User login
Several different currently approved immunomodulatory therapies ameliorated arthritis symptoms in chikungunya-infected mice in two studies that separate teams of researchers published online Feb. 1 in Science Translational Medicine.
The first team, led by Jonathan J. Miner, MD, PhD, of Washington University in St. Louis tested six different approved oral and biologic antirheumatic agents (along with control agents) in chikungunya virus-infected mice with acute arthritis and foot swelling (Sci Transl Med. 2017;9:eaah3438).
When the researchers paired abatacept with an antiviral therapy (monoclonal anti-CHIKV human antibody) the combination “was highly effective at reducing joint inflammation, periarticular swelling, migration of inflammatory leukocytes, and infection, even when administered several days after virus inoculation,” Dr. Miner and his colleagues wrote.
The researchers concluded that a combination of anti-inflammatory and antibody-based antiviral therapy “may serve as a model for treating humans with arthritis caused by CHIKV or other related viruses.”
In the second study, researchers led by Teck-Hui Teo, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore, further elucidated the mechanisms by which CHIKV proteins act on T cells (Sci Transl Med. 2017;9:eaal1333). They also found that CHIKV-infected mice treated with fingolimod (Gilenya), a drug that blocks T-cell migration from the lymph nodes to the joints and is approved for the treatment of multiple sclerosis, saw reduced arthritis symptoms even without reduction of viral replication.
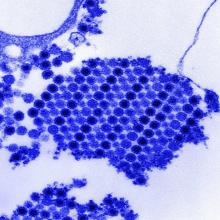
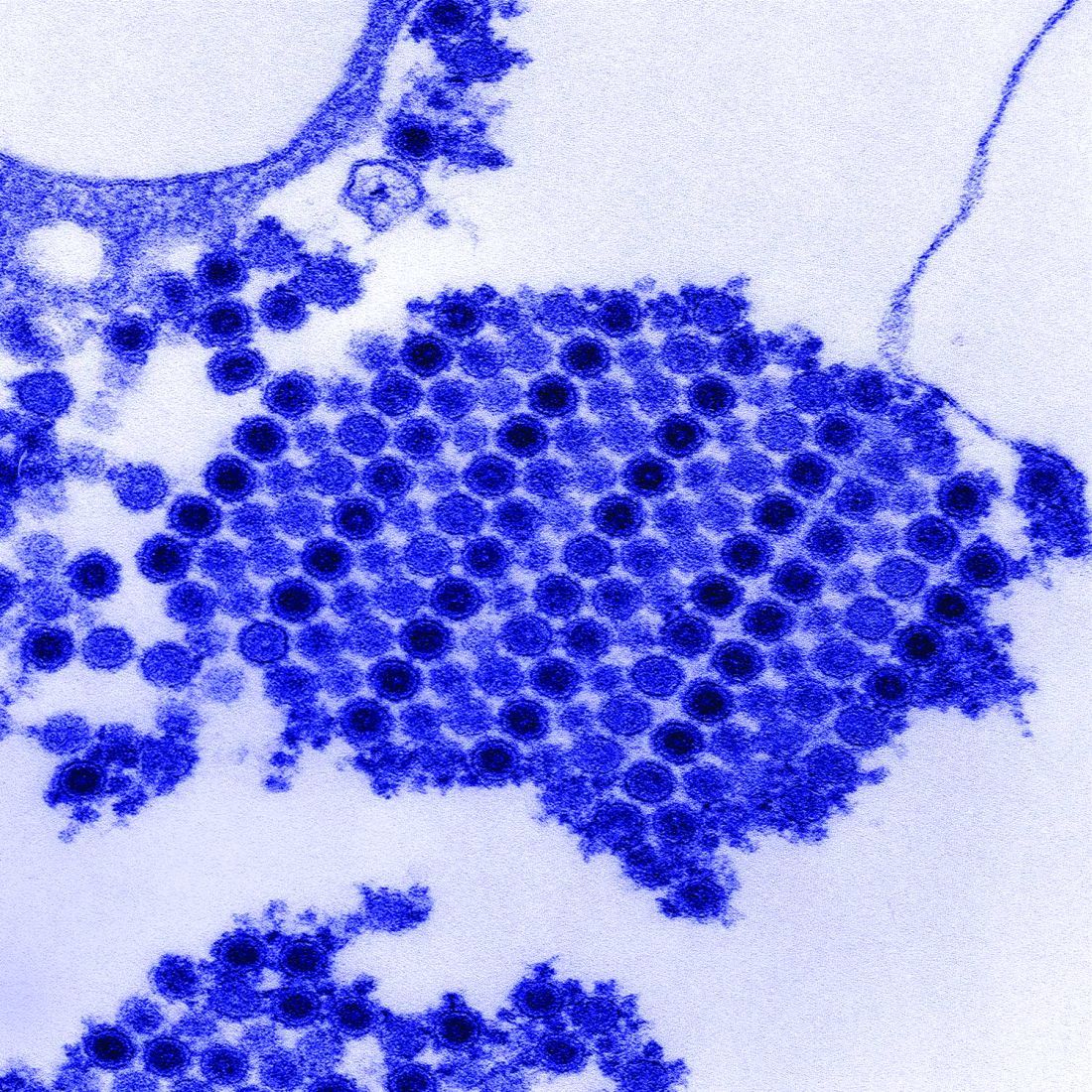
Infection with the chikungunya virus can produce arthritis that mimics symptoms of rheumatoid arthritis and may in some cases lead to joint damage. Though the mechanisms driving chikungunya-related arthritis are not well understood, preliminary studies have suggested a T-cell–mediated adverse response.
The Singapore team received funding from its own agency, A*STAR, while the Washington University researchers received grants from the National Institutes of Health and the Rheumatology Research Foundation. Two coauthors on the U.S. study reported extensive commercial conflicts, including consulting and advisory relationships with pharmaceutical and vaccine manufacturers, and one patent.
The studies by Dr. Miner and his colleagues and Dr. Teo and his associates demonstrate the potential value of combination therapies for ameliorating heightened T-cell responses and their pathogenic role in joint inflammation. They explored how T-cell responses could be blunted during ongoing viral replication to control overt inflammation, an approach that also may be valuable for treating immune-mediated tissue damage associated with other infectious agents.
Selective T-cell immunomodulatory therapies that offset damaging immune responses offer an attractive option for future pharmacologic interventions for treating chikungunya virus–induced inflammatory disease. The small market size and the rapid sporadic nature of outbreaks could be major obstacles to the development and deployment of virus-specific interventions such as therapeutic antiviral neutralizing monoclonal antibodies or even vaccines. Targeted drug and immunotherapy treatments are likely to offer practical and beneficial options for most patients with chikungunya.
Preliminary reports in humans have suggested that methotrexate may be effective for treating chikungunya virus–induced arthritis. In Dr. Miner and colleagues’ study, a low dose of methotrexate (0.3 mg/kg) was ineffective at treating acute joint swelling in mice. It remains to be addressed whether a higher dose of methotrexate for a longer time period could be of benefit in the setting of chronic chikungunya virus–induced arthritis.
Philippe Gasque, MD, PhD, is with the Université de La Réunion, Saint-Denis, Réunion, and Marie Christine Jaffar-Bandjee, MD, PhD, is with the Centre Hospitalier Universitaire Félix Guyon, Saint-Denis, Réunion. They made these remarks in an editorial (Sci Transl Med. 2017;9:eaam6567).
The studies by Dr. Miner and his colleagues and Dr. Teo and his associates demonstrate the potential value of combination therapies for ameliorating heightened T-cell responses and their pathogenic role in joint inflammation. They explored how T-cell responses could be blunted during ongoing viral replication to control overt inflammation, an approach that also may be valuable for treating immune-mediated tissue damage associated with other infectious agents.
Selective T-cell immunomodulatory therapies that offset damaging immune responses offer an attractive option for future pharmacologic interventions for treating chikungunya virus–induced inflammatory disease. The small market size and the rapid sporadic nature of outbreaks could be major obstacles to the development and deployment of virus-specific interventions such as therapeutic antiviral neutralizing monoclonal antibodies or even vaccines. Targeted drug and immunotherapy treatments are likely to offer practical and beneficial options for most patients with chikungunya.
Preliminary reports in humans have suggested that methotrexate may be effective for treating chikungunya virus–induced arthritis. In Dr. Miner and colleagues’ study, a low dose of methotrexate (0.3 mg/kg) was ineffective at treating acute joint swelling in mice. It remains to be addressed whether a higher dose of methotrexate for a longer time period could be of benefit in the setting of chronic chikungunya virus–induced arthritis.
Philippe Gasque, MD, PhD, is with the Université de La Réunion, Saint-Denis, Réunion, and Marie Christine Jaffar-Bandjee, MD, PhD, is with the Centre Hospitalier Universitaire Félix Guyon, Saint-Denis, Réunion. They made these remarks in an editorial (Sci Transl Med. 2017;9:eaam6567).
The studies by Dr. Miner and his colleagues and Dr. Teo and his associates demonstrate the potential value of combination therapies for ameliorating heightened T-cell responses and their pathogenic role in joint inflammation. They explored how T-cell responses could be blunted during ongoing viral replication to control overt inflammation, an approach that also may be valuable for treating immune-mediated tissue damage associated with other infectious agents.
Selective T-cell immunomodulatory therapies that offset damaging immune responses offer an attractive option for future pharmacologic interventions for treating chikungunya virus–induced inflammatory disease. The small market size and the rapid sporadic nature of outbreaks could be major obstacles to the development and deployment of virus-specific interventions such as therapeutic antiviral neutralizing monoclonal antibodies or even vaccines. Targeted drug and immunotherapy treatments are likely to offer practical and beneficial options for most patients with chikungunya.
Preliminary reports in humans have suggested that methotrexate may be effective for treating chikungunya virus–induced arthritis. In Dr. Miner and colleagues’ study, a low dose of methotrexate (0.3 mg/kg) was ineffective at treating acute joint swelling in mice. It remains to be addressed whether a higher dose of methotrexate for a longer time period could be of benefit in the setting of chronic chikungunya virus–induced arthritis.
Philippe Gasque, MD, PhD, is with the Université de La Réunion, Saint-Denis, Réunion, and Marie Christine Jaffar-Bandjee, MD, PhD, is with the Centre Hospitalier Universitaire Félix Guyon, Saint-Denis, Réunion. They made these remarks in an editorial (Sci Transl Med. 2017;9:eaam6567).
Several different currently approved immunomodulatory therapies ameliorated arthritis symptoms in chikungunya-infected mice in two studies that separate teams of researchers published online Feb. 1 in Science Translational Medicine.
The first team, led by Jonathan J. Miner, MD, PhD, of Washington University in St. Louis tested six different approved oral and biologic antirheumatic agents (along with control agents) in chikungunya virus-infected mice with acute arthritis and foot swelling (Sci Transl Med. 2017;9:eaah3438).
When the researchers paired abatacept with an antiviral therapy (monoclonal anti-CHIKV human antibody) the combination “was highly effective at reducing joint inflammation, periarticular swelling, migration of inflammatory leukocytes, and infection, even when administered several days after virus inoculation,” Dr. Miner and his colleagues wrote.
The researchers concluded that a combination of anti-inflammatory and antibody-based antiviral therapy “may serve as a model for treating humans with arthritis caused by CHIKV or other related viruses.”
In the second study, researchers led by Teck-Hui Teo, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore, further elucidated the mechanisms by which CHIKV proteins act on T cells (Sci Transl Med. 2017;9:eaal1333). They also found that CHIKV-infected mice treated with fingolimod (Gilenya), a drug that blocks T-cell migration from the lymph nodes to the joints and is approved for the treatment of multiple sclerosis, saw reduced arthritis symptoms even without reduction of viral replication.
Infection with the chikungunya virus can produce arthritis that mimics symptoms of rheumatoid arthritis and may in some cases lead to joint damage. Though the mechanisms driving chikungunya-related arthritis are not well understood, preliminary studies have suggested a T-cell–mediated adverse response.
The Singapore team received funding from its own agency, A*STAR, while the Washington University researchers received grants from the National Institutes of Health and the Rheumatology Research Foundation. Two coauthors on the U.S. study reported extensive commercial conflicts, including consulting and advisory relationships with pharmaceutical and vaccine manufacturers, and one patent.
Several different currently approved immunomodulatory therapies ameliorated arthritis symptoms in chikungunya-infected mice in two studies that separate teams of researchers published online Feb. 1 in Science Translational Medicine.
The first team, led by Jonathan J. Miner, MD, PhD, of Washington University in St. Louis tested six different approved oral and biologic antirheumatic agents (along with control agents) in chikungunya virus-infected mice with acute arthritis and foot swelling (Sci Transl Med. 2017;9:eaah3438).
When the researchers paired abatacept with an antiviral therapy (monoclonal anti-CHIKV human antibody) the combination “was highly effective at reducing joint inflammation, periarticular swelling, migration of inflammatory leukocytes, and infection, even when administered several days after virus inoculation,” Dr. Miner and his colleagues wrote.
The researchers concluded that a combination of anti-inflammatory and antibody-based antiviral therapy “may serve as a model for treating humans with arthritis caused by CHIKV or other related viruses.”
In the second study, researchers led by Teck-Hui Teo, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore, further elucidated the mechanisms by which CHIKV proteins act on T cells (Sci Transl Med. 2017;9:eaal1333). They also found that CHIKV-infected mice treated with fingolimod (Gilenya), a drug that blocks T-cell migration from the lymph nodes to the joints and is approved for the treatment of multiple sclerosis, saw reduced arthritis symptoms even without reduction of viral replication.
Infection with the chikungunya virus can produce arthritis that mimics symptoms of rheumatoid arthritis and may in some cases lead to joint damage. Though the mechanisms driving chikungunya-related arthritis are not well understood, preliminary studies have suggested a T-cell–mediated adverse response.
The Singapore team received funding from its own agency, A*STAR, while the Washington University researchers received grants from the National Institutes of Health and the Rheumatology Research Foundation. Two coauthors on the U.S. study reported extensive commercial conflicts, including consulting and advisory relationships with pharmaceutical and vaccine manufacturers, and one patent.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: Abatacept, tofacitinib, and fingolimod all reduced arthritis symptoms, compared with controls.
Data source: Two studies testing multiple immunomodulatory or antirheumatic agents in chikungunya virus–infected mice.
Disclosures: The Agency for Science, Technology and Research (A*STAR) funded the Singapore researchers, while grants from the NIH and the Rheumatology Research Foundation funded the U.S. team. Two coauthors on the U.S. study disclosed extensive financial relationships with multiple pharmaceutical and vaccine manufacturers.