User login
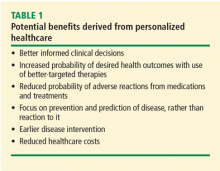
Personalized healthcare is the tailoring of medical management and patient care to the individual characteristics of each patient. This is achieved by incorporating the genetic and genomic makeup of an individual and his or her family medical history, environment, health-related behaviors, culture, and values into a complete health picture that can be used to customize care. Another level of personalization, often called personalized medicine, involves the selection of drug therapy through the use of tests to determine the genes and gene interactions that can reliably predict an individual’s response to a given therapy. This white paper focuses largely on the use of personalized healthcare as a risk prediction tool.
CURRENT STATUS OF PERSONALIZED HEALTHCARE
The case for personalized healthcare: Seeking value
To fully appreciate the need to advance the adoption of personalized healthcare into the delivery of medicine, one must consider the operation of our current healthcare system and its inefficiencies in terms of delivery and cost, its imprecision in the selection of therapies, and its inability to optimize outcomes. The framework of the US healthcare system as it is now constructed is expensive, disease-directed (instead of health- and wellness-directed), fragmented, and complex. While gross domestic product (GDP) in the United States has increased by approximately 3% per year,2 the compounded growth rate of healthcare expenditures is 6.1% per year. Healthcare in the aggregate now represents 17.6% of GDP and 27% of spending by the federal government and consumes 28% of the average household’s discretionary spending, surpassed only by housing.3
Personalized healthcare can potentially address the need for value consistent with the healthcare system’s prominent share of the US economy. The growth in healthcare spending is certain to be a target of the newly created Joint Select Committee on Deficit Reduction (created by the Budget Control Act of 2011), which is tasked with deficit reduction of at least $1.5 trillion over a 10-year period.
The need to address healthcare costs has been recognized in the Patient Protection and Affordable Care Act, a central feature of which is the creation of integrated health systems that pay for value based on quality, cost containment, and consumer experience. The legislation was enacted to transform healthcare in a variety of ways to make it more sustainable. The Patient Protection and Affordable Care Act seeks to end fragmentation by expanding the use of information technology to reorganize the delivery system and to prevent errors, shifting from volume-based incentives to incentives based on performance and outcomes, and rewarding effective healthcare delivery measures and good patient outcomes.
A shift from reactive to proactive
The premise behind personalized healthcare is the potential for more efficient healthcare, with the assumption that efficiency translates to lower cost and improved patient care.
Although healthcare reform is most often referred to in the context of improving access to care through insurance coverage mandates, true healthcare reform shifts current healthcare models from the practice of reactive medicine to the practice of proactive medicine, in which the tools of personalized healthcare (ie, genetics, genomics, and other molecular diagnostics) enable not only better quality of care but also less expensive care.
Several personalized tools have long been accepted into mainstream medicine. Two examples are the family history, which is the least expensive and most available genetic evaluation tool, and ABO blood typing for safe transfusions (as ABO blood types are alleles of a gene). In fact, much of what is now considered mainstream medical management was at one time considered new. To allow further evolution of medical practice, our challenge is to open our minds to the possibility that personalized proactive medicine can improve healthcare.
The new vision: More precise management
The trial-and-error approach to treating disease is inefficient and costly. Many drugs are effective for only about 50% of patients, often leading to switching or intensification of therapy that requires multiple patient visits.
Personalized medicine considers pharmacokinetic and other characteristics in selection of drug dosages. Genomic testing has the potential to provide clearer insight into the more successful use of currently available medicines. Treatment decisions (ie, drug and drug dosage choice) made on the basis of pharmacogenomic testing should increase adherence through greater effectiveness and fewer adverse drug reactions.
A massive amount of waste is related to pharmaceutical nonadherence and noncompliance. The New England Healthcare Institute has estimated that medication nonadherence costs the healthcare system $290 billion annually.4 Methodologies targeted at individual patients to improve adherence to drug regimens could save the healthcare system a tremendous amount of money.
Cancer management as a model for personalized healthcare. Personalization of therapy is especially suited to cancer management, given that the response to nonspecific cancer chemotherapy is suboptimal in most patients yet exposes them to adverse effects.5 Large-scale sequencing of human cancer genomes is rapidly changing the understanding of cancer biology and is identifying new targets in difficult-to-treat diseases and causes of drug resistance. Applying this information can achieve cost savings by avoiding the use of treatments that are ineffective in particular patients.
Overexpression of genetic mutations renders some cancers less susceptible to certain treatments, but has opened the door to individualized molecularly guided treatment strategies. For example, among patients with non–small cell lung cancer, mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain predict response to EGFR tyrosine kinase inhibitors, and anaplastic lymphoma kinase (ALK) inhibitors induce response in patients harboring a mutation in EML4-ALK genes. The recognition that human epidermal growth factor receptor (HER)-2 overexpression as a result of ERBB2 gene amplification occurs in as many as 20% of human breast cancers paved the way for the development of HER-2–targeted therapies. Patients with advanced colorectal cancer whose tumors express the KRAS gene mutation do not benefit from an EGFR inhibitor, whereas those with wild-type KRAS have improved survival with EGFR inhibitor treatment.6
BARRIERS TO THE APPLICATION OF PERSONALIZED HEALTHCARE
The availability and potential of personalized healthcare services and technology is not universally recognized or appreciated by consumers and clinicians. This lack of awareness contributes to a shortage of public support and limited demand for such services. Other barriers include misperceptions regarding the impact of personalized healthcare on disease management, limited incentives to use the available technology, and a knowledge gap among healthcare providers.
Lack of awareness and support
As applications of personalized healthcare advance to the point of clinical relevance, it is important to consider strategies for effective implementation into healthcare practice. Personalized healthcare, when more fully implemented, promises to accelerate the progress that healthcare reform hopes to achieve.
A major challenge to widespread adoption of personalized healthcare is limited recognition by the public and some healthcare providers that personalized healthcare can help to achieve better value. For personalized medicine to be embraced, the concept of “helix to health,” or translation of knowledge to the clinical setting, must resonate with the general public. Despite lack of public and provider awareness, the Personalized Medicine Coalition (PMC) has documented the existence of 56 personalized treatment and diagnostic products. Further, more than 200 product labels now recommend genetic testing prior to use to identify likely responders or inform of the influence of genetic variation on safety and effectiveness.
Consumers’ confidence in the efficacy and safety of medicines they take might contribute to the absence of public support for personalized healthcare. Similarly, despite the availability of genomic tests and tools, many physicians who might be advocates for personalized healthcare do not see the relevance of genomic medicine to their practices in terms of direct benefit to patient care.7
Apart from clinicians and consumers, support is also weak among health insurers and employers, even though the return on investment for personalized healthcare may be profound. Payers await the economic outcomes data that are crucial for their commitment to personalized healthcare. In addition, some have concerns about the ethical implications of personalized healthcare (see “Managing Genomic Information Responsibly”).
Perception of impact on treatment and prevention
A frequent criticism of genomics in medicine is that a genetic diagnosis does not help with patient management. In fact, surveillance and management of patients and family members often changes in response to a genetic diagnosis; knowing which gene is involved personalizes medical management. An example is the management of hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, which is the most common form of hereditary colon cancer. For a person with HNPCC, the lifetime risk of developing colorectal cancer is approximately 80%. Lynch syndrome is caused by germline mutations in one of three major mismatch repair (MMR) genes (MLH1, MSH2, and MSH6), and it predisposes to other cancers—uterine, stomach, and ovarian—as well. In women with Lynch syndrome, the lifetime risk for uterine cancer is 40%, compared with 4% in the general population.
At least 90% of patients with Lynch syndrome can be detected through MMR testing via microsatellite instability (MSI) or immunohistochemistry (IHC).8 MSI is a cellular phenotype that indicates a deficiency in at least one DNA MMR protein.
Although 5-fluorouracil–based chemo therapy is the standard of care for treatment of colorectal cancer, it confers no survival advantage in patients with MMR-IHC null (lack of expression of the gene) or MSI-high sporadic colorectal cancer.9,10 Knowing the status of MMR proteins, therefore, would alter the decision regarding neoadjuvant and adjuvant chemotherapy.
Perception of value
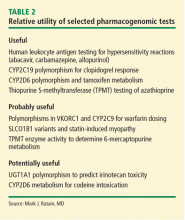
Implementation of pharmacogenomics into clinical practice has lagged. One major reason is the lack of an obvious business model for a product that may only be required once in an individual patient’s lifetime.11
A second barrier to integration lies in the limited demand for pharmacogenomics from physicians. This may be related partly to limited expertise in genetics among many physicians and to significant pushback from payers against today’s costs. Without reimbursement, little incentive exists for pharmacogenomics diagnostics. The incentive for physicians is further depressed, perhaps appropriately, when randomized controlled studies fail to demonstrate improved clinical outcomes with the use of pharmacogenomicbased treatment strategies. Two such examples are genotype-guided warfarin dosing, which failed in a randomized controlled trial to improve the proportion of international normalized ratios in the therapeutic range,12 and dosing of clopidogrel based on platelet reactivity, which did not improve outcomes after percutaneous coronary intervention compared with standard dosing in a randomized double-blind clinical trial.13
A significant delay in obtaining the results of pharmacogenomics testing, which also postpones the prescribing encounter, is another major drawback.
A knowledge gap persists

At present, delivery of personalized healthcare is not part of the usual training of physicians and other healthcare providers who are the gatekeepers of medicine. Few medical schools incorporate human and medical genetics, genomics, and pharmacogenomics into their curricula. Genetics is inadequately emphasized in residency curricula outside of pediatrics, family medicine, and obstetrics/gynecology.
The resulting knowledge gap is a fundamental factor in the lack of interest in using genomics in clinical medicine. Educating consumers and physicians at all levels, including specialty societies as well as insurers, will be key to expanding utilization of personalized healthcare. Educating payers and providing them with more data on economic outcomes associated with personalized healthcare will be necessary for adoption into clinical practice; implementation will lag as long as reimbursement decisions do not support personalized approaches to medicine.
As DNA sequencing technology has become less expensive and more powerful, companies have begun to market personal genomic testing. As a result, patients who use these services will increasingly want to discuss the results with their physicians. A significant number of clinicians are unfamiliar with personal genomic testing and emerging genetic testing options. In one survey of physicians who attended educational sessions that discussed recent developments in clinical genetics, only 37% indicated that they were familiar with recent genetic research that affected their patients.14
Targeted education will enhance physicians’ understanding of probabilities and risk estimates from the use of genomic testing; it will also improve recognition of potential causes of patient anxiety, gene variants of unknown significance, and follow-up tests and procedures that can add to expense. Nonphysician healthcare providers (ie, nurses and physician assistants) of direct care also will benefit from education.
INTEGRATING PERSONALIZED HEALTHCARE INTO CLINICAL PRACTICE
Practice standardization and an overhaul of the health information technology (HIT) infrastructure are needed if we are to reap the potential benefits of personalized healthcare. Creative approaches to practitioner education, which are being used in some institutions, must become more widespread. Similarly, the models for successful integration of personalized healthcare that have been achieved in some settings also can be implemented in other institutions.
Data collection and integration must be prioritized
Personalized healthcare can be both predictive and preventive, but moving past the disruptive phase of personalized healthcare will require a radical transformation of the healthcare “ecosystem” and HIT infrastructure.
Although data collection in the current system is extensive, data sharing and data management are inadequate. The pace at which HIT links clinical and genetic information must be accelerated. HIT will expedite innovation and implementation of personalized healthcare, allowing greater integration of data to permit improved data analysis capability. The ultimate goal is to create an interoperable system that connects these data across hospitals and clinicians to help clinicians interpret genomic and other risk information to better inform patient care.
Fully integrated health systems support better coordination of care and optimize the treatment of individual patients: linking research findings, treatment guidelines, treatment outcomes based on genetic profiles, and the individual patient’s own genetic profile will help to personalize treatments. Genomic information added to an individual’s electronic medical record along with improved data-sharing will facilitate clinicians’ ability to retrieve outcomes data based on patient characteristics.
Care models must be standardized, evidence-based practices must be executed, and care must be coordinated yet decentralized. In this way, clinicians can use the electronic medical record as an interoperable patient record to determine a personalized pathway to patient management. Standardization reduces variability in practice and permits seamless execution of care. Automation is imperative to achieving standardization, irrespective of the care supervisor. Investments must therefore be made to stimulate electronic medical record decision support.
In addition, larger data sets will be needed to identify the types of patients likely to respond to a treatment. Ideal data sets would be large enough to have adequate statistical power, be publicly available, standardize the collection of data with respect to response to therapy and toxicity, and contain data on concomitant collections of biologic samples.
Reimbursement must keep pace with medical advances
Payer willingness to reimburse for genomic tests and treatments will determine the pace of integration of personalized healthcare into clinical practice. Evidence that enhanced value can be derived from personalized approaches to medicine must be generated before personalized healthcare gains widespread acceptance by payers.
In addition, care-coordinated models must be developed to promote a value-based agenda that facilitates physician accountability and encourages clinical integration.
Innovative approaches are needed to educate providers
Development of point-of-care tools. Because information overload and lack of time are obstacles to clinicians’ efforts to incorporate genomic information into clinical practice, emphasis must be placed on genomic applications that have demonstrated utility. Engaging busy clinicians with point-of-care tools will maximize the relevance of the genomic information they receive and encourage effective use of their time. Decision-making should be supported through automatic risk assessment and management recommendations.
Educational tools. The National Coalition for Health Professional Education in Genetics (NCHPEG) was borne out of the recognition that the pace of genomic discovery far exceeds the pace at which healthcare providers can be educated. Its vision is to improve healthcare through informed use of genomic resources. NCHPEG is a member-based organization whose stakeholders include professional societies, hospitals, advocacy groups, and industry; it attempts to identify the specific educational needs for particular target audiences and then address these needs. It achieves its goals through the use of point-of-care tools and educational programs for continuing medical education credit.
One NCHPEG tool is the Pregnancy and Health Profile, which is a risk assessment and screening tool that attempts to improve the identification of women and babies at risk of developing genetic disease. It collects personal and family history information, performs a risk assessment for the clinician, and provides clinical decision support and education.
Another example of an educational tool is the “Genes to Society” curriculum initiated by The Johns Hopkins University School of Medicine in August 2009. The curriculum is being used as “the foundation for the scientific and clinical career development of future physicians.”15
Using personal genomic testing for education. The number of direct-to-consumer genomic tests is growing, and their market penetration will only increase as the cost of supplying a personal genome continues to decline. Whole genome scanning is being offered with the promise of identifying genetic predisposition to multiple diseases.
Participation in personal genomic testing may be a useful educational tool. Medical students, residents, and practicing physicians who participate in testing may be better equipped to advise patients about the processes involved and the potential utility and limitations of direct-to-consumer genotyping.14
Some companies that offer direct-to-consumer genomic testing provide telephone support from genetic counselors to help clients and their healthcare providers manage genetic information. Counselor services include identifying hereditary risks and reviewing diagnostic, preventive, and early-detection options.
Implementing pharmacogenomics into practice: Decision support systems are needed
A genomic decision support system that guides medication prescribing is needed to implement pharmacogenomic diagnostics. For such a system to achieve the goal of selecting the best medication for each individual, it must do the following:
- Test all polymorphisms relevant to the prescribing of any medication
- Be completed with no out-of-pocket cost to the patient
- Be performed before the patient requires the medication
- Provide results that will be interpreted as part of an individualized pharmacogenomics consult.11
The 1200 Patients Project, a pilot research study under way at the Center for Personalized Therapeutics at the University of Chicago, is attempting to demonstrate the feasibility of incorporating pharmacogenomic testing into routine clinical practice for medication treatment decisions. DNA samples from patients who are taking at least one prescription medication are being tested for differences in genes that may suggest greater effectiveness or an increased risk of side effects from certain medications.
Solutions in practice
Cleveland Clinic’s genetics-based management of Lynch syndrome, the integration of genetics services during patient appointments at Cleveland Clinic, and a coordinated approach at The Ohio State University Medical Center are examples of practical applications of personalized healthcare.
Colorectal cancer management. One example of a personalized approach to medicine that improves health outcome while achieving cost savings is the genetics-based approach to HNPCC (Lynch syndrome) at Cleveland Clinic.
Early identification of Lynch syndrome by screening all colorectal cancer patients has been shown to save $250,000 per life-year gained in the United States.16 All colorectal cancers resected at the Cleveland Clinic main campus are routinely screened for MSI and IHC, and the process is embedded into the routine pathology workflow. With the patients’ foreknowledge, a gastrointestinal cancer genetics counselor scans the list of MSI and IHC results each week. Patients who are MSI-high or IHC-null are invited to receive genetic counseling and consider germline single-gene testing guided by the IHC results. With this active approach, patient uptake is 80%; in comparison, with a passive approach (MSI/IHC results are placed in the pathology report), the uptake is 14%17 (B. Leach and C. Eng, unpublished data, 2011).The successful application of the active approach requires the close cooperation of multiple disciplines, including members of the Cleveland Clinic Genomic Medicine, Pathology & Laboratory Medicine, and Digestive Disease Institutes.18
Integrating genetics-based care at Cleveland Clinic. Time delays for genetics services and limited collaboration with managing physicians who are not genetics specialists reduces genetics-based access and availability. Broad access to genetics clinical services is a means of clinical integration of genetics-enabled care. Providing patients and healthcare providers with easy access and short wait times is vital for clinical integration of genetics-enabled personalized healthcare.
As part of a patient-centered focus on medicine, clinical genetics services have been integrated throughout Cleveland Clinic. The system has two genetics clinics at its main campus and has embedded multiple genetics satellites within its nongenetics clinics, easing access. Genetics counselors are stationed in the same areas of practice as referring providers. Although patient encounters have increased at the medical genetics clinic in the Genomic Medicine Institute, genetics consultations no longer require an extra trip to the clinic since they are integrated into existing appointments. With this approach, large numbers of patients can be seen with no wait times.
Coordinated care at The Ohio State University Medical Center. The Center for Personalized Health Care at The Ohio State University Medical Center (OSUMC) embraces a systems-based care-coordinated model that improves care by executing standardized processes and automating routine tasks. The Institute for Systems Biology, which was established to develop genomics, wellness, and chronic disease biomarkers, collaborates with OSUMC on pilot projects in chronic disease, including cancer.
The OSUMC has a closed system in which it is the payer, employer, and provider of healthcare. This closed system serves as an ideal testing ground for reform. Goals include intervention in disease before symptoms appear and maintenance of wellness. The data from these demonstration projects should facilitate adoption of personalized healthcare by improving physician acceptance of personalized approaches and satisfying payers that personalized healthcare is cost-effective.

- Personalized medicine. Coriell Institute for Medical Research Web site. http://www.coriell.org/personalized-medicine. Updated 2011. Accessed December 27, 2011.
- The 2012 Statistical Abstract. U.S. Census Bureau Web site. http://www.census.gov/compendia/statab/cats/income_expenditures_poverty_wealth/gross_domestic_product_gdp.html. Updated September 27, 2011. Accessed December 22, 2011.
- National health expenditure fact sheet. Center for Medicare & Medicaid Services (CMS) Web site. https://www.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Updated November 4, 2011. Accessed December 22, 2011.
- New England Healthcare Institute (NEHI). Thinking outside the pillbox: A system-wide approach to improving patient medication adherence for chronic disease. NEHI Web site. http://www.nehi.net/publications/44/thinking_outside_the_pillbox_a_systemwide_approach_to_improving_patient_medication_adherence_for_chronic_disease. Published August 12, 2009. Accessed December 22, 2011.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001; 7:201–204.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359:1757–1765.
- Feero WG, Green ED. Genomics education for healthcare professionals in the 21st century. JAMA 2011; 306:989–990.
- Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in flouropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001; 61:5193–5201.
- Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999; 117:123–131.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349:247–257.
- Ratain MJ. Personalized medicine: building the GPS to take us there. Clin Pharmacol Ther 2007; 81:321–322.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation [published online ahead of print November 7, 2007]. Circulation 2007; 116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312
- Price MJ, Angiolillo DJ, Teirstein PS, et al .Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial [published online ahead of print August 29, 2011]. Circulation 2011; 124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165
- Sharp RR, Goldlust ME, Eng C. Addressing gaps in physician education using personal genomic testing. Genet Med 2011; 13:750–751.
- Wiener CM, Thomas PA, Goodspeed E, Valle D, Nichols DG. “Genes to society”—the logic and process of the new curriculum for the Johns Hopkins University School of Medicine. Acad Med 2010; 85:498–506.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a costeffectiveness analysis. Ann Intern Med 2011; 155:69–79.
- Leach B, Eng C, Kalady M, et al. Sharing the responsibility: multidisciplinary model improves colorectal cancer microsatellite testing. Paper presented at: InSight 2009 Annual Conference: September 2009; Orlando, FL.
- Manolio TA, Chisolm R, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med Forthcoming.
Personalized healthcare is the tailoring of medical management and patient care to the individual characteristics of each patient. This is achieved by incorporating the genetic and genomic makeup of an individual and his or her family medical history, environment, health-related behaviors, culture, and values into a complete health picture that can be used to customize care. Another level of personalization, often called personalized medicine, involves the selection of drug therapy through the use of tests to determine the genes and gene interactions that can reliably predict an individual’s response to a given therapy. This white paper focuses largely on the use of personalized healthcare as a risk prediction tool.
CURRENT STATUS OF PERSONALIZED HEALTHCARE
The case for personalized healthcare: Seeking value
To fully appreciate the need to advance the adoption of personalized healthcare into the delivery of medicine, one must consider the operation of our current healthcare system and its inefficiencies in terms of delivery and cost, its imprecision in the selection of therapies, and its inability to optimize outcomes. The framework of the US healthcare system as it is now constructed is expensive, disease-directed (instead of health- and wellness-directed), fragmented, and complex. While gross domestic product (GDP) in the United States has increased by approximately 3% per year,2 the compounded growth rate of healthcare expenditures is 6.1% per year. Healthcare in the aggregate now represents 17.6% of GDP and 27% of spending by the federal government and consumes 28% of the average household’s discretionary spending, surpassed only by housing.3
Personalized healthcare can potentially address the need for value consistent with the healthcare system’s prominent share of the US economy. The growth in healthcare spending is certain to be a target of the newly created Joint Select Committee on Deficit Reduction (created by the Budget Control Act of 2011), which is tasked with deficit reduction of at least $1.5 trillion over a 10-year period.
The need to address healthcare costs has been recognized in the Patient Protection and Affordable Care Act, a central feature of which is the creation of integrated health systems that pay for value based on quality, cost containment, and consumer experience. The legislation was enacted to transform healthcare in a variety of ways to make it more sustainable. The Patient Protection and Affordable Care Act seeks to end fragmentation by expanding the use of information technology to reorganize the delivery system and to prevent errors, shifting from volume-based incentives to incentives based on performance and outcomes, and rewarding effective healthcare delivery measures and good patient outcomes.
A shift from reactive to proactive
The premise behind personalized healthcare is the potential for more efficient healthcare, with the assumption that efficiency translates to lower cost and improved patient care.
Although healthcare reform is most often referred to in the context of improving access to care through insurance coverage mandates, true healthcare reform shifts current healthcare models from the practice of reactive medicine to the practice of proactive medicine, in which the tools of personalized healthcare (ie, genetics, genomics, and other molecular diagnostics) enable not only better quality of care but also less expensive care.
Several personalized tools have long been accepted into mainstream medicine. Two examples are the family history, which is the least expensive and most available genetic evaluation tool, and ABO blood typing for safe transfusions (as ABO blood types are alleles of a gene). In fact, much of what is now considered mainstream medical management was at one time considered new. To allow further evolution of medical practice, our challenge is to open our minds to the possibility that personalized proactive medicine can improve healthcare.
The new vision: More precise management
The trial-and-error approach to treating disease is inefficient and costly. Many drugs are effective for only about 50% of patients, often leading to switching or intensification of therapy that requires multiple patient visits.
Personalized medicine considers pharmacokinetic and other characteristics in selection of drug dosages. Genomic testing has the potential to provide clearer insight into the more successful use of currently available medicines. Treatment decisions (ie, drug and drug dosage choice) made on the basis of pharmacogenomic testing should increase adherence through greater effectiveness and fewer adverse drug reactions.
A massive amount of waste is related to pharmaceutical nonadherence and noncompliance. The New England Healthcare Institute has estimated that medication nonadherence costs the healthcare system $290 billion annually.4 Methodologies targeted at individual patients to improve adherence to drug regimens could save the healthcare system a tremendous amount of money.
Cancer management as a model for personalized healthcare. Personalization of therapy is especially suited to cancer management, given that the response to nonspecific cancer chemotherapy is suboptimal in most patients yet exposes them to adverse effects.5 Large-scale sequencing of human cancer genomes is rapidly changing the understanding of cancer biology and is identifying new targets in difficult-to-treat diseases and causes of drug resistance. Applying this information can achieve cost savings by avoiding the use of treatments that are ineffective in particular patients.
Overexpression of genetic mutations renders some cancers less susceptible to certain treatments, but has opened the door to individualized molecularly guided treatment strategies. For example, among patients with non–small cell lung cancer, mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain predict response to EGFR tyrosine kinase inhibitors, and anaplastic lymphoma kinase (ALK) inhibitors induce response in patients harboring a mutation in EML4-ALK genes. The recognition that human epidermal growth factor receptor (HER)-2 overexpression as a result of ERBB2 gene amplification occurs in as many as 20% of human breast cancers paved the way for the development of HER-2–targeted therapies. Patients with advanced colorectal cancer whose tumors express the KRAS gene mutation do not benefit from an EGFR inhibitor, whereas those with wild-type KRAS have improved survival with EGFR inhibitor treatment.6
BARRIERS TO THE APPLICATION OF PERSONALIZED HEALTHCARE
The availability and potential of personalized healthcare services and technology is not universally recognized or appreciated by consumers and clinicians. This lack of awareness contributes to a shortage of public support and limited demand for such services. Other barriers include misperceptions regarding the impact of personalized healthcare on disease management, limited incentives to use the available technology, and a knowledge gap among healthcare providers.
Lack of awareness and support
As applications of personalized healthcare advance to the point of clinical relevance, it is important to consider strategies for effective implementation into healthcare practice. Personalized healthcare, when more fully implemented, promises to accelerate the progress that healthcare reform hopes to achieve.
A major challenge to widespread adoption of personalized healthcare is limited recognition by the public and some healthcare providers that personalized healthcare can help to achieve better value. For personalized medicine to be embraced, the concept of “helix to health,” or translation of knowledge to the clinical setting, must resonate with the general public. Despite lack of public and provider awareness, the Personalized Medicine Coalition (PMC) has documented the existence of 56 personalized treatment and diagnostic products. Further, more than 200 product labels now recommend genetic testing prior to use to identify likely responders or inform of the influence of genetic variation on safety and effectiveness.
Consumers’ confidence in the efficacy and safety of medicines they take might contribute to the absence of public support for personalized healthcare. Similarly, despite the availability of genomic tests and tools, many physicians who might be advocates for personalized healthcare do not see the relevance of genomic medicine to their practices in terms of direct benefit to patient care.7
Apart from clinicians and consumers, support is also weak among health insurers and employers, even though the return on investment for personalized healthcare may be profound. Payers await the economic outcomes data that are crucial for their commitment to personalized healthcare. In addition, some have concerns about the ethical implications of personalized healthcare (see “Managing Genomic Information Responsibly”).
Perception of impact on treatment and prevention
A frequent criticism of genomics in medicine is that a genetic diagnosis does not help with patient management. In fact, surveillance and management of patients and family members often changes in response to a genetic diagnosis; knowing which gene is involved personalizes medical management. An example is the management of hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, which is the most common form of hereditary colon cancer. For a person with HNPCC, the lifetime risk of developing colorectal cancer is approximately 80%. Lynch syndrome is caused by germline mutations in one of three major mismatch repair (MMR) genes (MLH1, MSH2, and MSH6), and it predisposes to other cancers—uterine, stomach, and ovarian—as well. In women with Lynch syndrome, the lifetime risk for uterine cancer is 40%, compared with 4% in the general population.
At least 90% of patients with Lynch syndrome can be detected through MMR testing via microsatellite instability (MSI) or immunohistochemistry (IHC).8 MSI is a cellular phenotype that indicates a deficiency in at least one DNA MMR protein.
Although 5-fluorouracil–based chemo therapy is the standard of care for treatment of colorectal cancer, it confers no survival advantage in patients with MMR-IHC null (lack of expression of the gene) or MSI-high sporadic colorectal cancer.9,10 Knowing the status of MMR proteins, therefore, would alter the decision regarding neoadjuvant and adjuvant chemotherapy.
Perception of value
Implementation of pharmacogenomics into clinical practice has lagged. One major reason is the lack of an obvious business model for a product that may only be required once in an individual patient’s lifetime.11
A second barrier to integration lies in the limited demand for pharmacogenomics from physicians. This may be related partly to limited expertise in genetics among many physicians and to significant pushback from payers against today’s costs. Without reimbursement, little incentive exists for pharmacogenomics diagnostics. The incentive for physicians is further depressed, perhaps appropriately, when randomized controlled studies fail to demonstrate improved clinical outcomes with the use of pharmacogenomicbased treatment strategies. Two such examples are genotype-guided warfarin dosing, which failed in a randomized controlled trial to improve the proportion of international normalized ratios in the therapeutic range,12 and dosing of clopidogrel based on platelet reactivity, which did not improve outcomes after percutaneous coronary intervention compared with standard dosing in a randomized double-blind clinical trial.13
A significant delay in obtaining the results of pharmacogenomics testing, which also postpones the prescribing encounter, is another major drawback.

A knowledge gap persists

At present, delivery of personalized healthcare is not part of the usual training of physicians and other healthcare providers who are the gatekeepers of medicine. Few medical schools incorporate human and medical genetics, genomics, and pharmacogenomics into their curricula. Genetics is inadequately emphasized in residency curricula outside of pediatrics, family medicine, and obstetrics/gynecology.
The resulting knowledge gap is a fundamental factor in the lack of interest in using genomics in clinical medicine. Educating consumers and physicians at all levels, including specialty societies as well as insurers, will be key to expanding utilization of personalized healthcare. Educating payers and providing them with more data on economic outcomes associated with personalized healthcare will be necessary for adoption into clinical practice; implementation will lag as long as reimbursement decisions do not support personalized approaches to medicine.
As DNA sequencing technology has become less expensive and more powerful, companies have begun to market personal genomic testing. As a result, patients who use these services will increasingly want to discuss the results with their physicians. A significant number of clinicians are unfamiliar with personal genomic testing and emerging genetic testing options. In one survey of physicians who attended educational sessions that discussed recent developments in clinical genetics, only 37% indicated that they were familiar with recent genetic research that affected their patients.14
Targeted education will enhance physicians’ understanding of probabilities and risk estimates from the use of genomic testing; it will also improve recognition of potential causes of patient anxiety, gene variants of unknown significance, and follow-up tests and procedures that can add to expense. Nonphysician healthcare providers (ie, nurses and physician assistants) of direct care also will benefit from education.
INTEGRATING PERSONALIZED HEALTHCARE INTO CLINICAL PRACTICE
Practice standardization and an overhaul of the health information technology (HIT) infrastructure are needed if we are to reap the potential benefits of personalized healthcare. Creative approaches to practitioner education, which are being used in some institutions, must become more widespread. Similarly, the models for successful integration of personalized healthcare that have been achieved in some settings also can be implemented in other institutions.
Data collection and integration must be prioritized
Personalized healthcare can be both predictive and preventive, but moving past the disruptive phase of personalized healthcare will require a radical transformation of the healthcare “ecosystem” and HIT infrastructure.
Although data collection in the current system is extensive, data sharing and data management are inadequate. The pace at which HIT links clinical and genetic information must be accelerated. HIT will expedite innovation and implementation of personalized healthcare, allowing greater integration of data to permit improved data analysis capability. The ultimate goal is to create an interoperable system that connects these data across hospitals and clinicians to help clinicians interpret genomic and other risk information to better inform patient care.
Fully integrated health systems support better coordination of care and optimize the treatment of individual patients: linking research findings, treatment guidelines, treatment outcomes based on genetic profiles, and the individual patient’s own genetic profile will help to personalize treatments. Genomic information added to an individual’s electronic medical record along with improved data-sharing will facilitate clinicians’ ability to retrieve outcomes data based on patient characteristics.
Care models must be standardized, evidence-based practices must be executed, and care must be coordinated yet decentralized. In this way, clinicians can use the electronic medical record as an interoperable patient record to determine a personalized pathway to patient management. Standardization reduces variability in practice and permits seamless execution of care. Automation is imperative to achieving standardization, irrespective of the care supervisor. Investments must therefore be made to stimulate electronic medical record decision support.
In addition, larger data sets will be needed to identify the types of patients likely to respond to a treatment. Ideal data sets would be large enough to have adequate statistical power, be publicly available, standardize the collection of data with respect to response to therapy and toxicity, and contain data on concomitant collections of biologic samples.
Reimbursement must keep pace with medical advances
Payer willingness to reimburse for genomic tests and treatments will determine the pace of integration of personalized healthcare into clinical practice. Evidence that enhanced value can be derived from personalized approaches to medicine must be generated before personalized healthcare gains widespread acceptance by payers.
In addition, care-coordinated models must be developed to promote a value-based agenda that facilitates physician accountability and encourages clinical integration.
Innovative approaches are needed to educate providers
Development of point-of-care tools. Because information overload and lack of time are obstacles to clinicians’ efforts to incorporate genomic information into clinical practice, emphasis must be placed on genomic applications that have demonstrated utility. Engaging busy clinicians with point-of-care tools will maximize the relevance of the genomic information they receive and encourage effective use of their time. Decision-making should be supported through automatic risk assessment and management recommendations.
Educational tools. The National Coalition for Health Professional Education in Genetics (NCHPEG) was borne out of the recognition that the pace of genomic discovery far exceeds the pace at which healthcare providers can be educated. Its vision is to improve healthcare through informed use of genomic resources. NCHPEG is a member-based organization whose stakeholders include professional societies, hospitals, advocacy groups, and industry; it attempts to identify the specific educational needs for particular target audiences and then address these needs. It achieves its goals through the use of point-of-care tools and educational programs for continuing medical education credit.
One NCHPEG tool is the Pregnancy and Health Profile, which is a risk assessment and screening tool that attempts to improve the identification of women and babies at risk of developing genetic disease. It collects personal and family history information, performs a risk assessment for the clinician, and provides clinical decision support and education.
Another example of an educational tool is the “Genes to Society” curriculum initiated by The Johns Hopkins University School of Medicine in August 2009. The curriculum is being used as “the foundation for the scientific and clinical career development of future physicians.”15
Using personal genomic testing for education. The number of direct-to-consumer genomic tests is growing, and their market penetration will only increase as the cost of supplying a personal genome continues to decline. Whole genome scanning is being offered with the promise of identifying genetic predisposition to multiple diseases.
Participation in personal genomic testing may be a useful educational tool. Medical students, residents, and practicing physicians who participate in testing may be better equipped to advise patients about the processes involved and the potential utility and limitations of direct-to-consumer genotyping.14
Some companies that offer direct-to-consumer genomic testing provide telephone support from genetic counselors to help clients and their healthcare providers manage genetic information. Counselor services include identifying hereditary risks and reviewing diagnostic, preventive, and early-detection options.
Implementing pharmacogenomics into practice: Decision support systems are needed
A genomic decision support system that guides medication prescribing is needed to implement pharmacogenomic diagnostics. For such a system to achieve the goal of selecting the best medication for each individual, it must do the following:
- Test all polymorphisms relevant to the prescribing of any medication
- Be completed with no out-of-pocket cost to the patient
- Be performed before the patient requires the medication
- Provide results that will be interpreted as part of an individualized pharmacogenomics consult.11
The 1200 Patients Project, a pilot research study under way at the Center for Personalized Therapeutics at the University of Chicago, is attempting to demonstrate the feasibility of incorporating pharmacogenomic testing into routine clinical practice for medication treatment decisions. DNA samples from patients who are taking at least one prescription medication are being tested for differences in genes that may suggest greater effectiveness or an increased risk of side effects from certain medications.
Solutions in practice
Cleveland Clinic’s genetics-based management of Lynch syndrome, the integration of genetics services during patient appointments at Cleveland Clinic, and a coordinated approach at The Ohio State University Medical Center are examples of practical applications of personalized healthcare.
Colorectal cancer management. One example of a personalized approach to medicine that improves health outcome while achieving cost savings is the genetics-based approach to HNPCC (Lynch syndrome) at Cleveland Clinic.
Early identification of Lynch syndrome by screening all colorectal cancer patients has been shown to save $250,000 per life-year gained in the United States.16 All colorectal cancers resected at the Cleveland Clinic main campus are routinely screened for MSI and IHC, and the process is embedded into the routine pathology workflow. With the patients’ foreknowledge, a gastrointestinal cancer genetics counselor scans the list of MSI and IHC results each week. Patients who are MSI-high or IHC-null are invited to receive genetic counseling and consider germline single-gene testing guided by the IHC results. With this active approach, patient uptake is 80%; in comparison, with a passive approach (MSI/IHC results are placed in the pathology report), the uptake is 14%17 (B. Leach and C. Eng, unpublished data, 2011).The successful application of the active approach requires the close cooperation of multiple disciplines, including members of the Cleveland Clinic Genomic Medicine, Pathology & Laboratory Medicine, and Digestive Disease Institutes.18
Integrating genetics-based care at Cleveland Clinic. Time delays for genetics services and limited collaboration with managing physicians who are not genetics specialists reduces genetics-based access and availability. Broad access to genetics clinical services is a means of clinical integration of genetics-enabled care. Providing patients and healthcare providers with easy access and short wait times is vital for clinical integration of genetics-enabled personalized healthcare.
As part of a patient-centered focus on medicine, clinical genetics services have been integrated throughout Cleveland Clinic. The system has two genetics clinics at its main campus and has embedded multiple genetics satellites within its nongenetics clinics, easing access. Genetics counselors are stationed in the same areas of practice as referring providers. Although patient encounters have increased at the medical genetics clinic in the Genomic Medicine Institute, genetics consultations no longer require an extra trip to the clinic since they are integrated into existing appointments. With this approach, large numbers of patients can be seen with no wait times.
Coordinated care at The Ohio State University Medical Center. The Center for Personalized Health Care at The Ohio State University Medical Center (OSUMC) embraces a systems-based care-coordinated model that improves care by executing standardized processes and automating routine tasks. The Institute for Systems Biology, which was established to develop genomics, wellness, and chronic disease biomarkers, collaborates with OSUMC on pilot projects in chronic disease, including cancer.
The OSUMC has a closed system in which it is the payer, employer, and provider of healthcare. This closed system serves as an ideal testing ground for reform. Goals include intervention in disease before symptoms appear and maintenance of wellness. The data from these demonstration projects should facilitate adoption of personalized healthcare by improving physician acceptance of personalized approaches and satisfying payers that personalized healthcare is cost-effective.

Personalized healthcare is the tailoring of medical management and patient care to the individual characteristics of each patient. This is achieved by incorporating the genetic and genomic makeup of an individual and his or her family medical history, environment, health-related behaviors, culture, and values into a complete health picture that can be used to customize care. Another level of personalization, often called personalized medicine, involves the selection of drug therapy through the use of tests to determine the genes and gene interactions that can reliably predict an individual’s response to a given therapy. This white paper focuses largely on the use of personalized healthcare as a risk prediction tool.
CURRENT STATUS OF PERSONALIZED HEALTHCARE
The case for personalized healthcare: Seeking value
To fully appreciate the need to advance the adoption of personalized healthcare into the delivery of medicine, one must consider the operation of our current healthcare system and its inefficiencies in terms of delivery and cost, its imprecision in the selection of therapies, and its inability to optimize outcomes. The framework of the US healthcare system as it is now constructed is expensive, disease-directed (instead of health- and wellness-directed), fragmented, and complex. While gross domestic product (GDP) in the United States has increased by approximately 3% per year,2 the compounded growth rate of healthcare expenditures is 6.1% per year. Healthcare in the aggregate now represents 17.6% of GDP and 27% of spending by the federal government and consumes 28% of the average household’s discretionary spending, surpassed only by housing.3
Personalized healthcare can potentially address the need for value consistent with the healthcare system’s prominent share of the US economy. The growth in healthcare spending is certain to be a target of the newly created Joint Select Committee on Deficit Reduction (created by the Budget Control Act of 2011), which is tasked with deficit reduction of at least $1.5 trillion over a 10-year period.
The need to address healthcare costs has been recognized in the Patient Protection and Affordable Care Act, a central feature of which is the creation of integrated health systems that pay for value based on quality, cost containment, and consumer experience. The legislation was enacted to transform healthcare in a variety of ways to make it more sustainable. The Patient Protection and Affordable Care Act seeks to end fragmentation by expanding the use of information technology to reorganize the delivery system and to prevent errors, shifting from volume-based incentives to incentives based on performance and outcomes, and rewarding effective healthcare delivery measures and good patient outcomes.
A shift from reactive to proactive
The premise behind personalized healthcare is the potential for more efficient healthcare, with the assumption that efficiency translates to lower cost and improved patient care.
Although healthcare reform is most often referred to in the context of improving access to care through insurance coverage mandates, true healthcare reform shifts current healthcare models from the practice of reactive medicine to the practice of proactive medicine, in which the tools of personalized healthcare (ie, genetics, genomics, and other molecular diagnostics) enable not only better quality of care but also less expensive care.
Several personalized tools have long been accepted into mainstream medicine. Two examples are the family history, which is the least expensive and most available genetic evaluation tool, and ABO blood typing for safe transfusions (as ABO blood types are alleles of a gene). In fact, much of what is now considered mainstream medical management was at one time considered new. To allow further evolution of medical practice, our challenge is to open our minds to the possibility that personalized proactive medicine can improve healthcare.
The new vision: More precise management
The trial-and-error approach to treating disease is inefficient and costly. Many drugs are effective for only about 50% of patients, often leading to switching or intensification of therapy that requires multiple patient visits.
Personalized medicine considers pharmacokinetic and other characteristics in selection of drug dosages. Genomic testing has the potential to provide clearer insight into the more successful use of currently available medicines. Treatment decisions (ie, drug and drug dosage choice) made on the basis of pharmacogenomic testing should increase adherence through greater effectiveness and fewer adverse drug reactions.
A massive amount of waste is related to pharmaceutical nonadherence and noncompliance. The New England Healthcare Institute has estimated that medication nonadherence costs the healthcare system $290 billion annually.4 Methodologies targeted at individual patients to improve adherence to drug regimens could save the healthcare system a tremendous amount of money.
Cancer management as a model for personalized healthcare. Personalization of therapy is especially suited to cancer management, given that the response to nonspecific cancer chemotherapy is suboptimal in most patients yet exposes them to adverse effects.5 Large-scale sequencing of human cancer genomes is rapidly changing the understanding of cancer biology and is identifying new targets in difficult-to-treat diseases and causes of drug resistance. Applying this information can achieve cost savings by avoiding the use of treatments that are ineffective in particular patients.
Overexpression of genetic mutations renders some cancers less susceptible to certain treatments, but has opened the door to individualized molecularly guided treatment strategies. For example, among patients with non–small cell lung cancer, mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain predict response to EGFR tyrosine kinase inhibitors, and anaplastic lymphoma kinase (ALK) inhibitors induce response in patients harboring a mutation in EML4-ALK genes. The recognition that human epidermal growth factor receptor (HER)-2 overexpression as a result of ERBB2 gene amplification occurs in as many as 20% of human breast cancers paved the way for the development of HER-2–targeted therapies. Patients with advanced colorectal cancer whose tumors express the KRAS gene mutation do not benefit from an EGFR inhibitor, whereas those with wild-type KRAS have improved survival with EGFR inhibitor treatment.6
BARRIERS TO THE APPLICATION OF PERSONALIZED HEALTHCARE
The availability and potential of personalized healthcare services and technology is not universally recognized or appreciated by consumers and clinicians. This lack of awareness contributes to a shortage of public support and limited demand for such services. Other barriers include misperceptions regarding the impact of personalized healthcare on disease management, limited incentives to use the available technology, and a knowledge gap among healthcare providers.
Lack of awareness and support
As applications of personalized healthcare advance to the point of clinical relevance, it is important to consider strategies for effective implementation into healthcare practice. Personalized healthcare, when more fully implemented, promises to accelerate the progress that healthcare reform hopes to achieve.
A major challenge to widespread adoption of personalized healthcare is limited recognition by the public and some healthcare providers that personalized healthcare can help to achieve better value. For personalized medicine to be embraced, the concept of “helix to health,” or translation of knowledge to the clinical setting, must resonate with the general public. Despite lack of public and provider awareness, the Personalized Medicine Coalition (PMC) has documented the existence of 56 personalized treatment and diagnostic products. Further, more than 200 product labels now recommend genetic testing prior to use to identify likely responders or inform of the influence of genetic variation on safety and effectiveness.
Consumers’ confidence in the efficacy and safety of medicines they take might contribute to the absence of public support for personalized healthcare. Similarly, despite the availability of genomic tests and tools, many physicians who might be advocates for personalized healthcare do not see the relevance of genomic medicine to their practices in terms of direct benefit to patient care.7
Apart from clinicians and consumers, support is also weak among health insurers and employers, even though the return on investment for personalized healthcare may be profound. Payers await the economic outcomes data that are crucial for their commitment to personalized healthcare. In addition, some have concerns about the ethical implications of personalized healthcare (see “Managing Genomic Information Responsibly”).
Perception of impact on treatment and prevention
A frequent criticism of genomics in medicine is that a genetic diagnosis does not help with patient management. In fact, surveillance and management of patients and family members often changes in response to a genetic diagnosis; knowing which gene is involved personalizes medical management. An example is the management of hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, which is the most common form of hereditary colon cancer. For a person with HNPCC, the lifetime risk of developing colorectal cancer is approximately 80%. Lynch syndrome is caused by germline mutations in one of three major mismatch repair (MMR) genes (MLH1, MSH2, and MSH6), and it predisposes to other cancers—uterine, stomach, and ovarian—as well. In women with Lynch syndrome, the lifetime risk for uterine cancer is 40%, compared with 4% in the general population.
At least 90% of patients with Lynch syndrome can be detected through MMR testing via microsatellite instability (MSI) or immunohistochemistry (IHC).8 MSI is a cellular phenotype that indicates a deficiency in at least one DNA MMR protein.
Although 5-fluorouracil–based chemo therapy is the standard of care for treatment of colorectal cancer, it confers no survival advantage in patients with MMR-IHC null (lack of expression of the gene) or MSI-high sporadic colorectal cancer.9,10 Knowing the status of MMR proteins, therefore, would alter the decision regarding neoadjuvant and adjuvant chemotherapy.
Perception of value
Implementation of pharmacogenomics into clinical practice has lagged. One major reason is the lack of an obvious business model for a product that may only be required once in an individual patient’s lifetime.11
A second barrier to integration lies in the limited demand for pharmacogenomics from physicians. This may be related partly to limited expertise in genetics among many physicians and to significant pushback from payers against today’s costs. Without reimbursement, little incentive exists for pharmacogenomics diagnostics. The incentive for physicians is further depressed, perhaps appropriately, when randomized controlled studies fail to demonstrate improved clinical outcomes with the use of pharmacogenomicbased treatment strategies. Two such examples are genotype-guided warfarin dosing, which failed in a randomized controlled trial to improve the proportion of international normalized ratios in the therapeutic range,12 and dosing of clopidogrel based on platelet reactivity, which did not improve outcomes after percutaneous coronary intervention compared with standard dosing in a randomized double-blind clinical trial.13
A significant delay in obtaining the results of pharmacogenomics testing, which also postpones the prescribing encounter, is another major drawback.

A knowledge gap persists

At present, delivery of personalized healthcare is not part of the usual training of physicians and other healthcare providers who are the gatekeepers of medicine. Few medical schools incorporate human and medical genetics, genomics, and pharmacogenomics into their curricula. Genetics is inadequately emphasized in residency curricula outside of pediatrics, family medicine, and obstetrics/gynecology.
The resulting knowledge gap is a fundamental factor in the lack of interest in using genomics in clinical medicine. Educating consumers and physicians at all levels, including specialty societies as well as insurers, will be key to expanding utilization of personalized healthcare. Educating payers and providing them with more data on economic outcomes associated with personalized healthcare will be necessary for adoption into clinical practice; implementation will lag as long as reimbursement decisions do not support personalized approaches to medicine.
As DNA sequencing technology has become less expensive and more powerful, companies have begun to market personal genomic testing. As a result, patients who use these services will increasingly want to discuss the results with their physicians. A significant number of clinicians are unfamiliar with personal genomic testing and emerging genetic testing options. In one survey of physicians who attended educational sessions that discussed recent developments in clinical genetics, only 37% indicated that they were familiar with recent genetic research that affected their patients.14
Targeted education will enhance physicians’ understanding of probabilities and risk estimates from the use of genomic testing; it will also improve recognition of potential causes of patient anxiety, gene variants of unknown significance, and follow-up tests and procedures that can add to expense. Nonphysician healthcare providers (ie, nurses and physician assistants) of direct care also will benefit from education.
INTEGRATING PERSONALIZED HEALTHCARE INTO CLINICAL PRACTICE
Practice standardization and an overhaul of the health information technology (HIT) infrastructure are needed if we are to reap the potential benefits of personalized healthcare. Creative approaches to practitioner education, which are being used in some institutions, must become more widespread. Similarly, the models for successful integration of personalized healthcare that have been achieved in some settings also can be implemented in other institutions.
Data collection and integration must be prioritized
Personalized healthcare can be both predictive and preventive, but moving past the disruptive phase of personalized healthcare will require a radical transformation of the healthcare “ecosystem” and HIT infrastructure.
Although data collection in the current system is extensive, data sharing and data management are inadequate. The pace at which HIT links clinical and genetic information must be accelerated. HIT will expedite innovation and implementation of personalized healthcare, allowing greater integration of data to permit improved data analysis capability. The ultimate goal is to create an interoperable system that connects these data across hospitals and clinicians to help clinicians interpret genomic and other risk information to better inform patient care.
Fully integrated health systems support better coordination of care and optimize the treatment of individual patients: linking research findings, treatment guidelines, treatment outcomes based on genetic profiles, and the individual patient’s own genetic profile will help to personalize treatments. Genomic information added to an individual’s electronic medical record along with improved data-sharing will facilitate clinicians’ ability to retrieve outcomes data based on patient characteristics.
Care models must be standardized, evidence-based practices must be executed, and care must be coordinated yet decentralized. In this way, clinicians can use the electronic medical record as an interoperable patient record to determine a personalized pathway to patient management. Standardization reduces variability in practice and permits seamless execution of care. Automation is imperative to achieving standardization, irrespective of the care supervisor. Investments must therefore be made to stimulate electronic medical record decision support.
In addition, larger data sets will be needed to identify the types of patients likely to respond to a treatment. Ideal data sets would be large enough to have adequate statistical power, be publicly available, standardize the collection of data with respect to response to therapy and toxicity, and contain data on concomitant collections of biologic samples.
Reimbursement must keep pace with medical advances
Payer willingness to reimburse for genomic tests and treatments will determine the pace of integration of personalized healthcare into clinical practice. Evidence that enhanced value can be derived from personalized approaches to medicine must be generated before personalized healthcare gains widespread acceptance by payers.
In addition, care-coordinated models must be developed to promote a value-based agenda that facilitates physician accountability and encourages clinical integration.
Innovative approaches are needed to educate providers
Development of point-of-care tools. Because information overload and lack of time are obstacles to clinicians’ efforts to incorporate genomic information into clinical practice, emphasis must be placed on genomic applications that have demonstrated utility. Engaging busy clinicians with point-of-care tools will maximize the relevance of the genomic information they receive and encourage effective use of their time. Decision-making should be supported through automatic risk assessment and management recommendations.
Educational tools. The National Coalition for Health Professional Education in Genetics (NCHPEG) was borne out of the recognition that the pace of genomic discovery far exceeds the pace at which healthcare providers can be educated. Its vision is to improve healthcare through informed use of genomic resources. NCHPEG is a member-based organization whose stakeholders include professional societies, hospitals, advocacy groups, and industry; it attempts to identify the specific educational needs for particular target audiences and then address these needs. It achieves its goals through the use of point-of-care tools and educational programs for continuing medical education credit.
One NCHPEG tool is the Pregnancy and Health Profile, which is a risk assessment and screening tool that attempts to improve the identification of women and babies at risk of developing genetic disease. It collects personal and family history information, performs a risk assessment for the clinician, and provides clinical decision support and education.
Another example of an educational tool is the “Genes to Society” curriculum initiated by The Johns Hopkins University School of Medicine in August 2009. The curriculum is being used as “the foundation for the scientific and clinical career development of future physicians.”15
Using personal genomic testing for education. The number of direct-to-consumer genomic tests is growing, and their market penetration will only increase as the cost of supplying a personal genome continues to decline. Whole genome scanning is being offered with the promise of identifying genetic predisposition to multiple diseases.
Participation in personal genomic testing may be a useful educational tool. Medical students, residents, and practicing physicians who participate in testing may be better equipped to advise patients about the processes involved and the potential utility and limitations of direct-to-consumer genotyping.14
Some companies that offer direct-to-consumer genomic testing provide telephone support from genetic counselors to help clients and their healthcare providers manage genetic information. Counselor services include identifying hereditary risks and reviewing diagnostic, preventive, and early-detection options.
Implementing pharmacogenomics into practice: Decision support systems are needed
A genomic decision support system that guides medication prescribing is needed to implement pharmacogenomic diagnostics. For such a system to achieve the goal of selecting the best medication for each individual, it must do the following:
- Test all polymorphisms relevant to the prescribing of any medication
- Be completed with no out-of-pocket cost to the patient
- Be performed before the patient requires the medication
- Provide results that will be interpreted as part of an individualized pharmacogenomics consult.11
The 1200 Patients Project, a pilot research study under way at the Center for Personalized Therapeutics at the University of Chicago, is attempting to demonstrate the feasibility of incorporating pharmacogenomic testing into routine clinical practice for medication treatment decisions. DNA samples from patients who are taking at least one prescription medication are being tested for differences in genes that may suggest greater effectiveness or an increased risk of side effects from certain medications.
Solutions in practice
Cleveland Clinic’s genetics-based management of Lynch syndrome, the integration of genetics services during patient appointments at Cleveland Clinic, and a coordinated approach at The Ohio State University Medical Center are examples of practical applications of personalized healthcare.
Colorectal cancer management. One example of a personalized approach to medicine that improves health outcome while achieving cost savings is the genetics-based approach to HNPCC (Lynch syndrome) at Cleveland Clinic.
Early identification of Lynch syndrome by screening all colorectal cancer patients has been shown to save $250,000 per life-year gained in the United States.16 All colorectal cancers resected at the Cleveland Clinic main campus are routinely screened for MSI and IHC, and the process is embedded into the routine pathology workflow. With the patients’ foreknowledge, a gastrointestinal cancer genetics counselor scans the list of MSI and IHC results each week. Patients who are MSI-high or IHC-null are invited to receive genetic counseling and consider germline single-gene testing guided by the IHC results. With this active approach, patient uptake is 80%; in comparison, with a passive approach (MSI/IHC results are placed in the pathology report), the uptake is 14%17 (B. Leach and C. Eng, unpublished data, 2011).The successful application of the active approach requires the close cooperation of multiple disciplines, including members of the Cleveland Clinic Genomic Medicine, Pathology & Laboratory Medicine, and Digestive Disease Institutes.18
Integrating genetics-based care at Cleveland Clinic. Time delays for genetics services and limited collaboration with managing physicians who are not genetics specialists reduces genetics-based access and availability. Broad access to genetics clinical services is a means of clinical integration of genetics-enabled care. Providing patients and healthcare providers with easy access and short wait times is vital for clinical integration of genetics-enabled personalized healthcare.
As part of a patient-centered focus on medicine, clinical genetics services have been integrated throughout Cleveland Clinic. The system has two genetics clinics at its main campus and has embedded multiple genetics satellites within its nongenetics clinics, easing access. Genetics counselors are stationed in the same areas of practice as referring providers. Although patient encounters have increased at the medical genetics clinic in the Genomic Medicine Institute, genetics consultations no longer require an extra trip to the clinic since they are integrated into existing appointments. With this approach, large numbers of patients can be seen with no wait times.
Coordinated care at The Ohio State University Medical Center. The Center for Personalized Health Care at The Ohio State University Medical Center (OSUMC) embraces a systems-based care-coordinated model that improves care by executing standardized processes and automating routine tasks. The Institute for Systems Biology, which was established to develop genomics, wellness, and chronic disease biomarkers, collaborates with OSUMC on pilot projects in chronic disease, including cancer.
The OSUMC has a closed system in which it is the payer, employer, and provider of healthcare. This closed system serves as an ideal testing ground for reform. Goals include intervention in disease before symptoms appear and maintenance of wellness. The data from these demonstration projects should facilitate adoption of personalized healthcare by improving physician acceptance of personalized approaches and satisfying payers that personalized healthcare is cost-effective.

- Personalized medicine. Coriell Institute for Medical Research Web site. http://www.coriell.org/personalized-medicine. Updated 2011. Accessed December 27, 2011.
- The 2012 Statistical Abstract. U.S. Census Bureau Web site. http://www.census.gov/compendia/statab/cats/income_expenditures_poverty_wealth/gross_domestic_product_gdp.html. Updated September 27, 2011. Accessed December 22, 2011.
- National health expenditure fact sheet. Center for Medicare & Medicaid Services (CMS) Web site. https://www.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Updated November 4, 2011. Accessed December 22, 2011.
- New England Healthcare Institute (NEHI). Thinking outside the pillbox: A system-wide approach to improving patient medication adherence for chronic disease. NEHI Web site. http://www.nehi.net/publications/44/thinking_outside_the_pillbox_a_systemwide_approach_to_improving_patient_medication_adherence_for_chronic_disease. Published August 12, 2009. Accessed December 22, 2011.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001; 7:201–204.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359:1757–1765.
- Feero WG, Green ED. Genomics education for healthcare professionals in the 21st century. JAMA 2011; 306:989–990.
- Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in flouropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001; 61:5193–5201.
- Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999; 117:123–131.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349:247–257.
- Ratain MJ. Personalized medicine: building the GPS to take us there. Clin Pharmacol Ther 2007; 81:321–322.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation [published online ahead of print November 7, 2007]. Circulation 2007; 116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312
- Price MJ, Angiolillo DJ, Teirstein PS, et al .Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial [published online ahead of print August 29, 2011]. Circulation 2011; 124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165
- Sharp RR, Goldlust ME, Eng C. Addressing gaps in physician education using personal genomic testing. Genet Med 2011; 13:750–751.
- Wiener CM, Thomas PA, Goodspeed E, Valle D, Nichols DG. “Genes to society”—the logic and process of the new curriculum for the Johns Hopkins University School of Medicine. Acad Med 2010; 85:498–506.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a costeffectiveness analysis. Ann Intern Med 2011; 155:69–79.
- Leach B, Eng C, Kalady M, et al. Sharing the responsibility: multidisciplinary model improves colorectal cancer microsatellite testing. Paper presented at: InSight 2009 Annual Conference: September 2009; Orlando, FL.
- Manolio TA, Chisolm R, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med Forthcoming.
- Personalized medicine. Coriell Institute for Medical Research Web site. http://www.coriell.org/personalized-medicine. Updated 2011. Accessed December 27, 2011.
- The 2012 Statistical Abstract. U.S. Census Bureau Web site. http://www.census.gov/compendia/statab/cats/income_expenditures_poverty_wealth/gross_domestic_product_gdp.html. Updated September 27, 2011. Accessed December 22, 2011.
- National health expenditure fact sheet. Center for Medicare & Medicaid Services (CMS) Web site. https://www.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Updated November 4, 2011. Accessed December 22, 2011.
- New England Healthcare Institute (NEHI). Thinking outside the pillbox: A system-wide approach to improving patient medication adherence for chronic disease. NEHI Web site. http://www.nehi.net/publications/44/thinking_outside_the_pillbox_a_systemwide_approach_to_improving_patient_medication_adherence_for_chronic_disease. Published August 12, 2009. Accessed December 22, 2011.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001; 7:201–204.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359:1757–1765.
- Feero WG, Green ED. Genomics education for healthcare professionals in the 21st century. JAMA 2011; 306:989–990.
- Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in flouropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001; 61:5193–5201.
- Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999; 117:123–131.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349:247–257.
- Ratain MJ. Personalized medicine: building the GPS to take us there. Clin Pharmacol Ther 2007; 81:321–322.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation [published online ahead of print November 7, 2007]. Circulation 2007; 116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312
- Price MJ, Angiolillo DJ, Teirstein PS, et al .Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial [published online ahead of print August 29, 2011]. Circulation 2011; 124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165
- Sharp RR, Goldlust ME, Eng C. Addressing gaps in physician education using personal genomic testing. Genet Med 2011; 13:750–751.
- Wiener CM, Thomas PA, Goodspeed E, Valle D, Nichols DG. “Genes to society”—the logic and process of the new curriculum for the Johns Hopkins University School of Medicine. Acad Med 2010; 85:498–506.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a costeffectiveness analysis. Ann Intern Med 2011; 155:69–79.
- Leach B, Eng C, Kalady M, et al. Sharing the responsibility: multidisciplinary model improves colorectal cancer microsatellite testing. Paper presented at: InSight 2009 Annual Conference: September 2009; Orlando, FL.
- Manolio TA, Chisolm R, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med Forthcoming.