User login
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
A syringe services program (SSP) is a harm reduction strategy designed to improve the quality of care provided to people who use drugs (PWUD). SSPs not only provide sterile syringes but establish a connection to medical services and resources for the safe disposal of syringes. By engaging with an SSP, patients may receive naloxone, condoms, fentanyl test strips, opioid use disorder medications, vaccinations, or testing for infectious diseases such as HIV and hepatitis C virus (HCV). Patients may also be connected to housing or social work services.
SSPs do not lead to increased drug use,1 increased improperly disposed supplies needed for drug use in the community, or increased crime.2,3 New users of SSPs are 5 times more likely to enter treatment for drug use than those who do not use SSPs.4-8 Further, SSPs have been found to reduce HIV and HCV transmission and are cost-effective in HIV prevention.9-11
Syringe Services Program
SSPs were implemented at the US Department of Veterans Affairs (VA) Alaska VA Healthcare System (AVAHCS) and VA Southern Oregon Healthcare System (VASOHCS). AVAHCS provides outpatient care across Alaska, with sites in Anchorage, Fairbanks, Homer, Juneau, Wasilla, and Soldotna. VASOHCS provides outpatient care to Southern Oregon and Northern California, with sites in White City, Grants Pass, and Klamath Falls, Oregon. Both are part of Veterans Integrated Service Network 20
Workgroups at AVAHCS and VASOHCS developed SSPs to reduce risks associated with drug use, promote positive outcomes for PWUD, and increase availability of harm reduction resources. During the July 2023 to June 2024 pharmacy residency cycle, an ambulatory care pharmacy resident from the Veterans Integrated Services Network 20 Clinical Resource Hub—a regional resource for clinical services—joined the workgroups. The workgroups established a goal that SSP resources would be made available to enrolled patients without any exclusions, prioritizing health equity.
SSP implementation needed buy-in from AVAHCS and VASOHCS leadership and key stakeholders who could participate in the workgroups. Following AVAHCS and VASOHCS leadership approval, each facility workgroup drafted standard operating procedures (SOPs). Both facilities planned to implement the program using prepackaged kits (sterile syringes, alcohol pads, cotton swabs, a sharps container, and an educational brochure on safe injection practices) supplied by the VA National Harm Reduction Program.
Each SSP offered patients direct links to additional care options at the time of kit distribution, including information regarding medications/supplies (ie, hepatitis A/B vaccines, HIV preexposure prophylaxis, substance use disorder pharmacotherapy, naloxone, and condoms), laboratory tests for infectious and sexually transmitted diseases, and referrals to substance use disorder treatment, social work, suicide prevention, mental health, and primary care.
The goal was to implement both SSPs during the July 2023 to June 2024 residency year. Other goals included tracking the quantity of supplies distributed, the number of patients reached, the impact of clinician education on the distribution of supplies, and comparing the implementation of the SSPs in the electronic health record (EHR) systems.
Alaska VA Healthcare System
An SOP was approved on December 20, 2023, and national supply kits were stocked in collaboration with the logistics department at the Anchorage AVAHCS campus. Social and behavioral health teams, primary care social workers, primary care clinicians, and nursing staff received training on the resources available through the SSP. A local adaptation of a template was created in the Computerized Patient Records System (CPRS) EHR. The template facilitates SSP kit distribution and patient screening for additional resources. Patients can engage with the SSP through any trained staff member. The staff member then completes the template and helps to distribute the SSP kit, in collaboration with the logistics department. The SSP does not operate in a dedicated physical space. The behavioral health team is most actively engaged in the SSP. The goal of SSP is to have resources available anywhere a patient requests services, including primary care and specialty clinics and to empower staff to meet patients’ needs. One patient has utilized the SSP as of June 2025.
Southern Oregon Healthcare System
Kits were ordered and stocked as pharmacy items in preparation for dispensing while awaiting medical center policy approval. Education began with the primary care mental health integration team. After initial education, an interdisciplinary presentation was given to VASOHCS clinicians to increase knowledge of the SSP. To enable documentation of SSP engagement, a local template was developed in the Cerner EHR to be shared among care team members at the facility. Similar to AVAHCS, the SSP does not have a physical space. All trained facility staff may engage in the SSP and distribute SSP kits. The workgroup that implemented this program remains available to support staff. Five patients have accessed the SSP since November 2024 and 7 SSP kits have been distributed as of June 2025.
Discussion
The SSP workgroups sought to expand the program through additional education. A number of factors should be considered when implementing an SSP. Across facilities, program implementation can be time-consuming and the timeline for administrative processes may be long. The workgroups met weekly or monthly depending on the status of the program and the administrative processes. Materials developed included SOP and MCP documents, a 1-page educational handout on SSP offerings, and a PowerPoint presentation for initial clinician education. Involving a pharmacy resident supported professional development and accelerated implementation timelines.
The facilities differed in implementation. AVAHCS collaborated with the logistics department to distribute kits, while VASOHCS worked with the Pharmacy service. A benefit of collaborating with logistics is that patients can receive a kit at the point of contact with the health care system, receiving it directly from the clinic the patient is visiting while eliminating the need to make an additional stop at the pharmacy. Conversely, partnering with the Pharmacy service allowed supply kits to be distributed by mail, enabling patients direct access to kits without having to present in-person. This is particularly valuable considering the large geographical area and remote care services available at VASOHCS.
Implementation varied significantly because AVAHCS operated on CPRS while VASOHCS used Cerner, a newer EHR. AVAHCS adapted a national template produced for CPRS sites, while VASOHCS had to prepare a local template (auto-text) for SSP documentation. Future plans at AVAHCS may include adding fentanyl test strips as an orderable item in the EHR given that AVAHCS has a local instance of CPRS; however, VASOHCS cannot order fentanyl test strips through the Pharmacy service due to legal restrictions. While Oregon permits fentanyl test strip use, the Cerner instance used by VA is a national program, and therefore the addition of fentanyl test strips as an orderable item in the EHR would carry national implications, including for VA health care systems in states where fentanyl test strip legality is variable. Despite the challenges, efforts to include fentanyl test strips in both SSPs are ongoing.
No significant EHR changes were needed to make the national supply kits available in the Cerner EHR through the VASOHCS Pharmacy service. To have national supply kits available through the AVAHCS Pharmacy service, the EHR would need to be manipulated by adding a local drug file in CPRS. Differences between the EHRs often facilitated the need for adaptation from existing models of SSPs within VA, which were all based in CPRS.
Conclusions
The implementation of SSPs at AVAHCS and VASOHCS enable clinicians to provide quality harm reduction services to PWUD. Despite variations in EHR systems, AVAHCS and VASOHCS implemented SSP within 1 year. Tracking of program engagement via the number of patients interacting with the program and the number of SSP kits distributed will continue. SSP implementation in states where it is permitted may help provide optimal patient care for PWUD.
- Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19(3):247-252. doi:10.1016/s0740-5472(00)00104-5
- Marx MA, Crape B, Brookmeyer RS, et al. Trends in crime and the introduction of a needle exchange program. Am J Public Health. 2000;90(12):1933-1936. doi:10.2105/ajph.90.12.1933
- Galea S, Ahern J, Fuller C, Freudenberg N, Vlahov D. Needle exchange programs and experience of violence in an inner city neighborhood. J Acquir Immune Defic Syndr. 2001;28(3):282-288. doi:10.1097/00042560-200111010-00014
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337-1341. doi:10.15585/ mmwr.mm6448a3
- Tookes HE, Kral AH, Wenger LD, et al. A comparison of syringe disposal practices among injection drug users in a city with versus a city without needle and syringe programs. Drug Alcohol Depend. 2012;123(1-3):255-259. doi:10.1016/j.drugalcdep.2011.12.001
- Klein SJ, Candelas AR, Cooper JG, et al. Increasing safe syringe collection sites in New York State. Public Health Rep. 2008;123(4):433-440. doi:10.1177/003335490812300404
- de Montigny L, Vernez Moudon A, Leigh B, Kim SY. Assessing a drop box programme: a spatial analysis of discarded needles. Int J Drug Policy. 2010;21(3):208-214. doi:10.1016/j.drugpo.2009.07.003
- Bluthenthal RN, Anderson R, Flynn NM, Kral AH. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug Alcohol Depend. 2007;89(2-3):214-222. doi:10.1016/j.drugalcdep.2006.12.035
- Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. doi:10.1002/14651858.CD012021.pub2
- Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe programmes in people who inject drugs — an overview of systematic reviews. BMC Public Health. 2017;17(1):309. doi:10.1186/s12889-017-4210-2
- Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. doi:10.1371/journal.pmed.1002312
A syringe services program (SSP) is a harm reduction strategy designed to improve the quality of care provided to people who use drugs (PWUD). SSPs not only provide sterile syringes but establish a connection to medical services and resources for the safe disposal of syringes. By engaging with an SSP, patients may receive naloxone, condoms, fentanyl test strips, opioid use disorder medications, vaccinations, or testing for infectious diseases such as HIV and hepatitis C virus (HCV). Patients may also be connected to housing or social work services.
SSPs do not lead to increased drug use,1 increased improperly disposed supplies needed for drug use in the community, or increased crime.2,3 New users of SSPs are 5 times more likely to enter treatment for drug use than those who do not use SSPs.4-8 Further, SSPs have been found to reduce HIV and HCV transmission and are cost-effective in HIV prevention.9-11
Syringe Services Program
SSPs were implemented at the US Department of Veterans Affairs (VA) Alaska VA Healthcare System (AVAHCS) and VA Southern Oregon Healthcare System (VASOHCS). AVAHCS provides outpatient care across Alaska, with sites in Anchorage, Fairbanks, Homer, Juneau, Wasilla, and Soldotna. VASOHCS provides outpatient care to Southern Oregon and Northern California, with sites in White City, Grants Pass, and Klamath Falls, Oregon. Both are part of Veterans Integrated Service Network 20
Workgroups at AVAHCS and VASOHCS developed SSPs to reduce risks associated with drug use, promote positive outcomes for PWUD, and increase availability of harm reduction resources. During the July 2023 to June 2024 pharmacy residency cycle, an ambulatory care pharmacy resident from the Veterans Integrated Services Network 20 Clinical Resource Hub—a regional resource for clinical services—joined the workgroups. The workgroups established a goal that SSP resources would be made available to enrolled patients without any exclusions, prioritizing health equity.
SSP implementation needed buy-in from AVAHCS and VASOHCS leadership and key stakeholders who could participate in the workgroups. Following AVAHCS and VASOHCS leadership approval, each facility workgroup drafted standard operating procedures (SOPs). Both facilities planned to implement the program using prepackaged kits (sterile syringes, alcohol pads, cotton swabs, a sharps container, and an educational brochure on safe injection practices) supplied by the VA National Harm Reduction Program.
Each SSP offered patients direct links to additional care options at the time of kit distribution, including information regarding medications/supplies (ie, hepatitis A/B vaccines, HIV preexposure prophylaxis, substance use disorder pharmacotherapy, naloxone, and condoms), laboratory tests for infectious and sexually transmitted diseases, and referrals to substance use disorder treatment, social work, suicide prevention, mental health, and primary care.
The goal was to implement both SSPs during the July 2023 to June 2024 residency year. Other goals included tracking the quantity of supplies distributed, the number of patients reached, the impact of clinician education on the distribution of supplies, and comparing the implementation of the SSPs in the electronic health record (EHR) systems.
Alaska VA Healthcare System
An SOP was approved on December 20, 2023, and national supply kits were stocked in collaboration with the logistics department at the Anchorage AVAHCS campus. Social and behavioral health teams, primary care social workers, primary care clinicians, and nursing staff received training on the resources available through the SSP. A local adaptation of a template was created in the Computerized Patient Records System (CPRS) EHR. The template facilitates SSP kit distribution and patient screening for additional resources. Patients can engage with the SSP through any trained staff member. The staff member then completes the template and helps to distribute the SSP kit, in collaboration with the logistics department. The SSP does not operate in a dedicated physical space. The behavioral health team is most actively engaged in the SSP. The goal of SSP is to have resources available anywhere a patient requests services, including primary care and specialty clinics and to empower staff to meet patients’ needs. One patient has utilized the SSP as of June 2025.
Southern Oregon Healthcare System
Kits were ordered and stocked as pharmacy items in preparation for dispensing while awaiting medical center policy approval. Education began with the primary care mental health integration team. After initial education, an interdisciplinary presentation was given to VASOHCS clinicians to increase knowledge of the SSP. To enable documentation of SSP engagement, a local template was developed in the Cerner EHR to be shared among care team members at the facility. Similar to AVAHCS, the SSP does not have a physical space. All trained facility staff may engage in the SSP and distribute SSP kits. The workgroup that implemented this program remains available to support staff. Five patients have accessed the SSP since November 2024 and 7 SSP kits have been distributed as of June 2025.
Discussion
The SSP workgroups sought to expand the program through additional education. A number of factors should be considered when implementing an SSP. Across facilities, program implementation can be time-consuming and the timeline for administrative processes may be long. The workgroups met weekly or monthly depending on the status of the program and the administrative processes. Materials developed included SOP and MCP documents, a 1-page educational handout on SSP offerings, and a PowerPoint presentation for initial clinician education. Involving a pharmacy resident supported professional development and accelerated implementation timelines.
The facilities differed in implementation. AVAHCS collaborated with the logistics department to distribute kits, while VASOHCS worked with the Pharmacy service. A benefit of collaborating with logistics is that patients can receive a kit at the point of contact with the health care system, receiving it directly from the clinic the patient is visiting while eliminating the need to make an additional stop at the pharmacy. Conversely, partnering with the Pharmacy service allowed supply kits to be distributed by mail, enabling patients direct access to kits without having to present in-person. This is particularly valuable considering the large geographical area and remote care services available at VASOHCS.
Implementation varied significantly because AVAHCS operated on CPRS while VASOHCS used Cerner, a newer EHR. AVAHCS adapted a national template produced for CPRS sites, while VASOHCS had to prepare a local template (auto-text) for SSP documentation. Future plans at AVAHCS may include adding fentanyl test strips as an orderable item in the EHR given that AVAHCS has a local instance of CPRS; however, VASOHCS cannot order fentanyl test strips through the Pharmacy service due to legal restrictions. While Oregon permits fentanyl test strip use, the Cerner instance used by VA is a national program, and therefore the addition of fentanyl test strips as an orderable item in the EHR would carry national implications, including for VA health care systems in states where fentanyl test strip legality is variable. Despite the challenges, efforts to include fentanyl test strips in both SSPs are ongoing.
No significant EHR changes were needed to make the national supply kits available in the Cerner EHR through the VASOHCS Pharmacy service. To have national supply kits available through the AVAHCS Pharmacy service, the EHR would need to be manipulated by adding a local drug file in CPRS. Differences between the EHRs often facilitated the need for adaptation from existing models of SSPs within VA, which were all based in CPRS.
Conclusions
The implementation of SSPs at AVAHCS and VASOHCS enable clinicians to provide quality harm reduction services to PWUD. Despite variations in EHR systems, AVAHCS and VASOHCS implemented SSP within 1 year. Tracking of program engagement via the number of patients interacting with the program and the number of SSP kits distributed will continue. SSP implementation in states where it is permitted may help provide optimal patient care for PWUD.
A syringe services program (SSP) is a harm reduction strategy designed to improve the quality of care provided to people who use drugs (PWUD). SSPs not only provide sterile syringes but establish a connection to medical services and resources for the safe disposal of syringes. By engaging with an SSP, patients may receive naloxone, condoms, fentanyl test strips, opioid use disorder medications, vaccinations, or testing for infectious diseases such as HIV and hepatitis C virus (HCV). Patients may also be connected to housing or social work services.
SSPs do not lead to increased drug use,1 increased improperly disposed supplies needed for drug use in the community, or increased crime.2,3 New users of SSPs are 5 times more likely to enter treatment for drug use than those who do not use SSPs.4-8 Further, SSPs have been found to reduce HIV and HCV transmission and are cost-effective in HIV prevention.9-11
Syringe Services Program
SSPs were implemented at the US Department of Veterans Affairs (VA) Alaska VA Healthcare System (AVAHCS) and VA Southern Oregon Healthcare System (VASOHCS). AVAHCS provides outpatient care across Alaska, with sites in Anchorage, Fairbanks, Homer, Juneau, Wasilla, and Soldotna. VASOHCS provides outpatient care to Southern Oregon and Northern California, with sites in White City, Grants Pass, and Klamath Falls, Oregon. Both are part of Veterans Integrated Service Network 20
Workgroups at AVAHCS and VASOHCS developed SSPs to reduce risks associated with drug use, promote positive outcomes for PWUD, and increase availability of harm reduction resources. During the July 2023 to June 2024 pharmacy residency cycle, an ambulatory care pharmacy resident from the Veterans Integrated Services Network 20 Clinical Resource Hub—a regional resource for clinical services—joined the workgroups. The workgroups established a goal that SSP resources would be made available to enrolled patients without any exclusions, prioritizing health equity.
SSP implementation needed buy-in from AVAHCS and VASOHCS leadership and key stakeholders who could participate in the workgroups. Following AVAHCS and VASOHCS leadership approval, each facility workgroup drafted standard operating procedures (SOPs). Both facilities planned to implement the program using prepackaged kits (sterile syringes, alcohol pads, cotton swabs, a sharps container, and an educational brochure on safe injection practices) supplied by the VA National Harm Reduction Program.
Each SSP offered patients direct links to additional care options at the time of kit distribution, including information regarding medications/supplies (ie, hepatitis A/B vaccines, HIV preexposure prophylaxis, substance use disorder pharmacotherapy, naloxone, and condoms), laboratory tests for infectious and sexually transmitted diseases, and referrals to substance use disorder treatment, social work, suicide prevention, mental health, and primary care.
The goal was to implement both SSPs during the July 2023 to June 2024 residency year. Other goals included tracking the quantity of supplies distributed, the number of patients reached, the impact of clinician education on the distribution of supplies, and comparing the implementation of the SSPs in the electronic health record (EHR) systems.
Alaska VA Healthcare System
An SOP was approved on December 20, 2023, and national supply kits were stocked in collaboration with the logistics department at the Anchorage AVAHCS campus. Social and behavioral health teams, primary care social workers, primary care clinicians, and nursing staff received training on the resources available through the SSP. A local adaptation of a template was created in the Computerized Patient Records System (CPRS) EHR. The template facilitates SSP kit distribution and patient screening for additional resources. Patients can engage with the SSP through any trained staff member. The staff member then completes the template and helps to distribute the SSP kit, in collaboration with the logistics department. The SSP does not operate in a dedicated physical space. The behavioral health team is most actively engaged in the SSP. The goal of SSP is to have resources available anywhere a patient requests services, including primary care and specialty clinics and to empower staff to meet patients’ needs. One patient has utilized the SSP as of June 2025.
Southern Oregon Healthcare System
Kits were ordered and stocked as pharmacy items in preparation for dispensing while awaiting medical center policy approval. Education began with the primary care mental health integration team. After initial education, an interdisciplinary presentation was given to VASOHCS clinicians to increase knowledge of the SSP. To enable documentation of SSP engagement, a local template was developed in the Cerner EHR to be shared among care team members at the facility. Similar to AVAHCS, the SSP does not have a physical space. All trained facility staff may engage in the SSP and distribute SSP kits. The workgroup that implemented this program remains available to support staff. Five patients have accessed the SSP since November 2024 and 7 SSP kits have been distributed as of June 2025.
Discussion
The SSP workgroups sought to expand the program through additional education. A number of factors should be considered when implementing an SSP. Across facilities, program implementation can be time-consuming and the timeline for administrative processes may be long. The workgroups met weekly or monthly depending on the status of the program and the administrative processes. Materials developed included SOP and MCP documents, a 1-page educational handout on SSP offerings, and a PowerPoint presentation for initial clinician education. Involving a pharmacy resident supported professional development and accelerated implementation timelines.
The facilities differed in implementation. AVAHCS collaborated with the logistics department to distribute kits, while VASOHCS worked with the Pharmacy service. A benefit of collaborating with logistics is that patients can receive a kit at the point of contact with the health care system, receiving it directly from the clinic the patient is visiting while eliminating the need to make an additional stop at the pharmacy. Conversely, partnering with the Pharmacy service allowed supply kits to be distributed by mail, enabling patients direct access to kits without having to present in-person. This is particularly valuable considering the large geographical area and remote care services available at VASOHCS.
Implementation varied significantly because AVAHCS operated on CPRS while VASOHCS used Cerner, a newer EHR. AVAHCS adapted a national template produced for CPRS sites, while VASOHCS had to prepare a local template (auto-text) for SSP documentation. Future plans at AVAHCS may include adding fentanyl test strips as an orderable item in the EHR given that AVAHCS has a local instance of CPRS; however, VASOHCS cannot order fentanyl test strips through the Pharmacy service due to legal restrictions. While Oregon permits fentanyl test strip use, the Cerner instance used by VA is a national program, and therefore the addition of fentanyl test strips as an orderable item in the EHR would carry national implications, including for VA health care systems in states where fentanyl test strip legality is variable. Despite the challenges, efforts to include fentanyl test strips in both SSPs are ongoing.
No significant EHR changes were needed to make the national supply kits available in the Cerner EHR through the VASOHCS Pharmacy service. To have national supply kits available through the AVAHCS Pharmacy service, the EHR would need to be manipulated by adding a local drug file in CPRS. Differences between the EHRs often facilitated the need for adaptation from existing models of SSPs within VA, which were all based in CPRS.
Conclusions
The implementation of SSPs at AVAHCS and VASOHCS enable clinicians to provide quality harm reduction services to PWUD. Despite variations in EHR systems, AVAHCS and VASOHCS implemented SSP within 1 year. Tracking of program engagement via the number of patients interacting with the program and the number of SSP kits distributed will continue. SSP implementation in states where it is permitted may help provide optimal patient care for PWUD.
- Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19(3):247-252. doi:10.1016/s0740-5472(00)00104-5
- Marx MA, Crape B, Brookmeyer RS, et al. Trends in crime and the introduction of a needle exchange program. Am J Public Health. 2000;90(12):1933-1936. doi:10.2105/ajph.90.12.1933
- Galea S, Ahern J, Fuller C, Freudenberg N, Vlahov D. Needle exchange programs and experience of violence in an inner city neighborhood. J Acquir Immune Defic Syndr. 2001;28(3):282-288. doi:10.1097/00042560-200111010-00014
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337-1341. doi:10.15585/ mmwr.mm6448a3
- Tookes HE, Kral AH, Wenger LD, et al. A comparison of syringe disposal practices among injection drug users in a city with versus a city without needle and syringe programs. Drug Alcohol Depend. 2012;123(1-3):255-259. doi:10.1016/j.drugalcdep.2011.12.001
- Klein SJ, Candelas AR, Cooper JG, et al. Increasing safe syringe collection sites in New York State. Public Health Rep. 2008;123(4):433-440. doi:10.1177/003335490812300404
- de Montigny L, Vernez Moudon A, Leigh B, Kim SY. Assessing a drop box programme: a spatial analysis of discarded needles. Int J Drug Policy. 2010;21(3):208-214. doi:10.1016/j.drugpo.2009.07.003
- Bluthenthal RN, Anderson R, Flynn NM, Kral AH. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug Alcohol Depend. 2007;89(2-3):214-222. doi:10.1016/j.drugalcdep.2006.12.035
- Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. doi:10.1002/14651858.CD012021.pub2
- Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe programmes in people who inject drugs — an overview of systematic reviews. BMC Public Health. 2017;17(1):309. doi:10.1186/s12889-017-4210-2
- Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. doi:10.1371/journal.pmed.1002312
- Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19(3):247-252. doi:10.1016/s0740-5472(00)00104-5
- Marx MA, Crape B, Brookmeyer RS, et al. Trends in crime and the introduction of a needle exchange program. Am J Public Health. 2000;90(12):1933-1936. doi:10.2105/ajph.90.12.1933
- Galea S, Ahern J, Fuller C, Freudenberg N, Vlahov D. Needle exchange programs and experience of violence in an inner city neighborhood. J Acquir Immune Defic Syndr. 2001;28(3):282-288. doi:10.1097/00042560-200111010-00014
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337-1341. doi:10.15585/ mmwr.mm6448a3
- Tookes HE, Kral AH, Wenger LD, et al. A comparison of syringe disposal practices among injection drug users in a city with versus a city without needle and syringe programs. Drug Alcohol Depend. 2012;123(1-3):255-259. doi:10.1016/j.drugalcdep.2011.12.001
- Klein SJ, Candelas AR, Cooper JG, et al. Increasing safe syringe collection sites in New York State. Public Health Rep. 2008;123(4):433-440. doi:10.1177/003335490812300404
- de Montigny L, Vernez Moudon A, Leigh B, Kim SY. Assessing a drop box programme: a spatial analysis of discarded needles. Int J Drug Policy. 2010;21(3):208-214. doi:10.1016/j.drugpo.2009.07.003
- Bluthenthal RN, Anderson R, Flynn NM, Kral AH. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug Alcohol Depend. 2007;89(2-3):214-222. doi:10.1016/j.drugalcdep.2006.12.035
- Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. doi:10.1002/14651858.CD012021.pub2
- Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe programmes in people who inject drugs — an overview of systematic reviews. BMC Public Health. 2017;17(1):309. doi:10.1186/s12889-017-4210-2
- Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. doi:10.1371/journal.pmed.1002312
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
Nine VA Facilities to Open Research Trials for Psychedelics
Nine VA Facilities to Open Research Trials for Psychedelics
On Nov. 22, 2014, 8 years after he came back from Iraq with “crippling” posttraumatic stress disorder (PTSD), Jonathan Lubecky took his first dose of the psychedelic compound methylenedioxymethamphetamine (MDMA). Lubecky, a Marine, Army, and National Guard veteran, described his path to MDMA therapy in in the New Horizons in Health podcast.
After 5 suicide attempts and “the hundreds of times I thought about it or stood on a bridge or had a plan,” he felt he had run out of options. Then, in a counseling session, a psychiatric intern slid a piece of paper across the table to him. It read “Google MDMA PTSD.”
Luckily for Lubecky, a space in a clinical trial opened up, in which he had 8 hours of talk therapy with specially trained therapists, combined with MDMA. “MDMA is a tool that opens up the mind, body and spirit,” he said, “so you can heal and process all those memories and traumas that are causing yourissues. It puts you in a middle place where you can talk about trauma without having panic attacks, without your body betraying you, and look at it from a different perspective.” said he added, “It’s like doing therapy while being hugged by everyone who loves you in a bathtub full of puppies licking your face.” In 2023, 9 years after that first dose, Lubecky said, “I’ve been PTSD free longer than I had it.”
And now, in 2025, the research into psychedelic therapy for veterans like Lubecky is taking another step forward according to a report by Military.com. Nine VA facilities, in the Bronx, Los Angeles, Omaha, Palo Alto, Portland (Oregon), San Diego, San Francisco, West Haven, and White River Junction, are participating in long-term studies to test the safety and clinical impact of psychedelic compounds for PTSD, treatment-resistant depression, and anxiety disorders.
Early trials from Johns Hopkins University, the Multidisciplinary Association for Psychedelic Studies (MAPS), and others found significant symptom reductions for some participants with chronic PTSD. MAPP2, the multisite phase 3 study that extended the findings of MAPP1, found that MDMA-assisted therapy significantly improved PTSD symptoms and functional impairment, compared with placebo-assisted therapy. Notably, of the 52 participants (including 16 veterans) 45 (86%) achieved a clinically meaningful benefit, and 37 (71%) no longer met criteria for PTSD by study end. Despite the promising findings, a US Food and Drug Administration (FDA) advisory panel recommended against approving the treatment.
In 2024 the VA issued a request for applications for proposals from its network of VA researchers and academic institutions to gather “definitive scientific evidence” on the potential efficacy and safety of psychedelic compounds, such as MDMA and psilocybin, when used in conjunction with psychotherapy. It would be the first time since the 1960s that the VA had funded research on such compounds.
Funding proposals for such research have cycled in and out of Congress for years, but have gathered more steam in the last few years. The 2024 National Defense Authorization Act directed the US Department of Defense to establish a process for funding clinical research into the use of certain psychedelic substances to treat PTSD and traumatic brain injury. In April 2024, Representatives Lou Correa (D-CA) and Jack Bergman (R-MI), cochairs of the Psychedelics Advancing Therapies (PATH) caucus, introduced the Innovative Therapies Centers of Excellence Act of 2025, bipartisan legislation that would increase federally funded research on innovative therapies to treat veterans with PTSD, substance use disorder, and depression. It would also, if enacted, direct the VA to create ≥ 5 dedicated centers of excellence to study the therapeutic uses of psychedelic substances. The bill has also been endorsed by the American Legion, Veterans of Foreign Wars, Iraq and Afghanistan Veterans of America, Disabled American Veterans, and the Wounded Warrior Project.
The current administration has two strong high-level supporters of psychedelics research: VA Secretary Doug Collins and US Department of Health and Human Service Secretary Robert F. Kennedy Jr. Sec. Kennedy has castigated the FDA for what he calls “aggressive suppression” of alternative and complementary treatments, including psychedelics. This, although the FDA granted breakthrough therapy status for MDMA for treating PTSD and psilocybin for treating depression in 2018 and 2019, respectively, as well a pivotal draft guidance in 2023 for the development of psychedelic drugs for psychiatric disorders, substance use disorders, and various medical conditions.
Collins, citing an “eye-opening” discussion with Kennedy, enthusiastically backs the research into psychedelics. In a May 2025 hearing that was mainly a series of testy exchanges about his proposed budget slashing, he emphasized the importance of keeping and expanding VA programs and studies on psychedelic treatments, something he has been advocating for since the beginning of his appointment. “We want to make sure we’re not closing off any outlet for a veteran who could be helped by these programs,” he said.
Taking the intern’s advice to look into MDMA, Jonathan Lubecky said, was one of the best decisions he’d ever made. But “it’s not the MDMA that fixes you,” he said. “It’s the therapy. It’s the therapist working with you and you doing the hard work.”
On Nov. 22, 2014, 8 years after he came back from Iraq with “crippling” posttraumatic stress disorder (PTSD), Jonathan Lubecky took his first dose of the psychedelic compound methylenedioxymethamphetamine (MDMA). Lubecky, a Marine, Army, and National Guard veteran, described his path to MDMA therapy in in the New Horizons in Health podcast.
After 5 suicide attempts and “the hundreds of times I thought about it or stood on a bridge or had a plan,” he felt he had run out of options. Then, in a counseling session, a psychiatric intern slid a piece of paper across the table to him. It read “Google MDMA PTSD.”
Luckily for Lubecky, a space in a clinical trial opened up, in which he had 8 hours of talk therapy with specially trained therapists, combined with MDMA. “MDMA is a tool that opens up the mind, body and spirit,” he said, “so you can heal and process all those memories and traumas that are causing yourissues. It puts you in a middle place where you can talk about trauma without having panic attacks, without your body betraying you, and look at it from a different perspective.” said he added, “It’s like doing therapy while being hugged by everyone who loves you in a bathtub full of puppies licking your face.” In 2023, 9 years after that first dose, Lubecky said, “I’ve been PTSD free longer than I had it.”
And now, in 2025, the research into psychedelic therapy for veterans like Lubecky is taking another step forward according to a report by Military.com. Nine VA facilities, in the Bronx, Los Angeles, Omaha, Palo Alto, Portland (Oregon), San Diego, San Francisco, West Haven, and White River Junction, are participating in long-term studies to test the safety and clinical impact of psychedelic compounds for PTSD, treatment-resistant depression, and anxiety disorders.
Early trials from Johns Hopkins University, the Multidisciplinary Association for Psychedelic Studies (MAPS), and others found significant symptom reductions for some participants with chronic PTSD. MAPP2, the multisite phase 3 study that extended the findings of MAPP1, found that MDMA-assisted therapy significantly improved PTSD symptoms and functional impairment, compared with placebo-assisted therapy. Notably, of the 52 participants (including 16 veterans) 45 (86%) achieved a clinically meaningful benefit, and 37 (71%) no longer met criteria for PTSD by study end. Despite the promising findings, a US Food and Drug Administration (FDA) advisory panel recommended against approving the treatment.
In 2024 the VA issued a request for applications for proposals from its network of VA researchers and academic institutions to gather “definitive scientific evidence” on the potential efficacy and safety of psychedelic compounds, such as MDMA and psilocybin, when used in conjunction with psychotherapy. It would be the first time since the 1960s that the VA had funded research on such compounds.
Funding proposals for such research have cycled in and out of Congress for years, but have gathered more steam in the last few years. The 2024 National Defense Authorization Act directed the US Department of Defense to establish a process for funding clinical research into the use of certain psychedelic substances to treat PTSD and traumatic brain injury. In April 2024, Representatives Lou Correa (D-CA) and Jack Bergman (R-MI), cochairs of the Psychedelics Advancing Therapies (PATH) caucus, introduced the Innovative Therapies Centers of Excellence Act of 2025, bipartisan legislation that would increase federally funded research on innovative therapies to treat veterans with PTSD, substance use disorder, and depression. It would also, if enacted, direct the VA to create ≥ 5 dedicated centers of excellence to study the therapeutic uses of psychedelic substances. The bill has also been endorsed by the American Legion, Veterans of Foreign Wars, Iraq and Afghanistan Veterans of America, Disabled American Veterans, and the Wounded Warrior Project.
The current administration has two strong high-level supporters of psychedelics research: VA Secretary Doug Collins and US Department of Health and Human Service Secretary Robert F. Kennedy Jr. Sec. Kennedy has castigated the FDA for what he calls “aggressive suppression” of alternative and complementary treatments, including psychedelics. This, although the FDA granted breakthrough therapy status for MDMA for treating PTSD and psilocybin for treating depression in 2018 and 2019, respectively, as well a pivotal draft guidance in 2023 for the development of psychedelic drugs for psychiatric disorders, substance use disorders, and various medical conditions.
Collins, citing an “eye-opening” discussion with Kennedy, enthusiastically backs the research into psychedelics. In a May 2025 hearing that was mainly a series of testy exchanges about his proposed budget slashing, he emphasized the importance of keeping and expanding VA programs and studies on psychedelic treatments, something he has been advocating for since the beginning of his appointment. “We want to make sure we’re not closing off any outlet for a veteran who could be helped by these programs,” he said.
Taking the intern’s advice to look into MDMA, Jonathan Lubecky said, was one of the best decisions he’d ever made. But “it’s not the MDMA that fixes you,” he said. “It’s the therapy. It’s the therapist working with you and you doing the hard work.”
On Nov. 22, 2014, 8 years after he came back from Iraq with “crippling” posttraumatic stress disorder (PTSD), Jonathan Lubecky took his first dose of the psychedelic compound methylenedioxymethamphetamine (MDMA). Lubecky, a Marine, Army, and National Guard veteran, described his path to MDMA therapy in in the New Horizons in Health podcast.
After 5 suicide attempts and “the hundreds of times I thought about it or stood on a bridge or had a plan,” he felt he had run out of options. Then, in a counseling session, a psychiatric intern slid a piece of paper across the table to him. It read “Google MDMA PTSD.”
Luckily for Lubecky, a space in a clinical trial opened up, in which he had 8 hours of talk therapy with specially trained therapists, combined with MDMA. “MDMA is a tool that opens up the mind, body and spirit,” he said, “so you can heal and process all those memories and traumas that are causing yourissues. It puts you in a middle place where you can talk about trauma without having panic attacks, without your body betraying you, and look at it from a different perspective.” said he added, “It’s like doing therapy while being hugged by everyone who loves you in a bathtub full of puppies licking your face.” In 2023, 9 years after that first dose, Lubecky said, “I’ve been PTSD free longer than I had it.”
And now, in 2025, the research into psychedelic therapy for veterans like Lubecky is taking another step forward according to a report by Military.com. Nine VA facilities, in the Bronx, Los Angeles, Omaha, Palo Alto, Portland (Oregon), San Diego, San Francisco, West Haven, and White River Junction, are participating in long-term studies to test the safety and clinical impact of psychedelic compounds for PTSD, treatment-resistant depression, and anxiety disorders.
Early trials from Johns Hopkins University, the Multidisciplinary Association for Psychedelic Studies (MAPS), and others found significant symptom reductions for some participants with chronic PTSD. MAPP2, the multisite phase 3 study that extended the findings of MAPP1, found that MDMA-assisted therapy significantly improved PTSD symptoms and functional impairment, compared with placebo-assisted therapy. Notably, of the 52 participants (including 16 veterans) 45 (86%) achieved a clinically meaningful benefit, and 37 (71%) no longer met criteria for PTSD by study end. Despite the promising findings, a US Food and Drug Administration (FDA) advisory panel recommended against approving the treatment.
In 2024 the VA issued a request for applications for proposals from its network of VA researchers and academic institutions to gather “definitive scientific evidence” on the potential efficacy and safety of psychedelic compounds, such as MDMA and psilocybin, when used in conjunction with psychotherapy. It would be the first time since the 1960s that the VA had funded research on such compounds.
Funding proposals for such research have cycled in and out of Congress for years, but have gathered more steam in the last few years. The 2024 National Defense Authorization Act directed the US Department of Defense to establish a process for funding clinical research into the use of certain psychedelic substances to treat PTSD and traumatic brain injury. In April 2024, Representatives Lou Correa (D-CA) and Jack Bergman (R-MI), cochairs of the Psychedelics Advancing Therapies (PATH) caucus, introduced the Innovative Therapies Centers of Excellence Act of 2025, bipartisan legislation that would increase federally funded research on innovative therapies to treat veterans with PTSD, substance use disorder, and depression. It would also, if enacted, direct the VA to create ≥ 5 dedicated centers of excellence to study the therapeutic uses of psychedelic substances. The bill has also been endorsed by the American Legion, Veterans of Foreign Wars, Iraq and Afghanistan Veterans of America, Disabled American Veterans, and the Wounded Warrior Project.
The current administration has two strong high-level supporters of psychedelics research: VA Secretary Doug Collins and US Department of Health and Human Service Secretary Robert F. Kennedy Jr. Sec. Kennedy has castigated the FDA for what he calls “aggressive suppression” of alternative and complementary treatments, including psychedelics. This, although the FDA granted breakthrough therapy status for MDMA for treating PTSD and psilocybin for treating depression in 2018 and 2019, respectively, as well a pivotal draft guidance in 2023 for the development of psychedelic drugs for psychiatric disorders, substance use disorders, and various medical conditions.
Collins, citing an “eye-opening” discussion with Kennedy, enthusiastically backs the research into psychedelics. In a May 2025 hearing that was mainly a series of testy exchanges about his proposed budget slashing, he emphasized the importance of keeping and expanding VA programs and studies on psychedelic treatments, something he has been advocating for since the beginning of his appointment. “We want to make sure we’re not closing off any outlet for a veteran who could be helped by these programs,” he said.
Taking the intern’s advice to look into MDMA, Jonathan Lubecky said, was one of the best decisions he’d ever made. But “it’s not the MDMA that fixes you,” he said. “It’s the therapy. It’s the therapist working with you and you doing the hard work.”
Nine VA Facilities to Open Research Trials for Psychedelics
Nine VA Facilities to Open Research Trials for Psychedelics
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Cancer patients experience depression at rates > 5 times that of the general population.1-11 Despite an increase in palliative care use, depression rates continued to rise.2-4 Between 5% to 16% of outpatients, 4% to 14% of inpatients, and up to 49% of patients receiving palliative care experience depression.5 This issue also impacts families and caregivers.1 A 2021 meta-analysis found that 23% of active military personnel and 20% of veterans experience depression.11
Antidepressants approved by the US Food and Drug Administration (FDA) target the serotonin, norepinephrine, or dopamine systems and include boxed warnings about an increased risk of suicidal thoughts in adults aged 18 to 24 years.12,13 These medications are categorized into several classes: monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), tetracyclic antidepressants (TeCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), serotonin receptor modulators (SRMs), serotonin-melatonin receptor antagonists (SMRAs), and N—methyl-D-aspartate receptor antagonists (NMDARAs).14,15 The first FDA-approved antidepressants, iproniazid (an MAOI) and imipramine (a TCA) laid the foundation for the development of newer classes like SSRIs and SNRIs.15-17
Older antidepressants such as MAOIs and TCAs are used less due to their adverse effects (AEs) and drug interactions. MAOIs, such as iproniazid, selegiline, moclobemide, tranylcypromine, isocarboxazid, and phenelzine, have numerous AEs and drug interactions, making them unsuitable for first- or second-line treatment of depression.14,18-21 TCAs such as doxepin, amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, trimipramine, protriptyline, maprotiline, and amoxapine have a narrow therapeutic index requiring careful monitoring for signs of toxicity such as QRS widening, tremors, or confusion. Despite the issues, TCAs are generally classified as second-line agents for major depressive disorder (MDD). TCAs have off-label uses for migraine prophylaxis, treatment of obsessive-compulsive disorder (OCD), insomnia, and chronic pain management first-line.14,22-29
Newer antidepressants, including TeCAs and NDRIs, are typically more effective, but also come with safety concerns. TeCAs like mirtazapine interact with several medications, including MAOIs, serotonin-increasing drugs, alcohol, cannabidiol, and marijuana. Mirtazapine is FDA-approved for the treatment of moderate to severe depression in adults. It is also used off-label to treat insomnia, panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), headaches, and migraines. Compared to other antidepressants, mirtazapine is effective for all stages of depression and addresses a broad range of related symptoms.14,30-34 NDRIs, such as bupropion, also interact with various medications, including MAOIs, other antidepressants, stimulants, and alcohol. Bupropion is FDA-approved for smoking cessation and to treat depression and SAD. It is also used off-label for depression- related bipolar disorder or sexual dysfunction, attention-deficit/hyperactivity disorder (ADHD), and obesity.14,35-42
SSRIs, SNRIs, and SRMs should be used with caution. SSRIs such as sertraline, citalopram, escitalopram, fluoxetine, paroxetine, and fluvoxamine are first-line treatments for depression and various psychiatric disorders due to their safety and efficacy. Common AEs of SSRIs include sexual dysfunction, sleep disturbances, weight changes, and gastrointestinal (GI) issues. SSRIs can prolong the QT interval, posing a risk of life-threatening arrhythmia, and may interact with other medications, necessitating treatment adjustments. The FDA approved SSRIs for MDD, GAD, bulimia nervosa, bipolar depression, OCD, panic disorder, premenstrual dysphoric disorder, treatment-resistant depression, PTSD, and SAD. Off-label uses include binge eating disorder, body dysmorphic disorder, fibromyalgia, premature ejaculation, paraphilias, autism, Raynaud phenomenon, and vasomotor symptoms associated with menopause. Among SSRIs, sertraline and escitalopram are noted for their effectiveness and tolerability.14,43-53
SNRIs, including duloxetine, venlafaxine, desvenlafaxine, milnacipran, and levomilnacipran, may increase bleeding risk, especially when taken with blood thinners. They can also elevate blood pressure, which may worsen if combined with stimulants. SNRIs may interact with other medications that affect serotonin levels, increasing the risk of serotonin syndrome when taken with triptans, pain medications, or other antidepressants.14 Desvenlafaxine has been approved by the FDA (but not by the European Medicines Agency).54-56 Duloxetine is FDA-approved for the treatment of depression, neuropathic pain, anxiety disorders, fibromyalgia, and musculoskeletal disorders. It is used off-label to treat chemotherapy-induced peripheral neuropathy and stress urinary incontinence.57-61 Venlafaxine is FDA-approved for depression, SAD, and panic disorder, and is prescribed off-label to treat ADHD, neuropathy, fibromyalgia, cataplexy, and PTSD, either alone or in combination with other medications.62,63 Milnacipran is not approved for MDD; levomilnacipran received approval in 2013.64
SRMs such as trazodone, nefazodone, vilazodone, and vortioxetine also function as serotonin reuptake inhibitors.14,15 Trazodone is FDA-approved for MDD. It has been used off-label to treat anxiety, Alzheimer disease, substance misuse, bulimia nervosa, insomnia, fibromyalgia, and PTSD when first-line SSRIs are ineffective. A notable AE of trazodone is orthostatic hypotension, which can lead to dizziness and increase the risk of falls, especially in geriatric patients.65-70 Nefazodone was discontinued in Europe in 2003 due to rare cases of liver toxicity but remains available in the US.71-74 Vilazodone and vortioxetine are FDA-approved.
The latest classes of antidepressants include SMRAs and NMDARAs.14 Agomelatine, an SMRA, was approved in Europe in 2009 but rejected by the FDA in 2011 due to liver toxicity.75 NMDARAs like esketamine and a combination of dextromethorphan and bupropion received FDA approval in 2019 and 2022, respectively.76,77
This retrospective study analyzes noncancer drugs used during systemic chemotherapy based on a dataset of 14 antineoplastic agents. It sought to identify the most dispensed noncancer drug groups, discuss findings, compare patients with and without antidepressant prescriptions, and examine trends in antidepressant use from 2002 to 2023. This analysis expands on prior research.78-81
Methods
The Walter Reed National Military Medical Center Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and Military Health System (MHS) data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Data Sources
The JPC DoD Cancer Registry Program contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in CAPER represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2024. PDTS records are available from 2002 to 2004. Each observation in PDTS represents a prescription filled for an MHS beneficiary, excluding those filled at international civilian pharmacies and inpatient pharmacy prescriptions.
This cross-sectional analysis requested data extraction for specific cancer drugs from the DoD Cancer Registry, focusing on treatment details, diagnosis dates, patient demographics, and physicians’ comments on AEs. After identifying patients, CAPER was used to identify additional health conditions. PDTS was used to compile a list of prescription medications filled during systemic cancer treatment or < 2 years postdiagnosis.
The 2016 Surveillance, Epidemiology, and End Results Program Coding and Staging Manual and International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.82,83 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the subgroup divided by the total number of patients or data variables. To compare the mean number of dispensed antidepressants to those without antidepressants, a 2-tailed, 2-sample z test was used to calculate the P value and determine statistical significance (P < .05) using socscistatistics.com.
Data were extracted 3 times between 2021 and 2023. The initial 2021 protocol focused on erlotinib and gefitinib. A modified protocol in 2022 added paclitaxel, cisplatin, docetaxel, pemetrexed, and crizotinib; further modification in 2023 included 8 new antineoplastic agents and 2 anticoagulants. Sotorasib has not been prescribed in the MHS, and JPC lacks records for noncancer drugs. The 2023 dataset comprised 2210 patients with cancer treated with 14 antineoplastic agents; 2104 had documented diagnoses and 2113 had recorded prescriptions. Data for erlotinib, gefitinib, and paclitaxel have been published previously.78,79
Results
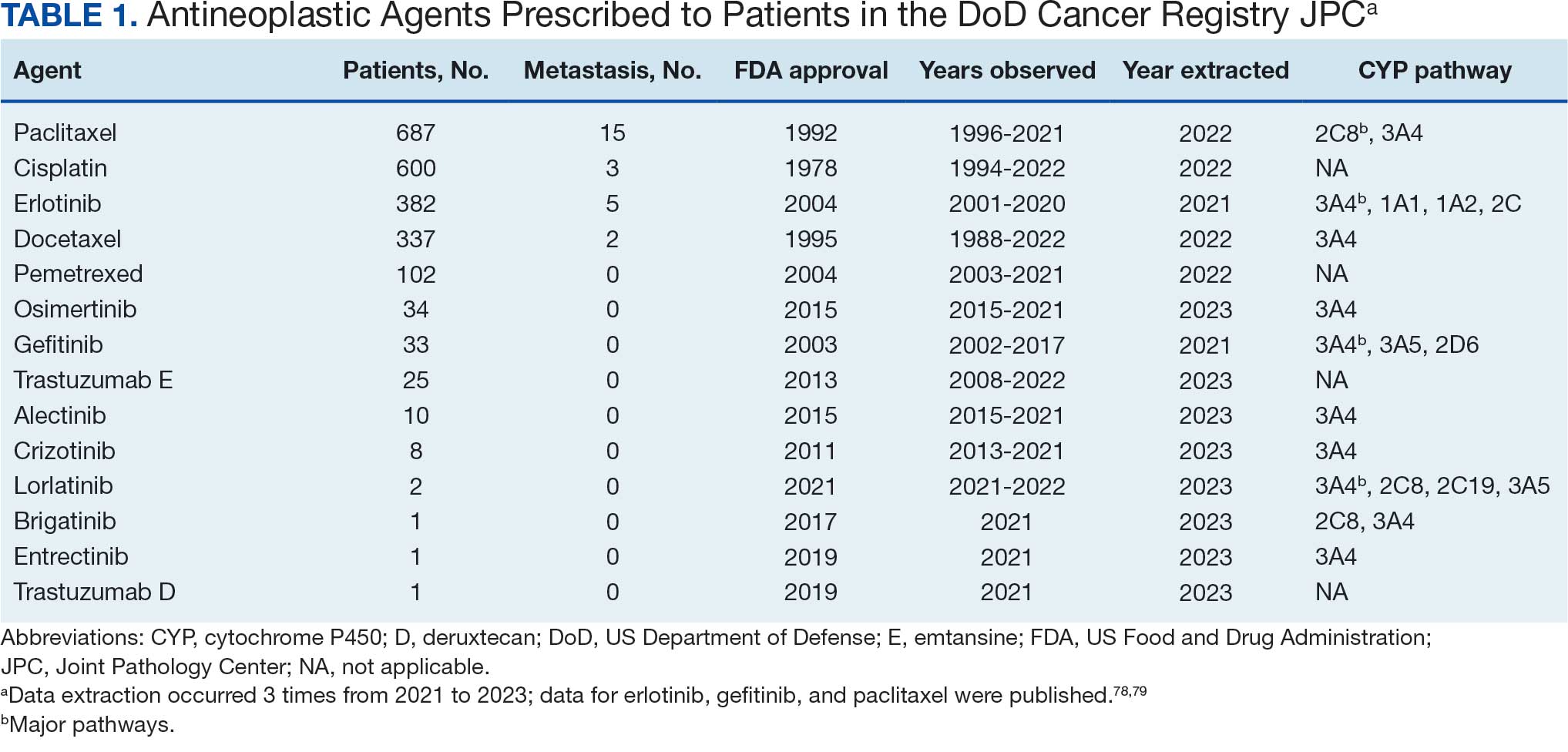
Of 2113 patients with recorded prescriptions, 1297 patients (61.4%) received 109 cancer drugs, including 96 antineoplastics, 7 disease-modifying antirheumatic agents, 4 biologic response modifiers, and 2 calcitonin gene-related peptides. Fourteen antineoplastic agents had complete data from JPC, while others were noted for combination therapies or treatment switches from the PDTS (Table 1). Seventy-six cancer drugs were prescribed with antidepressants in 489 patients (eAppendix).
The JPC provided 2242 entries for 2210 patients, ranging in age from 2 months to 88 years (mean, 56 years), documenting treatment from September 1988 to January 2023. Thirty-two patients had duplicate entries due to multiple cancer locations or occurrences. Of the 2242 patients, 1541 (68.7%) were aged > 50 years, 975 patients (43.5%) had cancers that were stage III or IV, and 1267 (56.5%) had cancers that were stage 0, I, II, or not applicable/unknown. There were 51 different types of cancer: breast, lung, testicular, endometrial, and ovarian were most common (n ≥ 100 patients). Forty-two cancer types were documented among 750 patients prescribed antidepressants (Table 2).
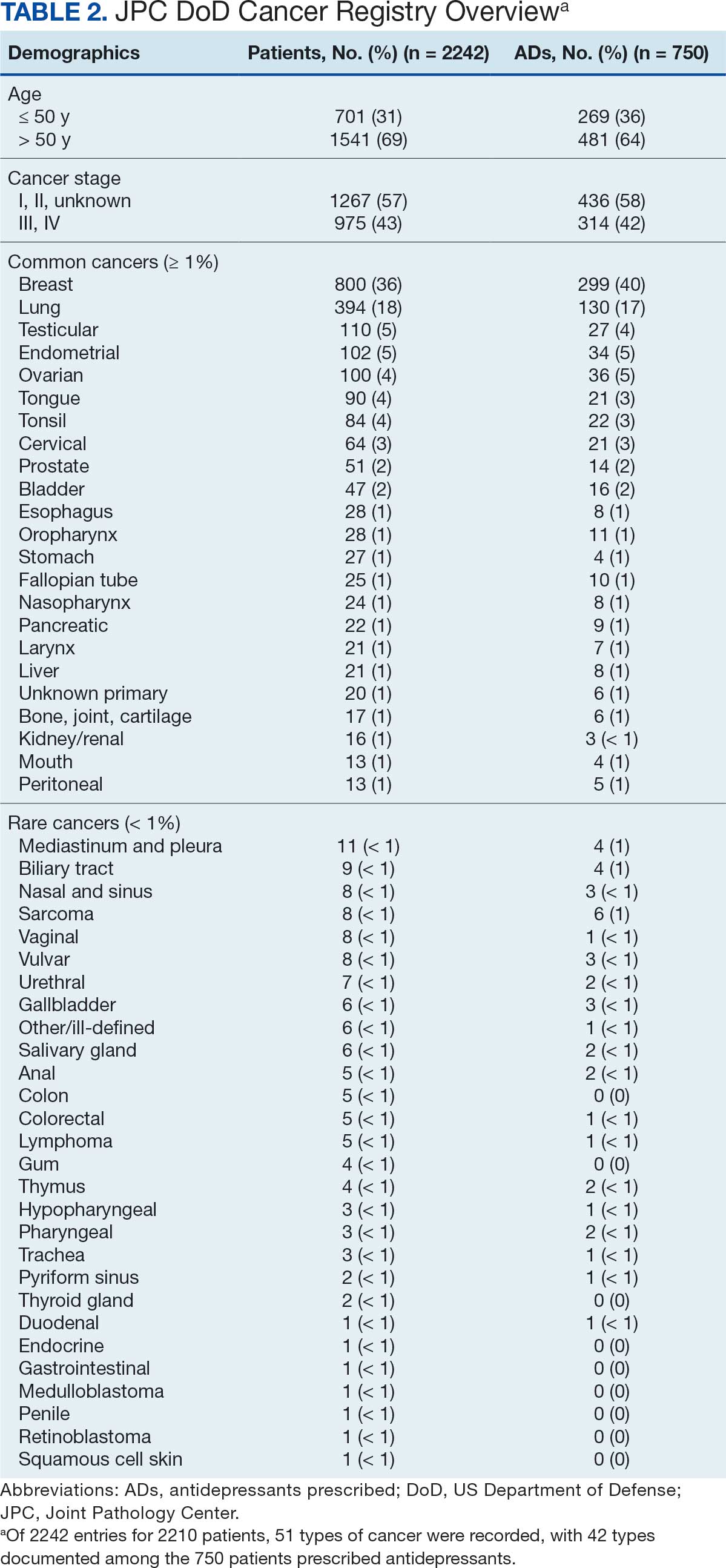
The CAPER database recorded 8882 unique diagnoses for 2104 patients, while PDTS noted 1089 unique prescriptions within 273 therapeutic codes for 2113 patients. Nine therapeutic codes (opiate agonists, adrenals, cathartics-laxatives, nonsteroidal anti-inflammatory agents, antihistamines for GI conditions, 5-HT3 receptor antagonists, analgesics and antipyretic miscellanea, antineoplastic agents, and proton-pump inhibitors) and 8 drugs (dexamethasone, prochlorperazine, ondansetron, docusate, acetaminophen, ibuprofen, oxycodone, and polyethylene glycol 3350) were associated with > 1000 patients (≥ 50%). Patients had between 1 and 275 unique health conditions and filled 1 to 108 prescriptions. The mean (SD) number of diagnoses and prescriptions was 50 (28) and 29 (12), respectively. Of the 273 therapeutic codes, 30 groups were analyzed, with others categorized into miscellaneous groups such as lotions, vaccines, and devices. Significant differences in mean number of prescriptions were found for patients taking antidepressants compared to those not (P < .05), except for anticonvulsants and antipsychotics (P = .12 and .09, respectively) (Table 3).
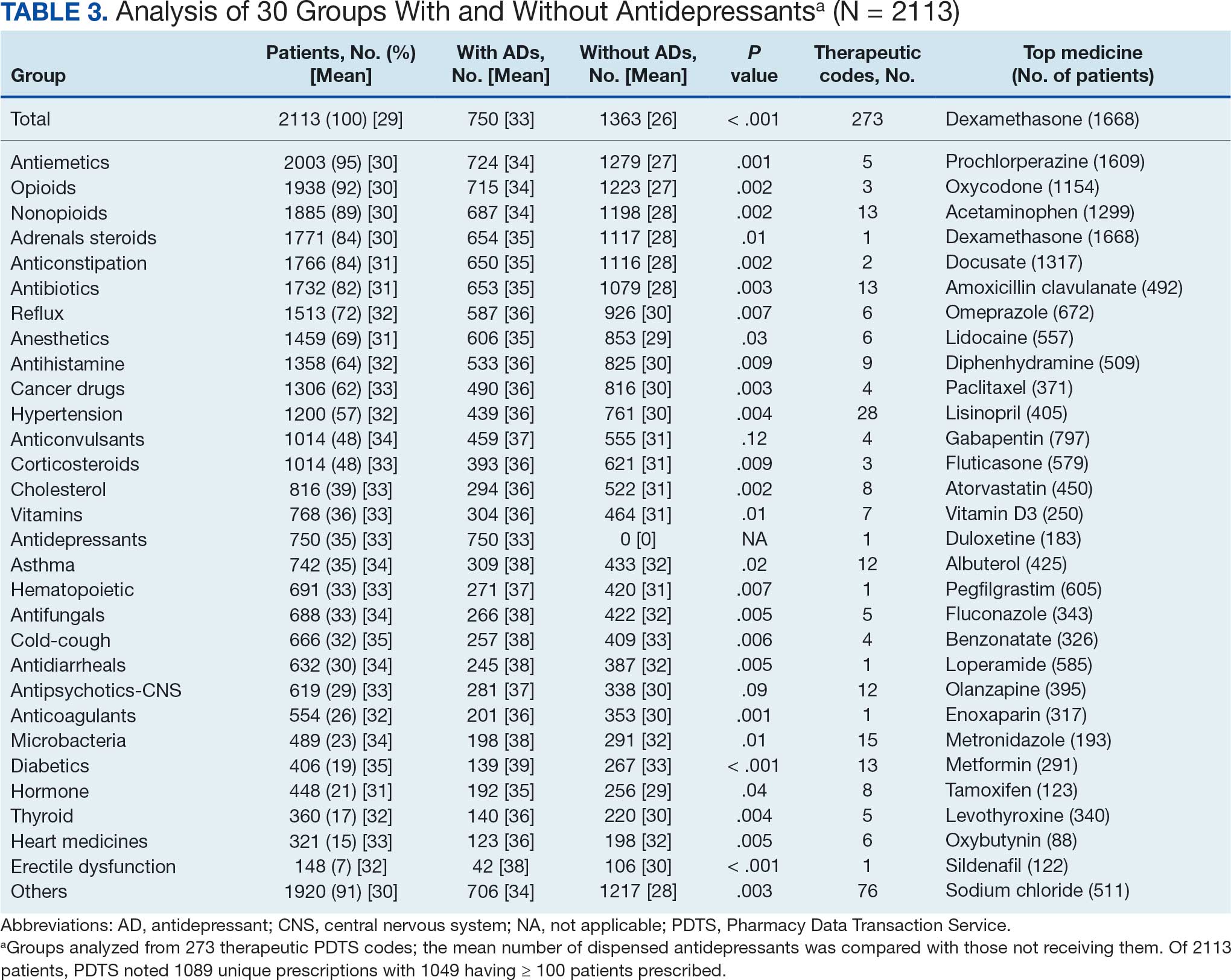
Antidepressants
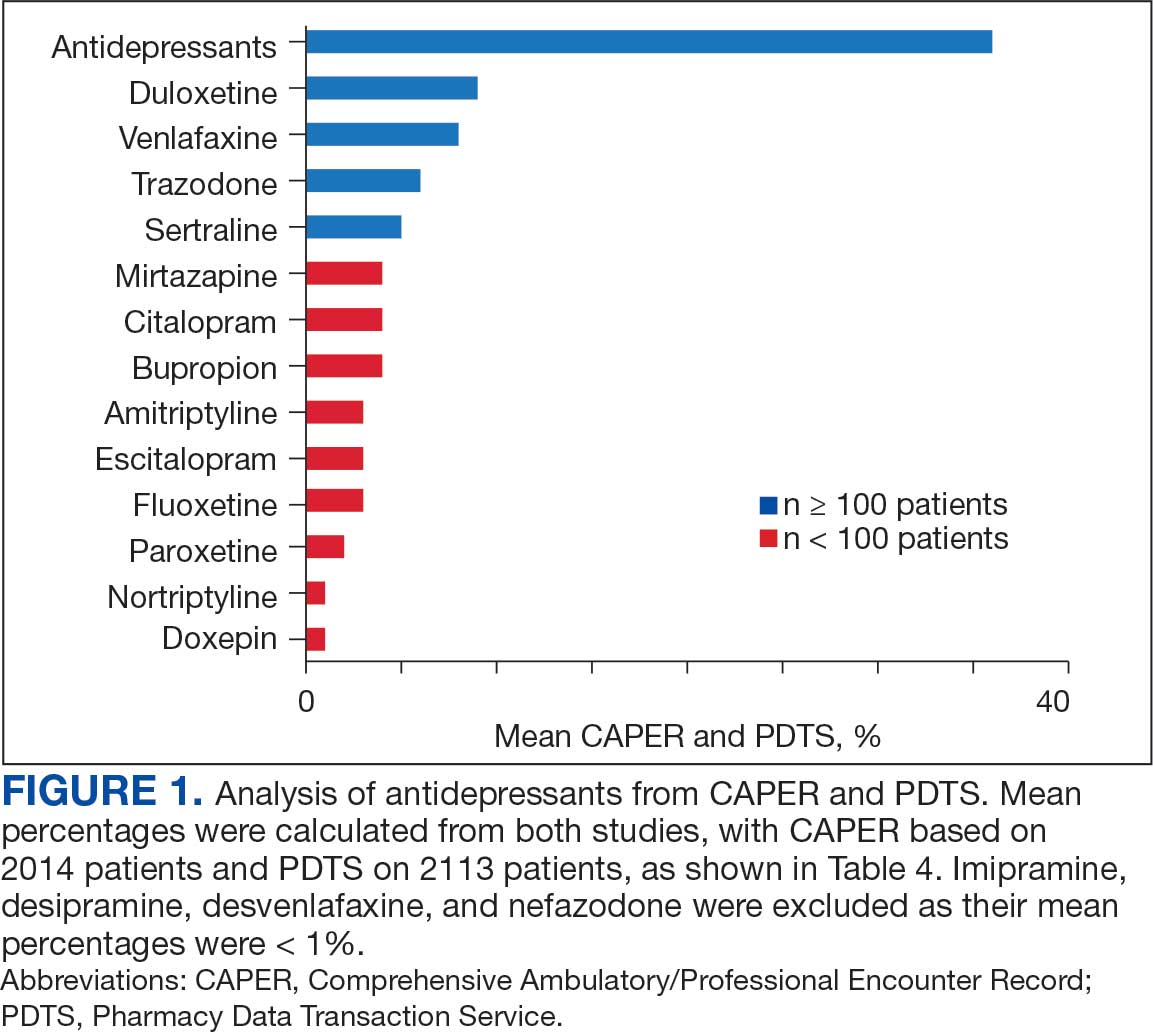
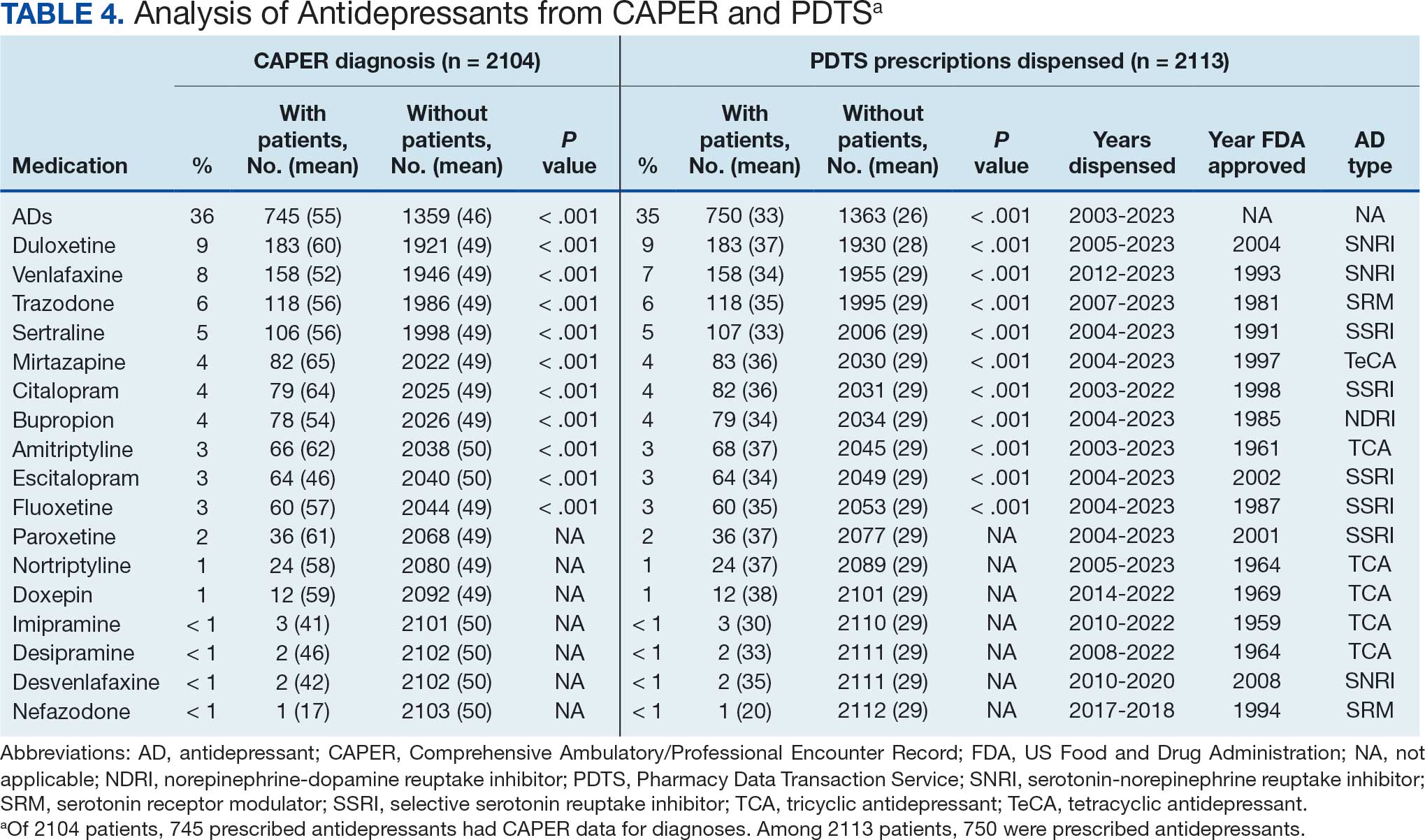
Of the 2113 patients with recorded prescriptions, 750 (35.5%) were dispensed 17 different antidepressants. Among these 17 antidepressants, 183 (8.7%) patients received duloxetine, 158 (7.5%) received venlafaxine, 118 (5.6%) received trazodone, and 107 (5.1%) received sertraline (Figure 1, Table 4). Of the 750 patients, 509 (67.9%) received 1 antidepressant, 168 (22.4%) received 2, 60 (8.0%) received 3, and 13 (1.7%) received > 3. Combinations varied, but only duloxetine and trazodone were prescribed to > 10 patients.
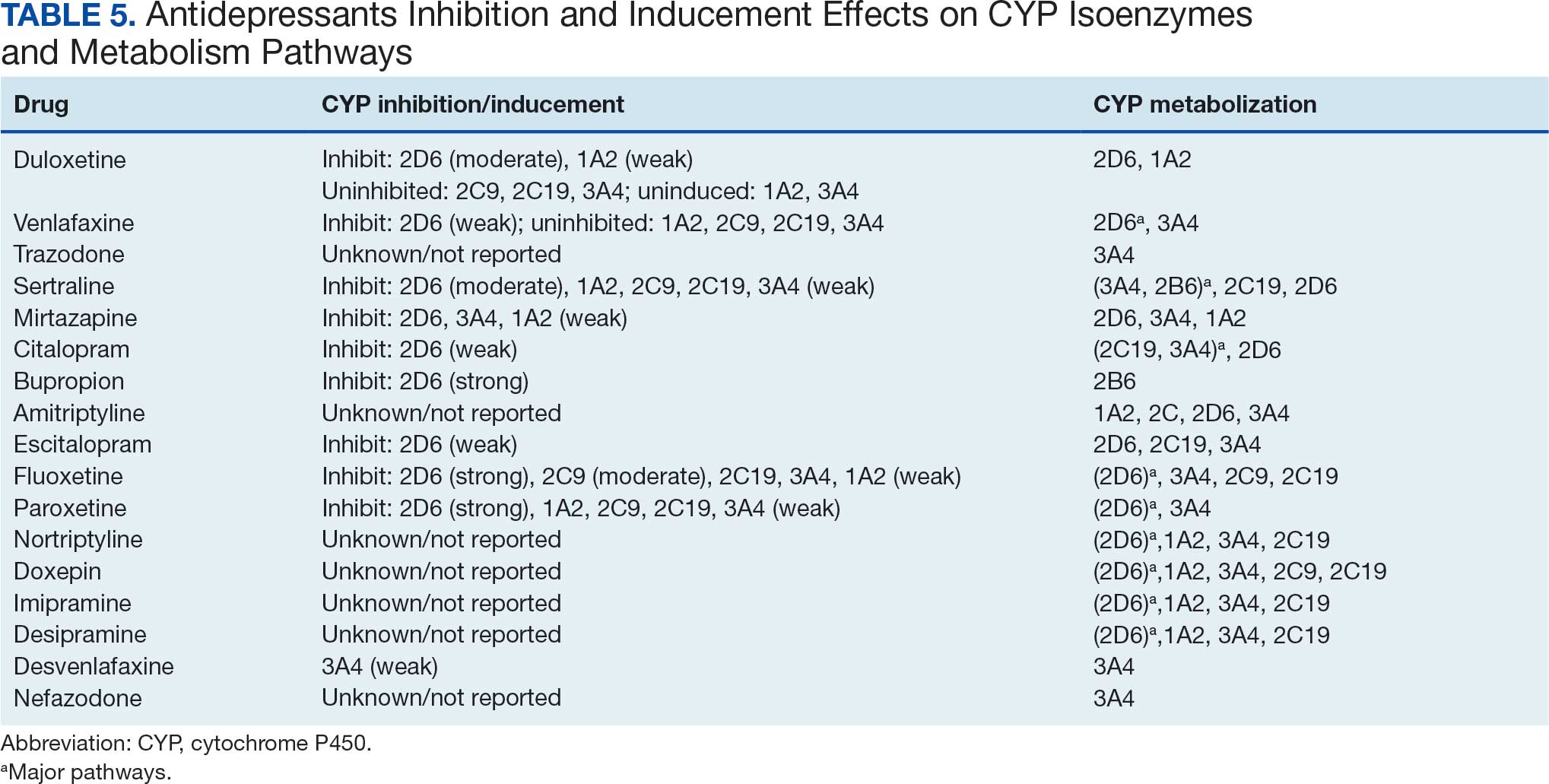
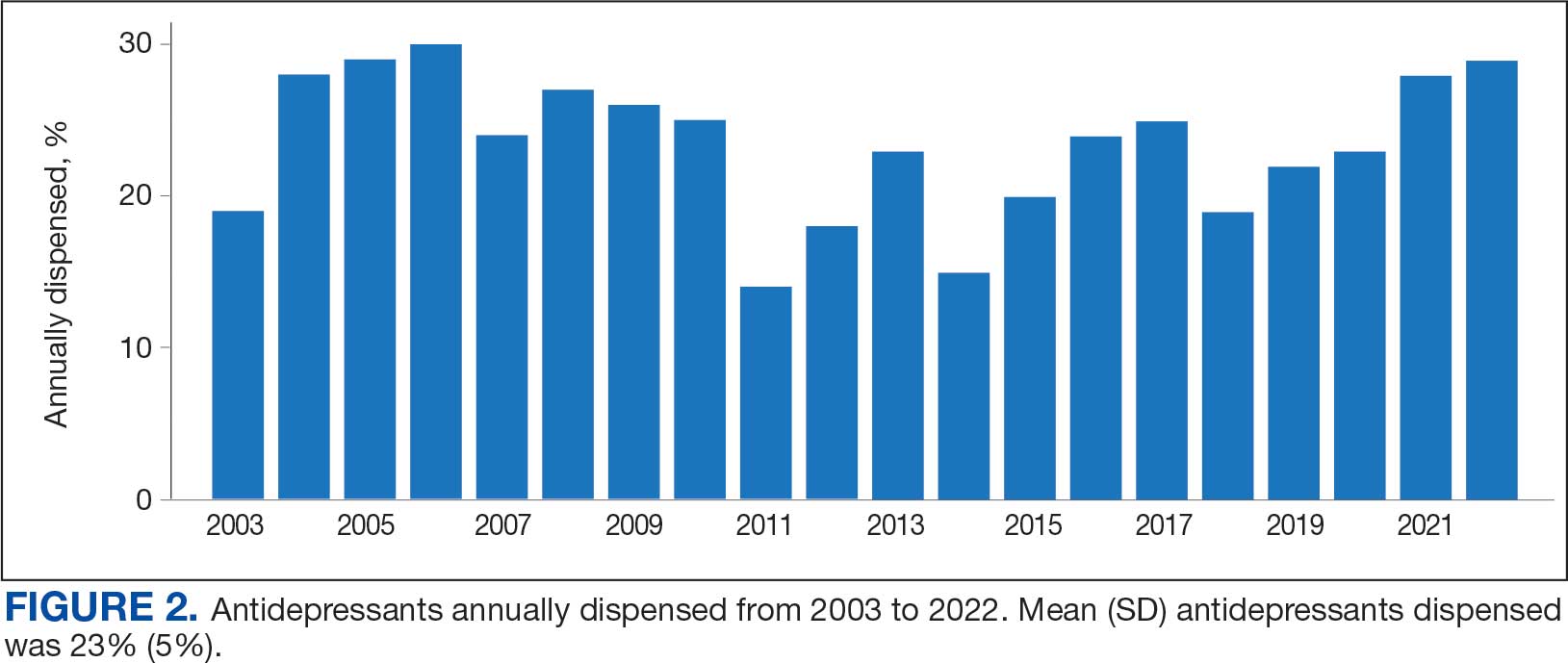
Antidepressants were prescribed annually at an overall mean (SD) rate of 23% (5%) from 2003 to 2022 (Figure 2). Patients on antidepressants during systemic therapy had a greater number of diagnosed medical conditions and received more prescription medications compared to those not taking antidepressants (P < .001) (Figure 3). The 745 patients taking antidepressants in CAPER data had between 1 and 275 diagnosed medical issues, with a mean (SD) of 55 (31) vs a range of 1 to 209 and a mean (SD) of 46 (26) for the 1359 patients not taking antidepressants. The 750 patients on antidepressants in PDTS data had between 8 and 108 prescriptions dispensed, with a mean (SD) of 32 (12), vs a range of 1 to 65 prescriptions and a mean (SD) of 29 (12) for 1363 patients not taking antidepressants.
Discussion
The JPC DoD Cancer Registry includes information on cancer types, stages, treatment regimens, and physicians’ notes, while noncancer drugs are sourced from the PDTS database. The pharmacy uses a different documentation system, leading to varied classifications.
Database reliance has its drawbacks. For example, megestrol is coded as a cancer drug, although it’s primarily used for endometrial or gynecologic cancers. Many drugs have multiple therapeutic codes assigned to them, including 10 antineoplastic agents: diclofenac, Bacillus Calmette-Guérin (BCG), megestrol acetate, tamoxifen, anastrozole, letrozole, leuprolide, goserelin, degarelix, and fluorouracil. Diclofenac, BCG, and mitomycin have been repurposed for cancer treatment.84-87 From 2003 to 2023, diclofenac was prescribed to 350 patients for mild-to-moderate pain, with only 2 patients receiving it for cancer in 2018. FDA-approved for bladder cancer in 1990, BCG was prescribed for cancer treatment for 1 patient in 2021 after being used for vaccines between 2003 and 2018. Tamoxifen, used for hormone receptor-positive breast cancer from 2004 to 2017 with 53 patients, switched to estrogen agonist-antagonists from 2017 to 2023 with 123 patients. Only a few of the 168 patients were prescribed tamoxifen using both codes.88-91 Anastrozole and letrozole were coded as antiestrogens for 7 and 18 patients, respectively, while leuprolide and goserelin were coded as gonadotropins for 59 and 18 patients. Degarelix was coded as antigonadotropins, fluorouracil as skin and mucous membrane agents miscellaneous, and megestrol acetate as progestins for 7, 6, and 3 patients, respectively. Duloxetine was given to 186 patients, primarily for depression from 2005 to 2023, with 7 patients treated for fibromyalgia from 2022 to 2023.
Antidepressants Observed
Tables 1 and 5 provide insight into the FDA approval of 14 antineoplastics and antidepressants and their CYP metabolic pathways.92-122 In Table 4, the most prescribed antidepressant classes are SNRIs, SRMs, SSRIs, TeCAs, NDRIs, and TCAs. This trend highlights a preference for newer medications with weak CYP inhibition. A total of 349 patients were prescribed SSRIs, 343 SNRIs, 119 SRMs, 109 TCAs, 83 TeCAs, and 79 NDRIs. MAOIs, SMRAs, and NMDARAs were not observed in this dataset. While there are instances of dextromethorphan-bupropion and sertraline-escitalopram being dispensed together, it remains unclear whether these were NMDARA combinations.
Among the 14 specific antineoplastic agents, 10 are metabolized by CYP isoenzymes, primarily CYP3A4. Duloxetine neither inhibits nor is metabolized by CYP3A4, a reason it is often recommended, following venlafaxine.
Both duloxetine and venlafaxine are used off-label for chemotherapy-induced peripheral neuropathy related to paclitaxel and docetaxel. According to the CYP metabolized pathway, duloxetine tends to have more favorable DDIs than venlafaxine. In PDTS data, 371 patients were treated with paclitaxel and 180 with docetaxel, with respective antidepressant prescriptions of 156 and 70. Of the 156 patients dispensed paclitaxel, 62 (40%) were dispensed with duloxetine compared to 43 (28%) with venlafaxine. Of the 70 patients dispensed docetaxel, 23 (33%) received duloxetine vs 24 (34%) with venlafaxine.
Of 85 patients prescribed duloxetine, 75 received it with either paclitaxel or docetaxel (5 received both). Five patients had documented AEs (1 neuropathy related). Of 67 patients prescribed venlafaxine, 66 received it with either paclitaxel or docetaxel. Two patients had documented AEs (1 was neuropathy related, the same patient who received duloxetine). Of the 687 patients treated with paclitaxel and 337 with docetaxel in all databases, 4 experienced neuropathic AEs from both medications.79
Antidepressants can increase the risk of bleeding, especially when combined with blood thinners, and may elevate blood pressure, particularly alongside stimulants. Of the 554 patients prescribed 9 different anticoagulants, enoxaparin, apixaban, and rivaroxaban were the most common (each > 100 patients). Among these, 201 patients (36%) received both anticoagulants and antidepressants: duloxetine for 64 patients, venlafaxine for 30, trazodone for 35, and sertraline for 26. There were no data available to assess bleeding rates related to the evaluation of DDIs between these medication classes.
Antidepressants can be prescribed for erectile dysfunction. Of the 148 patients prescribed an antidepressant for erectile dysfunction, duloxetine, trazodone, and mirtazapine were the most common. Antidepressant preferences varied by cancer type. Duloxetine was the only antidepressant used for all types of cancer. Venlafaxine, duloxetine, trazodone, sertraline, and escitalopram were the most prescribed antidepressants for breast cancer, while duloxetine, mirtazapine, citalopram, sertraline, and trazodone were the most prescribed for lung cancer. Sertraline, duloxetine, trazodone, amitriptyline, and escitalopram were most common for testicular cancer. Duloxetine, venlafaxine, trazodone, amitriptyline, and sertraline were the most prescribed for endometrial cancer, while duloxetine, venlafaxine, amitriptyline, citalopram, and sertraline were most prescribed for ovarian cancer.
The broadness of International Statistical Classification of Diseases, Tenth Revision codes made it challenging to identify nondepression diagnoses in the analyzed population. However, if all antidepressants were prescribed to treat depression, service members with cancer exhibited a higher depression rate (35%) than the general population (25%). Of 2104 patients, 191 (9.1%) had mood disorders, and 706 (33.6%) had mental disorders: 346 (49.0%) had 1 diagnosis, and 360 (51.0%) had multiple diagnoses. The percentage of diagnoses varied yearly, with notable drops in 2003, 2007, 2011, 2014, and 2018, and peaks in 2006, 2008, 2013, 2017, and 2022. This fluctuation was influenced by events like the establishment of PDTS in 2002, the 2008 economic recession, a hospital relocation in 2011, the 2014 Ebola outbreak, and the COVID-19 pandemic. Although the number of patients receiving antidepressants increased from 2019 to 2022, the overall percentage of patients receiving them did not significantly change from 2003 to 2022, aligning with previous research.5,125
Many medications have potential uses beyond what is detailed in the prescribing information. Antidepressants can relieve pain, while pain medications may help with depression. Opioids were once thought to effectively treat depression, but this perspective has changed with a greater understanding of their risks, including misuse.126-131 Pain is a severe and often unbearable AE of cancer. Of 2113 patients, 92% received opioids; 34% received both opioids and antidepressants; 2% received only antidepressants; and 7% received neither. This study didn’t clarify whether those on opioids alone recognized their depression or if those on both were aware of their dependence. While SSRIs are generally not addictive, they can lead to physical dependence, and any medication can be abused if not managed properly.132-134
Conclusions
This retrospective study analyzes data from antineoplastic agents used in systemic cancer treatment between 1988 and 2023, with a particular focus on the use of antidepressants. Data on antidepressant prescriptions are incomplete and specific to these agents, which means the findings cannot be generalized to all antidepressants. Hence, the results indicate that patients taking antidepressants had more diagnosed health issues and received more medications compared to patients who were not on these drugs.
This study underscores the need for further research into the effects of antidepressants on cancer treatment, utilizing all data from the DoD Cancer Registry. Future research should explore DDIs between antidepressants and other cancer and noncancer medications, as this study did not assess AE documentation, unlike in studies involving erlotinib, gefitinib, and paclitaxel.78,79 Further investigation is needed to evaluate the impact of discontinuing antidepressant use during cancer treatment. This comprehensive overview provides insights for clinicians to help them make informed decisions regarding the prescription of antidepressants in the context of cancer treatment.
- National Cancer Institute. Depression (PDQ)-Health Professional Version. Updated July 25, 2024. Accessed April 4, 2025. https://www.cancer.gov/about-cancer/coping/feelings/depression-hp-pdq
- Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130. doi:10.1002/pon.3409
- Xiang X, An R, Gehlert S. Trends in antidepressant use among U.S. cancer survivors, 1999-2012. Psychiatr Serv. 2015;66(6):564. doi:10.1176/appi.ps.201500007
- Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. doi:10.1016/j.ejca.2016.11.017
- Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900. doi:10.1093/annonc/mds575
- Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi:10.1136/bmj.k1415
- Kisely S, Alotiby MKN, Protani MM, Soole R, Arnautovska U, Siskind D. Breast cancer treatment disparities in patients with severe mental illness: a systematic review and meta-analysis. Psychooncology. 2023;32(5):651-662. doi:10.1002/pon.6120
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71. doi:10.1093/jncimonographs/lgh014
- Rodin G, Katz M, Lloyd N, Green E, Mackay JA, Wong RK. Treatment of depression in cancer patients. Curr Oncol. 2007;14(5):180-188. doi:10.3747/co.2007.146
- Wilson KG, Chochinov HM, Skirko MG, et al. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33(2):118-129. doi:10.1016/j.jpainsymman.2006.07.016
- Moradi Y, Dowran B, Sepandi M. The global prevalence of depression, suicide ideation, and attempts in the military forces: a systematic review and meta-analysis of cross sectional studies. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
- Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi:10.1136/bmj.g3596
- Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
- Sheffler ZM, Patel P, Abdijadid S. Antidepressants. In: StatPearls. StatPearls Publishing. Updated May 26, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK538182/
- Miller JJ. Antidepressants, Part 1: 100 Years and Counting. Accessed April 4, 2025. Psychiatric Times. https:// www.psychiatrictimes.com/view/antidepressants-part-1-100-years-and-counting
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21. doi:10.1037/a0038550
- Mitchell PB, Mitchell MS. The management of depression. Part 2. The place of the new antidepressants. Aust Fam Physician. 1994;23(9):1771-1781.
- Sabri MA, Saber-Ayad MM. MAO Inhibitors. In: Stat- Pearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557395/
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539848/
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248. doi:10.1097/00131746-200407000-00005
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
- Moraczewski J, Awosika AO, Aedma KK. Tricyclic Antidepressants. In: StatPearls. StatPearls Publishing. Updated August 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557791/
- Almasi A, Patel P, Meza CE. Doxepin. In: StatPearls. StatPearls Publishing. Updated February 14, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK542306/
- Thour A, Marwaha R. Amitriptyline. In: StatPearls. Stat- Pearls Publishing. Updated July 18, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK537225/
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
- Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680- 690. doi:10.1016/S0140-6736(22)01472-6
- Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network metaanalysis. JAMA Netw Open. 2022;5(5):e2212939. doi:10.1001/jamanetworkopen.2022.12939
- Merwar G, Gibbons JR, Hosseini SA, Saadabadi A. Nortriptyline. In: StatPearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482214/
- Fayez R, Gupta V. Imipramine. In: StatPearls. StatPearls Publishing. Updated May 22, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557656/
- Jilani TN, Gibbons JR, Faizy RM, Saadabadi A. Mirtazapine. In: StatPearls. StatPearls Publishing. Updated August 28, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519059/
- Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17 Suppl 1:S37-S41. doi:10.1002/hup.388
- Gandotra K, Chen P, Jaskiw GE, Konicki PE, Strohl KP. Effective treatment of insomnia with mirtazapine attenuates concomitant suicidal ideation. J Clin Sleep Med. 2018;14(5):901-902. doi:10.5664/jcsm.7142
- Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264. doi:10.1111/j.1527-3458.2001.tb00198.x
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101-112. doi:10.4068/cmj.2018.54.2.101
- Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls. StatPearls Publishing. Updated April 9, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470212/
- Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895- 905. doi:10.1111/obr.12835
- Hankosky ER, Bush HM, Dwoskin LP, et al. Retrospective analysis of health claims to evaluate pharmacotherapies with potential for repurposing: association of bupropion and stimulant use disorder remission. AMIA Annu Symp Proc. 2018;2018:1292-1299.
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Hajek P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2019; 2(2):CD003999. doi:10.1002/14651858.CD003999.pub5
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6(2):59-74. doi:10.1016/j.esxm.2018.01.004
- Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2017;10(10):CD009504. doi:10.1002/14651858.CD009504.pub2
- Ng QX. A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(2):112-116. doi:10.1089/cap.2016.0124
- Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113. doi:10.4088/pcc.v07n0305
- Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls. StatPearls Publishing. Updated May 1, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK554406/
- Singh HK, Saadabadi A. Sertraline. In: StatPearls. Stat- Pearls Publishing. Updated February 13, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK547689/
- MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7(1):1-24. doi:10.1111/j.1527-3458.2001.tb00188.x
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi:10.1016/S0140-6736(09)60046-5
- Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. Am J Psychiatry. 2006;163(11):1864-1866. doi:10.1176/ajp.2006.163.11.1864
- Sharbaf Shoar N, Fariba KA, Padhy RK. Citalopram. In: StatPearls. StatPearls Publishing. Updated November 7, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482222/
- Landy K, Rosani A, Estevez R. Escitalopram. In: Stat- Pearls. StatPearls Publishing. Updated November 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557734/
- Cavanah LR, Ray P, Goldhirsh JL, Huey LY, Piper BJ. Rise of escitalopram and the fall of citalopram. medRxiv. Preprint published online May 8, 2023. doi:10.1101/2023.05.07.23289632
- Sohel AJ, Shutter MC, Patel P, Molla M. Fluoxetine. In: StatPearls. StatPearls Publishing. Updated July 4, 2022. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4(9):764-774. doi:10.1038/nrd1821
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. In: Stat- Pearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526022/
- Naseeruddin R, Rosani A, Marwaha R. Desvenlafaxine. In: StatPearls. StatPearls Publishing. Updated July 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534829
- Lieberman DZ, Massey SH. Desvenlafaxine in major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2010;4:67-82. doi:10.2147/ce.s5998
- Withdrawal assessment report for Ellefore (desvenlafaxine). European Medicine Agency. 2009. Accessed April 4, 2025. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-ellefore_en.pdf
- Dhaliwal JS, Spurling BC, Molla M. Duloxetine. In: Stat- Pearls. Statpearls Publishing. Updated May 29, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967. doi:10.1200/JCO.2013.54.0914
- Sommer C, Häuser W, Alten R, et al. Medikamentöse Therapie des Fibromyalgiesyndroms. Systematische Übersicht und Metaanalyse [Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline]. Schmerz. 2012;26(3):297-310. doi:10.1007/s00482-012-1172-2
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi:10.1212/WNL.0b013e3182166ebe
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. doi:10.1111/j.1468-1331.2010.02999.x
- Singh D, Saadabadi A. Venlafaxine. In: StatPearls. StatePearls Publishing. Updated February 26, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Sertraline and venlafaxine: new indication. Prevention of recurrent depression: no advance. Prescrire Int. 2005;14(75):19-20.
- Bruno A, Morabito P, S p i n a E , M u s c a t ello MRl. The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuro pharmacol. 2016;14(2):191-199. doi:10.2174/1570159x14666151117122458
- Shin JJ, Saadabadi A. Trazodone. In: StatPearls. StatPearls Publishing. Updated February 29, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470560/
- Khouzam HR. A review of trazodone use in psychiatric and medical conditions. Postgrad Med. 2017;129(1):140-148. doi:10.1080/00325481.2017.1249265
- Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758-764. doi:10.1513/AnnalsATS.201408-399OC
- Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811-819. doi:10.5665/sleep.3596
- Schatzberg AF, Nemeroff CB, eds. The American Psychiat ric Association Publishing Textbook of Psychopharmacology. 4th ed. American Psychiatric Publishing; 2009.
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and allcause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209-220. doi:10.1111/bcp.12617
- Nefazodone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. Updated March 6, 2020. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK548179/
- Drugs of Current Interest: Nefazodone. WHO Pharmaceuticals Newsletter:(1). 2003(1):7. https://web.archive.org/web/20150403165029/http:/apps.who.int/medicinedocs/en/d/Js4944e/3.html
- Choi S. Nefazodone (serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169(11):1187.
- Teva Nefazodone Statement. News release. Teva USA. December 20, 2021. Accessed April 4, 2025. https:// www.tevausa.com/news-and-media/press-releases/teva-nefazodone-statement/
- Levitan MN, Papelbaum M, Nardi AE. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2015;11:1149- 1155. doi:10.2147/NDT.S67470
- Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi:10.4088/JCP.19m13191
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4): 21m14345. doi:10.4088/JCP.21m14345
- Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the Military Health System. Fed Pract. 2023;40(Suppl 3):S24-S34. doi:10.12788/fp.0401
- Luong TT, Shou KJ, Reinhard BJ, Kigelman OF, Greenfield KM. Paclitaxel drug-drug interactions in the Military Health System. Fed Pract. 2024;41(Suppl 3):S70-S82. doi:10.12788/fp.0499
- Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Preclinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
- Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217- 224. doi:10.1016/j.crtox.2021.05.006,
- Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed April 4, 2025. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
- World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed, 1st revision. World Health Organization; 2013. Accessed April 4, 2025. https://apps.who.int/iris/handle/10665/96612
- Simon S. FDA approves Jelmyto (mitomycin gel) for urothelial cancer. American Cancer Society. April 20, 2020. Accessed April 4, 2025. https://www.cancer.org/cancer/latest-news/fda-approves-jelmyto-mitomycin-gel-for-urothelial-cancer.html
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)- diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi:10.3332/ecancer.2016.610
- Choi S, Kim S, Park J, Lee SE, Kim C, Kang D. Diclofenac: a nonsteroidal anti-inflammatory drug inducing cancer cell death by inhibiting microtubule polymerization and autophagy flux. Antioxidants (Basel). 2022;11(5):1009. doi:10.3390/antiox11051009
- Tontonoz M. The ABCs of BCG: oldest approved immunotherapy gets new explanation. Memorial Sloan Kettering Cancer Center. July 17, 2020. Accessed April 4, 2025. https://www.mskcc.org/news/oldest-approved-immunotherapy-gets-new-explanation
- Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21(3):R235-R246. doi:10.1530/ERC-14-0092
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270-275. doi:10.1038/bjc.1971.33
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205-213. doi:10.1038/nrd1031
- Maximov PY, McDaniel RE, Jordan VC. Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Basel; 2013. Accessed April 4, 2025. https://link.springer.com/book/10.1007/978-3-0348-0664-0
- Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb Company; 2011. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
- Tarceva (erlotinib). Prescribing information. OSI Pharmaceuticals, LLC; 2016. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
- Docetaxel. Prescribing information. Sichuan Hyiyu Pharmaceutical Co.; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215813s000lbl.pdf
- Alimta (pemetrexed). Prescribing information. Teva Pharmaceuticals; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208419s004lbl.pdf
- Tagrisso (osimertinib). Prescribing information. Astra- Zeneca Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
- Iressa (gefitinib). Prescribing information. AstraZeneca Pharmaceuticals; 2018. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
- Kadcyla (ado-trastuzumab emtansine). Prescribing information. Genentech, Inc.; 2013. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf
- Alecensa (alectinib). Prescribing information. Genetech, Inc.; 2017. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf
- Xalkori (crizotinib). Prescribing information. Pfizer Laboratories; 2022. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2022/202570s033lbl.pdf
- Lorbrena (lorlatinib). Prescribing information. Pfizer Laboratories; 2018. Accessed April 14, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Alunbrig (brigatinib). Prescribing information. Takeda Pharmaceutical Company; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf
- Rozlytrek (entrectinib). Prescribing information. Genentech, Inc.; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf
- Herceptin (trastuzumab). Prescribing information. Genentech, Inc.; 2010. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf
- Cybalta (duloxetine). Prescribing information. Eli Lilly and Company; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021427s049lbl.pdf
- Effexor XR (venlafaxine). Prescribing information. Pfizer Wyeth Pharmaceuticals Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020699s112lbl.pdf
- Desyrel (trazodone hydrochloride). Prescribing information. Pragma Pharmaceuticals; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
- Sertraline hydrochloride. Prescribing information. Almatica Pharma LLC; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215133s000lbl.pdf
- Remeron (mirtazapine). Prescribing information. Merck & Co. Inc; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
- Celexa (citalopram). Prescribing information. Allergan USA Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- information. GlaxoSmithKline; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020358s066lbl.pdf
- Amitriptyline hydrochloride tablet. Prescribing information. Quality Care Products LLC; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0f12f50f-7087-46e7-a2e6-356b4c566c9f/0f12f50f-7087-46e7-a2e6-356b4c566c9f.xml
- Lexapro (escitalopram). Prescribing information. AbbVie Inc; 2023. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021323s055,021365s039lbl.pdf
- Fluoxetine. Prescribing information. Edgemont Pharmaceutical, LLC; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202133s004s005lbl.pdf
- Paxil (paroxetine). Prescribing Information. Apotex Inc; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
- Pamelor (nortriptyline HCl). Prescribing information. Mallinckrodt, Inc; 2012. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
- Silenor (doxepin). Prescribing information. Currax Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022036s006lbl.pdf
- Tofranil-PM (imipramine pamote). Prescribing information. Mallinckrodt, Inc; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/017090s078lbl.pdf
- Norpramin (desipramine hydrochloride). Prescribing information. Sanofi-aventis U.S. LLC; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/014399s069lbl.pdf
- Khedezla (desvenlafaxine). Prescribing information. Osmotical Pharmaceutical US LLC; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204683s006lbl.pdf
- Nefazodone hydrochloride. Prescribing information. Bryant Ranch Prepack; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0bd4c34a-4f43-4c84-8b98-1d074cba97d5/0bd4c34a-4f43-4c84-8b98-1d074cba97d5.xml
- Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol. 2018;29(1):101-111. doi:10.1093/annonc/mdx526
- Lee E, Park Y, Li D, Rodriguez-Fuguet A, Wang X, Zhang WC. Antidepressant use and lung cancer risk and survival: a meta-analysis of observational studies. Cancer Res Commun. 2023;3(6):1013-1025. doi:10.1158/2767-9764.CRC-23-0003
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848 -856. doi:10.1001/archgenpsychiatry.2009.81
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311. doi:10.1370/afm.1371
- Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4(4):340-351. doi:10.1111/j.1526-4637.2003.03038.x
- Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344-350. doi:10.1016/s1526-5900(03)00638-2
- Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8. doi:10.1097/AJP.0b013e3181b99f35
- Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339. doi:10.1016/j.pain.2010.05.020
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
- Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300- 307. doi:10.1177/026988119901300321
- Lakeview Health Staff. America’s most abused antidepressants. Lakeview Health. January 24, 2004. Accessed April 4, 2025. https://www.lakeviewhealth.com/blog/us-most-abused-antidepressants/
- Greenhouse Treatment Center Editorial Staff. Addiction to antidepressants: is it possible? America Addiction Centers: Greenhouse Treatment Center. Updated April 23, 2024. Accessed April 4, 2025. https://greenhousetreatment.com/prescription-medication/antidepressants/
Cancer patients experience depression at rates > 5 times that of the general population.1-11 Despite an increase in palliative care use, depression rates continued to rise.2-4 Between 5% to 16% of outpatients, 4% to 14% of inpatients, and up to 49% of patients receiving palliative care experience depression.5 This issue also impacts families and caregivers.1 A 2021 meta-analysis found that 23% of active military personnel and 20% of veterans experience depression.11
Antidepressants approved by the US Food and Drug Administration (FDA) target the serotonin, norepinephrine, or dopamine systems and include boxed warnings about an increased risk of suicidal thoughts in adults aged 18 to 24 years.12,13 These medications are categorized into several classes: monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), tetracyclic antidepressants (TeCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), serotonin receptor modulators (SRMs), serotonin-melatonin receptor antagonists (SMRAs), and N—methyl-D-aspartate receptor antagonists (NMDARAs).14,15 The first FDA-approved antidepressants, iproniazid (an MAOI) and imipramine (a TCA) laid the foundation for the development of newer classes like SSRIs and SNRIs.15-17
Older antidepressants such as MAOIs and TCAs are used less due to their adverse effects (AEs) and drug interactions. MAOIs, such as iproniazid, selegiline, moclobemide, tranylcypromine, isocarboxazid, and phenelzine, have numerous AEs and drug interactions, making them unsuitable for first- or second-line treatment of depression.14,18-21 TCAs such as doxepin, amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, trimipramine, protriptyline, maprotiline, and amoxapine have a narrow therapeutic index requiring careful monitoring for signs of toxicity such as QRS widening, tremors, or confusion. Despite the issues, TCAs are generally classified as second-line agents for major depressive disorder (MDD). TCAs have off-label uses for migraine prophylaxis, treatment of obsessive-compulsive disorder (OCD), insomnia, and chronic pain management first-line.14,22-29
Newer antidepressants, including TeCAs and NDRIs, are typically more effective, but also come with safety concerns. TeCAs like mirtazapine interact with several medications, including MAOIs, serotonin-increasing drugs, alcohol, cannabidiol, and marijuana. Mirtazapine is FDA-approved for the treatment of moderate to severe depression in adults. It is also used off-label to treat insomnia, panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), headaches, and migraines. Compared to other antidepressants, mirtazapine is effective for all stages of depression and addresses a broad range of related symptoms.14,30-34 NDRIs, such as bupropion, also interact with various medications, including MAOIs, other antidepressants, stimulants, and alcohol. Bupropion is FDA-approved for smoking cessation and to treat depression and SAD. It is also used off-label for depression- related bipolar disorder or sexual dysfunction, attention-deficit/hyperactivity disorder (ADHD), and obesity.14,35-42
SSRIs, SNRIs, and SRMs should be used with caution. SSRIs such as sertraline, citalopram, escitalopram, fluoxetine, paroxetine, and fluvoxamine are first-line treatments for depression and various psychiatric disorders due to their safety and efficacy. Common AEs of SSRIs include sexual dysfunction, sleep disturbances, weight changes, and gastrointestinal (GI) issues. SSRIs can prolong the QT interval, posing a risk of life-threatening arrhythmia, and may interact with other medications, necessitating treatment adjustments. The FDA approved SSRIs for MDD, GAD, bulimia nervosa, bipolar depression, OCD, panic disorder, premenstrual dysphoric disorder, treatment-resistant depression, PTSD, and SAD. Off-label uses include binge eating disorder, body dysmorphic disorder, fibromyalgia, premature ejaculation, paraphilias, autism, Raynaud phenomenon, and vasomotor symptoms associated with menopause. Among SSRIs, sertraline and escitalopram are noted for their effectiveness and tolerability.14,43-53
SNRIs, including duloxetine, venlafaxine, desvenlafaxine, milnacipran, and levomilnacipran, may increase bleeding risk, especially when taken with blood thinners. They can also elevate blood pressure, which may worsen if combined with stimulants. SNRIs may interact with other medications that affect serotonin levels, increasing the risk of serotonin syndrome when taken with triptans, pain medications, or other antidepressants.14 Desvenlafaxine has been approved by the FDA (but not by the European Medicines Agency).54-56 Duloxetine is FDA-approved for the treatment of depression, neuropathic pain, anxiety disorders, fibromyalgia, and musculoskeletal disorders. It is used off-label to treat chemotherapy-induced peripheral neuropathy and stress urinary incontinence.57-61 Venlafaxine is FDA-approved for depression, SAD, and panic disorder, and is prescribed off-label to treat ADHD, neuropathy, fibromyalgia, cataplexy, and PTSD, either alone or in combination with other medications.62,63 Milnacipran is not approved for MDD; levomilnacipran received approval in 2013.64
SRMs such as trazodone, nefazodone, vilazodone, and vortioxetine also function as serotonin reuptake inhibitors.14,15 Trazodone is FDA-approved for MDD. It has been used off-label to treat anxiety, Alzheimer disease, substance misuse, bulimia nervosa, insomnia, fibromyalgia, and PTSD when first-line SSRIs are ineffective. A notable AE of trazodone is orthostatic hypotension, which can lead to dizziness and increase the risk of falls, especially in geriatric patients.65-70 Nefazodone was discontinued in Europe in 2003 due to rare cases of liver toxicity but remains available in the US.71-74 Vilazodone and vortioxetine are FDA-approved.
The latest classes of antidepressants include SMRAs and NMDARAs.14 Agomelatine, an SMRA, was approved in Europe in 2009 but rejected by the FDA in 2011 due to liver toxicity.75 NMDARAs like esketamine and a combination of dextromethorphan and bupropion received FDA approval in 2019 and 2022, respectively.76,77
This retrospective study analyzes noncancer drugs used during systemic chemotherapy based on a dataset of 14 antineoplastic agents. It sought to identify the most dispensed noncancer drug groups, discuss findings, compare patients with and without antidepressant prescriptions, and examine trends in antidepressant use from 2002 to 2023. This analysis expands on prior research.78-81
Methods
The Walter Reed National Military Medical Center Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and Military Health System (MHS) data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Data Sources
The JPC DoD Cancer Registry Program contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in CAPER represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2024. PDTS records are available from 2002 to 2004. Each observation in PDTS represents a prescription filled for an MHS beneficiary, excluding those filled at international civilian pharmacies and inpatient pharmacy prescriptions.
This cross-sectional analysis requested data extraction for specific cancer drugs from the DoD Cancer Registry, focusing on treatment details, diagnosis dates, patient demographics, and physicians’ comments on AEs. After identifying patients, CAPER was used to identify additional health conditions. PDTS was used to compile a list of prescription medications filled during systemic cancer treatment or < 2 years postdiagnosis.
The 2016 Surveillance, Epidemiology, and End Results Program Coding and Staging Manual and International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.82,83 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the subgroup divided by the total number of patients or data variables. To compare the mean number of dispensed antidepressants to those without antidepressants, a 2-tailed, 2-sample z test was used to calculate the P value and determine statistical significance (P < .05) using socscistatistics.com.
Data were extracted 3 times between 2021 and 2023. The initial 2021 protocol focused on erlotinib and gefitinib. A modified protocol in 2022 added paclitaxel, cisplatin, docetaxel, pemetrexed, and crizotinib; further modification in 2023 included 8 new antineoplastic agents and 2 anticoagulants. Sotorasib has not been prescribed in the MHS, and JPC lacks records for noncancer drugs. The 2023 dataset comprised 2210 patients with cancer treated with 14 antineoplastic agents; 2104 had documented diagnoses and 2113 had recorded prescriptions. Data for erlotinib, gefitinib, and paclitaxel have been published previously.78,79
Results
Of 2113 patients with recorded prescriptions, 1297 patients (61.4%) received 109 cancer drugs, including 96 antineoplastics, 7 disease-modifying antirheumatic agents, 4 biologic response modifiers, and 2 calcitonin gene-related peptides. Fourteen antineoplastic agents had complete data from JPC, while others were noted for combination therapies or treatment switches from the PDTS (Table 1). Seventy-six cancer drugs were prescribed with antidepressants in 489 patients (eAppendix).

The JPC provided 2242 entries for 2210 patients, ranging in age from 2 months to 88 years (mean, 56 years), documenting treatment from September 1988 to January 2023. Thirty-two patients had duplicate entries due to multiple cancer locations or occurrences. Of the 2242 patients, 1541 (68.7%) were aged > 50 years, 975 patients (43.5%) had cancers that were stage III or IV, and 1267 (56.5%) had cancers that were stage 0, I, II, or not applicable/unknown. There were 51 different types of cancer: breast, lung, testicular, endometrial, and ovarian were most common (n ≥ 100 patients). Forty-two cancer types were documented among 750 patients prescribed antidepressants (Table 2).

The CAPER database recorded 8882 unique diagnoses for 2104 patients, while PDTS noted 1089 unique prescriptions within 273 therapeutic codes for 2113 patients. Nine therapeutic codes (opiate agonists, adrenals, cathartics-laxatives, nonsteroidal anti-inflammatory agents, antihistamines for GI conditions, 5-HT3 receptor antagonists, analgesics and antipyretic miscellanea, antineoplastic agents, and proton-pump inhibitors) and 8 drugs (dexamethasone, prochlorperazine, ondansetron, docusate, acetaminophen, ibuprofen, oxycodone, and polyethylene glycol 3350) were associated with > 1000 patients (≥ 50%). Patients had between 1 and 275 unique health conditions and filled 1 to 108 prescriptions. The mean (SD) number of diagnoses and prescriptions was 50 (28) and 29 (12), respectively. Of the 273 therapeutic codes, 30 groups were analyzed, with others categorized into miscellaneous groups such as lotions, vaccines, and devices. Significant differences in mean number of prescriptions were found for patients taking antidepressants compared to those not (P < .05), except for anticonvulsants and antipsychotics (P = .12 and .09, respectively) (Table 3).

Antidepressants
Of the 2113 patients with recorded prescriptions, 750 (35.5%) were dispensed 17 different antidepressants. Among these 17 antidepressants, 183 (8.7%) patients received duloxetine, 158 (7.5%) received venlafaxine, 118 (5.6%) received trazodone, and 107 (5.1%) received sertraline (Figure 1, Table 4). Of the 750 patients, 509 (67.9%) received 1 antidepressant, 168 (22.4%) received 2, 60 (8.0%) received 3, and 13 (1.7%) received > 3. Combinations varied, but only duloxetine and trazodone were prescribed to > 10 patients.



Antidepressants were prescribed annually at an overall mean (SD) rate of 23% (5%) from 2003 to 2022 (Figure 2). Patients on antidepressants during systemic therapy had a greater number of diagnosed medical conditions and received more prescription medications compared to those not taking antidepressants (P < .001) (Figure 3). The 745 patients taking antidepressants in CAPER data had between 1 and 275 diagnosed medical issues, with a mean (SD) of 55 (31) vs a range of 1 to 209 and a mean (SD) of 46 (26) for the 1359 patients not taking antidepressants. The 750 patients on antidepressants in PDTS data had between 8 and 108 prescriptions dispensed, with a mean (SD) of 32 (12), vs a range of 1 to 65 prescriptions and a mean (SD) of 29 (12) for 1363 patients not taking antidepressants.


Discussion
The JPC DoD Cancer Registry includes information on cancer types, stages, treatment regimens, and physicians’ notes, while noncancer drugs are sourced from the PDTS database. The pharmacy uses a different documentation system, leading to varied classifications.
Database reliance has its drawbacks. For example, megestrol is coded as a cancer drug, although it’s primarily used for endometrial or gynecologic cancers. Many drugs have multiple therapeutic codes assigned to them, including 10 antineoplastic agents: diclofenac, Bacillus Calmette-Guérin (BCG), megestrol acetate, tamoxifen, anastrozole, letrozole, leuprolide, goserelin, degarelix, and fluorouracil. Diclofenac, BCG, and mitomycin have been repurposed for cancer treatment.84-87 From 2003 to 2023, diclofenac was prescribed to 350 patients for mild-to-moderate pain, with only 2 patients receiving it for cancer in 2018. FDA-approved for bladder cancer in 1990, BCG was prescribed for cancer treatment for 1 patient in 2021 after being used for vaccines between 2003 and 2018. Tamoxifen, used for hormone receptor-positive breast cancer from 2004 to 2017 with 53 patients, switched to estrogen agonist-antagonists from 2017 to 2023 with 123 patients. Only a few of the 168 patients were prescribed tamoxifen using both codes.88-91 Anastrozole and letrozole were coded as antiestrogens for 7 and 18 patients, respectively, while leuprolide and goserelin were coded as gonadotropins for 59 and 18 patients. Degarelix was coded as antigonadotropins, fluorouracil as skin and mucous membrane agents miscellaneous, and megestrol acetate as progestins for 7, 6, and 3 patients, respectively. Duloxetine was given to 186 patients, primarily for depression from 2005 to 2023, with 7 patients treated for fibromyalgia from 2022 to 2023.
Antidepressants Observed
Tables 1 and 5 provide insight into the FDA approval of 14 antineoplastics and antidepressants and their CYP metabolic pathways.92-122 In Table 4, the most prescribed antidepressant classes are SNRIs, SRMs, SSRIs, TeCAs, NDRIs, and TCAs. This trend highlights a preference for newer medications with weak CYP inhibition. A total of 349 patients were prescribed SSRIs, 343 SNRIs, 119 SRMs, 109 TCAs, 83 TeCAs, and 79 NDRIs. MAOIs, SMRAs, and NMDARAs were not observed in this dataset. While there are instances of dextromethorphan-bupropion and sertraline-escitalopram being dispensed together, it remains unclear whether these were NMDARA combinations.
Among the 14 specific antineoplastic agents, 10 are metabolized by CYP isoenzymes, primarily CYP3A4. Duloxetine neither inhibits nor is metabolized by CYP3A4, a reason it is often recommended, following venlafaxine.
Both duloxetine and venlafaxine are used off-label for chemotherapy-induced peripheral neuropathy related to paclitaxel and docetaxel. According to the CYP metabolized pathway, duloxetine tends to have more favorable DDIs than venlafaxine. In PDTS data, 371 patients were treated with paclitaxel and 180 with docetaxel, with respective antidepressant prescriptions of 156 and 70. Of the 156 patients dispensed paclitaxel, 62 (40%) were dispensed with duloxetine compared to 43 (28%) with venlafaxine. Of the 70 patients dispensed docetaxel, 23 (33%) received duloxetine vs 24 (34%) with venlafaxine.
Of 85 patients prescribed duloxetine, 75 received it with either paclitaxel or docetaxel (5 received both). Five patients had documented AEs (1 neuropathy related). Of 67 patients prescribed venlafaxine, 66 received it with either paclitaxel or docetaxel. Two patients had documented AEs (1 was neuropathy related, the same patient who received duloxetine). Of the 687 patients treated with paclitaxel and 337 with docetaxel in all databases, 4 experienced neuropathic AEs from both medications.79
Antidepressants can increase the risk of bleeding, especially when combined with blood thinners, and may elevate blood pressure, particularly alongside stimulants. Of the 554 patients prescribed 9 different anticoagulants, enoxaparin, apixaban, and rivaroxaban were the most common (each > 100 patients). Among these, 201 patients (36%) received both anticoagulants and antidepressants: duloxetine for 64 patients, venlafaxine for 30, trazodone for 35, and sertraline for 26. There were no data available to assess bleeding rates related to the evaluation of DDIs between these medication classes.
Antidepressants can be prescribed for erectile dysfunction. Of the 148 patients prescribed an antidepressant for erectile dysfunction, duloxetine, trazodone, and mirtazapine were the most common. Antidepressant preferences varied by cancer type. Duloxetine was the only antidepressant used for all types of cancer. Venlafaxine, duloxetine, trazodone, sertraline, and escitalopram were the most prescribed antidepressants for breast cancer, while duloxetine, mirtazapine, citalopram, sertraline, and trazodone were the most prescribed for lung cancer. Sertraline, duloxetine, trazodone, amitriptyline, and escitalopram were most common for testicular cancer. Duloxetine, venlafaxine, trazodone, amitriptyline, and sertraline were the most prescribed for endometrial cancer, while duloxetine, venlafaxine, amitriptyline, citalopram, and sertraline were most prescribed for ovarian cancer.
The broadness of International Statistical Classification of Diseases, Tenth Revision codes made it challenging to identify nondepression diagnoses in the analyzed population. However, if all antidepressants were prescribed to treat depression, service members with cancer exhibited a higher depression rate (35%) than the general population (25%). Of 2104 patients, 191 (9.1%) had mood disorders, and 706 (33.6%) had mental disorders: 346 (49.0%) had 1 diagnosis, and 360 (51.0%) had multiple diagnoses. The percentage of diagnoses varied yearly, with notable drops in 2003, 2007, 2011, 2014, and 2018, and peaks in 2006, 2008, 2013, 2017, and 2022. This fluctuation was influenced by events like the establishment of PDTS in 2002, the 2008 economic recession, a hospital relocation in 2011, the 2014 Ebola outbreak, and the COVID-19 pandemic. Although the number of patients receiving antidepressants increased from 2019 to 2022, the overall percentage of patients receiving them did not significantly change from 2003 to 2022, aligning with previous research.5,125
Many medications have potential uses beyond what is detailed in the prescribing information. Antidepressants can relieve pain, while pain medications may help with depression. Opioids were once thought to effectively treat depression, but this perspective has changed with a greater understanding of their risks, including misuse.126-131 Pain is a severe and often unbearable AE of cancer. Of 2113 patients, 92% received opioids; 34% received both opioids and antidepressants; 2% received only antidepressants; and 7% received neither. This study didn’t clarify whether those on opioids alone recognized their depression or if those on both were aware of their dependence. While SSRIs are generally not addictive, they can lead to physical dependence, and any medication can be abused if not managed properly.132-134
Conclusions
This retrospective study analyzes data from antineoplastic agents used in systemic cancer treatment between 1988 and 2023, with a particular focus on the use of antidepressants. Data on antidepressant prescriptions are incomplete and specific to these agents, which means the findings cannot be generalized to all antidepressants. Hence, the results indicate that patients taking antidepressants had more diagnosed health issues and received more medications compared to patients who were not on these drugs.
This study underscores the need for further research into the effects of antidepressants on cancer treatment, utilizing all data from the DoD Cancer Registry. Future research should explore DDIs between antidepressants and other cancer and noncancer medications, as this study did not assess AE documentation, unlike in studies involving erlotinib, gefitinib, and paclitaxel.78,79 Further investigation is needed to evaluate the impact of discontinuing antidepressant use during cancer treatment. This comprehensive overview provides insights for clinicians to help them make informed decisions regarding the prescription of antidepressants in the context of cancer treatment.
Cancer patients experience depression at rates > 5 times that of the general population.1-11 Despite an increase in palliative care use, depression rates continued to rise.2-4 Between 5% to 16% of outpatients, 4% to 14% of inpatients, and up to 49% of patients receiving palliative care experience depression.5 This issue also impacts families and caregivers.1 A 2021 meta-analysis found that 23% of active military personnel and 20% of veterans experience depression.11
Antidepressants approved by the US Food and Drug Administration (FDA) target the serotonin, norepinephrine, or dopamine systems and include boxed warnings about an increased risk of suicidal thoughts in adults aged 18 to 24 years.12,13 These medications are categorized into several classes: monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), tetracyclic antidepressants (TeCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), serotonin receptor modulators (SRMs), serotonin-melatonin receptor antagonists (SMRAs), and N—methyl-D-aspartate receptor antagonists (NMDARAs).14,15 The first FDA-approved antidepressants, iproniazid (an MAOI) and imipramine (a TCA) laid the foundation for the development of newer classes like SSRIs and SNRIs.15-17
Older antidepressants such as MAOIs and TCAs are used less due to their adverse effects (AEs) and drug interactions. MAOIs, such as iproniazid, selegiline, moclobemide, tranylcypromine, isocarboxazid, and phenelzine, have numerous AEs and drug interactions, making them unsuitable for first- or second-line treatment of depression.14,18-21 TCAs such as doxepin, amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, trimipramine, protriptyline, maprotiline, and amoxapine have a narrow therapeutic index requiring careful monitoring for signs of toxicity such as QRS widening, tremors, or confusion. Despite the issues, TCAs are generally classified as second-line agents for major depressive disorder (MDD). TCAs have off-label uses for migraine prophylaxis, treatment of obsessive-compulsive disorder (OCD), insomnia, and chronic pain management first-line.14,22-29
Newer antidepressants, including TeCAs and NDRIs, are typically more effective, but also come with safety concerns. TeCAs like mirtazapine interact with several medications, including MAOIs, serotonin-increasing drugs, alcohol, cannabidiol, and marijuana. Mirtazapine is FDA-approved for the treatment of moderate to severe depression in adults. It is also used off-label to treat insomnia, panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), headaches, and migraines. Compared to other antidepressants, mirtazapine is effective for all stages of depression and addresses a broad range of related symptoms.14,30-34 NDRIs, such as bupropion, also interact with various medications, including MAOIs, other antidepressants, stimulants, and alcohol. Bupropion is FDA-approved for smoking cessation and to treat depression and SAD. It is also used off-label for depression- related bipolar disorder or sexual dysfunction, attention-deficit/hyperactivity disorder (ADHD), and obesity.14,35-42
SSRIs, SNRIs, and SRMs should be used with caution. SSRIs such as sertraline, citalopram, escitalopram, fluoxetine, paroxetine, and fluvoxamine are first-line treatments for depression and various psychiatric disorders due to their safety and efficacy. Common AEs of SSRIs include sexual dysfunction, sleep disturbances, weight changes, and gastrointestinal (GI) issues. SSRIs can prolong the QT interval, posing a risk of life-threatening arrhythmia, and may interact with other medications, necessitating treatment adjustments. The FDA approved SSRIs for MDD, GAD, bulimia nervosa, bipolar depression, OCD, panic disorder, premenstrual dysphoric disorder, treatment-resistant depression, PTSD, and SAD. Off-label uses include binge eating disorder, body dysmorphic disorder, fibromyalgia, premature ejaculation, paraphilias, autism, Raynaud phenomenon, and vasomotor symptoms associated with menopause. Among SSRIs, sertraline and escitalopram are noted for their effectiveness and tolerability.14,43-53
SNRIs, including duloxetine, venlafaxine, desvenlafaxine, milnacipran, and levomilnacipran, may increase bleeding risk, especially when taken with blood thinners. They can also elevate blood pressure, which may worsen if combined with stimulants. SNRIs may interact with other medications that affect serotonin levels, increasing the risk of serotonin syndrome when taken with triptans, pain medications, or other antidepressants.14 Desvenlafaxine has been approved by the FDA (but not by the European Medicines Agency).54-56 Duloxetine is FDA-approved for the treatment of depression, neuropathic pain, anxiety disorders, fibromyalgia, and musculoskeletal disorders. It is used off-label to treat chemotherapy-induced peripheral neuropathy and stress urinary incontinence.57-61 Venlafaxine is FDA-approved for depression, SAD, and panic disorder, and is prescribed off-label to treat ADHD, neuropathy, fibromyalgia, cataplexy, and PTSD, either alone or in combination with other medications.62,63 Milnacipran is not approved for MDD; levomilnacipran received approval in 2013.64
SRMs such as trazodone, nefazodone, vilazodone, and vortioxetine also function as serotonin reuptake inhibitors.14,15 Trazodone is FDA-approved for MDD. It has been used off-label to treat anxiety, Alzheimer disease, substance misuse, bulimia nervosa, insomnia, fibromyalgia, and PTSD when first-line SSRIs are ineffective. A notable AE of trazodone is orthostatic hypotension, which can lead to dizziness and increase the risk of falls, especially in geriatric patients.65-70 Nefazodone was discontinued in Europe in 2003 due to rare cases of liver toxicity but remains available in the US.71-74 Vilazodone and vortioxetine are FDA-approved.
The latest classes of antidepressants include SMRAs and NMDARAs.14 Agomelatine, an SMRA, was approved in Europe in 2009 but rejected by the FDA in 2011 due to liver toxicity.75 NMDARAs like esketamine and a combination of dextromethorphan and bupropion received FDA approval in 2019 and 2022, respectively.76,77
This retrospective study analyzes noncancer drugs used during systemic chemotherapy based on a dataset of 14 antineoplastic agents. It sought to identify the most dispensed noncancer drug groups, discuss findings, compare patients with and without antidepressant prescriptions, and examine trends in antidepressant use from 2002 to 2023. This analysis expands on prior research.78-81
Methods
The Walter Reed National Military Medical Center Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and Military Health System (MHS) data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Data Sources
The JPC DoD Cancer Registry Program contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in CAPER represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2024. PDTS records are available from 2002 to 2004. Each observation in PDTS represents a prescription filled for an MHS beneficiary, excluding those filled at international civilian pharmacies and inpatient pharmacy prescriptions.
This cross-sectional analysis requested data extraction for specific cancer drugs from the DoD Cancer Registry, focusing on treatment details, diagnosis dates, patient demographics, and physicians’ comments on AEs. After identifying patients, CAPER was used to identify additional health conditions. PDTS was used to compile a list of prescription medications filled during systemic cancer treatment or < 2 years postdiagnosis.
The 2016 Surveillance, Epidemiology, and End Results Program Coding and Staging Manual and International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.82,83 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the subgroup divided by the total number of patients or data variables. To compare the mean number of dispensed antidepressants to those without antidepressants, a 2-tailed, 2-sample z test was used to calculate the P value and determine statistical significance (P < .05) using socscistatistics.com.
Data were extracted 3 times between 2021 and 2023. The initial 2021 protocol focused on erlotinib and gefitinib. A modified protocol in 2022 added paclitaxel, cisplatin, docetaxel, pemetrexed, and crizotinib; further modification in 2023 included 8 new antineoplastic agents and 2 anticoagulants. Sotorasib has not been prescribed in the MHS, and JPC lacks records for noncancer drugs. The 2023 dataset comprised 2210 patients with cancer treated with 14 antineoplastic agents; 2104 had documented diagnoses and 2113 had recorded prescriptions. Data for erlotinib, gefitinib, and paclitaxel have been published previously.78,79
Results
Of 2113 patients with recorded prescriptions, 1297 patients (61.4%) received 109 cancer drugs, including 96 antineoplastics, 7 disease-modifying antirheumatic agents, 4 biologic response modifiers, and 2 calcitonin gene-related peptides. Fourteen antineoplastic agents had complete data from JPC, while others were noted for combination therapies or treatment switches from the PDTS (Table 1). Seventy-six cancer drugs were prescribed with antidepressants in 489 patients (eAppendix).

The JPC provided 2242 entries for 2210 patients, ranging in age from 2 months to 88 years (mean, 56 years), documenting treatment from September 1988 to January 2023. Thirty-two patients had duplicate entries due to multiple cancer locations or occurrences. Of the 2242 patients, 1541 (68.7%) were aged > 50 years, 975 patients (43.5%) had cancers that were stage III or IV, and 1267 (56.5%) had cancers that were stage 0, I, II, or not applicable/unknown. There were 51 different types of cancer: breast, lung, testicular, endometrial, and ovarian were most common (n ≥ 100 patients). Forty-two cancer types were documented among 750 patients prescribed antidepressants (Table 2).

The CAPER database recorded 8882 unique diagnoses for 2104 patients, while PDTS noted 1089 unique prescriptions within 273 therapeutic codes for 2113 patients. Nine therapeutic codes (opiate agonists, adrenals, cathartics-laxatives, nonsteroidal anti-inflammatory agents, antihistamines for GI conditions, 5-HT3 receptor antagonists, analgesics and antipyretic miscellanea, antineoplastic agents, and proton-pump inhibitors) and 8 drugs (dexamethasone, prochlorperazine, ondansetron, docusate, acetaminophen, ibuprofen, oxycodone, and polyethylene glycol 3350) were associated with > 1000 patients (≥ 50%). Patients had between 1 and 275 unique health conditions and filled 1 to 108 prescriptions. The mean (SD) number of diagnoses and prescriptions was 50 (28) and 29 (12), respectively. Of the 273 therapeutic codes, 30 groups were analyzed, with others categorized into miscellaneous groups such as lotions, vaccines, and devices. Significant differences in mean number of prescriptions were found for patients taking antidepressants compared to those not (P < .05), except for anticonvulsants and antipsychotics (P = .12 and .09, respectively) (Table 3).

Antidepressants
Of the 2113 patients with recorded prescriptions, 750 (35.5%) were dispensed 17 different antidepressants. Among these 17 antidepressants, 183 (8.7%) patients received duloxetine, 158 (7.5%) received venlafaxine, 118 (5.6%) received trazodone, and 107 (5.1%) received sertraline (Figure 1, Table 4). Of the 750 patients, 509 (67.9%) received 1 antidepressant, 168 (22.4%) received 2, 60 (8.0%) received 3, and 13 (1.7%) received > 3. Combinations varied, but only duloxetine and trazodone were prescribed to > 10 patients.



Antidepressants were prescribed annually at an overall mean (SD) rate of 23% (5%) from 2003 to 2022 (Figure 2). Patients on antidepressants during systemic therapy had a greater number of diagnosed medical conditions and received more prescription medications compared to those not taking antidepressants (P < .001) (Figure 3). The 745 patients taking antidepressants in CAPER data had between 1 and 275 diagnosed medical issues, with a mean (SD) of 55 (31) vs a range of 1 to 209 and a mean (SD) of 46 (26) for the 1359 patients not taking antidepressants. The 750 patients on antidepressants in PDTS data had between 8 and 108 prescriptions dispensed, with a mean (SD) of 32 (12), vs a range of 1 to 65 prescriptions and a mean (SD) of 29 (12) for 1363 patients not taking antidepressants.


Discussion
The JPC DoD Cancer Registry includes information on cancer types, stages, treatment regimens, and physicians’ notes, while noncancer drugs are sourced from the PDTS database. The pharmacy uses a different documentation system, leading to varied classifications.
Database reliance has its drawbacks. For example, megestrol is coded as a cancer drug, although it’s primarily used for endometrial or gynecologic cancers. Many drugs have multiple therapeutic codes assigned to them, including 10 antineoplastic agents: diclofenac, Bacillus Calmette-Guérin (BCG), megestrol acetate, tamoxifen, anastrozole, letrozole, leuprolide, goserelin, degarelix, and fluorouracil. Diclofenac, BCG, and mitomycin have been repurposed for cancer treatment.84-87 From 2003 to 2023, diclofenac was prescribed to 350 patients for mild-to-moderate pain, with only 2 patients receiving it for cancer in 2018. FDA-approved for bladder cancer in 1990, BCG was prescribed for cancer treatment for 1 patient in 2021 after being used for vaccines between 2003 and 2018. Tamoxifen, used for hormone receptor-positive breast cancer from 2004 to 2017 with 53 patients, switched to estrogen agonist-antagonists from 2017 to 2023 with 123 patients. Only a few of the 168 patients were prescribed tamoxifen using both codes.88-91 Anastrozole and letrozole were coded as antiestrogens for 7 and 18 patients, respectively, while leuprolide and goserelin were coded as gonadotropins for 59 and 18 patients. Degarelix was coded as antigonadotropins, fluorouracil as skin and mucous membrane agents miscellaneous, and megestrol acetate as progestins for 7, 6, and 3 patients, respectively. Duloxetine was given to 186 patients, primarily for depression from 2005 to 2023, with 7 patients treated for fibromyalgia from 2022 to 2023.
Antidepressants Observed
Tables 1 and 5 provide insight into the FDA approval of 14 antineoplastics and antidepressants and their CYP metabolic pathways.92-122 In Table 4, the most prescribed antidepressant classes are SNRIs, SRMs, SSRIs, TeCAs, NDRIs, and TCAs. This trend highlights a preference for newer medications with weak CYP inhibition. A total of 349 patients were prescribed SSRIs, 343 SNRIs, 119 SRMs, 109 TCAs, 83 TeCAs, and 79 NDRIs. MAOIs, SMRAs, and NMDARAs were not observed in this dataset. While there are instances of dextromethorphan-bupropion and sertraline-escitalopram being dispensed together, it remains unclear whether these were NMDARA combinations.
Among the 14 specific antineoplastic agents, 10 are metabolized by CYP isoenzymes, primarily CYP3A4. Duloxetine neither inhibits nor is metabolized by CYP3A4, a reason it is often recommended, following venlafaxine.
Both duloxetine and venlafaxine are used off-label for chemotherapy-induced peripheral neuropathy related to paclitaxel and docetaxel. According to the CYP metabolized pathway, duloxetine tends to have more favorable DDIs than venlafaxine. In PDTS data, 371 patients were treated with paclitaxel and 180 with docetaxel, with respective antidepressant prescriptions of 156 and 70. Of the 156 patients dispensed paclitaxel, 62 (40%) were dispensed with duloxetine compared to 43 (28%) with venlafaxine. Of the 70 patients dispensed docetaxel, 23 (33%) received duloxetine vs 24 (34%) with venlafaxine.
Of 85 patients prescribed duloxetine, 75 received it with either paclitaxel or docetaxel (5 received both). Five patients had documented AEs (1 neuropathy related). Of 67 patients prescribed venlafaxine, 66 received it with either paclitaxel or docetaxel. Two patients had documented AEs (1 was neuropathy related, the same patient who received duloxetine). Of the 687 patients treated with paclitaxel and 337 with docetaxel in all databases, 4 experienced neuropathic AEs from both medications.79
Antidepressants can increase the risk of bleeding, especially when combined with blood thinners, and may elevate blood pressure, particularly alongside stimulants. Of the 554 patients prescribed 9 different anticoagulants, enoxaparin, apixaban, and rivaroxaban were the most common (each > 100 patients). Among these, 201 patients (36%) received both anticoagulants and antidepressants: duloxetine for 64 patients, venlafaxine for 30, trazodone for 35, and sertraline for 26. There were no data available to assess bleeding rates related to the evaluation of DDIs between these medication classes.
Antidepressants can be prescribed for erectile dysfunction. Of the 148 patients prescribed an antidepressant for erectile dysfunction, duloxetine, trazodone, and mirtazapine were the most common. Antidepressant preferences varied by cancer type. Duloxetine was the only antidepressant used for all types of cancer. Venlafaxine, duloxetine, trazodone, sertraline, and escitalopram were the most prescribed antidepressants for breast cancer, while duloxetine, mirtazapine, citalopram, sertraline, and trazodone were the most prescribed for lung cancer. Sertraline, duloxetine, trazodone, amitriptyline, and escitalopram were most common for testicular cancer. Duloxetine, venlafaxine, trazodone, amitriptyline, and sertraline were the most prescribed for endometrial cancer, while duloxetine, venlafaxine, amitriptyline, citalopram, and sertraline were most prescribed for ovarian cancer.
The broadness of International Statistical Classification of Diseases, Tenth Revision codes made it challenging to identify nondepression diagnoses in the analyzed population. However, if all antidepressants were prescribed to treat depression, service members with cancer exhibited a higher depression rate (35%) than the general population (25%). Of 2104 patients, 191 (9.1%) had mood disorders, and 706 (33.6%) had mental disorders: 346 (49.0%) had 1 diagnosis, and 360 (51.0%) had multiple diagnoses. The percentage of diagnoses varied yearly, with notable drops in 2003, 2007, 2011, 2014, and 2018, and peaks in 2006, 2008, 2013, 2017, and 2022. This fluctuation was influenced by events like the establishment of PDTS in 2002, the 2008 economic recession, a hospital relocation in 2011, the 2014 Ebola outbreak, and the COVID-19 pandemic. Although the number of patients receiving antidepressants increased from 2019 to 2022, the overall percentage of patients receiving them did not significantly change from 2003 to 2022, aligning with previous research.5,125
Many medications have potential uses beyond what is detailed in the prescribing information. Antidepressants can relieve pain, while pain medications may help with depression. Opioids were once thought to effectively treat depression, but this perspective has changed with a greater understanding of their risks, including misuse.126-131 Pain is a severe and often unbearable AE of cancer. Of 2113 patients, 92% received opioids; 34% received both opioids and antidepressants; 2% received only antidepressants; and 7% received neither. This study didn’t clarify whether those on opioids alone recognized their depression or if those on both were aware of their dependence. While SSRIs are generally not addictive, they can lead to physical dependence, and any medication can be abused if not managed properly.132-134
Conclusions
This retrospective study analyzes data from antineoplastic agents used in systemic cancer treatment between 1988 and 2023, with a particular focus on the use of antidepressants. Data on antidepressant prescriptions are incomplete and specific to these agents, which means the findings cannot be generalized to all antidepressants. Hence, the results indicate that patients taking antidepressants had more diagnosed health issues and received more medications compared to patients who were not on these drugs.
This study underscores the need for further research into the effects of antidepressants on cancer treatment, utilizing all data from the DoD Cancer Registry. Future research should explore DDIs between antidepressants and other cancer and noncancer medications, as this study did not assess AE documentation, unlike in studies involving erlotinib, gefitinib, and paclitaxel.78,79 Further investigation is needed to evaluate the impact of discontinuing antidepressant use during cancer treatment. This comprehensive overview provides insights for clinicians to help them make informed decisions regarding the prescription of antidepressants in the context of cancer treatment.
- National Cancer Institute. Depression (PDQ)-Health Professional Version. Updated July 25, 2024. Accessed April 4, 2025. https://www.cancer.gov/about-cancer/coping/feelings/depression-hp-pdq
- Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130. doi:10.1002/pon.3409
- Xiang X, An R, Gehlert S. Trends in antidepressant use among U.S. cancer survivors, 1999-2012. Psychiatr Serv. 2015;66(6):564. doi:10.1176/appi.ps.201500007
- Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. doi:10.1016/j.ejca.2016.11.017
- Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900. doi:10.1093/annonc/mds575
- Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi:10.1136/bmj.k1415
- Kisely S, Alotiby MKN, Protani MM, Soole R, Arnautovska U, Siskind D. Breast cancer treatment disparities in patients with severe mental illness: a systematic review and meta-analysis. Psychooncology. 2023;32(5):651-662. doi:10.1002/pon.6120
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71. doi:10.1093/jncimonographs/lgh014
- Rodin G, Katz M, Lloyd N, Green E, Mackay JA, Wong RK. Treatment of depression in cancer patients. Curr Oncol. 2007;14(5):180-188. doi:10.3747/co.2007.146
- Wilson KG, Chochinov HM, Skirko MG, et al. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33(2):118-129. doi:10.1016/j.jpainsymman.2006.07.016
- Moradi Y, Dowran B, Sepandi M. The global prevalence of depression, suicide ideation, and attempts in the military forces: a systematic review and meta-analysis of cross sectional studies. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
- Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi:10.1136/bmj.g3596
- Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
- Sheffler ZM, Patel P, Abdijadid S. Antidepressants. In: StatPearls. StatPearls Publishing. Updated May 26, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK538182/
- Miller JJ. Antidepressants, Part 1: 100 Years and Counting. Accessed April 4, 2025. Psychiatric Times. https:// www.psychiatrictimes.com/view/antidepressants-part-1-100-years-and-counting
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21. doi:10.1037/a0038550
- Mitchell PB, Mitchell MS. The management of depression. Part 2. The place of the new antidepressants. Aust Fam Physician. 1994;23(9):1771-1781.
- Sabri MA, Saber-Ayad MM. MAO Inhibitors. In: Stat- Pearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557395/
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539848/
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248. doi:10.1097/00131746-200407000-00005
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
- Moraczewski J, Awosika AO, Aedma KK. Tricyclic Antidepressants. In: StatPearls. StatPearls Publishing. Updated August 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557791/
- Almasi A, Patel P, Meza CE. Doxepin. In: StatPearls. StatPearls Publishing. Updated February 14, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK542306/
- Thour A, Marwaha R. Amitriptyline. In: StatPearls. Stat- Pearls Publishing. Updated July 18, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK537225/
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
- Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680- 690. doi:10.1016/S0140-6736(22)01472-6
- Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network metaanalysis. JAMA Netw Open. 2022;5(5):e2212939. doi:10.1001/jamanetworkopen.2022.12939
- Merwar G, Gibbons JR, Hosseini SA, Saadabadi A. Nortriptyline. In: StatPearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482214/
- Fayez R, Gupta V. Imipramine. In: StatPearls. StatPearls Publishing. Updated May 22, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557656/
- Jilani TN, Gibbons JR, Faizy RM, Saadabadi A. Mirtazapine. In: StatPearls. StatPearls Publishing. Updated August 28, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519059/
- Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17 Suppl 1:S37-S41. doi:10.1002/hup.388
- Gandotra K, Chen P, Jaskiw GE, Konicki PE, Strohl KP. Effective treatment of insomnia with mirtazapine attenuates concomitant suicidal ideation. J Clin Sleep Med. 2018;14(5):901-902. doi:10.5664/jcsm.7142
- Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264. doi:10.1111/j.1527-3458.2001.tb00198.x
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101-112. doi:10.4068/cmj.2018.54.2.101
- Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls. StatPearls Publishing. Updated April 9, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470212/
- Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895- 905. doi:10.1111/obr.12835
- Hankosky ER, Bush HM, Dwoskin LP, et al. Retrospective analysis of health claims to evaluate pharmacotherapies with potential for repurposing: association of bupropion and stimulant use disorder remission. AMIA Annu Symp Proc. 2018;2018:1292-1299.
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Hajek P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2019; 2(2):CD003999. doi:10.1002/14651858.CD003999.pub5
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6(2):59-74. doi:10.1016/j.esxm.2018.01.004
- Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2017;10(10):CD009504. doi:10.1002/14651858.CD009504.pub2
- Ng QX. A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(2):112-116. doi:10.1089/cap.2016.0124
- Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113. doi:10.4088/pcc.v07n0305
- Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls. StatPearls Publishing. Updated May 1, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK554406/
- Singh HK, Saadabadi A. Sertraline. In: StatPearls. Stat- Pearls Publishing. Updated February 13, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK547689/
- MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7(1):1-24. doi:10.1111/j.1527-3458.2001.tb00188.x
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi:10.1016/S0140-6736(09)60046-5
- Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. Am J Psychiatry. 2006;163(11):1864-1866. doi:10.1176/ajp.2006.163.11.1864
- Sharbaf Shoar N, Fariba KA, Padhy RK. Citalopram. In: StatPearls. StatPearls Publishing. Updated November 7, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482222/
- Landy K, Rosani A, Estevez R. Escitalopram. In: Stat- Pearls. StatPearls Publishing. Updated November 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557734/
- Cavanah LR, Ray P, Goldhirsh JL, Huey LY, Piper BJ. Rise of escitalopram and the fall of citalopram. medRxiv. Preprint published online May 8, 2023. doi:10.1101/2023.05.07.23289632
- Sohel AJ, Shutter MC, Patel P, Molla M. Fluoxetine. In: StatPearls. StatPearls Publishing. Updated July 4, 2022. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4(9):764-774. doi:10.1038/nrd1821
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. In: Stat- Pearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526022/
- Naseeruddin R, Rosani A, Marwaha R. Desvenlafaxine. In: StatPearls. StatPearls Publishing. Updated July 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534829
- Lieberman DZ, Massey SH. Desvenlafaxine in major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2010;4:67-82. doi:10.2147/ce.s5998
- Withdrawal assessment report for Ellefore (desvenlafaxine). European Medicine Agency. 2009. Accessed April 4, 2025. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-ellefore_en.pdf
- Dhaliwal JS, Spurling BC, Molla M. Duloxetine. In: Stat- Pearls. Statpearls Publishing. Updated May 29, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967. doi:10.1200/JCO.2013.54.0914
- Sommer C, Häuser W, Alten R, et al. Medikamentöse Therapie des Fibromyalgiesyndroms. Systematische Übersicht und Metaanalyse [Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline]. Schmerz. 2012;26(3):297-310. doi:10.1007/s00482-012-1172-2
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi:10.1212/WNL.0b013e3182166ebe
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. doi:10.1111/j.1468-1331.2010.02999.x
- Singh D, Saadabadi A. Venlafaxine. In: StatPearls. StatePearls Publishing. Updated February 26, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Sertraline and venlafaxine: new indication. Prevention of recurrent depression: no advance. Prescrire Int. 2005;14(75):19-20.
- Bruno A, Morabito P, S p i n a E , M u s c a t ello MRl. The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuro pharmacol. 2016;14(2):191-199. doi:10.2174/1570159x14666151117122458
- Shin JJ, Saadabadi A. Trazodone. In: StatPearls. StatPearls Publishing. Updated February 29, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470560/
- Khouzam HR. A review of trazodone use in psychiatric and medical conditions. Postgrad Med. 2017;129(1):140-148. doi:10.1080/00325481.2017.1249265
- Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758-764. doi:10.1513/AnnalsATS.201408-399OC
- Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811-819. doi:10.5665/sleep.3596
- Schatzberg AF, Nemeroff CB, eds. The American Psychiat ric Association Publishing Textbook of Psychopharmacology. 4th ed. American Psychiatric Publishing; 2009.
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and allcause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209-220. doi:10.1111/bcp.12617
- Nefazodone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. Updated March 6, 2020. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK548179/
- Drugs of Current Interest: Nefazodone. WHO Pharmaceuticals Newsletter:(1). 2003(1):7. https://web.archive.org/web/20150403165029/http:/apps.who.int/medicinedocs/en/d/Js4944e/3.html
- Choi S. Nefazodone (serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169(11):1187.
- Teva Nefazodone Statement. News release. Teva USA. December 20, 2021. Accessed April 4, 2025. https:// www.tevausa.com/news-and-media/press-releases/teva-nefazodone-statement/
- Levitan MN, Papelbaum M, Nardi AE. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2015;11:1149- 1155. doi:10.2147/NDT.S67470
- Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi:10.4088/JCP.19m13191
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4): 21m14345. doi:10.4088/JCP.21m14345
- Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the Military Health System. Fed Pract. 2023;40(Suppl 3):S24-S34. doi:10.12788/fp.0401
- Luong TT, Shou KJ, Reinhard BJ, Kigelman OF, Greenfield KM. Paclitaxel drug-drug interactions in the Military Health System. Fed Pract. 2024;41(Suppl 3):S70-S82. doi:10.12788/fp.0499
- Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Preclinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
- Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217- 224. doi:10.1016/j.crtox.2021.05.006,
- Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed April 4, 2025. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
- World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed, 1st revision. World Health Organization; 2013. Accessed April 4, 2025. https://apps.who.int/iris/handle/10665/96612
- Simon S. FDA approves Jelmyto (mitomycin gel) for urothelial cancer. American Cancer Society. April 20, 2020. Accessed April 4, 2025. https://www.cancer.org/cancer/latest-news/fda-approves-jelmyto-mitomycin-gel-for-urothelial-cancer.html
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)- diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi:10.3332/ecancer.2016.610
- Choi S, Kim S, Park J, Lee SE, Kim C, Kang D. Diclofenac: a nonsteroidal anti-inflammatory drug inducing cancer cell death by inhibiting microtubule polymerization and autophagy flux. Antioxidants (Basel). 2022;11(5):1009. doi:10.3390/antiox11051009
- Tontonoz M. The ABCs of BCG: oldest approved immunotherapy gets new explanation. Memorial Sloan Kettering Cancer Center. July 17, 2020. Accessed April 4, 2025. https://www.mskcc.org/news/oldest-approved-immunotherapy-gets-new-explanation
- Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21(3):R235-R246. doi:10.1530/ERC-14-0092
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270-275. doi:10.1038/bjc.1971.33
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205-213. doi:10.1038/nrd1031
- Maximov PY, McDaniel RE, Jordan VC. Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Basel; 2013. Accessed April 4, 2025. https://link.springer.com/book/10.1007/978-3-0348-0664-0
- Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb Company; 2011. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
- Tarceva (erlotinib). Prescribing information. OSI Pharmaceuticals, LLC; 2016. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
- Docetaxel. Prescribing information. Sichuan Hyiyu Pharmaceutical Co.; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215813s000lbl.pdf
- Alimta (pemetrexed). Prescribing information. Teva Pharmaceuticals; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208419s004lbl.pdf
- Tagrisso (osimertinib). Prescribing information. Astra- Zeneca Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
- Iressa (gefitinib). Prescribing information. AstraZeneca Pharmaceuticals; 2018. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
- Kadcyla (ado-trastuzumab emtansine). Prescribing information. Genentech, Inc.; 2013. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf
- Alecensa (alectinib). Prescribing information. Genetech, Inc.; 2017. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf
- Xalkori (crizotinib). Prescribing information. Pfizer Laboratories; 2022. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2022/202570s033lbl.pdf
- Lorbrena (lorlatinib). Prescribing information. Pfizer Laboratories; 2018. Accessed April 14, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Alunbrig (brigatinib). Prescribing information. Takeda Pharmaceutical Company; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf
- Rozlytrek (entrectinib). Prescribing information. Genentech, Inc.; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf
- Herceptin (trastuzumab). Prescribing information. Genentech, Inc.; 2010. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf
- Cybalta (duloxetine). Prescribing information. Eli Lilly and Company; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021427s049lbl.pdf
- Effexor XR (venlafaxine). Prescribing information. Pfizer Wyeth Pharmaceuticals Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020699s112lbl.pdf
- Desyrel (trazodone hydrochloride). Prescribing information. Pragma Pharmaceuticals; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
- Sertraline hydrochloride. Prescribing information. Almatica Pharma LLC; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215133s000lbl.pdf
- Remeron (mirtazapine). Prescribing information. Merck & Co. Inc; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
- Celexa (citalopram). Prescribing information. Allergan USA Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- information. GlaxoSmithKline; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020358s066lbl.pdf
- Amitriptyline hydrochloride tablet. Prescribing information. Quality Care Products LLC; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0f12f50f-7087-46e7-a2e6-356b4c566c9f/0f12f50f-7087-46e7-a2e6-356b4c566c9f.xml
- Lexapro (escitalopram). Prescribing information. AbbVie Inc; 2023. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021323s055,021365s039lbl.pdf
- Fluoxetine. Prescribing information. Edgemont Pharmaceutical, LLC; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202133s004s005lbl.pdf
- Paxil (paroxetine). Prescribing Information. Apotex Inc; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
- Pamelor (nortriptyline HCl). Prescribing information. Mallinckrodt, Inc; 2012. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
- Silenor (doxepin). Prescribing information. Currax Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022036s006lbl.pdf
- Tofranil-PM (imipramine pamote). Prescribing information. Mallinckrodt, Inc; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/017090s078lbl.pdf
- Norpramin (desipramine hydrochloride). Prescribing information. Sanofi-aventis U.S. LLC; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/014399s069lbl.pdf
- Khedezla (desvenlafaxine). Prescribing information. Osmotical Pharmaceutical US LLC; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204683s006lbl.pdf
- Nefazodone hydrochloride. Prescribing information. Bryant Ranch Prepack; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0bd4c34a-4f43-4c84-8b98-1d074cba97d5/0bd4c34a-4f43-4c84-8b98-1d074cba97d5.xml
- Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol. 2018;29(1):101-111. doi:10.1093/annonc/mdx526
- Lee E, Park Y, Li D, Rodriguez-Fuguet A, Wang X, Zhang WC. Antidepressant use and lung cancer risk and survival: a meta-analysis of observational studies. Cancer Res Commun. 2023;3(6):1013-1025. doi:10.1158/2767-9764.CRC-23-0003
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848 -856. doi:10.1001/archgenpsychiatry.2009.81
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311. doi:10.1370/afm.1371
- Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4(4):340-351. doi:10.1111/j.1526-4637.2003.03038.x
- Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344-350. doi:10.1016/s1526-5900(03)00638-2
- Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8. doi:10.1097/AJP.0b013e3181b99f35
- Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339. doi:10.1016/j.pain.2010.05.020
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
- Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300- 307. doi:10.1177/026988119901300321
- Lakeview Health Staff. America’s most abused antidepressants. Lakeview Health. January 24, 2004. Accessed April 4, 2025. https://www.lakeviewhealth.com/blog/us-most-abused-antidepressants/
- Greenhouse Treatment Center Editorial Staff. Addiction to antidepressants: is it possible? America Addiction Centers: Greenhouse Treatment Center. Updated April 23, 2024. Accessed April 4, 2025. https://greenhousetreatment.com/prescription-medication/antidepressants/
- National Cancer Institute. Depression (PDQ)-Health Professional Version. Updated July 25, 2024. Accessed April 4, 2025. https://www.cancer.gov/about-cancer/coping/feelings/depression-hp-pdq
- Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130. doi:10.1002/pon.3409
- Xiang X, An R, Gehlert S. Trends in antidepressant use among U.S. cancer survivors, 1999-2012. Psychiatr Serv. 2015;66(6):564. doi:10.1176/appi.ps.201500007
- Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. doi:10.1016/j.ejca.2016.11.017
- Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900. doi:10.1093/annonc/mds575
- Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi:10.1136/bmj.k1415
- Kisely S, Alotiby MKN, Protani MM, Soole R, Arnautovska U, Siskind D. Breast cancer treatment disparities in patients with severe mental illness: a systematic review and meta-analysis. Psychooncology. 2023;32(5):651-662. doi:10.1002/pon.6120
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71. doi:10.1093/jncimonographs/lgh014
- Rodin G, Katz M, Lloyd N, Green E, Mackay JA, Wong RK. Treatment of depression in cancer patients. Curr Oncol. 2007;14(5):180-188. doi:10.3747/co.2007.146
- Wilson KG, Chochinov HM, Skirko MG, et al. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33(2):118-129. doi:10.1016/j.jpainsymman.2006.07.016
- Moradi Y, Dowran B, Sepandi M. The global prevalence of depression, suicide ideation, and attempts in the military forces: a systematic review and meta-analysis of cross sectional studies. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
- Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi:10.1136/bmj.g3596
- Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
- Sheffler ZM, Patel P, Abdijadid S. Antidepressants. In: StatPearls. StatPearls Publishing. Updated May 26, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK538182/
- Miller JJ. Antidepressants, Part 1: 100 Years and Counting. Accessed April 4, 2025. Psychiatric Times. https:// www.psychiatrictimes.com/view/antidepressants-part-1-100-years-and-counting
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21. doi:10.1037/a0038550
- Mitchell PB, Mitchell MS. The management of depression. Part 2. The place of the new antidepressants. Aust Fam Physician. 1994;23(9):1771-1781.
- Sabri MA, Saber-Ayad MM. MAO Inhibitors. In: Stat- Pearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557395/
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539848/
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248. doi:10.1097/00131746-200407000-00005
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
- Moraczewski J, Awosika AO, Aedma KK. Tricyclic Antidepressants. In: StatPearls. StatPearls Publishing. Updated August 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557791/
- Almasi A, Patel P, Meza CE. Doxepin. In: StatPearls. StatPearls Publishing. Updated February 14, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK542306/
- Thour A, Marwaha R. Amitriptyline. In: StatPearls. Stat- Pearls Publishing. Updated July 18, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK537225/
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
- Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680- 690. doi:10.1016/S0140-6736(22)01472-6
- Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network metaanalysis. JAMA Netw Open. 2022;5(5):e2212939. doi:10.1001/jamanetworkopen.2022.12939
- Merwar G, Gibbons JR, Hosseini SA, Saadabadi A. Nortriptyline. In: StatPearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482214/
- Fayez R, Gupta V. Imipramine. In: StatPearls. StatPearls Publishing. Updated May 22, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557656/
- Jilani TN, Gibbons JR, Faizy RM, Saadabadi A. Mirtazapine. In: StatPearls. StatPearls Publishing. Updated August 28, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519059/
- Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17 Suppl 1:S37-S41. doi:10.1002/hup.388
- Gandotra K, Chen P, Jaskiw GE, Konicki PE, Strohl KP. Effective treatment of insomnia with mirtazapine attenuates concomitant suicidal ideation. J Clin Sleep Med. 2018;14(5):901-902. doi:10.5664/jcsm.7142
- Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264. doi:10.1111/j.1527-3458.2001.tb00198.x
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101-112. doi:10.4068/cmj.2018.54.2.101
- Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls. StatPearls Publishing. Updated April 9, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470212/
- Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895- 905. doi:10.1111/obr.12835
- Hankosky ER, Bush HM, Dwoskin LP, et al. Retrospective analysis of health claims to evaluate pharmacotherapies with potential for repurposing: association of bupropion and stimulant use disorder remission. AMIA Annu Symp Proc. 2018;2018:1292-1299.
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Hajek P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2019; 2(2):CD003999. doi:10.1002/14651858.CD003999.pub5
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6(2):59-74. doi:10.1016/j.esxm.2018.01.004
- Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2017;10(10):CD009504. doi:10.1002/14651858.CD009504.pub2
- Ng QX. A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(2):112-116. doi:10.1089/cap.2016.0124
- Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113. doi:10.4088/pcc.v07n0305
- Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls. StatPearls Publishing. Updated May 1, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK554406/
- Singh HK, Saadabadi A. Sertraline. In: StatPearls. Stat- Pearls Publishing. Updated February 13, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK547689/
- MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7(1):1-24. doi:10.1111/j.1527-3458.2001.tb00188.x
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi:10.1016/S0140-6736(09)60046-5
- Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. Am J Psychiatry. 2006;163(11):1864-1866. doi:10.1176/ajp.2006.163.11.1864
- Sharbaf Shoar N, Fariba KA, Padhy RK. Citalopram. In: StatPearls. StatPearls Publishing. Updated November 7, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482222/
- Landy K, Rosani A, Estevez R. Escitalopram. In: Stat- Pearls. StatPearls Publishing. Updated November 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557734/
- Cavanah LR, Ray P, Goldhirsh JL, Huey LY, Piper BJ. Rise of escitalopram and the fall of citalopram. medRxiv. Preprint published online May 8, 2023. doi:10.1101/2023.05.07.23289632
- Sohel AJ, Shutter MC, Patel P, Molla M. Fluoxetine. In: StatPearls. StatPearls Publishing. Updated July 4, 2022. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4(9):764-774. doi:10.1038/nrd1821
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. In: Stat- Pearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526022/
- Naseeruddin R, Rosani A, Marwaha R. Desvenlafaxine. In: StatPearls. StatPearls Publishing. Updated July 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534829
- Lieberman DZ, Massey SH. Desvenlafaxine in major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2010;4:67-82. doi:10.2147/ce.s5998
- Withdrawal assessment report for Ellefore (desvenlafaxine). European Medicine Agency. 2009. Accessed April 4, 2025. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-ellefore_en.pdf
- Dhaliwal JS, Spurling BC, Molla M. Duloxetine. In: Stat- Pearls. Statpearls Publishing. Updated May 29, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967. doi:10.1200/JCO.2013.54.0914
- Sommer C, Häuser W, Alten R, et al. Medikamentöse Therapie des Fibromyalgiesyndroms. Systematische Übersicht und Metaanalyse [Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline]. Schmerz. 2012;26(3):297-310. doi:10.1007/s00482-012-1172-2
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi:10.1212/WNL.0b013e3182166ebe
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. doi:10.1111/j.1468-1331.2010.02999.x
- Singh D, Saadabadi A. Venlafaxine. In: StatPearls. StatePearls Publishing. Updated February 26, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Sertraline and venlafaxine: new indication. Prevention of recurrent depression: no advance. Prescrire Int. 2005;14(75):19-20.
- Bruno A, Morabito P, S p i n a E , M u s c a t ello MRl. The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuro pharmacol. 2016;14(2):191-199. doi:10.2174/1570159x14666151117122458
- Shin JJ, Saadabadi A. Trazodone. In: StatPearls. StatPearls Publishing. Updated February 29, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470560/
- Khouzam HR. A review of trazodone use in psychiatric and medical conditions. Postgrad Med. 2017;129(1):140-148. doi:10.1080/00325481.2017.1249265
- Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758-764. doi:10.1513/AnnalsATS.201408-399OC
- Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811-819. doi:10.5665/sleep.3596
- Schatzberg AF, Nemeroff CB, eds. The American Psychiat ric Association Publishing Textbook of Psychopharmacology. 4th ed. American Psychiatric Publishing; 2009.
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and allcause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209-220. doi:10.1111/bcp.12617
- Nefazodone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. Updated March 6, 2020. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK548179/
- Drugs of Current Interest: Nefazodone. WHO Pharmaceuticals Newsletter:(1). 2003(1):7. https://web.archive.org/web/20150403165029/http:/apps.who.int/medicinedocs/en/d/Js4944e/3.html
- Choi S. Nefazodone (serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169(11):1187.
- Teva Nefazodone Statement. News release. Teva USA. December 20, 2021. Accessed April 4, 2025. https:// www.tevausa.com/news-and-media/press-releases/teva-nefazodone-statement/
- Levitan MN, Papelbaum M, Nardi AE. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2015;11:1149- 1155. doi:10.2147/NDT.S67470
- Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi:10.4088/JCP.19m13191
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4): 21m14345. doi:10.4088/JCP.21m14345
- Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the Military Health System. Fed Pract. 2023;40(Suppl 3):S24-S34. doi:10.12788/fp.0401
- Luong TT, Shou KJ, Reinhard BJ, Kigelman OF, Greenfield KM. Paclitaxel drug-drug interactions in the Military Health System. Fed Pract. 2024;41(Suppl 3):S70-S82. doi:10.12788/fp.0499
- Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Preclinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
- Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217- 224. doi:10.1016/j.crtox.2021.05.006,
- Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed April 4, 2025. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
- World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed, 1st revision. World Health Organization; 2013. Accessed April 4, 2025. https://apps.who.int/iris/handle/10665/96612
- Simon S. FDA approves Jelmyto (mitomycin gel) for urothelial cancer. American Cancer Society. April 20, 2020. Accessed April 4, 2025. https://www.cancer.org/cancer/latest-news/fda-approves-jelmyto-mitomycin-gel-for-urothelial-cancer.html
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)- diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi:10.3332/ecancer.2016.610
- Choi S, Kim S, Park J, Lee SE, Kim C, Kang D. Diclofenac: a nonsteroidal anti-inflammatory drug inducing cancer cell death by inhibiting microtubule polymerization and autophagy flux. Antioxidants (Basel). 2022;11(5):1009. doi:10.3390/antiox11051009
- Tontonoz M. The ABCs of BCG: oldest approved immunotherapy gets new explanation. Memorial Sloan Kettering Cancer Center. July 17, 2020. Accessed April 4, 2025. https://www.mskcc.org/news/oldest-approved-immunotherapy-gets-new-explanation
- Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21(3):R235-R246. doi:10.1530/ERC-14-0092
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270-275. doi:10.1038/bjc.1971.33
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205-213. doi:10.1038/nrd1031
- Maximov PY, McDaniel RE, Jordan VC. Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Basel; 2013. Accessed April 4, 2025. https://link.springer.com/book/10.1007/978-3-0348-0664-0
- Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb Company; 2011. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
- Tarceva (erlotinib). Prescribing information. OSI Pharmaceuticals, LLC; 2016. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
- Docetaxel. Prescribing information. Sichuan Hyiyu Pharmaceutical Co.; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215813s000lbl.pdf
- Alimta (pemetrexed). Prescribing information. Teva Pharmaceuticals; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208419s004lbl.pdf
- Tagrisso (osimertinib). Prescribing information. Astra- Zeneca Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
- Iressa (gefitinib). Prescribing information. AstraZeneca Pharmaceuticals; 2018. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
- Kadcyla (ado-trastuzumab emtansine). Prescribing information. Genentech, Inc.; 2013. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf
- Alecensa (alectinib). Prescribing information. Genetech, Inc.; 2017. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf
- Xalkori (crizotinib). Prescribing information. Pfizer Laboratories; 2022. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2022/202570s033lbl.pdf
- Lorbrena (lorlatinib). Prescribing information. Pfizer Laboratories; 2018. Accessed April 14, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Alunbrig (brigatinib). Prescribing information. Takeda Pharmaceutical Company; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf
- Rozlytrek (entrectinib). Prescribing information. Genentech, Inc.; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf
- Herceptin (trastuzumab). Prescribing information. Genentech, Inc.; 2010. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf
- Cybalta (duloxetine). Prescribing information. Eli Lilly and Company; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021427s049lbl.pdf
- Effexor XR (venlafaxine). Prescribing information. Pfizer Wyeth Pharmaceuticals Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020699s112lbl.pdf
- Desyrel (trazodone hydrochloride). Prescribing information. Pragma Pharmaceuticals; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
- Sertraline hydrochloride. Prescribing information. Almatica Pharma LLC; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215133s000lbl.pdf
- Remeron (mirtazapine). Prescribing information. Merck & Co. Inc; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
- Celexa (citalopram). Prescribing information. Allergan USA Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- information. GlaxoSmithKline; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020358s066lbl.pdf
- Amitriptyline hydrochloride tablet. Prescribing information. Quality Care Products LLC; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0f12f50f-7087-46e7-a2e6-356b4c566c9f/0f12f50f-7087-46e7-a2e6-356b4c566c9f.xml
- Lexapro (escitalopram). Prescribing information. AbbVie Inc; 2023. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021323s055,021365s039lbl.pdf
- Fluoxetine. Prescribing information. Edgemont Pharmaceutical, LLC; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202133s004s005lbl.pdf
- Paxil (paroxetine). Prescribing Information. Apotex Inc; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
- Pamelor (nortriptyline HCl). Prescribing information. Mallinckrodt, Inc; 2012. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
- Silenor (doxepin). Prescribing information. Currax Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022036s006lbl.pdf
- Tofranil-PM (imipramine pamote). Prescribing information. Mallinckrodt, Inc; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/017090s078lbl.pdf
- Norpramin (desipramine hydrochloride). Prescribing information. Sanofi-aventis U.S. LLC; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/014399s069lbl.pdf
- Khedezla (desvenlafaxine). Prescribing information. Osmotical Pharmaceutical US LLC; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204683s006lbl.pdf
- Nefazodone hydrochloride. Prescribing information. Bryant Ranch Prepack; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0bd4c34a-4f43-4c84-8b98-1d074cba97d5/0bd4c34a-4f43-4c84-8b98-1d074cba97d5.xml
- Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol. 2018;29(1):101-111. doi:10.1093/annonc/mdx526
- Lee E, Park Y, Li D, Rodriguez-Fuguet A, Wang X, Zhang WC. Antidepressant use and lung cancer risk and survival: a meta-analysis of observational studies. Cancer Res Commun. 2023;3(6):1013-1025. doi:10.1158/2767-9764.CRC-23-0003
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848 -856. doi:10.1001/archgenpsychiatry.2009.81
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311. doi:10.1370/afm.1371
- Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4(4):340-351. doi:10.1111/j.1526-4637.2003.03038.x
- Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344-350. doi:10.1016/s1526-5900(03)00638-2
- Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8. doi:10.1097/AJP.0b013e3181b99f35
- Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339. doi:10.1016/j.pain.2010.05.020
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
- Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300- 307. doi:10.1177/026988119901300321
- Lakeview Health Staff. America’s most abused antidepressants. Lakeview Health. January 24, 2004. Accessed April 4, 2025. https://www.lakeviewhealth.com/blog/us-most-abused-antidepressants/
- Greenhouse Treatment Center Editorial Staff. Addiction to antidepressants: is it possible? America Addiction Centers: Greenhouse Treatment Center. Updated April 23, 2024. Accessed April 4, 2025. https://greenhousetreatment.com/prescription-medication/antidepressants/
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).
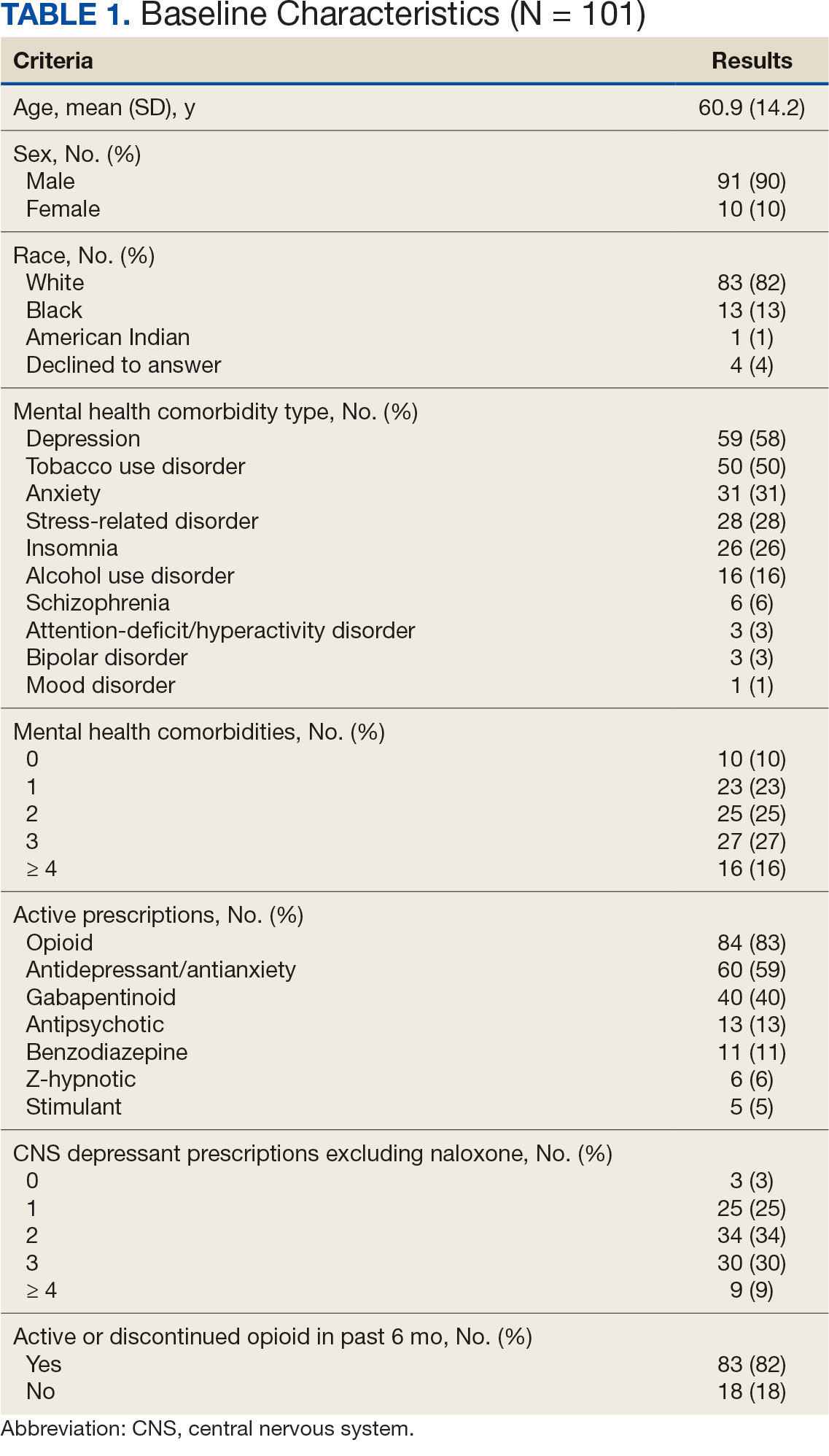
The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).
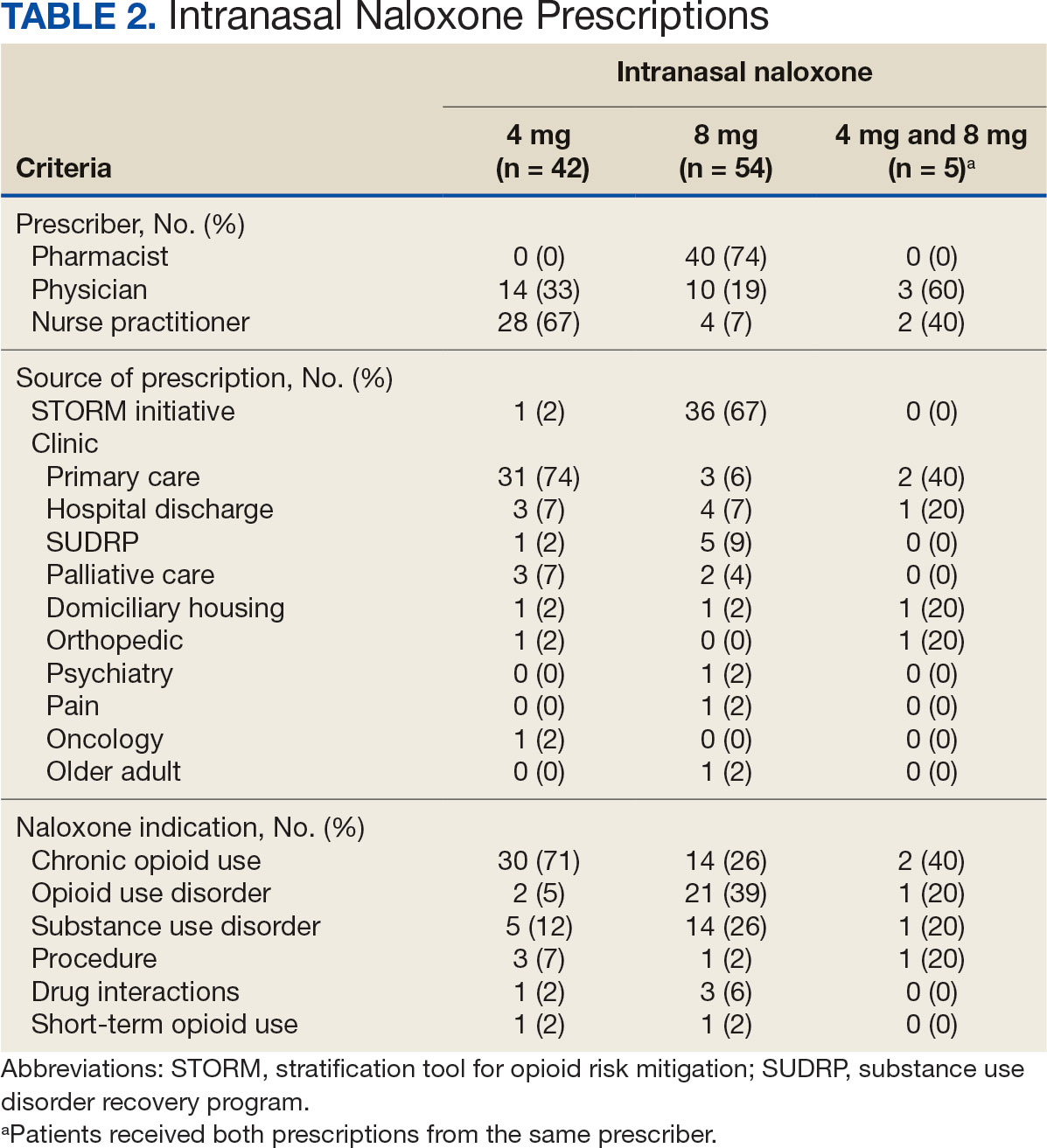
Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).


The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).

Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).


The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).

Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.
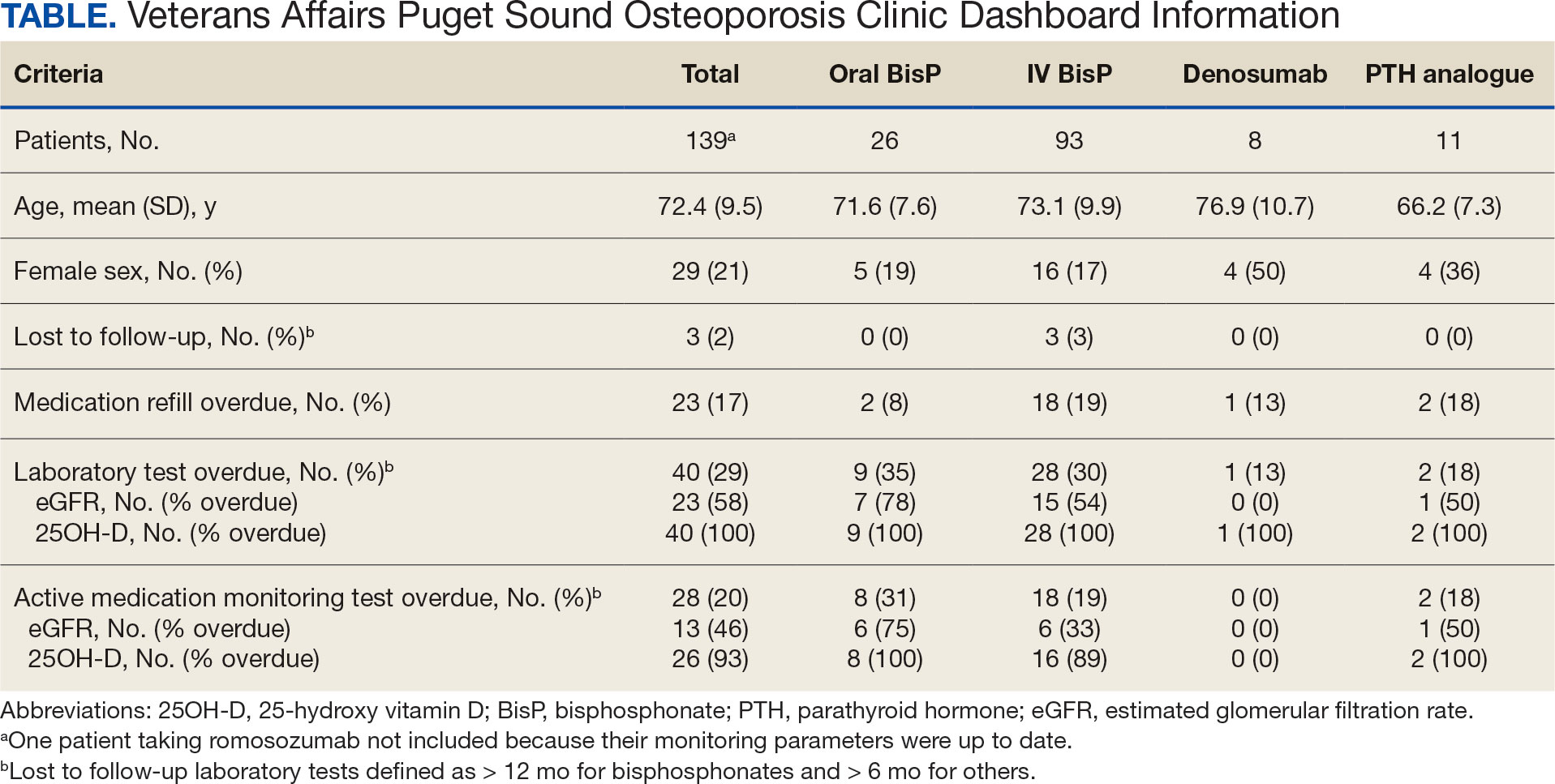
The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Meet the JCOM Author with Dr. Barkoudah: EHR Interventions to Improve Glucagon Prescription Rates for Individuals With T1DM
Glucagon Prescription Rates for Individuals With Type 1 Diabetes Mellitus Following Implementation of an Electronic Health Records Intervention
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.
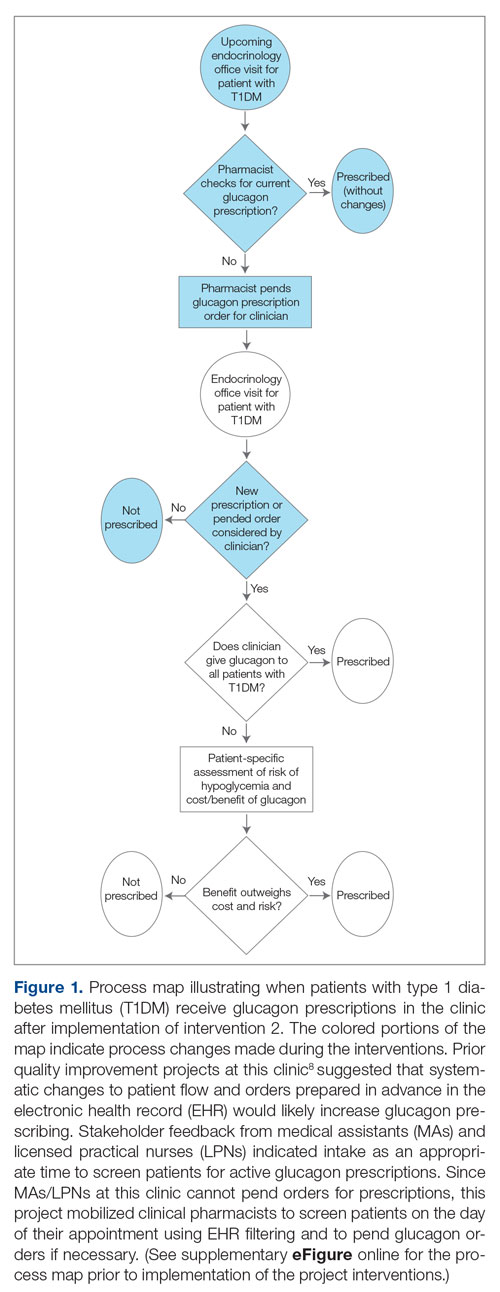
In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).
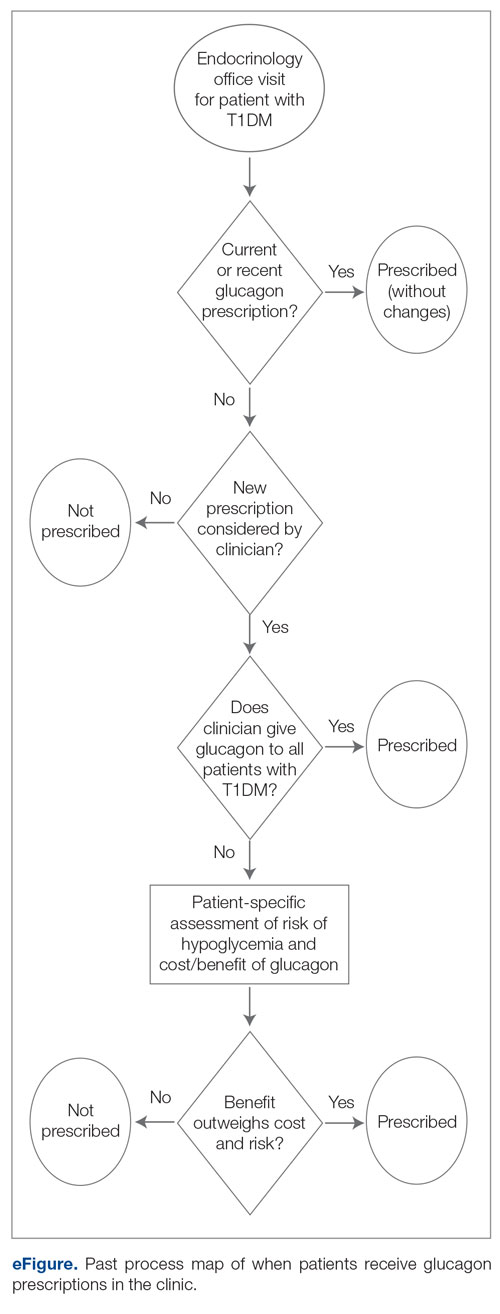
This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).
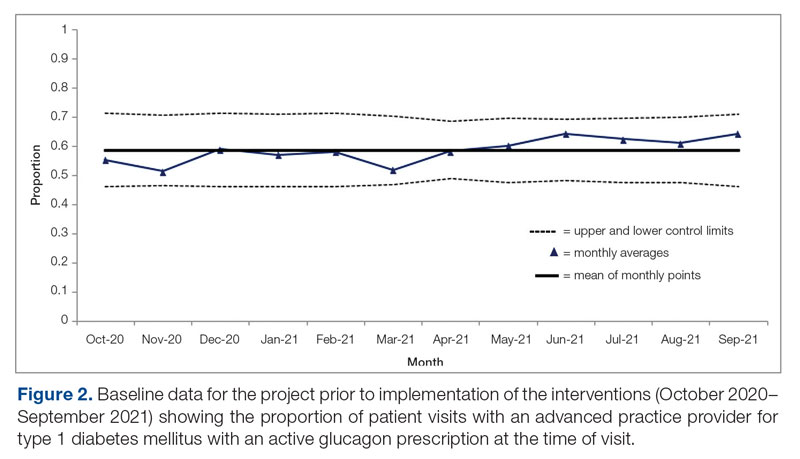
Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.
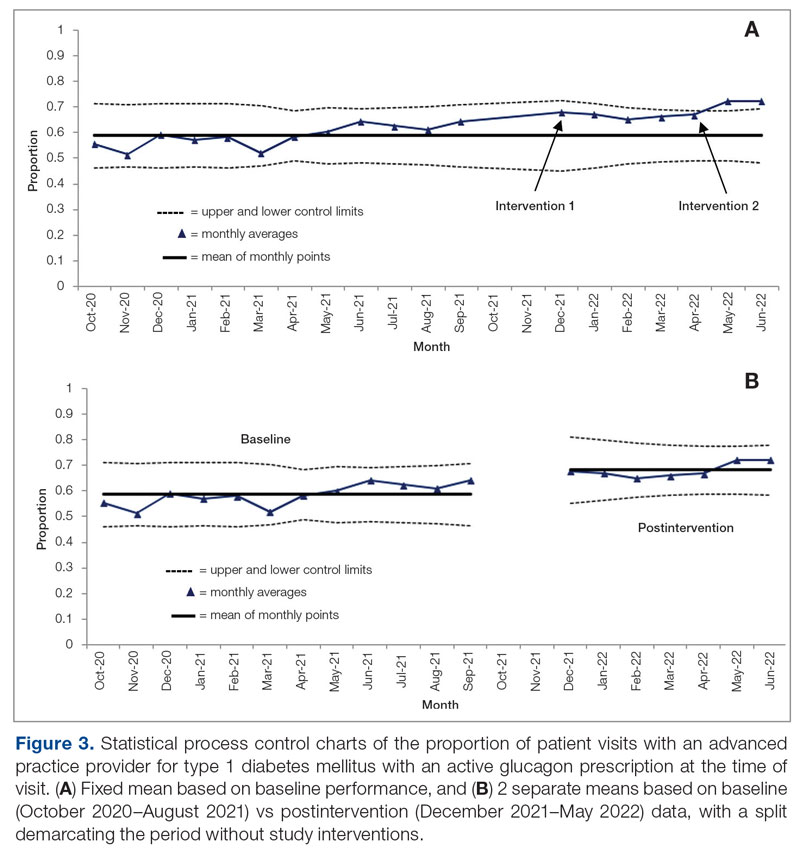
Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
Patient Safety in Transitions of Care: Addressing Discharge Communication Gaps and the Potential of the Teach-Back Method
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
Meet the JCOM Author with Dr. Barkoudah: A Multidisciplinary Team–Based Clinical Care Pathway for Infective Endocarditis
Implementation of a Multidisciplinary Team–Based Clinical Care Pathway Is Associated With Increased Surgery Rates for Infective Endocarditis
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338