User login
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
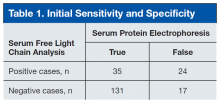
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
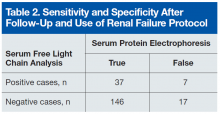
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
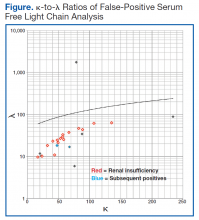
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.