. A chest x-ray (FIGURE 2). showed an anterior and middle mediastinal soft tissue mass with a left-sided pleural effusion.
The patient was admitted to the hospital for further evaluation.
FIGURE 1
Induration, erythema, and diffuse edema of left breast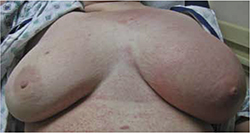
FIGURE 2
Chest x-ray reveals left pleural effusion, mediastinal mass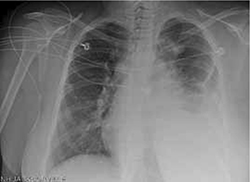
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diffuse large B-cell lymphoma
This patient had Stage IV-B diffuse large B-cell lymphoma (DLBCL), diagnosed by computed tomography (CT)-guided biopsy of the mediastinal mass. DLBCL is the most common histological subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of NHL cases.1,2
Patients with DLBCL typically present with a rapidly enlarging mass in the neck or abdomen. Thirty percent of patients will have systemic “B” symptoms, including fever, weight loss, and night sweats.3 DLBCL can be highly invasive and may cause compression of the airway and lymphatic or circulatory vessels. While dysphagia, hoarseness, breast swelling, chest pain, and cough can be among the presenting symptoms, superior vena cava syndrome is the most common complication and occurs in 30% of patients.3 The lymphatic obstruction seen in lymphoproliferative disease can produce lymphedema.
In this patient, diffuse lymphadenopathy in the subpectoral and axillary region caused lymphatic obstruction and breast edema. Extra-nodal disease occurs in up to one-third of cases.4 DLBCL can be involved in virtually any tissue, including breast, bone, testes, skin, liver, central nervous system, uterus, and gastrointestinal tract.5
The National Cancer Institute reports that between 2004 and 2008, the US age-adjusted incidence rate for non-Hodgkin lymphoma was 19.8 per 100,000 men and women per year.6 Incidence varies by ethnicity (Caucasians have the highest rate) and increases with age (median age at diagnosis is 66 years with a male predominance).6
Consider infection early on in the differential
The differential diagnosis for unilateral breast swelling and lymphadenopathy includes infection and malignancies. Potential infectious etiologies include acute bacterial mastitis, cat scratch disease, and breast abcess. Malignant causes include inflammatory breast cancer and primary malignant lymphoma. Primary malignant lymphoma of the breast is rare, with an estimated prevalence of 0.04% to 1.1% of all breast tumors and 1.7% to 2.2% of all extranodal non-Hodgkin lymphomas.7
Initial evaluation should be directed toward the most likely etiologies of the symptoms and should include breast ultrasound, blood cultures, and serologies—such as Bartonella—as history dictates.
Once more common causes of unilateral breast swelling are excluded, less common etiologies, such as duct ectasia, mammary adenosis or fat necrosis, and blunt force trauma, should be considered.
Due to the age of our patient, mastitis was initially high on the differential; however, when she did not respond to antibiotic therapy, other causes for her symptoms had to be considered. An ultrasound (noted earlier) ruled out a breast abscess. Blood cultures and serologies were negative. Punch skin biopsies of the patient’s breast showed no evidence of carcinoma, making inflammatory breast cancer less likely.
Imaging studies to start, then biopsy
Many imaging modalities can aid in the diagnosis of DLBCL and help determine the extent of involvement for staging of the disease. These modalities include plain radiograph; CT, magnetic resonance imaging, and positron emission tomography (PET) scans; and lymphangiograms. Confirmatory diagnosis of DLBCL is best made by excisional tissue biopsy. Bone marrow biopsy is also used to help determine staging and management after initial diagnosis.8
Survival hinges on chemotherapy
Survival without treatment in patients with aggressive DLBCL can be measured in months. Combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (the CHOP regimen) increases the disease-free survival rate to between 35% and 45% at 4 years.9 The addition of rituximab has further increased survival in adult patients.10 While the optimal number of treatment cycles remains unclear, 6 to 8 cycles are typically given prior to PET imaging to assess for response.
Consider radiation. Some patients benefit from the addition of radiation to their chemotherapy regimen—particularly those with bulky disease.1 After completion of the planned treatment of DLBCL, a one-month follow-up physical exam with labs and a 2-month follow-up PET/CT scan should be obtained to evaluate response.
It took many scans and tests to arrive at a Dx
The patient’s CT scan showed a large anterior mediastinal mass with central necrosis, diffuse lymphadenopathy, a large left-sided pleural effusion, and multiple pulmonary nodules. Cytologic evaluation of pleural fluid did not show evidence of carcinoma. A fine needle aspiration of the substernal mass under radiologic guidance was performed, and histology was consistent with DLBCL. Further testing confirmed that disease was present both above and below the diaphragm, leading to the diagnosis of Stage IV-B DLBCL.
Our patient. The patient was treated with a chemotherapy regimen of rituximab-cyclophosphamide, vincristine, prednisone, and doxorubicin every 2 weeks for a total of 5 cycles.
Vincristine was discontinued after 2 weeks when the patient developed a Grade 2 peripheral neuropathy at her fingertips. The patient is currently in remission.
CORRESPONDENCE
Larissa Buccolo, MD, Naval Hospital Family Practice Clinic, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Nguyen LN, Ha CS, Hess M, et al. The outcome of combined-modality treatments for stage I and II primary large B-cell lymphoma of the mediastinum. Int J Radiat Oncol Biol Phys. 2000;47:1281-1285.
2. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124:151-159.
3. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphoma: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795.
4. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999;22:357-364.
5. Aviles A, Neri N, Huerta-Guzman J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol. 2002;80:111-115.
6. National Cancer Institute. Surveillance epidemiology and end results. SEER stat fact sheets: non-Hodgkin lymphoma. Available at: (http://seer.cancer.gov/statfacts/html/nhl.html.) Accessed September 28, 2011.
7. Gholam D, Bibeau F, El Welshi A, et al. Primary breast lymphoma. Leuk Lymphoma. 2003;44:1173-1178.
8. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-1006.
9. Pond GD, Castellini RA, Horning S, et al. Non-Hodgkin lymphoma: influence of lymphography, CT, and bone marrow biopsy on staging and management. Radiology. 1989;170:159-164.
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027-5033.
A 19-YEAR-OLD NULLIPAROUS CAUCASIAN WOMAN came into our family practice clinic; she was concerned about redness and swelling that had developed in her left breast 2 weeks earlier. She’d gone to a local emergency department 8 days ago with similar complaints, and was treated with azithromycin and cephalexin for presumptive mastitis and possible cat scratch disease. Despite the antibiotics, she said that her symptoms had worsened; she’d developed a dry cough, dyspnea, general malaise, and a fever of 100.6°F.
The patient was a former smoker and had asthma as a child. She said her mother had been diagnosed with premenopausal breast cancer at age 31. She also indicated that she had a cat and that it might have scratched her arm prior to the onset of symptoms.
On exam, the patient was afebrile but tachycardic at 140 beats per minute. She had left axillary lymphadenopathy. Her left breast was indurated, erythematous, and generally edematous (FIGURE 1). There was no nipple discharge or evidence of trauma to the skin. The patient had decreased breath sounds at the bases.
A breast ultrasound (obtained to evaluate for possible abscess) showed only diffuse edema—but no abscess. A chest x-ray (FIGURE 2). showed an anterior and middle mediastinal soft tissue mass with a left-sided pleural effusion.
The patient was admitted to the hospital for further evaluation.
FIGURE 1
Induration, erythema, and diffuse edema of left breast
FIGURE 2
Chest x-ray reveals left pleural effusion, mediastinal mass
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diffuse large B-cell lymphoma
This patient had Stage IV-B diffuse large B-cell lymphoma (DLBCL), diagnosed by computed tomography (CT)-guided biopsy of the mediastinal mass. DLBCL is the most common histological subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of NHL cases.1,2
Patients with DLBCL typically present with a rapidly enlarging mass in the neck or abdomen. Thirty percent of patients will have systemic “B” symptoms, including fever, weight loss, and night sweats.3 DLBCL can be highly invasive and may cause compression of the airway and lymphatic or circulatory vessels. While dysphagia, hoarseness, breast swelling, chest pain, and cough can be among the presenting symptoms, superior vena cava syndrome is the most common complication and occurs in 30% of patients.3 The lymphatic obstruction seen in lymphoproliferative disease can produce lymphedema.
In this patient, diffuse lymphadenopathy in the subpectoral and axillary region caused lymphatic obstruction and breast edema. Extra-nodal disease occurs in up to one-third of cases.4 DLBCL can be involved in virtually any tissue, including breast, bone, testes, skin, liver, central nervous system, uterus, and gastrointestinal tract.5
The National Cancer Institute reports that between 2004 and 2008, the US age-adjusted incidence rate for non-Hodgkin lymphoma was 19.8 per 100,000 men and women per year.6 Incidence varies by ethnicity (Caucasians have the highest rate) and increases with age (median age at diagnosis is 66 years with a male predominance).6
Consider infection early on in the differential
The differential diagnosis for unilateral breast swelling and lymphadenopathy includes infection and malignancies. Potential infectious etiologies include acute bacterial mastitis, cat scratch disease, and breast abcess. Malignant causes include inflammatory breast cancer and primary malignant lymphoma. Primary malignant lymphoma of the breast is rare, with an estimated prevalence of 0.04% to 1.1% of all breast tumors and 1.7% to 2.2% of all extranodal non-Hodgkin lymphomas.7
Initial evaluation should be directed toward the most likely etiologies of the symptoms and should include breast ultrasound, blood cultures, and serologies—such as Bartonella—as history dictates.
Once more common causes of unilateral breast swelling are excluded, less common etiologies, such as duct ectasia, mammary adenosis or fat necrosis, and blunt force trauma, should be considered.
Due to the age of our patient, mastitis was initially high on the differential; however, when she did not respond to antibiotic therapy, other causes for her symptoms had to be considered. An ultrasound (noted earlier) ruled out a breast abscess. Blood cultures and serologies were negative. Punch skin biopsies of the patient’s breast showed no evidence of carcinoma, making inflammatory breast cancer less likely.
Imaging studies to start, then biopsy
Many imaging modalities can aid in the diagnosis of DLBCL and help determine the extent of involvement for staging of the disease. These modalities include plain radiograph; CT, magnetic resonance imaging, and positron emission tomography (PET) scans; and lymphangiograms. Confirmatory diagnosis of DLBCL is best made by excisional tissue biopsy. Bone marrow biopsy is also used to help determine staging and management after initial diagnosis.8
Survival hinges on chemotherapy
Survival without treatment in patients with aggressive DLBCL can be measured in months. Combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (the CHOP regimen) increases the disease-free survival rate to between 35% and 45% at 4 years.9 The addition of rituximab has further increased survival in adult patients.10 While the optimal number of treatment cycles remains unclear, 6 to 8 cycles are typically given prior to PET imaging to assess for response.
Consider radiation. Some patients benefit from the addition of radiation to their chemotherapy regimen—particularly those with bulky disease.1 After completion of the planned treatment of DLBCL, a one-month follow-up physical exam with labs and a 2-month follow-up PET/CT scan should be obtained to evaluate response.
It took many scans and tests to arrive at a Dx
The patient’s CT scan showed a large anterior mediastinal mass with central necrosis, diffuse lymphadenopathy, a large left-sided pleural effusion, and multiple pulmonary nodules. Cytologic evaluation of pleural fluid did not show evidence of carcinoma. A fine needle aspiration of the substernal mass under radiologic guidance was performed, and histology was consistent with DLBCL. Further testing confirmed that disease was present both above and below the diaphragm, leading to the diagnosis of Stage IV-B DLBCL.
Our patient. The patient was treated with a chemotherapy regimen of rituximab-cyclophosphamide, vincristine, prednisone, and doxorubicin every 2 weeks for a total of 5 cycles.
Vincristine was discontinued after 2 weeks when the patient developed a Grade 2 peripheral neuropathy at her fingertips. The patient is currently in remission.
CORRESPONDENCE
Larissa Buccolo, MD, Naval Hospital Family Practice Clinic, 2080 Child Street, Jacksonville, FL 32214; [email protected]
A 19-YEAR-OLD NULLIPAROUS CAUCASIAN WOMAN came into our family practice clinic; she was concerned about redness and swelling that had developed in her left breast 2 weeks earlier. She’d gone to a local emergency department 8 days ago with similar complaints, and was treated with azithromycin and cephalexin for presumptive mastitis and possible cat scratch disease. Despite the antibiotics, she said that her symptoms had worsened; she’d developed a dry cough, dyspnea, general malaise, and a fever of 100.6°F.
The patient was a former smoker and had asthma as a child. She said her mother had been diagnosed with premenopausal breast cancer at age 31. She also indicated that she had a cat and that it might have scratched her arm prior to the onset of symptoms.
On exam, the patient was afebrile but tachycardic at 140 beats per minute. She had left axillary lymphadenopathy. Her left breast was indurated, erythematous, and generally edematous (FIGURE 1). There was no nipple discharge or evidence of trauma to the skin. The patient had decreased breath sounds at the bases.
A breast ultrasound (obtained to evaluate for possible abscess) showed only diffuse edema—but no abscess. A chest x-ray (FIGURE 2). showed an anterior and middle mediastinal soft tissue mass with a left-sided pleural effusion.
The patient was admitted to the hospital for further evaluation.
FIGURE 1
Induration, erythema, and diffuse edema of left breast
FIGURE 2
Chest x-ray reveals left pleural effusion, mediastinal mass
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diffuse large B-cell lymphoma
This patient had Stage IV-B diffuse large B-cell lymphoma (DLBCL), diagnosed by computed tomography (CT)-guided biopsy of the mediastinal mass. DLBCL is the most common histological subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of NHL cases.1,2
Patients with DLBCL typically present with a rapidly enlarging mass in the neck or abdomen. Thirty percent of patients will have systemic “B” symptoms, including fever, weight loss, and night sweats.3 DLBCL can be highly invasive and may cause compression of the airway and lymphatic or circulatory vessels. While dysphagia, hoarseness, breast swelling, chest pain, and cough can be among the presenting symptoms, superior vena cava syndrome is the most common complication and occurs in 30% of patients.3 The lymphatic obstruction seen in lymphoproliferative disease can produce lymphedema.
In this patient, diffuse lymphadenopathy in the subpectoral and axillary region caused lymphatic obstruction and breast edema. Extra-nodal disease occurs in up to one-third of cases.4 DLBCL can be involved in virtually any tissue, including breast, bone, testes, skin, liver, central nervous system, uterus, and gastrointestinal tract.5
The National Cancer Institute reports that between 2004 and 2008, the US age-adjusted incidence rate for non-Hodgkin lymphoma was 19.8 per 100,000 men and women per year.6 Incidence varies by ethnicity (Caucasians have the highest rate) and increases with age (median age at diagnosis is 66 years with a male predominance).6
Consider infection early on in the differential
The differential diagnosis for unilateral breast swelling and lymphadenopathy includes infection and malignancies. Potential infectious etiologies include acute bacterial mastitis, cat scratch disease, and breast abcess. Malignant causes include inflammatory breast cancer and primary malignant lymphoma. Primary malignant lymphoma of the breast is rare, with an estimated prevalence of 0.04% to 1.1% of all breast tumors and 1.7% to 2.2% of all extranodal non-Hodgkin lymphomas.7
Initial evaluation should be directed toward the most likely etiologies of the symptoms and should include breast ultrasound, blood cultures, and serologies—such as Bartonella—as history dictates.
Once more common causes of unilateral breast swelling are excluded, less common etiologies, such as duct ectasia, mammary adenosis or fat necrosis, and blunt force trauma, should be considered.
Due to the age of our patient, mastitis was initially high on the differential; however, when she did not respond to antibiotic therapy, other causes for her symptoms had to be considered. An ultrasound (noted earlier) ruled out a breast abscess. Blood cultures and serologies were negative. Punch skin biopsies of the patient’s breast showed no evidence of carcinoma, making inflammatory breast cancer less likely.
Imaging studies to start, then biopsy
Many imaging modalities can aid in the diagnosis of DLBCL and help determine the extent of involvement for staging of the disease. These modalities include plain radiograph; CT, magnetic resonance imaging, and positron emission tomography (PET) scans; and lymphangiograms. Confirmatory diagnosis of DLBCL is best made by excisional tissue biopsy. Bone marrow biopsy is also used to help determine staging and management after initial diagnosis.8
Survival hinges on chemotherapy
Survival without treatment in patients with aggressive DLBCL can be measured in months. Combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (the CHOP regimen) increases the disease-free survival rate to between 35% and 45% at 4 years.9 The addition of rituximab has further increased survival in adult patients.10 While the optimal number of treatment cycles remains unclear, 6 to 8 cycles are typically given prior to PET imaging to assess for response.
Consider radiation. Some patients benefit from the addition of radiation to their chemotherapy regimen—particularly those with bulky disease.1 After completion of the planned treatment of DLBCL, a one-month follow-up physical exam with labs and a 2-month follow-up PET/CT scan should be obtained to evaluate response.
It took many scans and tests to arrive at a Dx
The patient’s CT scan showed a large anterior mediastinal mass with central necrosis, diffuse lymphadenopathy, a large left-sided pleural effusion, and multiple pulmonary nodules. Cytologic evaluation of pleural fluid did not show evidence of carcinoma. A fine needle aspiration of the substernal mass under radiologic guidance was performed, and histology was consistent with DLBCL. Further testing confirmed that disease was present both above and below the diaphragm, leading to the diagnosis of Stage IV-B DLBCL.
Our patient. The patient was treated with a chemotherapy regimen of rituximab-cyclophosphamide, vincristine, prednisone, and doxorubicin every 2 weeks for a total of 5 cycles.
Vincristine was discontinued after 2 weeks when the patient developed a Grade 2 peripheral neuropathy at her fingertips. The patient is currently in remission.
CORRESPONDENCE
Larissa Buccolo, MD, Naval Hospital Family Practice Clinic, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Nguyen LN, Ha CS, Hess M, et al. The outcome of combined-modality treatments for stage I and II primary large B-cell lymphoma of the mediastinum. Int J Radiat Oncol Biol Phys. 2000;47:1281-1285.
2. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124:151-159.
3. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphoma: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795.
4. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999;22:357-364.
5. Aviles A, Neri N, Huerta-Guzman J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol. 2002;80:111-115.
6. National Cancer Institute. Surveillance epidemiology and end results. SEER stat fact sheets: non-Hodgkin lymphoma. Available at: (http://seer.cancer.gov/statfacts/html/nhl.html.) Accessed September 28, 2011.
7. Gholam D, Bibeau F, El Welshi A, et al. Primary breast lymphoma. Leuk Lymphoma. 2003;44:1173-1178.
8. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-1006.
9. Pond GD, Castellini RA, Horning S, et al. Non-Hodgkin lymphoma: influence of lymphography, CT, and bone marrow biopsy on staging and management. Radiology. 1989;170:159-164.
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027-5033.
1. Nguyen LN, Ha CS, Hess M, et al. The outcome of combined-modality treatments for stage I and II primary large B-cell lymphoma of the mediastinum. Int J Radiat Oncol Biol Phys. 2000;47:1281-1285.
2. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124:151-159.
3. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphoma: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795.
4. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999;22:357-364.
5. Aviles A, Neri N, Huerta-Guzman J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol. 2002;80:111-115.
6. National Cancer Institute. Surveillance epidemiology and end results. SEER stat fact sheets: non-Hodgkin lymphoma. Available at: (http://seer.cancer.gov/statfacts/html/nhl.html.) Accessed September 28, 2011.
7. Gholam D, Bibeau F, El Welshi A, et al. Primary breast lymphoma. Leuk Lymphoma. 2003;44:1173-1178.
8. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-1006.
9. Pond GD, Castellini RA, Horning S, et al. Non-Hodgkin lymphoma: influence of lymphography, CT, and bone marrow biopsy on staging and management. Radiology. 1989;170:159-164.
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027-5033.
Teen with a diffuse erythematous, pruritic eruption
An 18-Year-old caucasian female sought care at our dermatology clinic for a progressive, erythematous eruption on her face, neck, trunk, and extremities (FIGURES 1A AND 1B). She noted that the eruption had developed suddenly and that it was itchy.
The patient had no significant past medical history and denied being sexually active. The only medication she was taking was mestranol/norethisterone.
The patient denied any new exposures to medications, detergents, or foods. Upon questioning, she did note that about 1 to 2 weeks prior to the skin eruption, she had a mild sore throat and cough. However, her upper respiratory symptoms had resolved by the time she arrived at the clinic.
On physical exam, the patient had multiple erythematous papules and plaques with a fine scale over her face, neck, trunk, and lower legs (FIGURE2). There were areas of confluence on her face and neck. Her palms, soles, nails, and intertriginous areas were spared.
The patient’s mucous membranes were moist and there was no erythema or tonsillar exudate in her pharynx. A complete blood count, basic metabolic panel, and urinalysis were all within normal limits; a rapid plasma reagin (RPR) was nonreactive.
FIGURE 1
Papules and plaques with fine scale
This 18-year-old patient had multiple, erythematous papules and plaques with a fine scale over her face, neck, trunk, and lower legs. We performed a punch biopsy on her left posterior shoulder.
FIGURE 2
Papules and plaques on lower legs
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
We diagnosed guttate psoriasis in this patient based on her history and physical exam, a throat culture that was positive for group A beta-hemolytic streptococci, and blood work that showed an elevated antistreptolysin O titer. Further confirmation was obtained via punch biopsy.
Guttate psoriasis is a fairly uncommon form of psoriasis, affecting approximately 2% of patients with psoriasis.1 It is characterized by the abrupt onset of pruritic, salmon-pink 1- to 10-mm drop-like lesions with fine scale that may spread to the face, but spare the palms and soles. It’s uncommon for this sub-type of psoriasis to involve the nails.
Guttate psoriasis affects individuals younger than 30 years of age; there appears to be no gender predilection.2 The rash usually appears 2 to 3 weeks after an upper respiratory group A beta-hemolytic streptococci infection. Although less common, there have also been reports of guttate psoriasis associated with perianal streptococcal disease.2
While the pathophysiology is unclear, recent evidence suggests that a genetic autoimmune-mediated reaction to a recent streptococcal infection in immunologically susceptible hosts is at work. It’s thought that T-cell stimulation from a streptococcal superantigen is responsible for the acute cutaneous eruption. Various human leukocyte antigens, including HLA-Bw17, HLA-B13, and HLA-Cw6, have been identified and appear to confer a genetic predisposition to the development of guttate psoriasis.3
Differential includes secondary syphilis
The differential diagnosis of guttate psoriasis includes lichen planus, pityriasis rosea, and secondary syphilis.
Lichen planus is characterized by pruritic, planar, polyangular purple papules with a reticular pattern of criss-crossed whitish lines called “Wickham’s striae,” which are areas of epidermal thickening.1
Pityriasis rosea typically presents in a “Christmas-tree” distribution in which the long axis of these oval plaques are oriented along skin lines. Also, the lesions have a distinctive collarette scale, appearing as a fine, wrinkled tissue-like scale surrounding the plaque borders.
Secondary syphilis has numerous signs and symptoms, including rash. In this case, we ruled it out because our patient had no history of sexual contacts and a negative RPR.
Testing confirms suspicions, identifies organism
The diagnosis of guttate psoriasis is established from clinical presentation. While there is no laboratory test specific for diagnosis, as many as 80% of patients will have clinical or laboratory evidence suggestive of a streptococcal infection—specifically tonsillopharyngitis.4 Utilize bacteriologic throat or perianal cultures to isolate the organism. Levels of antibodies to streptolysin O, hyaluronidase, and deoxyribonuclease B may be elevated.4
Urinalysis can be used to rule out associated streptococcal complications, such as poststreptococcal glomerulonephritis. Further serologic evaluation may also include RPR testing to exclude secondary syphilis.
Biopsy is rarely needed to establish the diagnosis, but may be used for confirmation of complicated presentations or to rule out other concerning diagnoses. Histologic findings demonstrate epidermal hyperplasia with small foci of parakeratosis and dermal layer capillary dilation and edema. Infiltrating lymphocytes, macrophages, and polymorpho-nuclear leukocytes may be found at all dermal levels.5
Symptoms dictate treatment strategies
Guttate psoriasis is usually self-limiting and resolves within a few weeks to months. One small study, however, suggests that 33% of patients will eventually develop chronic plaque disease.6 A systematic review of treatments for guttate psoriasis failed to show firm evidence in favor of any specific treatment.7 Treatment strategies are thus based on symptomatology and may include emollients or, less commonly, low-potency topical corticosteroids.
Phototherapy, via direct sunlight exposure or by a short course of UV-B phototherapy, has been used to help clear lesions, but care must be taken to avoid burns, which can exacerbate the eruption. More resistant cases may benefit from oral psoralen plus exposure to ultraviolet A radiation.7
Due to the clear association between guttate psoriasis and streptococcal disease, appropriate testing should be done and antistreptococcal antibiotics initiated. While erythromycin and penicillin VK have been used in the past as first-line agents (with the addition of rifampin usually reserved for more resistant cases or chronic carrier states), a small case-controlled study failed to find statistically significant improvement with a course of penicillin or erythromycin.8 For patients with recurrent or chronic guttate psoriasis, tonsillectomy for poststreptococcal tonsillitis may be offered, although a systematic review failed to show a benefit.9
A chronic problem for our patient
We initially treated our patient with a 10-day course of penicillin VK and UV-B phototherapy. Eight weeks later, she had 90% resolution of her lesions. However, our patient subsequently experienced a flare, suggesting that she might go on to develop chronic plaque psoriasis.
CORRESPONDENCE
Christopher R. Worley, DO, LT, MC, USN, Naval Hospital Jacksonville, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004;209-239
2. Honig PJ. Guttate psoriasis associated with perianal streptococcal disease. J Pediatr. 1988;113:1037-1039.
3. Holm SJ, Sakuraba K, Mallbris L, et al. Distinct HLA-C/KIR genotype profile associates with guttate psoriasis. J Invest Dermatol. 2005;125:721-730.
4. Telfer NR, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
5. Brody I. Dermal and epidermal involvement in the evolution of acute eruptive guttate psoriasis vulgaris. J Invest Dermatol. 1984;82:465-470.
6. Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
7. Chalmers RJ, O’Sullivan T, Owen CM, et al. A systematic review of treatments for guttate psoriasis. Br J Dermatol. 2001;145:891-894.
8. Dogan B, Karabudak O, Harmanyeri Y. Antistreptococcal treatment of guttate psoriasis: a controlled study. Int J Dermatol. 2008;47:950-952.
9. Owen CM, Chalmers RJ, O’Sullivan T, et al. A systematic review of antistreptococcal interventions for guttate and chronic plaque psoriasis. Br J Dermatol. 2001;145:886-890.
An 18-Year-old caucasian female sought care at our dermatology clinic for a progressive, erythematous eruption on her face, neck, trunk, and extremities (FIGURES 1A AND 1B). She noted that the eruption had developed suddenly and that it was itchy.
The patient had no significant past medical history and denied being sexually active. The only medication she was taking was mestranol/norethisterone.
The patient denied any new exposures to medications, detergents, or foods. Upon questioning, she did note that about 1 to 2 weeks prior to the skin eruption, she had a mild sore throat and cough. However, her upper respiratory symptoms had resolved by the time she arrived at the clinic.
On physical exam, the patient had multiple erythematous papules and plaques with a fine scale over her face, neck, trunk, and lower legs (FIGURE2). There were areas of confluence on her face and neck. Her palms, soles, nails, and intertriginous areas were spared.
The patient’s mucous membranes were moist and there was no erythema or tonsillar exudate in her pharynx. A complete blood count, basic metabolic panel, and urinalysis were all within normal limits; a rapid plasma reagin (RPR) was nonreactive.
FIGURE 1
Papules and plaques with fine scale
This 18-year-old patient had multiple, erythematous papules and plaques with a fine scale over her face, neck, trunk, and lower legs. We performed a punch biopsy on her left posterior shoulder.
FIGURE 2
Papules and plaques on lower legs
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
We diagnosed guttate psoriasis in this patient based on her history and physical exam, a throat culture that was positive for group A beta-hemolytic streptococci, and blood work that showed an elevated antistreptolysin O titer. Further confirmation was obtained via punch biopsy.
Guttate psoriasis is a fairly uncommon form of psoriasis, affecting approximately 2% of patients with psoriasis.1 It is characterized by the abrupt onset of pruritic, salmon-pink 1- to 10-mm drop-like lesions with fine scale that may spread to the face, but spare the palms and soles. It’s uncommon for this sub-type of psoriasis to involve the nails.
Guttate psoriasis affects individuals younger than 30 years of age; there appears to be no gender predilection.2 The rash usually appears 2 to 3 weeks after an upper respiratory group A beta-hemolytic streptococci infection. Although less common, there have also been reports of guttate psoriasis associated with perianal streptococcal disease.2
While the pathophysiology is unclear, recent evidence suggests that a genetic autoimmune-mediated reaction to a recent streptococcal infection in immunologically susceptible hosts is at work. It’s thought that T-cell stimulation from a streptococcal superantigen is responsible for the acute cutaneous eruption. Various human leukocyte antigens, including HLA-Bw17, HLA-B13, and HLA-Cw6, have been identified and appear to confer a genetic predisposition to the development of guttate psoriasis.3
Differential includes secondary syphilis
The differential diagnosis of guttate psoriasis includes lichen planus, pityriasis rosea, and secondary syphilis.
Lichen planus is characterized by pruritic, planar, polyangular purple papules with a reticular pattern of criss-crossed whitish lines called “Wickham’s striae,” which are areas of epidermal thickening.1
Pityriasis rosea typically presents in a “Christmas-tree” distribution in which the long axis of these oval plaques are oriented along skin lines. Also, the lesions have a distinctive collarette scale, appearing as a fine, wrinkled tissue-like scale surrounding the plaque borders.
Secondary syphilis has numerous signs and symptoms, including rash. In this case, we ruled it out because our patient had no history of sexual contacts and a negative RPR.
Testing confirms suspicions, identifies organism
The diagnosis of guttate psoriasis is established from clinical presentation. While there is no laboratory test specific for diagnosis, as many as 80% of patients will have clinical or laboratory evidence suggestive of a streptococcal infection—specifically tonsillopharyngitis.4 Utilize bacteriologic throat or perianal cultures to isolate the organism. Levels of antibodies to streptolysin O, hyaluronidase, and deoxyribonuclease B may be elevated.4
Urinalysis can be used to rule out associated streptococcal complications, such as poststreptococcal glomerulonephritis. Further serologic evaluation may also include RPR testing to exclude secondary syphilis.
Biopsy is rarely needed to establish the diagnosis, but may be used for confirmation of complicated presentations or to rule out other concerning diagnoses. Histologic findings demonstrate epidermal hyperplasia with small foci of parakeratosis and dermal layer capillary dilation and edema. Infiltrating lymphocytes, macrophages, and polymorpho-nuclear leukocytes may be found at all dermal levels.5
Symptoms dictate treatment strategies
Guttate psoriasis is usually self-limiting and resolves within a few weeks to months. One small study, however, suggests that 33% of patients will eventually develop chronic plaque disease.6 A systematic review of treatments for guttate psoriasis failed to show firm evidence in favor of any specific treatment.7 Treatment strategies are thus based on symptomatology and may include emollients or, less commonly, low-potency topical corticosteroids.
Phototherapy, via direct sunlight exposure or by a short course of UV-B phototherapy, has been used to help clear lesions, but care must be taken to avoid burns, which can exacerbate the eruption. More resistant cases may benefit from oral psoralen plus exposure to ultraviolet A radiation.7
Due to the clear association between guttate psoriasis and streptococcal disease, appropriate testing should be done and antistreptococcal antibiotics initiated. While erythromycin and penicillin VK have been used in the past as first-line agents (with the addition of rifampin usually reserved for more resistant cases or chronic carrier states), a small case-controlled study failed to find statistically significant improvement with a course of penicillin or erythromycin.8 For patients with recurrent or chronic guttate psoriasis, tonsillectomy for poststreptococcal tonsillitis may be offered, although a systematic review failed to show a benefit.9
A chronic problem for our patient
We initially treated our patient with a 10-day course of penicillin VK and UV-B phototherapy. Eight weeks later, she had 90% resolution of her lesions. However, our patient subsequently experienced a flare, suggesting that she might go on to develop chronic plaque psoriasis.
CORRESPONDENCE
Christopher R. Worley, DO, LT, MC, USN, Naval Hospital Jacksonville, 2080 Child Street, Jacksonville, FL 32214; [email protected]
An 18-Year-old caucasian female sought care at our dermatology clinic for a progressive, erythematous eruption on her face, neck, trunk, and extremities (FIGURES 1A AND 1B). She noted that the eruption had developed suddenly and that it was itchy.
The patient had no significant past medical history and denied being sexually active. The only medication she was taking was mestranol/norethisterone.
The patient denied any new exposures to medications, detergents, or foods. Upon questioning, she did note that about 1 to 2 weeks prior to the skin eruption, she had a mild sore throat and cough. However, her upper respiratory symptoms had resolved by the time she arrived at the clinic.
On physical exam, the patient had multiple erythematous papules and plaques with a fine scale over her face, neck, trunk, and lower legs (FIGURE2). There were areas of confluence on her face and neck. Her palms, soles, nails, and intertriginous areas were spared.
The patient’s mucous membranes were moist and there was no erythema or tonsillar exudate in her pharynx. A complete blood count, basic metabolic panel, and urinalysis were all within normal limits; a rapid plasma reagin (RPR) was nonreactive.
FIGURE 1
Papules and plaques with fine scale
This 18-year-old patient had multiple, erythematous papules and plaques with a fine scale over her face, neck, trunk, and lower legs. We performed a punch biopsy on her left posterior shoulder.
FIGURE 2
Papules and plaques on lower legs
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
We diagnosed guttate psoriasis in this patient based on her history and physical exam, a throat culture that was positive for group A beta-hemolytic streptococci, and blood work that showed an elevated antistreptolysin O titer. Further confirmation was obtained via punch biopsy.
Guttate psoriasis is a fairly uncommon form of psoriasis, affecting approximately 2% of patients with psoriasis.1 It is characterized by the abrupt onset of pruritic, salmon-pink 1- to 10-mm drop-like lesions with fine scale that may spread to the face, but spare the palms and soles. It’s uncommon for this sub-type of psoriasis to involve the nails.
Guttate psoriasis affects individuals younger than 30 years of age; there appears to be no gender predilection.2 The rash usually appears 2 to 3 weeks after an upper respiratory group A beta-hemolytic streptococci infection. Although less common, there have also been reports of guttate psoriasis associated with perianal streptococcal disease.2
While the pathophysiology is unclear, recent evidence suggests that a genetic autoimmune-mediated reaction to a recent streptococcal infection in immunologically susceptible hosts is at work. It’s thought that T-cell stimulation from a streptococcal superantigen is responsible for the acute cutaneous eruption. Various human leukocyte antigens, including HLA-Bw17, HLA-B13, and HLA-Cw6, have been identified and appear to confer a genetic predisposition to the development of guttate psoriasis.3
Differential includes secondary syphilis
The differential diagnosis of guttate psoriasis includes lichen planus, pityriasis rosea, and secondary syphilis.
Lichen planus is characterized by pruritic, planar, polyangular purple papules with a reticular pattern of criss-crossed whitish lines called “Wickham’s striae,” which are areas of epidermal thickening.1
Pityriasis rosea typically presents in a “Christmas-tree” distribution in which the long axis of these oval plaques are oriented along skin lines. Also, the lesions have a distinctive collarette scale, appearing as a fine, wrinkled tissue-like scale surrounding the plaque borders.
Secondary syphilis has numerous signs and symptoms, including rash. In this case, we ruled it out because our patient had no history of sexual contacts and a negative RPR.
Testing confirms suspicions, identifies organism
The diagnosis of guttate psoriasis is established from clinical presentation. While there is no laboratory test specific for diagnosis, as many as 80% of patients will have clinical or laboratory evidence suggestive of a streptococcal infection—specifically tonsillopharyngitis.4 Utilize bacteriologic throat or perianal cultures to isolate the organism. Levels of antibodies to streptolysin O, hyaluronidase, and deoxyribonuclease B may be elevated.4
Urinalysis can be used to rule out associated streptococcal complications, such as poststreptococcal glomerulonephritis. Further serologic evaluation may also include RPR testing to exclude secondary syphilis.
Biopsy is rarely needed to establish the diagnosis, but may be used for confirmation of complicated presentations or to rule out other concerning diagnoses. Histologic findings demonstrate epidermal hyperplasia with small foci of parakeratosis and dermal layer capillary dilation and edema. Infiltrating lymphocytes, macrophages, and polymorpho-nuclear leukocytes may be found at all dermal levels.5
Symptoms dictate treatment strategies
Guttate psoriasis is usually self-limiting and resolves within a few weeks to months. One small study, however, suggests that 33% of patients will eventually develop chronic plaque disease.6 A systematic review of treatments for guttate psoriasis failed to show firm evidence in favor of any specific treatment.7 Treatment strategies are thus based on symptomatology and may include emollients or, less commonly, low-potency topical corticosteroids.
Phototherapy, via direct sunlight exposure or by a short course of UV-B phototherapy, has been used to help clear lesions, but care must be taken to avoid burns, which can exacerbate the eruption. More resistant cases may benefit from oral psoralen plus exposure to ultraviolet A radiation.7
Due to the clear association between guttate psoriasis and streptococcal disease, appropriate testing should be done and antistreptococcal antibiotics initiated. While erythromycin and penicillin VK have been used in the past as first-line agents (with the addition of rifampin usually reserved for more resistant cases or chronic carrier states), a small case-controlled study failed to find statistically significant improvement with a course of penicillin or erythromycin.8 For patients with recurrent or chronic guttate psoriasis, tonsillectomy for poststreptococcal tonsillitis may be offered, although a systematic review failed to show a benefit.9
A chronic problem for our patient
We initially treated our patient with a 10-day course of penicillin VK and UV-B phototherapy. Eight weeks later, she had 90% resolution of her lesions. However, our patient subsequently experienced a flare, suggesting that she might go on to develop chronic plaque psoriasis.
CORRESPONDENCE
Christopher R. Worley, DO, LT, MC, USN, Naval Hospital Jacksonville, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004;209-239
2. Honig PJ. Guttate psoriasis associated with perianal streptococcal disease. J Pediatr. 1988;113:1037-1039.
3. Holm SJ, Sakuraba K, Mallbris L, et al. Distinct HLA-C/KIR genotype profile associates with guttate psoriasis. J Invest Dermatol. 2005;125:721-730.
4. Telfer NR, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
5. Brody I. Dermal and epidermal involvement in the evolution of acute eruptive guttate psoriasis vulgaris. J Invest Dermatol. 1984;82:465-470.
6. Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
7. Chalmers RJ, O’Sullivan T, Owen CM, et al. A systematic review of treatments for guttate psoriasis. Br J Dermatol. 2001;145:891-894.
8. Dogan B, Karabudak O, Harmanyeri Y. Antistreptococcal treatment of guttate psoriasis: a controlled study. Int J Dermatol. 2008;47:950-952.
9. Owen CM, Chalmers RJ, O’Sullivan T, et al. A systematic review of antistreptococcal interventions for guttate and chronic plaque psoriasis. Br J Dermatol. 2001;145:886-890.
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004;209-239
2. Honig PJ. Guttate psoriasis associated with perianal streptococcal disease. J Pediatr. 1988;113:1037-1039.
3. Holm SJ, Sakuraba K, Mallbris L, et al. Distinct HLA-C/KIR genotype profile associates with guttate psoriasis. J Invest Dermatol. 2005;125:721-730.
4. Telfer NR, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
5. Brody I. Dermal and epidermal involvement in the evolution of acute eruptive guttate psoriasis vulgaris. J Invest Dermatol. 1984;82:465-470.
6. Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
7. Chalmers RJ, O’Sullivan T, Owen CM, et al. A systematic review of treatments for guttate psoriasis. Br J Dermatol. 2001;145:891-894.
8. Dogan B, Karabudak O, Harmanyeri Y. Antistreptococcal treatment of guttate psoriasis: a controlled study. Int J Dermatol. 2008;47:950-952.
9. Owen CM, Chalmers RJ, O’Sullivan T, et al. A systematic review of antistreptococcal interventions for guttate and chronic plaque psoriasis. Br J Dermatol. 2001;145:886-890.
Severe rash after dermatitis
An 18-year-old Caucasian woman came to the emergency department with a pruritic rash and localized swelling, most marked in the periorbital area. The rash had started 5 days earlier on her upper lip and subsequently spread to her face and upper chest.
Two days before, the patient was treated with amoxicillin/clavulanate (Augmentin), prednisone, and hydroxyzine (Atarax), but her symptoms worsened. She said she felt feverish but did not have any visual disturbances. She had no contacts with others ill with herpes or Varicella, although she did admit to having an unprotected sexual encounter 2 weeks before the rash’s onset. Her medical history was significant for untreated atopic dermatitis.
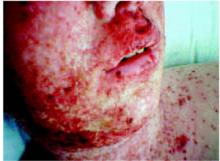
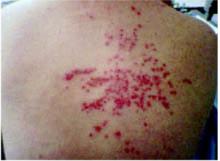
On exam, the patient was afebrile and had a diffuse maculopapular rash with areas of confluence over her face and hands (Figure 1). The face and hands also showed crusting and scaling. Discrete lesions were found on her upper and lower back (Figure 2), chest, volar wrist, and popliteal fossa. No vesicular lesions were present, although some isolated scabbed areas suggested previous vesicular lesions. The facial lesions were tender to palpation, and there was periorbital edema. No oral lesions were seen. Wound cultures grew methicillin-resistant Staphylococcus aureus.
FIGURE 1
Rash covering the face
FIGURE 2
Lesions on the upper back
What is the diagnosis?
How can the diagnosis be confirmed?
How should this disease be treated?
Diagnosis: Eczema herpeticum
Eczema herpeticum is an overwhelming herpesvirus infection on skin already affected by atopic dermatitis. It is a dermatologic emergency—untreated infections may lead to complications, including herpes keratitis and disseminated herpes simplex virus (HSV) infections with visceral involvement. Mortality is 1%–9%,1 although before antiviral therapy it was as high as 75%.2
The rash begins as dome-shaped vesicles, which subsequently disappear and become punched-out excoriations, crusts, and erythematous plaques. The head, neck, and trunk are the most commonly affected areas. Systemic symptoms such as fever and malaise usually accompany the rash.
Causes of eczema herpeticum
The cause of eczema herpeticum is always HSV type I.3 The exact pathophysiology is unknown, but it is thought to involve HSV entering the skin when skin barrier function is compromised due to dermatitis. Defective cytokine secretion in the affected skin also plays an important role.3
The severity of preexisting eczema does not seem to dictate the severity of eczema herpeticum.4 Secondary bacterial skin infections are very common. A mixture of aerobic and anaerobic bacteria are commonly isolated, the most common being S aureus, Group A β-hemolytic Streptococcus, Pseudomonas, and Peptostreptococcus.6,7
Risks
It is not clear which patients with atopic dermatitis are more at risk for developing eczema herpeticum. High total serum immunoglobulin E (IgE) and early age of onset are 2 risk factors that have been identified.7,8
Some researchers have suggested that use of topical corticosteroids predisposes those with atopic dermatitis to develop eczema herpeticum, but larger studies do not support this.8 However, topical calcineurin inhibitors do seem to pose a higher risk and are thus contraindicated during an eczema herpeticum infection.9
Differential diagnosis
Kaposi’s varicelliform eruption is a disseminated eruption of HSV on skin already affected with another dermatitis; eczema herpeticum refers specifically to the occurrence of an eruption on skin affected by atopic dermatitis. Thus, other types of Kaposi’s varicelliform eruption should be considered in the differential diagnosis. These include HSV infections on skin affected by Darier-White disease, pemphigus foliaceus, and mycosis fungoides. A good history taken from the patient regarding coexisting skin disorders makes the difference clear.
Other generalized vesicular eruptions such as Varicella should also be considered before making the diagnosis. Since distinct vesicles are not often present by the time the patient presents for care, the rash may also be confused with impetigo or other bacterial infections.
Laboratory tests
Several tests are available to detect the presence of HSV in the skin lesions of eczema herpeticum. These include polymerase chain reaction (PCR), immunofluorescence, and electron microscopy. Electron microscopy is not widely available and PCR can take several days, so often it is helpful to do direct fluorescent antibody testing while PCR results are pending. Light microscopy can be used to do a Tzanck test, which looks for multinucleated giant cells in blister fluid.
Serologies are often ordered, but results are nonspecific. Viral cultures are not very sensitive and take a while to get results. Aerobic and anaerobic bacterial cultures should be done because superinfection is common.
Treatment: systemic antivirals, antibiotics
Systemic antiviral medications are the mainstay of treatment for eczema herpeticum. Before the advent of acyclovir, the mortality rate of eczema herpeticum was 75%.2
The nucleoside analogs, which work by inhibiting viral DNA replication, are the most commonly used antiviral agents. Most of the studies on the treatment of eczema herpeticum have been on acyclovir; a 7-day course of IV therapy is typical. Alternatives such as valacyclovir have also been shown to be effective and have better oral bioavailability. Topical antivirals are often used for prophylaxis of keratoconjunctivitis if lesions are found around eyelids.
Antibiotics are often necessary to treat bacterial superinfection. Topical or systemic corticosteroids are not generally recommended and may worsen disease by attenuating the immune response.
Patient follow-up
This patient was initially treated with levofloxacin (Levaquin) for a presumed diagnosis of dermatitis with bacterial superinfection. Vancomycin was added on hospital day 2 when the rash was not improving. Due to a high suspicion for eczema herpeticum, a direct fluorescent antibody test sent for herpes, When the test results came back positive, the patient was started on intravenous acyclovir. A HSV PCR also had positive results. An ophthalmology specialist found no evidence for herpes keratoconjunctivitis and made no further recommendations.
The patient responded to the treatment and was discharged on hospital day 5. She was given a 10-day course of valacyclovir and a 21-day course of levofloxacin to complete at home. The patient fully recovered from the acute infections with some residual scarring.
1. Atherton DJ, Marshall WC. Eczema herpeticum. Practitioner 1982;226:971-973.
2. Wollenberg A, Wetzel S, Burgdorf W, Haas J. Viral infections in atopic dermatitis: Pathogenic aspects and clinical management. J Allergy Clin Immunol 2003;112:667-674.
3. Goodyear HM, et al. Growth of herpes simplex type 1 on skin explants of atopic eczema. Clin Exp Dermatol 1996;21:185-189.
4. Harindra V, Paffett MC. Recurrent eczema herpeticum: an underrecognised condition. Sex Transm Infect 2001;77:76.-
5. Brook I, Frazier EH, Yeager JK. Microbiology of infected eczema herpeticum. J Am Acad Dermatol 1998;38:627-629.
6. Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol 2002;51:808-812.
7. Bork K, Brauninger W. Increasing evidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol 1988;19:1024-1029.
8. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum—a retrospective analysis of 100 cases. J Amer Acad Dermatol 2003;49:198-205.
9. Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110:e2.
An 18-year-old Caucasian woman came to the emergency department with a pruritic rash and localized swelling, most marked in the periorbital area. The rash had started 5 days earlier on her upper lip and subsequently spread to her face and upper chest.
Two days before, the patient was treated with amoxicillin/clavulanate (Augmentin), prednisone, and hydroxyzine (Atarax), but her symptoms worsened. She said she felt feverish but did not have any visual disturbances. She had no contacts with others ill with herpes or Varicella, although she did admit to having an unprotected sexual encounter 2 weeks before the rash’s onset. Her medical history was significant for untreated atopic dermatitis.
On exam, the patient was afebrile and had a diffuse maculopapular rash with areas of confluence over her face and hands (Figure 1). The face and hands also showed crusting and scaling. Discrete lesions were found on her upper and lower back (Figure 2), chest, volar wrist, and popliteal fossa. No vesicular lesions were present, although some isolated scabbed areas suggested previous vesicular lesions. The facial lesions were tender to palpation, and there was periorbital edema. No oral lesions were seen. Wound cultures grew methicillin-resistant Staphylococcus aureus.
FIGURE 1
Rash covering the face
FIGURE 2
Lesions on the upper back
What is the diagnosis?
How can the diagnosis be confirmed?
How should this disease be treated?
Diagnosis: Eczema herpeticum
Eczema herpeticum is an overwhelming herpesvirus infection on skin already affected by atopic dermatitis. It is a dermatologic emergency—untreated infections may lead to complications, including herpes keratitis and disseminated herpes simplex virus (HSV) infections with visceral involvement. Mortality is 1%–9%,1 although before antiviral therapy it was as high as 75%.2
The rash begins as dome-shaped vesicles, which subsequently disappear and become punched-out excoriations, crusts, and erythematous plaques. The head, neck, and trunk are the most commonly affected areas. Systemic symptoms such as fever and malaise usually accompany the rash.
Causes of eczema herpeticum
The cause of eczema herpeticum is always HSV type I.3 The exact pathophysiology is unknown, but it is thought to involve HSV entering the skin when skin barrier function is compromised due to dermatitis. Defective cytokine secretion in the affected skin also plays an important role.3
The severity of preexisting eczema does not seem to dictate the severity of eczema herpeticum.4 Secondary bacterial skin infections are very common. A mixture of aerobic and anaerobic bacteria are commonly isolated, the most common being S aureus, Group A β-hemolytic Streptococcus, Pseudomonas, and Peptostreptococcus.6,7
Risks
It is not clear which patients with atopic dermatitis are more at risk for developing eczema herpeticum. High total serum immunoglobulin E (IgE) and early age of onset are 2 risk factors that have been identified.7,8
Some researchers have suggested that use of topical corticosteroids predisposes those with atopic dermatitis to develop eczema herpeticum, but larger studies do not support this.8 However, topical calcineurin inhibitors do seem to pose a higher risk and are thus contraindicated during an eczema herpeticum infection.9
Differential diagnosis
Kaposi’s varicelliform eruption is a disseminated eruption of HSV on skin already affected with another dermatitis; eczema herpeticum refers specifically to the occurrence of an eruption on skin affected by atopic dermatitis. Thus, other types of Kaposi’s varicelliform eruption should be considered in the differential diagnosis. These include HSV infections on skin affected by Darier-White disease, pemphigus foliaceus, and mycosis fungoides. A good history taken from the patient regarding coexisting skin disorders makes the difference clear.
Other generalized vesicular eruptions such as Varicella should also be considered before making the diagnosis. Since distinct vesicles are not often present by the time the patient presents for care, the rash may also be confused with impetigo or other bacterial infections.
Laboratory tests
Several tests are available to detect the presence of HSV in the skin lesions of eczema herpeticum. These include polymerase chain reaction (PCR), immunofluorescence, and electron microscopy. Electron microscopy is not widely available and PCR can take several days, so often it is helpful to do direct fluorescent antibody testing while PCR results are pending. Light microscopy can be used to do a Tzanck test, which looks for multinucleated giant cells in blister fluid.
Serologies are often ordered, but results are nonspecific. Viral cultures are not very sensitive and take a while to get results. Aerobic and anaerobic bacterial cultures should be done because superinfection is common.
Treatment: systemic antivirals, antibiotics
Systemic antiviral medications are the mainstay of treatment for eczema herpeticum. Before the advent of acyclovir, the mortality rate of eczema herpeticum was 75%.2
The nucleoside analogs, which work by inhibiting viral DNA replication, are the most commonly used antiviral agents. Most of the studies on the treatment of eczema herpeticum have been on acyclovir; a 7-day course of IV therapy is typical. Alternatives such as valacyclovir have also been shown to be effective and have better oral bioavailability. Topical antivirals are often used for prophylaxis of keratoconjunctivitis if lesions are found around eyelids.
Antibiotics are often necessary to treat bacterial superinfection. Topical or systemic corticosteroids are not generally recommended and may worsen disease by attenuating the immune response.
Patient follow-up
This patient was initially treated with levofloxacin (Levaquin) for a presumed diagnosis of dermatitis with bacterial superinfection. Vancomycin was added on hospital day 2 when the rash was not improving. Due to a high suspicion for eczema herpeticum, a direct fluorescent antibody test sent for herpes, When the test results came back positive, the patient was started on intravenous acyclovir. A HSV PCR also had positive results. An ophthalmology specialist found no evidence for herpes keratoconjunctivitis and made no further recommendations.
The patient responded to the treatment and was discharged on hospital day 5. She was given a 10-day course of valacyclovir and a 21-day course of levofloxacin to complete at home. The patient fully recovered from the acute infections with some residual scarring.
An 18-year-old Caucasian woman came to the emergency department with a pruritic rash and localized swelling, most marked in the periorbital area. The rash had started 5 days earlier on her upper lip and subsequently spread to her face and upper chest.
Two days before, the patient was treated with amoxicillin/clavulanate (Augmentin), prednisone, and hydroxyzine (Atarax), but her symptoms worsened. She said she felt feverish but did not have any visual disturbances. She had no contacts with others ill with herpes or Varicella, although she did admit to having an unprotected sexual encounter 2 weeks before the rash’s onset. Her medical history was significant for untreated atopic dermatitis.
On exam, the patient was afebrile and had a diffuse maculopapular rash with areas of confluence over her face and hands (Figure 1). The face and hands also showed crusting and scaling. Discrete lesions were found on her upper and lower back (Figure 2), chest, volar wrist, and popliteal fossa. No vesicular lesions were present, although some isolated scabbed areas suggested previous vesicular lesions. The facial lesions were tender to palpation, and there was periorbital edema. No oral lesions were seen. Wound cultures grew methicillin-resistant Staphylococcus aureus.
FIGURE 1
Rash covering the face
FIGURE 2
Lesions on the upper back
What is the diagnosis?
How can the diagnosis be confirmed?
How should this disease be treated?
Diagnosis: Eczema herpeticum
Eczema herpeticum is an overwhelming herpesvirus infection on skin already affected by atopic dermatitis. It is a dermatologic emergency—untreated infections may lead to complications, including herpes keratitis and disseminated herpes simplex virus (HSV) infections with visceral involvement. Mortality is 1%–9%,1 although before antiviral therapy it was as high as 75%.2
The rash begins as dome-shaped vesicles, which subsequently disappear and become punched-out excoriations, crusts, and erythematous plaques. The head, neck, and trunk are the most commonly affected areas. Systemic symptoms such as fever and malaise usually accompany the rash.
Causes of eczema herpeticum
The cause of eczema herpeticum is always HSV type I.3 The exact pathophysiology is unknown, but it is thought to involve HSV entering the skin when skin barrier function is compromised due to dermatitis. Defective cytokine secretion in the affected skin also plays an important role.3
The severity of preexisting eczema does not seem to dictate the severity of eczema herpeticum.4 Secondary bacterial skin infections are very common. A mixture of aerobic and anaerobic bacteria are commonly isolated, the most common being S aureus, Group A β-hemolytic Streptococcus, Pseudomonas, and Peptostreptococcus.6,7
Risks
It is not clear which patients with atopic dermatitis are more at risk for developing eczema herpeticum. High total serum immunoglobulin E (IgE) and early age of onset are 2 risk factors that have been identified.7,8
Some researchers have suggested that use of topical corticosteroids predisposes those with atopic dermatitis to develop eczema herpeticum, but larger studies do not support this.8 However, topical calcineurin inhibitors do seem to pose a higher risk and are thus contraindicated during an eczema herpeticum infection.9
Differential diagnosis
Kaposi’s varicelliform eruption is a disseminated eruption of HSV on skin already affected with another dermatitis; eczema herpeticum refers specifically to the occurrence of an eruption on skin affected by atopic dermatitis. Thus, other types of Kaposi’s varicelliform eruption should be considered in the differential diagnosis. These include HSV infections on skin affected by Darier-White disease, pemphigus foliaceus, and mycosis fungoides. A good history taken from the patient regarding coexisting skin disorders makes the difference clear.
Other generalized vesicular eruptions such as Varicella should also be considered before making the diagnosis. Since distinct vesicles are not often present by the time the patient presents for care, the rash may also be confused with impetigo or other bacterial infections.
Laboratory tests
Several tests are available to detect the presence of HSV in the skin lesions of eczema herpeticum. These include polymerase chain reaction (PCR), immunofluorescence, and electron microscopy. Electron microscopy is not widely available and PCR can take several days, so often it is helpful to do direct fluorescent antibody testing while PCR results are pending. Light microscopy can be used to do a Tzanck test, which looks for multinucleated giant cells in blister fluid.
Serologies are often ordered, but results are nonspecific. Viral cultures are not very sensitive and take a while to get results. Aerobic and anaerobic bacterial cultures should be done because superinfection is common.
Treatment: systemic antivirals, antibiotics
Systemic antiviral medications are the mainstay of treatment for eczema herpeticum. Before the advent of acyclovir, the mortality rate of eczema herpeticum was 75%.2
The nucleoside analogs, which work by inhibiting viral DNA replication, are the most commonly used antiviral agents. Most of the studies on the treatment of eczema herpeticum have been on acyclovir; a 7-day course of IV therapy is typical. Alternatives such as valacyclovir have also been shown to be effective and have better oral bioavailability. Topical antivirals are often used for prophylaxis of keratoconjunctivitis if lesions are found around eyelids.
Antibiotics are often necessary to treat bacterial superinfection. Topical or systemic corticosteroids are not generally recommended and may worsen disease by attenuating the immune response.
Patient follow-up
This patient was initially treated with levofloxacin (Levaquin) for a presumed diagnosis of dermatitis with bacterial superinfection. Vancomycin was added on hospital day 2 when the rash was not improving. Due to a high suspicion for eczema herpeticum, a direct fluorescent antibody test sent for herpes, When the test results came back positive, the patient was started on intravenous acyclovir. A HSV PCR also had positive results. An ophthalmology specialist found no evidence for herpes keratoconjunctivitis and made no further recommendations.
The patient responded to the treatment and was discharged on hospital day 5. She was given a 10-day course of valacyclovir and a 21-day course of levofloxacin to complete at home. The patient fully recovered from the acute infections with some residual scarring.
1. Atherton DJ, Marshall WC. Eczema herpeticum. Practitioner 1982;226:971-973.
2. Wollenberg A, Wetzel S, Burgdorf W, Haas J. Viral infections in atopic dermatitis: Pathogenic aspects and clinical management. J Allergy Clin Immunol 2003;112:667-674.
3. Goodyear HM, et al. Growth of herpes simplex type 1 on skin explants of atopic eczema. Clin Exp Dermatol 1996;21:185-189.
4. Harindra V, Paffett MC. Recurrent eczema herpeticum: an underrecognised condition. Sex Transm Infect 2001;77:76.-
5. Brook I, Frazier EH, Yeager JK. Microbiology of infected eczema herpeticum. J Am Acad Dermatol 1998;38:627-629.
6. Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol 2002;51:808-812.
7. Bork K, Brauninger W. Increasing evidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol 1988;19:1024-1029.
8. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum—a retrospective analysis of 100 cases. J Amer Acad Dermatol 2003;49:198-205.
9. Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110:e2.
1. Atherton DJ, Marshall WC. Eczema herpeticum. Practitioner 1982;226:971-973.
2. Wollenberg A, Wetzel S, Burgdorf W, Haas J. Viral infections in atopic dermatitis: Pathogenic aspects and clinical management. J Allergy Clin Immunol 2003;112:667-674.
3. Goodyear HM, et al. Growth of herpes simplex type 1 on skin explants of atopic eczema. Clin Exp Dermatol 1996;21:185-189.
4. Harindra V, Paffett MC. Recurrent eczema herpeticum: an underrecognised condition. Sex Transm Infect 2001;77:76.-
5. Brook I, Frazier EH, Yeager JK. Microbiology of infected eczema herpeticum. J Am Acad Dermatol 1998;38:627-629.
6. Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol 2002;51:808-812.
7. Bork K, Brauninger W. Increasing evidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol 1988;19:1024-1029.
8. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum—a retrospective analysis of 100 cases. J Amer Acad Dermatol 2003;49:198-205.
9. Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110:e2.