User login
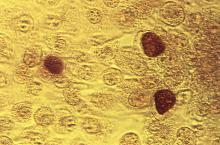
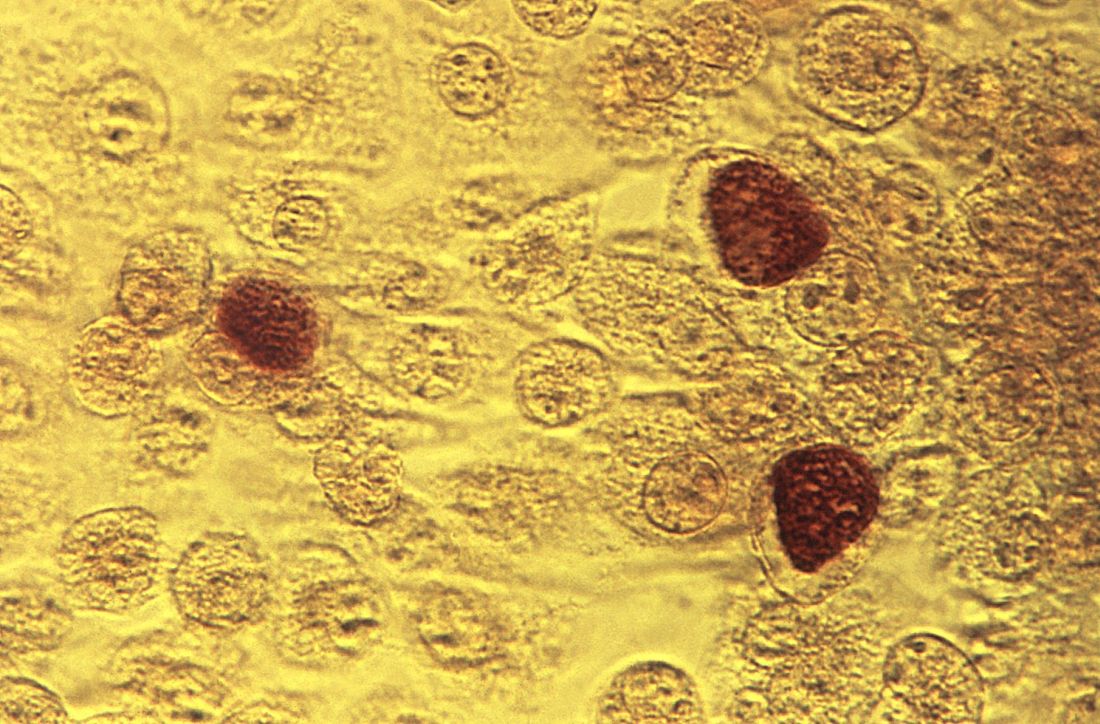
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
FROM CLINICAL INFECTIOUS DISEASES