User login
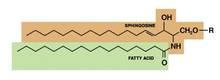
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at [email protected].
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at [email protected].
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at [email protected].